Abstract
Purpose
To compare prediction of disease outcome, severity, and patient triage in coronavirus disease 2019 (COVID-19) pneumonia with whole lung radiomics, radiologists’ interpretation, and clinical variables.
Materials and Methods
This institutional review board-approved retrospective study included 315 adult patients (mean age, 56 years [range, 21–100 years], 190 men, 125 women) with COVID-19 pneumonia who underwent noncontrast chest CT. All patients (inpatients, n = 210; outpatients, n = 105) were followed-up for at least 2 weeks to record disease outcome. Clinical variables, such as presenting symptoms, laboratory data, peripheral oxygen saturation, and comorbid diseases, were recorded. Two radiologists assessed each CT in consensus and graded the extent of pulmonary involvement (by percentage of involved lobe) and type of opacities within each lobe. Radiomics were obtained for the entire lung, and multiple logistic regression analyses with areas under the curve (AUCs) as outputs were performed.
Results
Most patients (276/315, 88%) recovered from COVID-19 pneumonia; 36/315 patients (11%) died, and 3/315 patients (1%) remained admitted in the hospital. Radiomics differentiated chest CT in outpatient versus inpatient with an AUC of 0.84 (P < .005), while radiologists’ interpretations of disease extent and opacity type had an AUC of 0.69 (P < .0001). Whole lung radiomics were superior to the radiologists’ interpretation for predicting patient outcome in terms of intensive care unit (ICU) admission (AUC: 0.75 vs 0.68) and death (AUC: 0.81 vs 0.68) (P < .002). The addition of clinical variables to radiomics improved the AUC to 0.84 for predicting ICU admission.
Conclusion
Radiomics from noncontrast chest CT were superior to radiologists’ assessment of extent and type of pulmonary opacities in predicting COVID-19 pneumonia outcome, disease severity, and patient triage.
© RSNA, 2020
Summary
CT radiomics are superior to radiologists’ visual assessment and the combination of patient demographics, symptoms, and laboratory data for predicting severity of lung involvement, disease outcome, and patient triage in COVID-19 pneumonia.
Key Points
■ Radiomics from noncontrast chest CT scan accurately measure the extent of pulmonary involvement, assign to intensive care unit, inpatient, or outpatient care, and predict disease outcome in COVID-19 pneumonia.
■ Whole lung radiomics were superior to subjective assessment of radiologists in predicting patient outcome and disease severity in COVID-19 pneumonia.
■ Adding clinical variables to radiomics resulted in modest improvement in patient outcome prediction but did not improve prediction of disease severity.
Introduction
Severe acute respiratory syndrome coronavirus 2, the seventh known coronavirus in humans, is an enveloped, single-strand RNA virus responsible for the global coronavirus disease 2019 (COVID-19) pandemic since the beginning of 2020 (1). With millions of confirmed cases in 187 countries within 5 months, the pandemic has taken a huge toll on health and economic status, particularly in the most vulnerable, developing countries, such as Iran.
Although any individual can get COVID-19 infection regardless of age and sex, elderly persons and those with underlying comorbidities such as obesity, hypertension, and diabetes mellitus are at higher risk of severe COVID-19 pneumonia, complications, and multiorgan failure (2). Reverse-transcriptase polymerase chain reaction (RT-PCR) assay from nasopharyngeal swab or bronchoalveolar lavage is the preferred test for diagnosis of COVID-19 infection (3,4). In the first 10 days of infection, the RT-PCR assay for COVID-19 infection has lower sensitivity (60%–70%) than in the later stage of infection (5–7). Although chest CT images can display findings of early COVID-19 pneumonia, about 20% of chest CT scans do not show any findings (8). Thus, many organizations recommend limiting chest CT to patients with moderate to severe COVID-19 pneumonia or in those with unexplained deterioration of respiratory status (9–12).
In resource-starved sites with limited access to RT-PCR assays and in high-risk patients with a negative initial RT-PCR result, chest CT is frequently used for diagnosis and severity assessment of COVID-19 pneumonia (13,14). Subjective grading for assessing disease severity from lobar extent and type of pulmonary opacities is time-consuming, and therefore has limited clinical applications (15–20). Some studies have explored radiomics for screening and diagnosis of patients with COVID-19 (21,22), but to the best of our knowledge, no peer-reviewed studies have compared radiomics, radiologists’ interpretation, and clinical variables for predicting disease outcome, severity, and patient triage in COVID-19 pneumonia. We compared prediction of disease outcome, severity, and patient triage in COVID-19 pneumonia with whole lung radiomics, radiologists’ interpretation, and clinical variables.
Materials and Methods
Approvals and Disclosures
The institutional review board approved our retrospective Health Insurance Portability and Accountability Act–compliant human subject study with waiver of informed consent from the study subjects. We have no financial disclosures pertaining to this article. Our institution received research grants from Siemens Healthineers, Lunit, and Riverain Technologies for unrelated projects.
Patients
We identified 350 consecutive adult patients who presented with symptoms compatible with COVID-19 pneumonia and underwent noncontrast chest CT in a teaching hospital in Tehran, Iran, between February 20, 2020 and April 10, 2020.
Per the hospital policies for management of COVID-19 pneumonia, all patients with dyspnea, hypoxemia (peripheral capillary oxygen saturation [Spo2] < 93%), rapid respiratory rate (> 30 breaths per minute), fever (temperature ≥ 37.8°C) with risk factors (cardiovascular disease, hypertension, diabetes mellitus, underlying pulmonary diseases, and body mass index > 40 kg/m2), or immunodeficiencies (corticosteroid therapy, chemotherapy, malignancies, organ transplants, and human immunodeficiency virus infection) underwent noncontrast low-dose chest CT. Patients with at least one of the following criteria were admitted to the hospital: dyspnea (Spo2 < 93% or respiratory rate > 30 breaths per minute), positive CT findings in patients with the previously mentioned risk factors or immunodeficiencies, fever (temperature ≥ 37.8°C), and discretion of attending physician for patients with positive radiologic findings for pneumonia. Patients with resistant hypoxemia, altered mental status, hemodynamic instability, or hypercapnia (or respiratory failure) were transferred to the intensive care unit (ICU).
Two study coauthors (R.B., H.K.M.) reviewed medical records and digital imaging archive (in the hospital picture archiving and communication system) to identify 350 adult patients which included 230 patients admitted to the hospital and 120 patients managed in outpatient settings. All patients had clinical findings and chest CT findings compatible with COVID-19 pneumonia.
Inpatients.—The recorded clinical variables for inpatients included age, sex, presenting symptoms (such as fever, chills, fatigue, myalgia, cough, sputum production, sore throat, hemoptysis, chest pain, shortness of breath, headache, anorexia, nausea and vomiting, diarrhea, and loss of consciousness), symptomatic days before hospital admission, temperature and Spo2 on hospital admission, and presence of any comorbidities and immunodeficiencies. Results of the following laboratory tests were recorded: total white blood count, differential white blood counts, platelets count, C-reactive protein level, erythrocyte sedimentation rate, and lactate dehydrogenase level. Information pertaining to patient outcome (discharged, deceased, or still admitted and under treatment at the time of data analysis) was recorded.
Outpatients.—Patient symptoms, comorbidities, and past medical history for outpatients were not available because of lack of electronic medical records. Results of RT-PCR assays, when performed, were recorded for all patients. All patients (outpatients and inpatients) had a 2-week televisit following their discharge or outpatient visit.
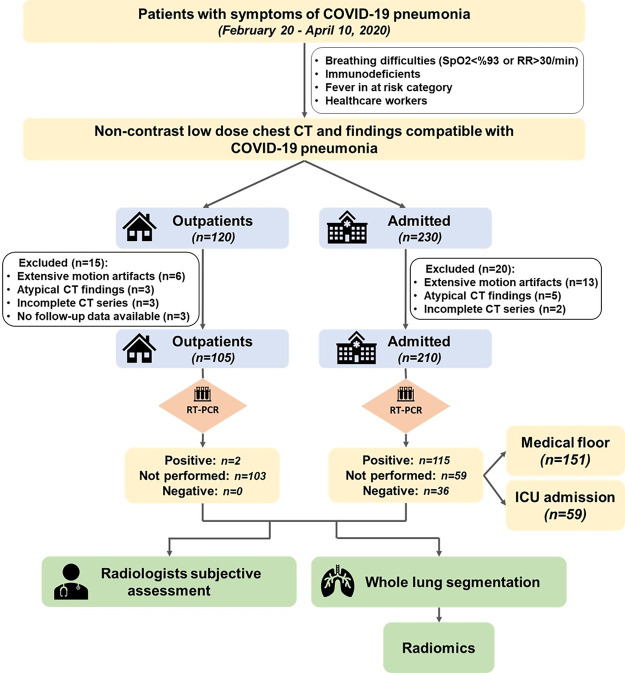
We excluded 20 inpatients and 15 outpatients because of the presence of extensive motion artifacts on the chest CT images (13 and six patients, respectively), coexisting or atypical CT findings suggestive of other abnormalities (five and three patients, respectively), incomplete CT datasets (two and three patients, respectively), and lack of follow-up (three patients in outpatient setting). Thus, the final sample size included 315 adult patients (210 inpatients and 105 outpatients; mean age, 56 years [range, 21 to 100]; 190 men, 125 women) who met our inclusion and exclusion criteria (Fig 1).
Figure 1:
Flowchart shows details of patients included in our study. ICU = intensive care unit, RR = respiratory rate, RT-PCR = reverse-transcription polymerase chain reaction.
Noncontrast Chest CT
All included patients underwent a standard-of-care, chest CT examination without oral and intravenous contrast material administration on admission. Chest CT scans were performed on a 16-slice multidetector CT (SOMATOM Emotion 16; Siemens Healthineers, Forchheim, Germany) at 110–130 kV, fixed tube current of 30–50 mA, 1-second gantry rotation time, 16 × 1.2-mm detector configuration, 19.2-mm x-ray beam collimation, and 1.5:1 pitch. Two- and 5-mm section thicknesses were reconstructed with filtered back projection reconstruction technique with B20f (standard soft-tissue kernel) and B70f reconstruction (high spatial frequency sharp) kernels. De-identified Digital Imaging and Communications in Medicine CT images were used for subjective evaluation and radiomics.
Subjective Assessment of Chest CT
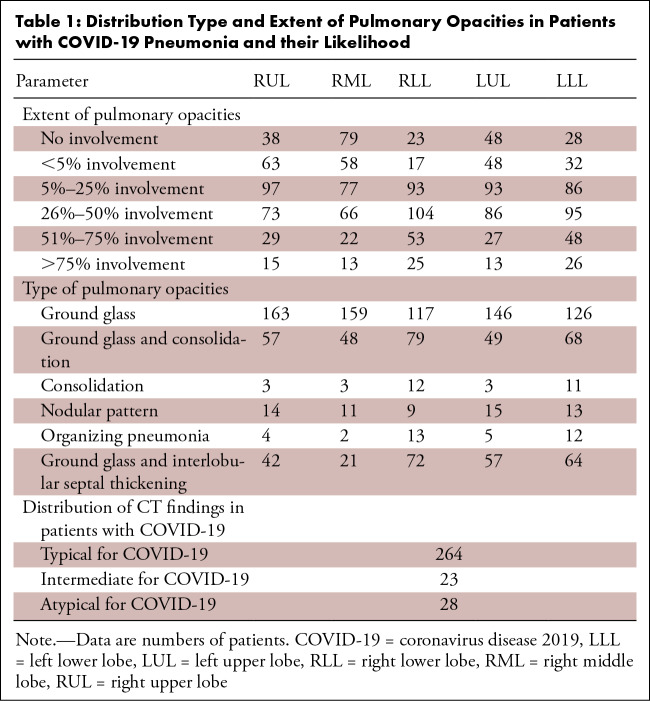
Two experienced thoracic radiologists (S.R.D., with 16 years of experience; M.K.K., with 14 years of experience) reviewed each CT examination in consensus (MicroDicom DICOM Viewer, Sofia, Bulgaria) in lung windows (at modifiable window width 1500 HU, window level −600 HU). The radiologists excluded cases with extensive motion artifacts on their chest CT images (n = 19), coexisting or atypical CT findings (n = 8), and incomplete CT image series (n = 5). In consensus, they recorded the type of pulmonary opacities (ground-glass, mixed ground-glass and consolidation, consolidation, organizing pneumonia [reverse halo sign with ground-glass opacity surrounded by consolidation], nodular, or ground-glass with septal thickening [crazy paving appearance]) and the percentage of each lobe (right upper, right middle, right lower, left upper, and left lower) affected by the opacities (score 0: 0% involvement; score 1: < 5% involvement; score 2: 5%–25% involvement; score 3: 26%–50% involvement; score 4: 51%–75% involvement; score 5: > 75% lobar involvement) (15,23). Total lung involvement (labeled as subjective severity score) was estimated by adding the scores for all lobes (minimum score 0; maximum score 25). We classified this subjective severity score or total lung involvement into two groups (extensive: total score ≥ 15; nonextensive: total score < 15). CT findings were classified into typical, intermediate, atypical, and negative based on the published guidelines for COVID-19 pneumonia (Table 1) (24).
Table 1:
Distribution Type and Extent of Pulmonary Opacities in Patients with COVID-19 Pneumonia and their Likelihood
Radiomics
Radiomics were estimated from the 2-mm image series. First, we segmented both lungs (entire lung volume) with a semiautomatic approach with Radiomics 3D Slicer Extension program (25). Regions beyond the lung were edited out to exclude mediastinal, hilar, or pleural structures and abnormalities from the lung volumes (F.H., 2-year postdoctoral research experience in radiology). Then, we derived a total of 1690 radiomics over the segmented entire lung volumes which included both the abnormal and normal lung parenchyma. These radiomics included first order (n = 18), shape (n = 17), gray-level co-occurrence matrix (GLCM) (n = 24), gray-level run-length matrix (GLRLM) (n = 16), gray-level size-zone matrix (GLSZM) (n = 16), neighboring gray tone difference matrix (n = 5), and gray-level dependence matrix (GLDM) (n = 14) features (total n = 110) as described extensively in prior publications (26). Squares, square roots, logarithms, and exponentials of these features (n = 372) were also obtained in addition to three-dimensional (3D) wavelet transform (multidimensional decomposition of image – multidimensional signal processing) parameters (n = 744) and Log with five (0–4 mm) sigma levels (n = 465) of the previously mentioned features.
Statistical Analysis
Data were analyzed with Excel (Microsoft, Redmond, Wash) and R Statistical Computing (https://www.R-project.org, R Foundation for Statistical Computing, Vienna, Austria, Accessed 4.15.2020). We performed multiple logistic regression tests for data analyses and obtained both the area under the curve (AUC) and P values as outputs. The multiple logistic regression used a stepwise procedure and displayed AUCs and P values for each combination of input variables (no variables were excluded from the model). From these, we identified the combination of input variables with best AUCs. For prediction of type of pulmonary opacities with radiomics, we classified the opacities into two groups (group 1: ground-glass opacities; group 2: other types of opacities including consolidation, nodular, ground-glass opacities mixed with consolidation or interlobular septal thickening). P values < .05 were considered statistically significant.
Results
Of the 210 inpatients, 115 (55%) patients had positive RT-PCR assays, 59 (28%) did not have any RT-PCR, and 36 (17%) had a negative single RT-PCR assay. Just two patients in the outpatient setting had positive RT-PCR, and the rest were not tested because of a shortage of test kits and a large number of patients suspected of having COVID-19 infection.
Entire Patient Cohort
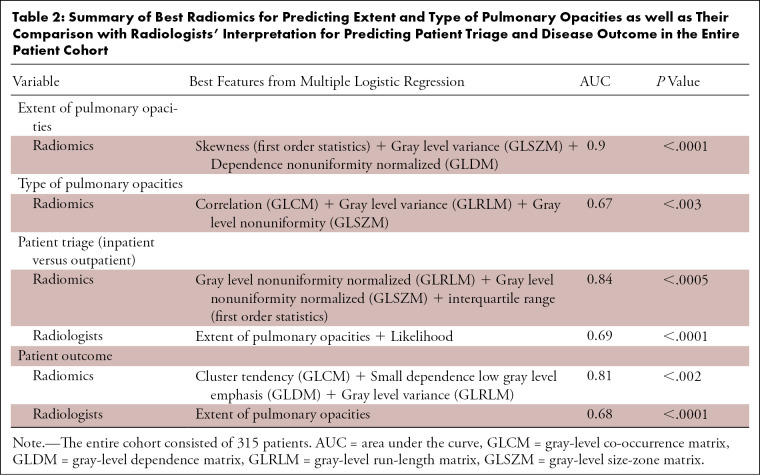
Of 315 patients included in our study, 227 patients (72%) had a subjective severity score < 15 (nonextensive pulmonary opacities), and 88 patients (28%) had extensive pulmonary opacities (subjective severity score ≥ 15) from COVID-19 pneumonia. The 3D wavelet transforms of skewness (first order statistics), 3D log sigma of gray level variance (GLSZM feature), and dependence nonuniformity normalized (GLDM feature) in combination had the highest AUC (AUC 0.9; 95% CI AUC: 0.88, 0.91, P < .0001) for differentiating extensive and nonextensive pulmonary involvement.
About a third of the patients (110/315; 35%) had pure ground-glass opacities associated with early stage of the disease; the remaining 205/315 patients (65%) had other opacities such as consolidation, nodular opacities, and ground-glass with interlobular septal thickening or consolidation. Radiomics had a lower AUC (AUC 0.67; 95% CI AUC: 0.64, 0.7, P < .003) for differentiating type of pulmonary opacities (such as differentiating ground-glass, consolidative, and mixed opacities) from COVID-19 pneumonia. Only a few patients had mild emphysema, subsegmental or relaxation atelectasis, biapical scarring, or calcified granulomata on their chest CT images. A combination of square root of correlation (GLCM feature), 3D log sigma of gray-level variance (GLRLM feature), and 3D wavelet transforms of gray-level nonuniformity (GLSZM feature) had the highest AUC.
Close to half of all patients (151/315; 48%) were admitted on the non-ICU medical floors, a third (105/315; 33%) were managed as outpatients, and 19% (59/315) of patients needed ICU admission for management. A combination of three radiomics (gray-level nonuniformity normalized-GLRLM, 3D log sigma of gray-level nonuniformity normalized-GLSZM, and interquartile range) were able to discern chest CT features of outpatients versus inpatients (ICU and non-ICU) with an AUC of 0.84 (95% CI AUC: 0.83, 0.86, P < .005). Although statistically significant, the subjective severity scores from the radiologists’ interpretation (AUC: 0.69, 95% CI: 0.69, P < .0001) did not have an AUC as high as radiomics for predicting hospitalization (AUC: 0.84, 95% CI: 0.82, 0.86, P < .005) (Fig 2). In 28 patients with atypical findings for COVID-19 pneumonia, radiomics predicted hospitalization with an AUC of 0.95 (95% CI: 0.92, 0.96), while subjective severity scores were not significantly different among inpatients and outpatients (AUC: 0.57, P > .6).
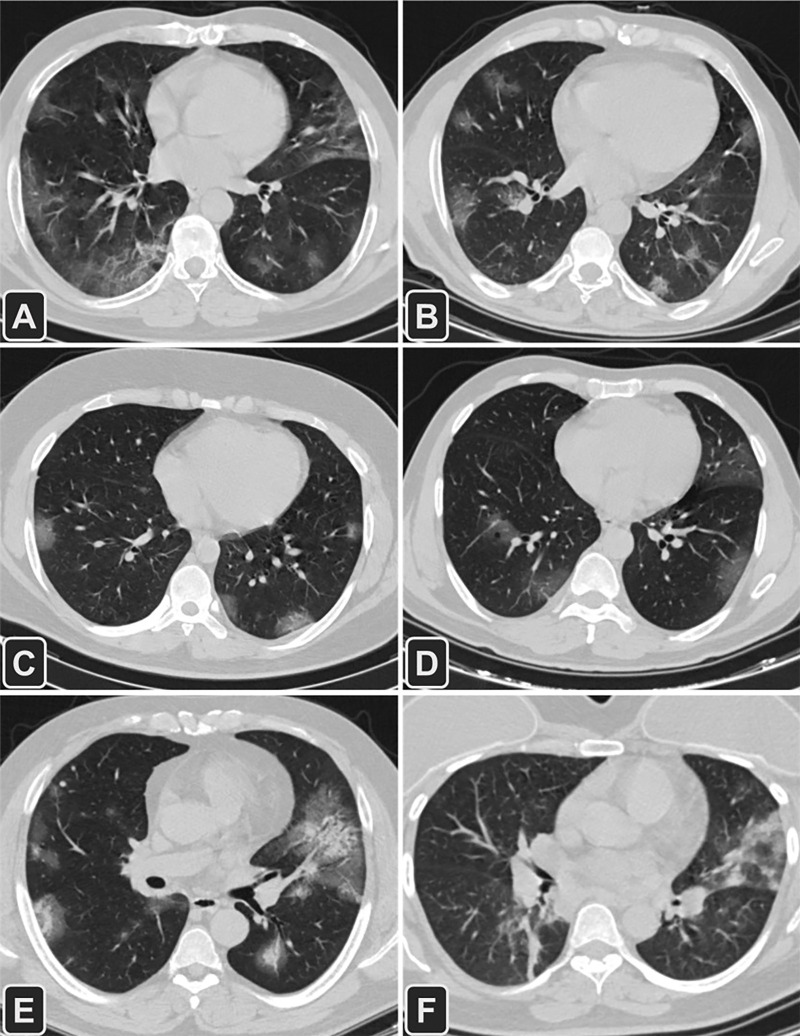
Figure 2:
Transverse CT images of six patients with different outcomes (A, B) and management (C−F) for COVID-19 pneumonia. A, A 52-year-old man with a medical history of diabetes mellitus, hypertension, and ischemic heart disease had a complete recovery and was discharged (CT severity score = 13); B, A 55-year-old man with diabetes mellitus died of complications related to COVID-19 infection (CT severity score = 12); C, A 25-year-old man who was treated in an outpatient setting (CT severity score = 11); D, A 63-year-old man with diabetes mellitus was admitted into the intensive care unit (ICU) for 9 days and then spent 5 days in a non-ICU medical unit (CT severity score = 14); E, A 49-year-old man with diabetes mellitus and hypertension was admitted to the medical floor (CT severity score = 17); F, A 54-year-old woman admitted to the ICU (CT severity score = 12).
Most patients (276/315; 88%) recovered from COVID-19 pneumonia, 36/215 patients (11%) died, and 3/315 patients (1%) remained admitted in the hospital at the time of writing of our manuscript. The combination of three radiomics (square root of cluster tendency-GLCM feature, 3D wavelet transforms of small dependence low gray-level emphasis-GLDM feature, and 3D log sigma of gray-level variance-GLRLM feature) had an AUC of 0.81 (95% CI AUC: 0.75, 0.84, P < .002) for predicting mortality associated with COVID-19 pneumonia as opposed to an AUC of 0.68 based on the radiologists’ assessment of chest CT images (Table 2). In 28 patients with atypical findings, radiomics predicted patient outcome with an AUC of 0.93 (95% CI: 0.83, 0.95), while subjective severity scores could not predict outcome (AUC: 0.59, P > .08).
Table 2:
Summary of Best Radiomics for Predicting Extent and Type of Pulmonary Opacities as well as Their Comparison with Radiologists’ Interpretation for Predicting Patient Triage and Disease Outcome in the Entire Patient Cohort
RT-PCR Positive COVID-19 Pneumonia in Inpatients
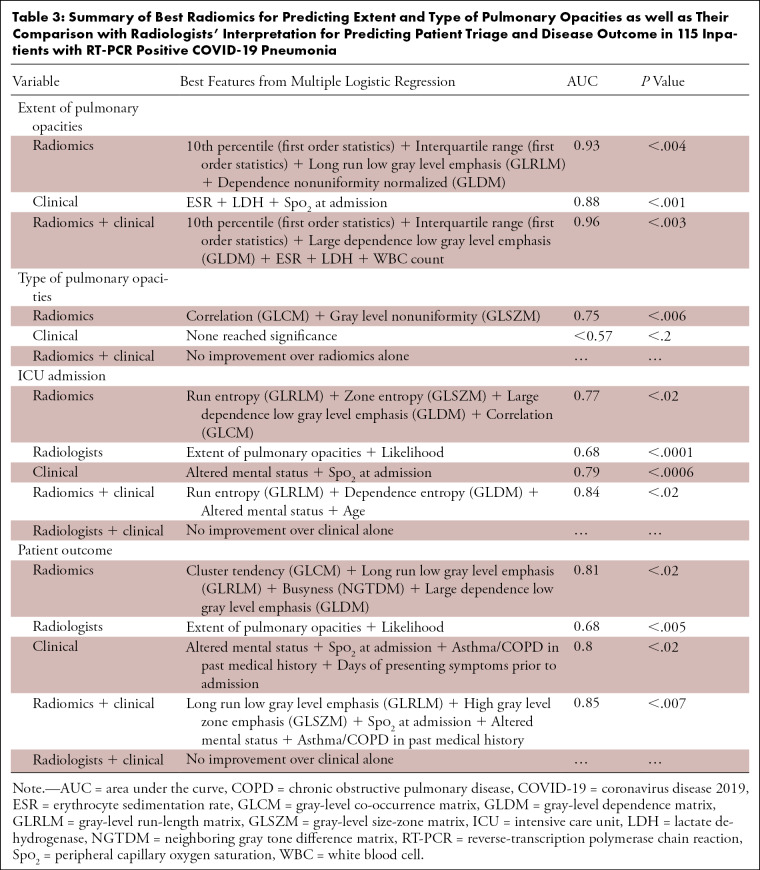
Among the 115 inpatients with positive RT-PCR assay for COVID-19 pneumonia, 40 patients (40/115; 35%) had extensive pulmonary opacities with a severity score ≥ 15, and 75 patients (75/115; 65%) had nonextensive pulmonary involvement and a severity score < 15. Radiomics differentiated these two groups with an AUC of 0.93 (95% CI AUC: 0.89, 0.94, P < .004) (Table 3). Clinical variables were inferior to radiomics for differentiating extensive and nonextensive lung involvement (AUC 0.88, 95% CI AUC: 0.8, 0.91, P < .0001). A combination of both clinical variables and radiomics resulted in improvement in differentiation of extensive and nonextensive pulmonary opacities (combined AUC 0.96, 95% CI: 0.9, 0.96, P < .003).
Table 3:
Summary of Best Radiomics for Predicting Extent and Type of Pulmonary Opacities as well as Their Comparison with Radiologists’ Interpretation for Predicting Patient Triage and Disease Outcome in 115 Inpatients with RT-PCR Positive COVID-19 Pneumonia
Most inpatients with positive RT-PCR assay for COVID-19 pneumonia also had mixed or consolidative opacities (78/115; 68%) as opposed to pure ground-glass opacities (37/115; 32%).
Radiomics distinguished different pulmonary opacities with an AUC of 0.75 (95% CI: 0.74, 0.75, P < .006). Square root of correlation (GLCM feature) plus exponential of gray-level nonuniformity (GLSZM feature) represented the best radiomics for such differentiation. The clinical variables (such as demographics, presenting symptoms, comorbidities, vital signs, and laboratory values) could not predict the type of pulmonary opacities (best AUC of up to 0.57, P > .2).
The combination of square root run entropy (GLRLM feature), 3D log sigma of zone entropy (GLSZM feature), and large dependence low gray-level emphasis (GLDM feature) (AUC 0.77, 95% CI: 0.73, 0.79, P < .02) was better than the radiologists’ assessment (AUC 0.68, 95% CI: 0.61, 0.68, P < .0001) for predicting ICU admission among inpatients. The clinical variables (such as altered mental status and low peripheral O2 saturation) had a slightly higher AUC (AUC 0.79, 95% CI: 0.62, 0.8, P < .0006) for predicting ICU admission compared with radiomics. The combination of these clinical variables and radiomics was associated with modest improvement in AUC for predicting need for ICU admission (AUC 0.84, 95% CI: 0.78, 0.85, P < .02) as well as for patient outcome (AUC 0.85, 95% CI: 0.73, 0.87, P < .007) (Table 3).
Finally, radiomics of patients with positive RT-PCR assay for COVID-19 pneumonia and those without RT-PCR confirmation were similar (AUC < 0.62, P > .06). None of the radiologists’ interpretation features or clinical variables differentiated patients with RT-PCR positive COVID-19 infection from those who did not have a RT-PCR (AUC < 0.56, P > .08).
Discussion
Our study demonstrated the value of whole lung radiomics from noncontrast chest CT in prediction of disease severity, outcome, and patient triage in COVID-19 pneumonia. Whole lung radiomics were superior to radiologists’ interpretation for patient triage (AUC 0.84 vs 0.69) and in predicting patient outcome (AUC 0.81 vs 0.68). The addition of clinical variables and laboratory data to radiomics did not improve the AUCs of radiomics alone for differentiating patients with extensive and nonextensive pulmonary involvement as well as early and advanced stages of disease (based on types of pulmonary opacities). Adding clinical variables to the whole lung radiomics improved prediction of patient outcome and those with ICU admission.
Although chest CT is used for diagnosis of COVID-19 pneumonia in sites with high disease prevalence and limited RT-PCR and immunoassays, several organizations such as the United States’ Centers for Disease Control and Prevention, Society of Thoracic Radiology, American College of Radiology, and Royal College of Radiology do not recommend routine use of imaging for diagnosis of COVID-19 pneumonia (9–12,27). Chest CT is frequently recommended for assessing moderate to severe infection, unexplained worsening of cardiorespiratory status, and disease complications (15–20).
Use of chest CT-based radiomics has been described for differentiating COVID-19 pneumonia from other pneumonias (21,22). Chen et al developed a diagnostic model based on clinical features and radiologic semantics for differentiating COVID-19 pneumonia (22). They trained their model with radiomics derived from lung opacities rather than the entire lung volume used in our study. Radiomics from pulmonary opacities can be tedious (with manual segmentation), subjective, and thus prone to interobserver variations. In comparison, semiautomatic entire lung segmentation used in our study reduced segmentation effort and interobserver variations while also obtaining radiomics information from both the normal and abnormal lung parenchyma. Likewise, the entire lung radiomics also account for other coexisting pulmonary abnormalities, such as emphysema and atelectasis, which can reduce the functional lung volume. A recent study from Colombi et al reported that quantification of well-aerated pulmonary parenchyma on hospital admission chest CT could predict ICU admission and death related to COVID-19 pneumonia (28).
The ability of whole lung radiomics to differentiate the type of pulmonary opacities can be explained on the basis of differences in CT numbers based on the type of pulmonary opacities (for example, ground-glass vs consolidation vs crazy-paving pattern). Likewise, success of radiomics for differentiating disease severity is likely related to changes in distribution of CT voxel values in patients with less or more extensive pulmonary opacities. Several prior studies have reported that radiomics help predict treatment response and prognosis in several malignancies, including lung cancer (29–32). In fact, radiomics signatures of more heterogeneous malignant lesions are associated with poor outcome. Although its application in an infectious disease like viral pneumonia such as in our study has not been assessed, the ability of radiomics to assess and quantify attenuation changes related to COVID-19 pneumonia like in patients with cancer explains why radiomics were useful for predicting disease outcome in our patient population.
Although prior studies have reported on the ability of visual severity score of COVID-19 pneumonia on chest CT (16,18,20), we found that such qualitative assessment was not as useful as radiomics in predicting ICU admission or patient outcome (recovery vs death). This may be related to difficulty in differentiating various opacity types and assigning scores based on percentage of individual lung lobe involved by pulmonary opacities. Although not formally quantified in prior studies (16,18,20) or in our study, it is challenging to assign reliable and reproducible scores based on a difference of 1% in terms of lung lobe involvement. For example, less than 4% lung lobe involvement gets a score of 1 and 5% is categorized with a score of 2. Such scoring is also not part of the clinical interpretation of chest CT. The inconsistencies in scoring lung involvement from visual inspection may explain why radiologists significantly underperformed compared with whole lung radiomics.
The main implication of our study was the demonstration of use of open access whole lung segmentation and radiomics tool in predicting patients requiring ICU admission and differentiating those with favorable and unfavorable outcome of COVID-19 pneumonia. Along with the clinical variables, whole lung radiomics can be a powerful tool for assessing diffuse pulmonary parenchymal diseases such as the viral pneumonia assessed in our study. Such prediction can help in allocation and planning of resources in high prevalence diseases such as the current COVID-19 pandemic. We believe that our study provides evidence for integration of information from whole lung radiomics into radiology reports. The automatic whole lung segmentation ability, available in both open access and commercial image processing platforms, can avoid or minimize any effort from radiologists in obtaining radiomics information. However, institutions would require datasets, such as in our study, to establish a training model so that individual prospective cases could be tested against such local models to account for local variations in CT scanners, scan parameters, and patient characteristics. For such integration to happen, both the radiologists and referring physicians need to understand the principles, strengths, and limitations of radiomics so that meaningful inferences can be drawn from a large amount of quantitative information generated from radiomics.
One of the limitations of our retrospective study was the lack of RT-PCR confirmation availability for all included cases because of the shortage of test kits in an extremely resource-constrained country with a high prevalence of COVID-19 pneumonia. However, neither radiomics nor subjective radiologist evaluation could differentiate between patients deemed to have COVID-19 pneumonia with or without RT-PCR. A recent study with 1014 patients reported positive rates of 88% for chest CT and 59% for RT-PCR assay for the diagnosis of suspected COVID-19 and a sensitivity of 97% for CT with RT-PCR as a reference standard (13). Another limitation of our study pertained to the fact that some patients may have been admitted to the hospital based on severity of symptoms, other comorbidities (such as immunodeficiencies), or positive CT findings rather than extensive lung changes related to COVID-19 pneumonia. In such cases, neither radiomics nor subjective severity scores can reliably predict hospitalization. We also included patients with negative RT-PCR but CT findings typical of COVID-19 pneumonia in our study. He et al has reported on the role of chest CT for clinically suspected COVID-19 pneumonia in patients with a negative RT-PCR (33). Prior studies have also included negative RT-PCR cases in part of their entire datasets (14). Another limitation of our study was the lack of a complete list of clinical variables for outpatients because of a lack of electronic medical records in the teaching hospital in Tehran, Iran. Our results may not be generalizable because of the use of a single CT scanner and data from a single institution. Further studies with multiscanner and multicenter data will be necessary to validate our study results.
In conclusion, whole lung radiomics derived from noncontrast chest CT can help identify patients in need of ICU admission and predict outcome in patients with COVID-19 pneumonia. Whole lung radiomics were superior to subjective assessment of radiologists in predicting patient outcome and disease severity in COVID-19 pneumonia. The addition of clinical features to the radiomics can further improve the prediction of patient outcome and the need for ICU admission.
Disclosures of Conflicts of Interest: F.H. disclosed no relevant relationships. S.E. disclosed no relevant relationships. R.B. disclosed no relevant relationships. H.K.M. disclosed no relevant relationships. E.Z. disclosed no relevant relationships. B.C.B disclosed no relevant relationships. I.M. disclosed no relevant relationships. S.R.D. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: author paid for lecture by Siemens; Siemens paid for travel fees for conference and speaking; institution receives payment for independent image analysis for hospital contracted clinical research trial programs by Merck, Pfizer, Bristol Myers Squibb, Novartis, Roche, Polaris, Cascadian, Abbvie, Gradalis, Clinical Bay, and Zai Laboratories; institution receives research funding by Iunit. Other relationships: disclosed no relevant relationships. M.K.K. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: institution received research grant from Siemens Healthineers for unrelated research projects. Other relationships: received research grant from Riverain for unrelated projects.
Abbreviations:
- AUC
- area under the curve
- COVID-19
- coronavirus disease 2019
- GLCM
- gray-level co-occurrence matrix
- GLDM
- gray-level dependence matrix
- GLRLM
- gray-level run-length matrix
- GLSZM
- gray-level size-zone matrix
- ICU
- intensive care unit
- RT-PCR
- reverse-transcription polymerase chain reaction
- Spo2
- peripheral capillary oxygen saturation
- 3D
- three dimensional
References
- 1.Oren O, Kopecky SL, Gluckman TJ, et al. Coronavirus Disease 2019 (COVID-19): Epidemiology, Clinical Spectrum and Implications for the Cardiovascular Clinician. (American College of Cardiology website; ). https://www.acc.org/latest-in-cardiology/articles/2020/04/06/11/08/COVID-19-epidemiology-clinical-spectrum-and-implications-for-the-cv-clinician. Published 2020. Accessed May 14, 2020. [Google Scholar]
- 2.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395(10223):497–506 [Published correction appears in Lancet 2020;395(10223):496.]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Accelerated Emergency Use Authorization (EUA) Summary. COVID-19 RT-PCR Test. (Laboratory Corporation of America). U.S. Food and Drug Administration. https://www.fda.gov/media/136151/download. Accessed May 14, 2020. [Google Scholar]
- 4.Corman VM, Landt O, Kaiser M, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill 2020;25(3):2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wölfel R, Corman VM, Guggemos W, et al. Virological assessment of hospitalized patients with COVID-2019. Nature 2020;581(7809):465–469. [DOI] [PubMed] [Google Scholar]
- 6.Green K, Allen J, Suklan J, et al. What is the role of imaging and biomarkers within the current testing strategy for the diagnosis of Covid-19? Centre for Evidence-Based Medicine. https://www.cebm.net/covid-19/what-is-the-role-of-imaging-and-biomarkers-within-the-current-testing-strategy-for-the-diagnosis-of-covid-19/. Published April 8, 2020. Accessed May 14, 2020.
- 7.Xu H, Yan L, Qiu CM, et al. Analysis and Prediction of False Negative Results for SARS-CoV-2 Detection with Pharyngeal Swab Specimen in COVID-19 Patients: A Retrospective Study. MedRxiv 2020. [preprint] Posted March 30, 2020. Accessed April 8, 2020. [Google Scholar]
- 8.Guan WJ, Ni ZY, Hu Y, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med 2020;382(18):1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention . Interim Clinical Guidance for Management of Patients with Confirmed Coronavirus Disease (COVID-19). https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html. Accessed May 10, 2020.
- 10.ACR recommendations for the use of Chest Radiography and Computed Tomography (CT) for Suspected COVID-19 Infection. https://www.acr.org/Advocacy-and-Economics/ACR-Position-Statements/Recommendations-for-Chest-Radiography-and-CT-for-Suspected-COVID19-Infection. Accessed May 10, 2020. [Google Scholar]
- 11.Coronavirus (COVID-19) clinical radiology resources. https://www.rcr.ac.uk/college/coronavirus-covid-19-what-rcr-doing/clinical-information/rcr-position-role-ct-patients. Accessed May 10, 2020. [Google Scholar]
- 12.Clinical Management of COVID-19 (Spanish). Spanish guidelines. https://seram.es/images/site/Recomendaciones_imagen_SERAM_COVID_19.pdf. Accessed 4.30.2020.
- 13.Ai T, Yang Z, Hou H, et al. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: A report of 1014 cases. Radiology 2020;296(2):E32–E40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caruso D, Zerunian M, Polici M, et al. Chest CT Features of COVID-19 in Rome, Italy. Radiology 2020;296(2):E79–E85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bernheim A, Mei X, Huang M, et al. Chest CT findings in coronavirus disease-19 (COVID-19): Relationship to duration of infection. Radiology 2020;295(3):200463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li K, Wu J, Wu F, et al. The clinical and chest CT features associated with severe and critical COVID-19 pneumonia. Invest Radiol 2020;55(6):327–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y, Dong C, Hu Y, et al. Temporal Changes of CT Findings in 90 Patients with COVID-19 Pneumonia: A Longitudinal Study. Radiology 2020;296(2):E55–E64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou Z, Guo D, Li C, et al. Coronavirus disease 2019: initial chest CT findings. Eur Radiol 2020;30(8):4398–4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li K, Fang Y, Li W, et al. CT image visual quantitative evaluation and clinical classification of coronavirus disease (COVID-19). Eur Radiol 2020;30(8):4407–4416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao W, Zhong Z, Xie X, Yu Q, Liu J. Relation Between Chest CT Findings and Clinical Conditions of Coronavirus Disease (COVID-19) Pneumonia: A Multicenter Study. AJR Am J Roentgenol 2020;214(5):1072–1077. [DOI] [PubMed] [Google Scholar]
- 21.Fang M, He B, Li L, et al. CT radiomics can help screen the Coronavirus disease 2019 (COVID-19): a preliminary study. Sci China Inf Sci 2020;63(7):172103. [Google Scholar]
- 22.Chen X, Tang Y, Mo Y, et al. A diagnostic model for coronavirus disease 2019 (COVID-19) based on radiological semantic and clinical features: a multi-center study. Eur Radiol 2020. 10.1007/s00330-020-06829-2. Published online April 16, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pan F, Ye T, Sun P, et al. Time Course of Lung Changes at Chest CT during Recovery from Coronavirus Disease 2019 (COVID-19). Radiology 2020;295(3):715–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simpson S, Kay FU, Abbara S, et al. Radiological Society of North America Expert Consensus Statement on Reporting Chest CT Findings Related to COVID-19. Endorsed by the Society of Thoracic Radiology, the American College of Radiology, and RSNA - Secondary Publication. J Thorac Imaging 2020;35(4):219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Slicer Radiomics. Computational Imaging & Bioinformatics Lab - Harvard Medical School. https://www.radiomics.io/slicerradiomics.html. Accessed April 1, 2020. [Google Scholar]
- 26.Radiomic Features. Pyradiomics. https://pyradiomics.readthedocs.io/en/latest/features.html. Accessed April 15, 2020.
- 27.Society of Thoracic Radiology Web site. https://thoracicrad.org/. Accessed May 14, 2020.
- 28.Colombi D, Bodini FC, Petrini M, et al. Well-aerated Lung on Admitting Chest CT to Predict Adverse Outcome in COVID-19 Pneumonia. Radiology 2020;296(2):E86–E96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klaassen R, Larue RTHM, Mearadji B, et al. Feasibility of CT radiomics to predict treatment response of individual liver metastases in esophagogastric cancer patients. PLoS One 2018;13(11):e0207362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li H, Galperin-Aizenberg M, Pryma D, Simone CB 2nd, Fan Y. Unsupervised machine learning of radiomic features for predicting treatment response and overall survival of early stage non-small cell lung cancer patients treated with stereotactic body radiation therapy. Radiother Oncol 2018;129(2):218–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bera K, Velcheti V, Madabhushi A. Novel Quantitative Imaging for Predicting Response to Therapy: Techniques and Clinical Applications. Am Soc Clin Oncol Educ Book 2018;38(38):1008–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nardone V, Tini P, Pastina P, et al. Radiomics predicts survival of patients with advanced non-small cell lung cancer undergoing PD-1 blockade using Nivolumab. Oncol Lett 2020;19(2):1559–1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.He JL, Luo L, Luo ZD, et al. Diagnostic performance between CT and initial real-time RT-PCR for clinically suspected 2019 coronavirus disease (COVID-19) patients outside Wuhan, China. Respir Med 2020;168:105980. [DOI] [PMC free article] [PubMed] [Google Scholar]