Abstract
D-Serine dehydratase from Escherichia coli is a member of the β-family (fold-type II) of the pyridoxal 5′-phosphate-dependent enzymes, catalyzing the conversion of D-serine to pyruvate and ammonia. The crystal structure of monomeric D-serine dehydratase has been solved to 1.97 Å-resolution for an orthorhombic data set by molecular replacement. In addition, the structure was refined in a monoclinic data set to 1.55 Å resolution. The structure of DSD reveals a larger pyridoxal 5′-phosphate-binding domain and a smaller domain. The active site of DSD is very similar to those of the other members of the β-family. Lys118 forms the Schiff base to PLP, the cofactor phosphate group is liganded to a tetraglycine cluster Gly279–Gly283, and the 3-hydroxyl group of PLP is liganded to Asn170 and N1 to Thr424, respectively. In the closed conformation the movement of the small domain blocks the entrance to active site of DSD. The domain movement plays an important role in the formation of the substrate recognition site and the catalysis of the enzyme. Modeling of D-serine into the active site of DSD suggests that the hydroxyl group of D-serine is coordinated to the carboxyl group of Asp238. The carboxyl oxygen of D-serine is coordinated to the hydroxyl group of Ser167 and the amide group of Leu171 (O1), whereas the O2 of the carboxyl group of D-serine is hydrogen-bonded to the hydroxyl group of Ser167 and the amide group of Thr168. A catalytic mechanism very similar to that proposed for L-serine dehydratase is discussed.
Keywords: D-Serine dehydratase; Pyridoxal 5′-phosphate; Type II fold; Open and closed conformation; α,β elimination
1. Introduction
D-Serine dehydratase (EC 4.2.1.18) (DSD) from Escherichia coli strain K12 catalyzes the degradation of D-serine to pyruvate and ammonia (Scheme 1) [1]. D-Serine is a competitive antagonist of β-alanine in the biosynthetic pathway of pantothenate and coenzyme A [2]. Therefore, DSD appears to serve as a detoxifying enzyme in most E. coli strains.
Scheme 1.
To our knowledge, DSD is the only PLP-dependent enzyme with a monomeric quaternary structure. D-Serine dehydratase consists of a single polypeptide chain (Mr 47,901) and has one catalytically essential pyridoxal 5′-phosphate (PLP) per protein molecule (Kd=0.03 μM) [1]. A second low affinity-binding site for the cofactor has also been reported (Kd~1 mM) [3]. D-Serine dehydratase is a member of the β-family of the PLP-dependent enzymes and belongs to the fold-type II family [4].
Even though DSD crystallizes readily, the microcrystals initially obtained were not suitable for structure determination due to unfavorable zwilling formation. Single crystals of DSD in complex with 3-amino-2-hydroxyproprionate were obtained to determine the space group [5].
Here, we report two three-dimensional structures of the holoform of DSD as determined by X-ray crystallography to a resolution of 1.97 Å and 1.55 Å, respectively. It is the first crystallographic structure of the holo form of the fold-type II DSD. The crystal structure of D-serine dehydratase from chicken kidney was reported by Tanaka and coworkers [6] instead presents a zinc-dependent enzyme similar to DSD from Saccharomyces cerevisiae [7]. The DSD from chicken kidney furthermore belongs to the fold-type III of PLP-dependent enzymes, thus its structure is very different from DSD from E. coli, described in this paper. At the same time, Bharath and coworkers [8] reported the crystal structure of D-serine deaminase from Salmonella typhimurium. The latter enzyme was crystallized in two structures, an open and closed conformation. However, for both structures no traceable electron density could be observed for the cofactor PLP, indicating that the enzyme likely has a low affinity for the cofactor under the crystallization conditions used. In the deaminase from S. typhimurium, modeling of the active site and substrate suggests that Thr 166 may be involved in the abstraction of a proton from the Cα of the substrate. However, the accuracy of the fitted substrate in the deaminase protein seems unclear considering that no unambiguous electron density is observed for PLP in the PLP-D-serine complex [8]. In contrast, our high-resolution structure data provides a highly defined electron density of the cofactor in DSD and is fully developed and all protein partners are established enabling us to propose a putative reaction mechanism for DSD.
2. Materials and methods
2.1. Enzyme
D-Serine dehydratase from E. coli was purified from a wild-type DSD expression plasmid [9] using the method described by Schiltz and Schnackerz [10]. 125 g E. coli cell paste was suspended in 280 ml potassium phosphate, pH 7.8, containing 1 mM DTE and 1 mM EDTA. The cell suspension was forced three-times through a French press at a pressure of 8000–9000 lb/in2. Cell debris removed by centrifugation at 27,500×g for 30 min. The supernatant crude extract was adjusted to pH 7.3 by slow addition of cold 2.5 M KOH. To remove nucleic acids and nucleoproteins 10% aqueous Polymin P solution was slowly added to bring the protein solution to a final concentration of 1% in Polymin P. After 15 min stirring the suspension was centrifuged for 20 min at 27,500×g. The next step was an ammonium sulfate precipitation at 35–55% saturation followed by centrifugation. The pellet was dissolved in a minimum amount of 20 mM potassium phosphate, 0.1 mM EDTA, 0.1 mM pyridoxal phosphate, 1 mM DTE, pH 7.8. Residual ammonium sulfate was removed by dialyzing the protein solution against three-1.5 liter changes of the same buffer. Insoluble material was removed by centrifugation and the clear solution applied to a DEAE-Cellulose column (3.6×30 cm) equilibrated with the dialysis buffer at 4 °C. The enzyme was eluted with a 500 ml gradient of 0–140 mM KCl dissolved in phosphate buffer. Fractions with specific activities greater than 30 units/ml were pooled and concentrated by precipitation with ammonium sulfate (80% saturation). The precipitated protein was dissolved in 70 mM potassium phosphate, 1 mM DTE, 5 μM PLP, 0.1 mM EDTA, pH 7.8. A molecular sieve chromatography on Biogel P-100 (1.6×85 cm) with a flow rate of 2 ml/h followed. Active fractions are pooled and concentrated using an Amicon ultrafiltration system. Further purification was accomplished by crystallization of the enzyme with saturated ammonium sulfate. Specific activities between 120 and 140 units/mg protein were obtained.
2.2. Enzyme assay
The enzymatic activity of DSD was determined at 25 °C as described by Dowhan and Snell [1]. The assay mixture contains 10 mM D-serine, 100 mM potassium phosphate, pH 7.8, 0.15 mM NADH, lactate dehydrogenase (0.1 mg/ml) and DSD. The decrease of NADH absorbance was measured at 334 nm. One unit is defined as the amount of enzyme required to form 1 μmol of pyruvate in 1 min at 25 °C under the above assay conditions.
2.3. Crystallization and data collection
Different crystal forms of DSD could be obtained. The crystal growth conditions are described in Table 1. Crystals were picked up by a fiber cryo-loop and frozen in a stream of liquid nitrogen for X-ray data collection. Diffraction data from crystals of orthorhombic and monclinic crystal forms were collected at the beamlines EMBL/DESY X31, MPG/DESY BW6 and SRS Daresbury beamline 10.1 using MAR 345 imaging plate, MAR CCD 165 mm, and MAR CCD 225 mm mosaic detectors, respectively. The data collection statistics of the two crystal forms of DSD are summarized in Table 2.
Table 1.
Crystal growth conditions.
| Orthorhombic P212121 |
Monoclinic P21 |
|---|---|
| Drop: | Drop: |
| DSD (10 mg/mL), 1 M Sodium formate in imidazole/maleate buffer, pH 7.0 | DSD (10 mg/mL), 0.35M Sodium citrate in 0.1 M imidazole/maleate buffer, pH 7.0 |
| Well: | Well: |
| 2.0 M formate in 0.1 M imidazole/maleate buffer, pH 7.0 | 0.7 M sodium citrate in 0.1 M imidazole/maleate buffer, pH 7.0 |
Table 2.
Summary of crystallographic data collection statistics.
| Crystal form | Orthorhombic | Monoclinic |
|---|---|---|
| X-ray source | BW6, MPG/DESY | 10.1, SRS |
| Wavelenght (A) | 1.05 | 0.979 |
| Space group | P21212 | P21 |
| a, b, c (A) | a = 143.16, b = 47.74, c = 72.30 | a = 73.77, b = 47.80,c = 75.19 |
| Resolution (A)a | 40–1.97 (2.0–1.97) | 50.0–1.55 (1.61–1.55) |
| Rsymb (%) | 9.7 (51.5) | 85.7 (41.7) |
| Number of unique reflections | 35756 | 73947 |
| Completeness (%) | 99.6 (99.4) | 95.7 (80.3) |
| Redundancy | 6.2 (2.9) | 4.7 (2.5) |
| <I/σ(I)> | 19.8 (1.8) | 15.9 (2.25) |
I/σ(I) is the ratio of the mean intensity to the mean standard deviation of intensity.
The data for the highest resolution shell are shown in parentheses.
Rsym = SI – <I>, where I is intensity of reflection and <I> is the intensity averaged from multiple observations of symmetry-related reflections.
2.4. Structure determination and refinement
The DSD structure was solved by molecular replacement in the orthorhombic space group using the program MOLREP [11]. A solution was found for a model comprising residues 29–317 of the catalytic subunit of allosteric threonine deaminase from E. coli (pdb code 1TDJ) [12] with 19% sequence identity to DSD.
The rotation function was calculated at 40–3 Å resolution using full atomic model with default MOLREP 8.2 options. The translation function was found for polyalanine model at 40–5 Å resolution. The solution had correlation of 38.8% and R-factor 59.0% while the wrong translation peaks for correct orientation had correlation not higher than 35.0% and R-factor not lower than 60.3%. For wrong orientations translation peaks had correlation not higher than 33.0% and R-factor not lower than 60.6%.
Next, the solution was subjected to 200 cycles of positional and B-factor refinement at 40–2.0 Å in REFMAC [11,13]. Although Free R went down only marginally, the refined model was successfully used for molecular replacement in the trigonal space group. The correct enantiomorph of space group was determined as P32 and three subunits related by pseudotranslation were successfully positioned in the unit cell using pseudotranslation vector option of MOLREP. Multicrystal averaging was performed by DMMULTI [14]. However, the resulting electron density was strongly model-biased and difficult to interpret. Therefore rigid body refinement was performed in the orthorhombic space group with original molecular replacement model using rigid body refinement option of MOLREP. The refinement of positions and orientations of the two domains was followed by refinement of the β-sheets of two domains and large helices as separate rigid bodies. The resulting model was subjected to positional and B-factor in REFMAC and multicrystal averaging by DMMULTI. The electron density after phased refinement in REFMAC with external DMMULTI phases allowed unambiguous side chain assignments for residues in the β-sheets. The model building employing the software Coot [15] was followed by further refinement and averaging. The resulting model has been refined to R-factor of 17.2% and free R-factor of 21.6%.
The coordinates of refined model in orthorhombic group symmetry were used for finding the solution of the monoclinic data set at 1.55 Å resolution by MOLREP following by refinement with the help of REFMAC program coupled with automated building and updating of the solvent structure using ARP [16]. Atomic model of the structure was rebuilt in the program O [17] between each round of refinement using 2Fo−Fc and Fo−Fc weighted electron density maps. Multiple conformations of the side chains were included in the model. Final statistics of the refinement is given in Table 3.
Table 3.
Summary of refinement statistics.
| Crystal form | Orthorhombic | Monoclinic |
|---|---|---|
| Resolution range (Å) | 39.84 – 1.97 | 72.74 – 1.55 |
| Rwork (%) | 17.2 | 15.9 |
| Rfree (%) | 21.6 | 19.4 |
| Number of protein atoms | 3303 | 3438 |
| Number of water molecules | 451 | 699 |
| RMSD bond length (Å) | 0.014 | 0.015 |
| RMSD bond angles (°) | 1.401 | 1.524 |
| Ramachandran Plot Statistics | ||
| Most favored regions (%) | 95.52 | 96.84 |
| Additional allowed regions (%) | 2.59 | 2.92 |
Rwork = Σhkl ||Fobs(hkl)| - |Fcalc(hkl)||/ Σhkl |Fobs|, Rfree is calculated with 5% of total number of reflections.
2.5. Coordinates
The atomic coordinates and the structure factors pdb code 3ss7 and 3ss9 for the monoclinic and the orthorhombic crystals, respectively, have been deposited in the Protein Data Bank, Research Collaboratory for Structural Bioinformatics, Rutgers University, New Brunswick, NJ (http://www.rcsb.org/).
3. Results
3.1. Structure determination and overall structure
3.1.1. Overall structure
The orthorhombic crystal contains one monomeric molecule of DSD per asymmetric unit. Fig. 1 illustrates the overall topology of the monomer of DSD. The DSD molecule has 20 α-helices and 11 β-sheets which are arranged in a large and a small domain. The PLP binding site is located at the interface of the two domains and is accessible for substrate or solvent by a large entrance cavity. The large domain is referred to as the catalytic domain since it contains the essential PLP cofactor. It contains 15 α-helices, and seven stranded β-sheets. The small domain folds as a twisted α/β structure consisting of a central four-stranded parallel β-sheet and five surrounding α-helices. In both reported structures, the electron density maps (2Fo−Fc, φc) contoured at 1 σ above the mean show continuous density for all main chain atoms with two exceptions. The first one is the absence of N-terminal residues 1 to 11 in the orthorhombic structure (1.97 Å) or 1 to 5 in the monoclinic structure (1.55 Å). Secondly, the segment with no or low electron density consists of residues 210–231 in the orthorhombic structure, whilst this segment shows continuous density in the monoclinic structure.
Fig. 1.
Stereo view of the overall structure of D-serine dehydratase from Escherichia coli in the open form. DSD is divided into two domains, a large PLP-binding domain (red), and a small domain (blue), PLP molecule as van der Waals spheres in atom colors.
3.1.2. Open-closed conformational change
The orthorhombic and the monoclinic structures of DSD show large differences in the arrangement of the small domain with respect to the large domain (Fig. 2). In the closed conformation the movement of the small domain blocks the entrance to the active site of DSD, the loop between the β-sheet 5 and α-helix 8 moves the furthest — distance of the Cα positions is 6.5 Å whereas the distance of the Cα positions of the loop between β-sheet 8 and α-helix 9 is 5.5 to 5.8 Å. With regard to the secondary structure elements the N-terminal half of α-helix 9 moves about 5.5 Å whereas the movement of the C-terminal half of β-sheet 5 is only 2 Å. The N-terminal half of α-helix 8 moves only by 1 Å. In the closed conformation, the entrance to the active site is blocked by the movement of the small domain.
Fig. 2.
(Upper panel) Open and closed conformation of DSD. The small domain is shown as ribbon plot with the open conformation in blue (parts that don’t move), red (<beta>5<alpha>5-loop) and orange (<alpha>8<alpha>9-loop) and the closed conformation in green, magenta and purple, respectively. The PLP-binding domain is shown as C<alpha>−trace in gray. Between both conformations the <alpha>8<alpha>9-loop moves together with helix 9, the helix axis is indicated. In contrast, for the <beta>5<alpha>8-loop only the loop element alters its conformation upon open the access to the PLP binding site, the PLP cofactor is shown as van-der-Waals spheres. Left figure side view, right figure rotated 90° around the y-axis. (Lower panel) Surface representation of DSD (orientation as in the upper right figure) with the open conformation on the left and the closed conformation on the right. Residues 1–11 do not show any density.
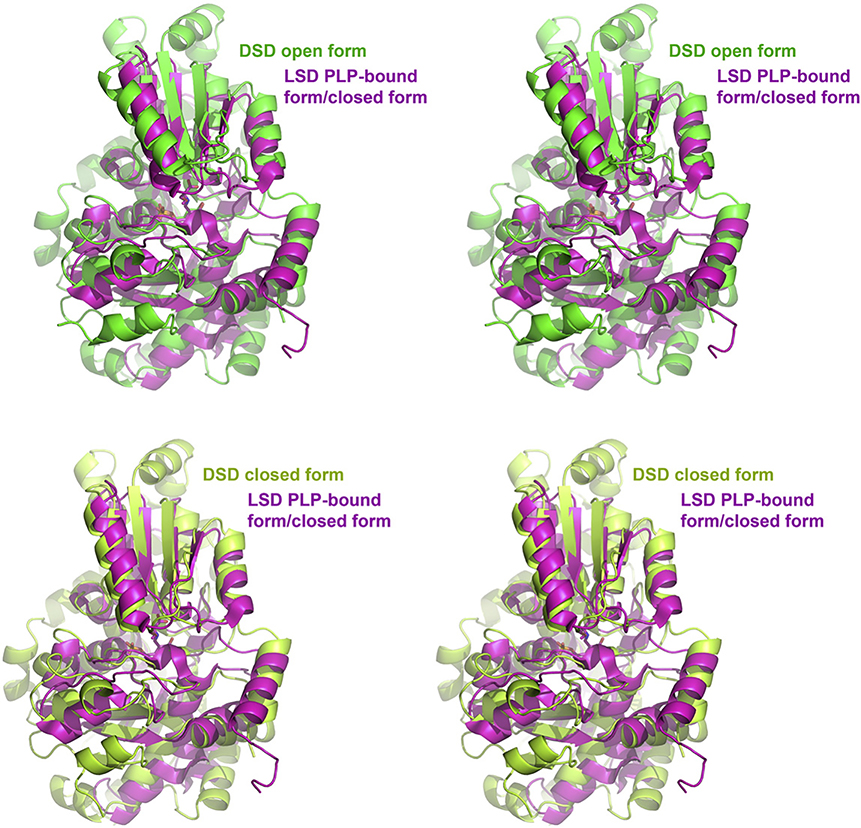
If one compares the open and closed conformations of DSD with holo-LSD, apo-LSD and the holo-tryptophan synthase β-subunit it becomes apparent that residues 188–238 belong to the moving part of the small domain with the open conformation of DSD being similar to the conformation of apo-LSD and consequently the closed conformation of DSD being similar to holo-LSD, holo-trpytophan synthase β-subunit and serine racemase.
3.1.3. Coenzyme binding site
The large deep groove of DSD formed at the domain interface is part of the long groove connecting the top and bottom of the molecule. The groove embraces the cofactor PLP in the central region and is filled with water molecules. The PLP cofactor forms a typical Schiff base bond with the NZ of the catalytic Lys118 (Fig. 3). The Schiff base of PLP with Lys118 shows the characteristic absorption band at 415 nm which is pH-independent [18].
Fig. 3.
View of the PLP binding site of DSD. Fo−Fc map showing the electron density peaks of PLP aldimine to Lys118.
Each of the polar substituents of PLP is coordinated to an appropriate functional group of the protein. The pyridinium nitrogen N1 is hydrogen-bonded to the side chain of Thr424, the hydroxyl group at C3 is hydrogen-bonded to the side chain of Asn170, and the phosphate group of PLP is coordinated by main chain amides to a tetraglycine loop (residues Gly279, Gly281, Gly282, and Gly283). The so-called asparagine loop (Ser167, Thr168, Gly169, Asn170) resides on the O3′ side of PLP and acts as the recognition site for the substrate carboxylate in the closed form. A similar arrangement of the active site residues has been found in serine racemase from rat liver and Schizosaccharomyces pombe [19].
31P NMR experiments of DSD revealed information about the properties of the DSD-bound cofactor phosphate. The pH-dependence of the 31P chemical shift of PLP bound to DSD suggests that the 5′-phosphate group of the enzyme-bound cofactor is exposed to solvent, i.e. in the open conformation (Fig. 2). The 31P line width of 13 Hz indicates that the cofactor is tightly bound to the protein and tumbles with the protein [18]. In the presence of the potent competitive substrate analog isoserine (D,L-3-amino-2-hydroxypropionate), a new Schiff base linkage is formed. The 31P NMR signal of the external Schiff base is pH-independent with a chemical shift of 4.2 ppm. Thus the phosphate group of the complex is locked in its dianionic form in the closed conformation (Fig. 2) [18].
3.1.4. Monovalent cation binding site
When the structure of the metal ion binding site of rat liver L-serine dehydratase [20] is used as a template for the corresponding site in DSD, a similar arrangement of amino acid residues found in L-serine dehydratase can be recognized in DSD (Fig. 4). Residues Cys311 (Ser200), Gly279 (Gly198), Glu305 (Glu194), Ser309 (Ala198), Leu340 (Leu233) and Val342 (Val225) form the binding site of the catalytically important potassium ion. The amino acids in parentheses are those found in L-serine dehydratase from rat liver. In the structure of DSD the electron density was found in the site identified as potassium binding site based on our NMR data [21]. In addition, the high probability of potassium binding is given due to the fact that protein expression and purification were executed under high excess of potassium ions.
Fig. 4.
Binding site of monovalent ion in rat liver L-serine dehydratase (LSD). The structures of D-serine dehydratase in its closed form (carbon atoms are colored in green) and L-serine dehydratase (carbon atoms are colored in magenta) were superimposed. In L-serine dehydratse a monovalent cation (K+) was identified which is coordinated by residues of the 711-loop, the 812- and the long1213-loop. The K+ cation is coordinated by five backbone carbonyl groups and one carboxylate group of Glu194 of LSD. The architecture of this cation-binding site is highly conserved in D-serine dehydratase, however, a water molecule occupies the cation-binding site in DSD.
Gly279 is not only part of the tetraglycine loop involved in the coordination of the phosphate group of PLP but also part of the monovalent cation (MVC) binding site, i.e., the MVC site is rather close to the catalytic site. It could be concluded that the mechanisms of action of MVC is coupled by way of the closeness of the MVC site to the active site of DSD. A similar situation is seen in serine racemase from Schizosaccharamyces pombe [19]. A magnesium ion is located beside the tetraglycine (Leu182–Gly186) loop and coordinated to the carbonyl groups of Leu182 and Gly183 via water molecules and the carbonyl group of Gly212 and the carboxylates of Glu208 and Asp214. The racemase loses its catalytic activity in the presence of the chelator EDTA. It recovers its activity on addition of Mg2+ and Ca2+. It was assumed that the divalent cation is not directly involved in the catalytic action but rather in stabilization of the folding of the protein since the metal ion is located outside of the active center [19]. The best characterized MVC-dependent, fold-type II, PLP-dependent protein is tryptophan synthase. Binding of an MVC is essential for switching between open (inactive) and closed (active) states of tryptophan synthase, and for maintaining of the correct conformation of the indole subsite at the β-subunit [22]. Furthermore, a MVC is essential for the activity of dialkylglycine decarboxylase. As in DSD, the MVC is hydrogen-bonded to an amino acid which is also part of the tetraglycine loop [41].
4. Discussion
D-Serine is a D-amino acid previously been thought to be restricted to some bacteria and insects, but was recently discovered in unusually high concentrations in mammalian brain [23], where it serves as an intrinsic co-agonist of the N-methyl-D-aspartate (NMDA) type glutamate receptor – a key excitatory neurotransmitter receptors in the brain – modulates brain functions, such as memory formation, synaptic plasticity and development [24]. NMDARs are unique in their requirement for more than one agonist to operate. Glutamate, the main NMDAR agonist, does not activate the receptor unless either one of two endogenous co-agonists, glycine or D-serine, are bound to the NMDAR “glycine modulatory site” at the NR1 subunit. D-Serine is now recognized as the physiological ligand of the NMDAR co-agonist site [25]. It has been reported that the D-serine concentration and the ratio of D-serine to the total serine concentration in the serum and spinal fluid of schizophrenic patients were significant lower than the corresponding values of the controls [26]. Since the relationship of D-serine to these neurological disorders much attention has been given to the D-serine biosynthesis. Serine racemases are known to be the major enzymes that produce D-serine [27–29]. The enzyme is expressed in glial cells and neurons. Amino acid racemase (EC 5.1.1.10) of Pseudomonas putida with low-substrate specificity also catalyzes the serine racemization [30]. In addition, VanT from vancomycin-resistent Enterococcus gallinarum BM4174 is a fold-type III serine racemase, whose structure completely differs from that of the mammalian serine racemase [31].
The phosphate group in the 5′-position of the cofactor in its dianionic form is essential as very well documented by 31P NMR experiments [18]. The absorption spectrum of highly purified DSD exhibits prominent absorbance maxima at 280 and 415 nm, the latter indicating a protonated Schiff base linkage of the formyl group of PLP to the ε-amino group of Lys118 [32–34]. Excitation of DSD at 296 nm causes an emission spectrum with maxima at 335 and 510 nm, respectively [33–35]. The emission maximum at 510 nm is due to energy transfer between a tryptophan residue and PLP [33]. The dehydratase is weakly fluorescent with an excitation maximum, corresponding to the absorbance maximum of the internal Schiff base and an emission maximum at 510 nm, typical for all PLP-dependent enzymes studied so far [9,36]. Upon addition of 0.5 M glycine or L-alanine the fluorescence intensity at 510 nm increased markedly, and the λmax shifts from 510 to 485 [36,37] similar to spectral changes observed for O-acetylserine sulfhydrylase [38]. When DSD is compared with other type II-fold PLP enzymes it can be deduced that in DSD Trp197 is the only tryptophan in the correct position for Förster energy transfer with PLP [39]. In an earlier study, the tryptophan residues of DSD were modified with the disulfonium salt of 2-hydroxy-5-nitro-benzyl bromide at pH 6.5 after masking reactive sulfhydryl groups with pCMB, concomitant with the loss of up to 90% of initial activity. Tryptic digestion of the modified DSD revealed after separation of tryptic peptides two modified tryptophan containing peptides, one of which was Ala-Trp197-Lys [40]. To obtain insight into hydroxyl group elimination and the stereo specificity of DSD for D-serine a high-resolution structure is required.
4.1. Comparison with other PLP enzymes of fold-type II
D-Serine dehydratase is to our knowledge the only monomeric PLP-dependent enzyme. DSD has a molecular mass of 47.92 kDa. Sequence comparisons revealed that DSD is a member of the fold-type II family of PLP enzymes, including the β-subunits of tryptophan synthase [43], O-acetylserine sulfhydrylase [44], allosteric threonine deaminase [12], L-serine dehydratases [20,45], cystationine-β-synthase [46] and threonine synthase [47].
Even though the sequence identity or sequence similarity of DSD when compared with the β-subunits of tryptophan synthase, O-acetylserine sulfhydrylase, allosteric threonine deaminase and L-serine dehydratases is less than 20%, all enzymes mentioned have the same fold-type II, i.e., have very similar active sites. The catalytic subunit of threonine deaminase has a sequence identity of 19% to DSD. It was used as template for a search model. Sequence alignment between TDH, LSD from rat and human liver and DSD revealed that at least two regions are conserved in all types of enzymes. One of them is the region around the lysine residue which forms the Schiff base with PLP and secondly the glycine-rich sequence which is thought to interact with the phosphate moiety, providing the anchor for the substrate-PLP complex (Fig. 5).
Fig. 5.
Sequence alignment of D-serine dehydratase from Escherichia coli (DSD) with other serine/threonine dehydratases. The first 55 amino acids of the DSD sequence [17] are not shown. The sequences displayed are human L-serine dehydratase (LSD human), rat liver L-serine dehydratase (LSD rat), threonine dehydratase from Escherichia coli (TDH) (P04968), serine racemase (SR) (AF 169974) and DSD. The amino acids in the red boxes represent the completely identical and conserved changed amino acid residues among all sequences. Green shades represent amino acids which are not seen in all five sequences. Asterisks represent amino acid residues in the active site of DSD. The secondary structure elements indicated corresponds to that of human LSD structure.
All fold-type II, PLP-dependent enzymes have a tetraglycine loop which is coordinated to the phosphate group of PLP. One of the glycine residues is at the same time coordinated to the MVC binding site. The substrate-binding site is surrounded by the “asparagine” loop, (Ser167-Thr168-Gly169-Asn170). These residues are responsible for the binding of carboxylate of D-serine. A similar loop has been regonized in L-serine dehydratase (Ser64-Ala65-Gly66-Asn67-Ala68).
The superposition of the structures of DSD and of the allosteric threonine deaminase is shown in Fig. 6. DSD covers only the catalytic domain of threonine deaminase. It should be noted that as the larger molecule DSD has some extra loops. The same is true when comparing DSD with L-serine dehydratase (LSD). Fig. 7 shows the overlay of DSD and of LSD from rat liver, the latter having only 3/4 of the molecular mass of DSD. In both cases the active sites are very similar to that of DSD.
Fig. 6.
View of the superposition of the backbone of D-serine dehydratase from Escherichia coli and allosteric threonine deaminase. DSD is shown in red and blue, threonine deaminase in gray and green. At the right, the view points towards PLP head-on, whereas on the left the view is rotated by 90°.
Fig. 7.
Stereo view of the superposition of the backbone residues of D-serine dehydratase (DSD) and of rat liver L-serine dehydratase (LSD rat) in the open (upper half) and closed (lower half) form. DSD is shown in green, LSD in cyan.
Serine racemases, the main enzyme source to produce D-serine in the brain is also a fold-type II protein. The large deep groove of serine racemase from Schizosaccharomyces pombe [20] formed at the domain interface is part of the long groove connecting the top and bottom of the molecule. The groove embraces the cofactor PLP in the central region and is filled with water molecules. Many of the interactions between PLP and active-site residues reflect those observed in PLP-dependent fold-type II enzymes including DSD.
In early publications of DSD, Snell’s group reported that monovalent ions, such as Na+ and K+, greatly influence the activity of DSD. K+ ions enhanced the activity, whereas Na+ ions inhibited the enzymatic activity of DSD [32]. Recently it could be shown that the 31P nucleus of the cofactor phosphate group senses which of the two monovalent ions is present [22]. The behavior of the 31P nucleus is very much the same as for dialkylglycine decarboxylase in which different coordination for potassium and sodium ions in the protein has been structurally established [41]. K+ and Na+ ions are bound to the same site but in different fashion — octahedral versus trigonal bipyramidal, respectively.
DSD has been crystallized in different forms. The crystal shape is unusual with one very long edge and two much smaller ones [5]. The crystal structure of DSD has some similarity to threonine deaminase which was used as a template to solve its structure by molecular replacement in the orthorhombic space group. DSD, with a molecular mass of 47,901 Da, one of the largest proteins performing a α,β-elimination reaction (see Table 4). It is, however, the only known monomeric species, all other α,β-eliminating proteins are either dimers, such as LSD, OASS, and tryptophan synthase (α2 β2) or tetramers like threonine deaminase and cystathionine β-synthase (CBS). Even though the sequence identity of the fold-type II PLP-enzymes is rather small, all enzymes have almost identical features with respect to their active site as summarized in Table 4. The active site features of DSD and threonine deaminase are nearly identical. All interactions of side chains of the protein with PLP are important especially for the correct positioning of the substrate-PLP Schiff base complex during catalysis. Previous notions of the importance of the tetraglycine loop were provided by site-directed mutagenesis experiments of the glycine-rich loop of DSD. Gly279Ala and Gly281Ala mutant DSD species showed a 33- and 22-fold lower affinity for PLP when compared with wild-type DSD and the turnover numbers with D-serine were 6 and 60% of normal, respectively [48]. The hydrogen bonding partners of C3 and N1 of the pyridine ring of PLP could be predicted from sequence alignment of DSD with LSD and threonine deaminase [12].
Table 4.
Hydrogen bonding partners of PLP in D-serine dehydratase (DSD), deaminase (TDH), L-serine dehydratase from E. coli and human liver (LSD), tryptophan synthase (β-subunit) (TPS), O-acetylserine sulfhydrylase (OASS), cystathionine β-synthase (CBS), serine racemase (SR).
| Enzyme | DSD | TDH | LDH | TPS | OASS | CBS | SR |
|---|---|---|---|---|---|---|---|
| Total number of amino acids per subunit | 442 | 494 | 327 | 391 | 315 | 435 | 340 |
| PLP-phosphate group | G282 | G191 | G171 | S235 | T180 | T260 | G185 |
| G281 | G190 | G170 | G234 | G178 | G268 | G186 | |
| G279 | G188 | G168 | G232 | G176 | G256 | G187 | |
| V280 | G189 | G169 | G233 | T177 | G257 | G188 | |
| O3 ligand | N170 | N89 | N67 | N71 | N149 | N86 | |
| N1 ligand | T424 | T315 | C303 | S377 | S272 | S349 | S308 |
| SB ligand | K118 | K62 | K41 | K87 | K41 | K119 | K57 |
Not much is known about the intermediates occurring during catalysis. Static absorbance measurements of DSD at 2 °C show that the absorption maximum of the Schiff base of the cofactor PLP is shifted from 415 to 442 nm during the reaction with D-serine [33]. The half-time of the absorbance changes occurring at 330 and 455 nm was found to be 6.5 ms, suggesting the observation of a single enzyme-bound intermediate. It was hypothesized that the structure of this intermediate is most likely the α-aminoacrylate–PLP complex. Incubating apo-dehydratase with 4-[2-methyl-3hydroxy-5-(phosphooxy)-methyl)-4-pyridinyl]-2-oxo-3-butenoic acid (obtained by aldol condensation of PLP and pyruvate) creates an absorbance shift from 415 to 460 nm and a shoulder centered at around 330 nm, very much like the transient occurrence of the α-aminoacrylate–PLP complex, further confirming evidence for the structure of the single intermediate. Only later the spectral features of the long-lived α-aminoacrylate–PLP intermediate in the reaction of O-acetylserine with O-acetylserine sulfhydrylase could be documented by Cook and coworkers [42]. L-Cysteine is formed upon addition of sulfide to the double bond of the intermediate.
4.2. Proposed catalytic mechanism
Since no crystallographic structure of a substrate or inhibitor complex with DSD is available D-serine was modeled into the active site of DSD using the L-serine dehydratase from rat liver as a template. Therefore, at present the catalytic mechanism of DSD has to be putative and the reported distances are only tentatively. The catalytic mechanism is, however, very likely to be very similar to that proposed for rat liver L-serine dehydratase [20] or serine racemase [19].
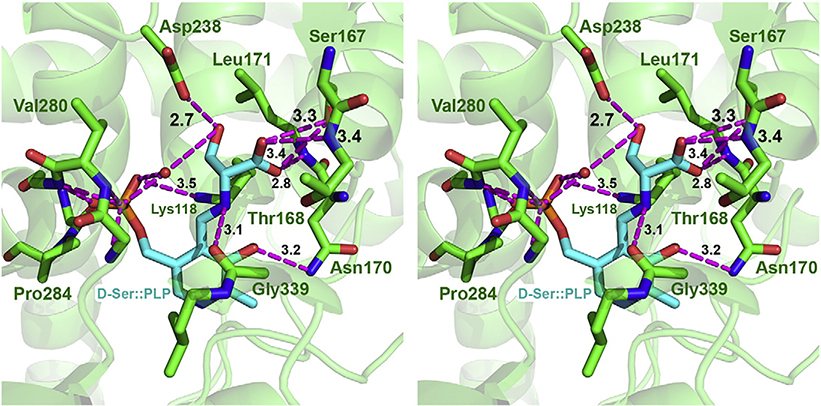
The phosphate group of PLP is located in a pocket coordinated to main chain amides of conserved glycines (279GlyValGlyGlyGly283). The substrate D-serine enters the active site from the re face with respect to the pyridine ring of the cofactor [49]. The amino group of the substrate, D-serine, forms a hydrogen bond with an oxygen of the cofactor phosphate (Fig. 9, 1). The phosphate becomes protonated and the amino group changes into a nucleophile, attacking the C4′ of the internal Schiff base by releasing the neutral Lys118 (Figs. 9, 2). The O1 of the carboxyl group of D-serine is coordinated to the hydroxyl group of Ser167 (3.3 and 2.8 Å, respectively) (Fig. 8) and the amide nitrogen of Leu171 (2.8 Å) via hydrogen bonds. The O2 of the carboxyl group of D-serine is hydrogen-bonded to the hydroxyl group of Ser167 (3.3 Å) and the amide nitrogen of Thr168 (3.4 Å). The hydroxyl group of D-serine is hydrogen-bonded to the carboxyl group of Asp238 (2.7 Å) and to the phosphate group of PLP via a water molecule (Fig. 8). The N1 of PLP is not protonated, the abstraction of the α-hydrogen from the Cα of D-serine cannot be executed by Lys118, because the ε-amino group resides in the si face whereas the α-proton of the substrate protudes to the re face of the D-serine-PLP Schiff base. It is very likely that the phosphate group of PLP can act as a general acid to donate a proton to the oxygen OG of D-serine and abstraction of the hydrogen from Cα by the hydroxyl group of Thr168 occurs in a concerted fashion (Fig. 9, 4). Water and the PLP–α-aminoacrylate Schiff base are released (Fig. 9, 4). Then, Lys118 attacks C4′ of the Schiff base of the PLP–α-aminoacrylate intermediate to form the PLP-Lys118 (internal) Schiff base (Fig. 9, 5) and releases α-aminoacrylate (Fig. 9, 6) which in a non-enzymatic hydrolysis is deaminated to pyruvate. The putative mechanism of the reaction is summarized in Fig. 9. The catalytic mechanism of DSD is very much like that of LSD [39] the only difference is that in the case of DSD Thr168 abstracts the proton from the Cα of D--serine whereas in LSD Lys 41 is responsible for the abstraction. The hydroxyl group of D-serine is forming a hydrogen bond to a water molecule (3.3 Å) and the distance from the water molecule to the oxygens of the cofactor phosphate is 2.6 and 3.2 Å, respectively. Other examples of the use of the cofactor phosphate group as acid– base catalyst are L-serine dehydratase [45] and glycogen phosphory lase [50].
Fig. 9.
Putative catalytic mechanism of DSD. Arrows indicate movements of electrons. Possible hydrogen bonds are represented by dashed lines. (A) A D-serine molecule enters the active site. The amino group of the substrate forms a hydrogen bond with the phosphate of the cofactor PLP. A proton moves towards the PLP phosphate. Thereby the substrate amino group becomes a nucleophile. (2) The amino group of the substrate attacks the DSD-PLP Schiff base carbon (C4′) and forms the geminal diamine intermediate. (3) K118 is released and the PLP-Serine aldimine is produced. The PLP phosphate acts as a general acid to donate the proton to the OG of the serine molecule. Abstraction of the α-hydrogen from Cα by Thr168 occurs in a concerted fashion, (4) A water molecule is released and the PLP-aminoacrylate intermediate is formed. (5) K118 attacks the C4′ and the ternary complex is produced. (6) Aminoacrylate is released and the DSD-PLP Schiff base is formed again to start the next catalytic cycle.
Fig. 8.
Modeling of the active site of DSD with the substrate D-serine. Model of the PLP cofactor loaded with D-serine (shown with C-atoms colored in cyan) in the active site of D-serine dehydratase. Surrounding residues of the dehydratase are shown as sticks (with C-atoms colored in green), hydrogen bonds between the cofactor::D-serine complex and the dehydratase are shown as stippled lines in magenta. Residues participating in the coordination of PLP and/or the D-serine residue are indicated. Aspartate238, involved in the dehydration mechanism is coordinated via a hydrogen bond with the hydroxyl group of the D-serine. L-serine attached to PLP would lack this hydrogen bond as its side chain is oriented away from the active site Asp238.
Alanine racemase is an example of a PLP-dependent enzyme with unique features of its active site. The pyridine nitrogen of the cofactor is unprotonated because it accepts a hydrogen bond from Arg219. This structural feature makes it difficult to form a charge-delocalized quinonoid intermediate as in other PLP-dependent enzymes, yet alanine racemase can still effectively lower the α-amino carbon acidity and enhance the reaction rate of proton transfer reactions [51,52].
In the two structures of D-serine deaminase from S. typhimurium reported by Bharath and coworkers [5], no significant electron density was observed for the cofactor PLP, indicating that the enzyme has a low affinity for the cofactor or even represents the apoenzyme form, under the crystallization conditions used. Electron density corresponding to a plausible sodium ion was seen near the active site of the closed conformation of the deaminase. It is not quite clear why the authors use sodium and not potassium ions during crystallization. The reason might be that the enzymatic activity of the S. typhimurium enzyme with D-serine as substrate is higher in the presence of Na+ than K+ ions. We used potassium in all steps of the protein purification of DSD from E. coli [53] and thus think that the electron densities observed for the monovalent cation binding sites are occupied by potassium. Future studies including site-directed mutagenesis of amino acid residues, such as Asp238, Thr168 and Ser167, will shed additional light on their involvement in the catalytic mechanism.
Acknowledgement
We thank Prof. Manfred Christl, Institute of Organic Chemistry, University of Würzburg, for his help with the construction of Fig. 9.
We acknowledge the use of the following synchrotron facilities: SRS Daresbury beamline 10.1, EMBL/DESY beamline X31 and MPG/DESY beamline BW6.
Abbreviations
- DSD
D-serine dehydratase
- PLP
pyridoxal 5′-phosphate
- LSD
rat liver L-serine dehydratase
- OASS
O-acetylserine sulfhydrylase
- TDH
L-threonine dehydratase
- SR
serine racemase
- TPS
tryptophan synthase
- CBS
cysteine β-synthase
- NMDA
N-methyl-D-aspartate
- DTE
dithioerythritol
- TAPS
N-Tris(hydroxymethyl) methyl-3-propane sulfonic acid
References
- [1].Dowhan W Jr., Snell EE, D-Serine dehydratase from Escherichia coli. II. Analytical studies and subunit structure, J. Biol. Chem. 245 (1970) 4618–4628. [PubMed] [Google Scholar]
- [2].Maas WK, Davis BD, Pantothenate studies. I. Interference by D-serine and L-aspartic acid with pantothenate synthesis in Escherichia coli, J. Bacteriol. 60 (1950) 733–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Huang YZ, Snell EE, D-Serine dehydratase from Escherichia coli. IV. Comparative sequences of pyridoxylpeptides derived from the active site and from the inhibitory site of the enzyme, J. Biol. Chem. 247 (1972) 7358–7364. [PubMed] [Google Scholar]
- [4].Grishin NV, Phillips MA, Goldsmith EJ, Modeling of the spatial structure of eukaryotic ornithine decarboxylases, Protein Sci. 4 (1995) 1291–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Obmolova G, Tepliakov A, Harutyunyan E, Wahler GG, Schnackerz KD, Crystallization and preliminary X-ray studies of D-serine dehydratase from Escherichia coli, J. Mol. Biol. 214 (1990) 641–642. [DOI] [PubMed] [Google Scholar]
- [6].Tanaka H, Senda M, Venugopalan N, Yamamoto A, Senda T, Ishida T, Horiike K, Crystal structure of a zinc-dependent D-serine dehydratase from chicken kidney, J. Biol. Chem. 286 (2011) 27548–27558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ito T, Hemmi H, Kataoka K, Mukai Y, Yoshimura T, A novel zinc-dependent D-serine dehydratase from Saccharomyces cerevisiae, Biochem. J. 409 (2008) 399–406. [DOI] [PubMed] [Google Scholar]
- [8].Bharath SR, Bisht S, Savithri HS, Murthy MRN, Crystal structures of open and closed forms of D-serine deaminase from Salmonella typhimurium — implications on substrate specificity and catalysis, FEBS J. 278 (2011) 2879–2891. [DOI] [PubMed] [Google Scholar]
- [9].Marceau M, McFall E, Lewis SD, Shafer JA, D-Serine dehydratase from Escherichia coli. DNA sequences and identification of catalytically inactive glycine to aspartic acid variants, J. Biol. Chem. 263 (1988) 16926–16933. [PubMed] [Google Scholar]
- [10].Schiltz E, Schnackerz KD, Sequence studies on D-serine dehydratase of Escherichia coli. Primary structure of the tryptic phosphopyridoxyl peptide and of the N-terminus, Eur. J. Biochem. 71 (1976) 109–116. [DOI] [PubMed] [Google Scholar]
- [11].Vagin AA, Teplyakov A, MOLREP: an automated program for molecular replacement, J. Appl. Crystallogr. 30 (1997) 1022. [Google Scholar]
- [12].Gallagher DT, Gilliland GL, Xiao G, Zondlo J, Fisher KE, Chinchilla D, Eisenstein E, Structure and control of pyridoxal phosphate dependent allosteric threonine deaminase, Structure 6 (1998) 465–475. [DOI] [PubMed] [Google Scholar]
- [13].CCP4. Collaborative Computational Project, Number 4, The CCP4 suite: programs for protein crystallography, Acta Crystallogr. D50 (1994) 760–763. [DOI] [PubMed] [Google Scholar]
- [14].Murshudov GN, Vagin AA, Dodson EJ, Refinement of macromolecular structures by the maximum-likelihood method, Acta Crystallogr. D Biol. Crystallogr. 53 (1997) 240–255. [DOI] [PubMed] [Google Scholar]
- [15].Cowtan K, Main P, Miscellaneous algorithms for density modification, Acta Crystallogr. D Biol. Crystallogr. 54 (1998) 487–493. [DOI] [PubMed] [Google Scholar]
- [16].Lamzin VS, Wilson KS, Automated refinement of protein models, Acta Crystallogr. D Biol. Crystallogr. 49 (1993) 129–147. [DOI] [PubMed] [Google Scholar]
- [17].Jones TA, Zou JY, Cowan SW, Kjeldgaard, Improved methods for building protein models in electron density maps and the location of errors in these models, Acta Crystallogr. A 47 (1991) 110–119. [DOI] [PubMed] [Google Scholar]
- [18].Schnackerz KD, Feldmann K, Hull WE, Phosphorus-31 nuclear magnetic resonance study of D-serine dehydratase: pyridoxal phosphate binding site, Biochemistry 18 (1979) 1536–1539. [DOI] [PubMed] [Google Scholar]
- [19].Goto M, Yamauchi T, Kamiya N, Miyahara I, Yoshimura T, Mihara H, Kurihara T, Hirotsu K, Esaki N, Crystal structure of a homolog of mammalian serine racemase from Schizosaccharomyces pombe, J. Biol. Chem. 284 (2009) 25944–25952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Yamada T, Komoto J, Takata Y, Ogawa H, Pitot HC, Takusagawa F, Crystal structure of serine dehydratase from rat liver, Biochemistry 42 (2003) 12854–12865. [DOI] [PubMed] [Google Scholar]
- [21].Schnackerz KD, Keller JW, Phillips RS, Toney MD, Ionization state of pyridoxal 5′-phosphate in D-serine dehydratase, dialkylglycine decarboxylase, and tyrosine phenol-lyase and the influence of monovalent cations as inferred by 31P NMR spectroscpopy, Biochim. Biophys. Acta 1764 (2006) 230–238. [DOI] [PubMed] [Google Scholar]
- [22].Diekers AT, Niks D, Schlichting I, Dunn MF, Tryptophan synthase: structure and function of the monovalent cation site, Biochemistry 48 (2009) 10997–11010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hashimoto A, Nishikawa T, Hayashi T, Fujii N, Harada K, Oka T, Takashi K, The presence of free D-serine in rat brain, FEBS Lett. 296 (1992) 33–36. [DOI] [PubMed] [Google Scholar]
- [24].Wolosker H, Dumin E, Balan L, Foltyn VN, D-Amino acids in the brain: D-serine in neurotransmission and neurodegeneration, FEBS J. 275 (2008) 3514–3526. [DOI] [PubMed] [Google Scholar]
- [25].Gustafson EC, Stevens ER, Wolosker H, Miller RF, Endogeneous D-serine contributes to NMDA-receptor-mediated light-evoked responses in the vertebrate retina, J. Neurophysiol. 98 (2007) 122–130. [DOI] [PubMed] [Google Scholar]
- [26].Hashimoto K, Fukushima T, Shimizu T, Komatsu N, Watanabe H, Shinoda N, Nakazato M, Kumakiri C, Okada S, Hasegawa H, Imai H, Iyo M, Decreased serum levels of D-serine in patients with schizophrenia: evidence in support of the N-methyl-D-aspartate receptor hypofunction hypothesis of schizophrenia, Arch. Gen. Psychiatry 60 (2003) 572–576. [DOI] [PubMed] [Google Scholar]
- [27].Wolosker H, Blackshaw S, Synder SH, Serine racemase: a glial enzyme synthesizing D-serine to regulate glutamate-N-methyl-D-aspartate neurotransmission, Proc. Natl. Acad. Sci. U. S. A. 96 (1999) 13409–13414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Yoshimura T, Goto M, D-Amino acids in the brain: structure and function of pyridoxal phosphate-dependent amino acid racemases, FEBS J. 275 (2008) 3527–3537. [DOI] [PubMed] [Google Scholar]
- [29].Smith M, Mack V, Ebneth A, Moraes I, Felicetti B, Wood M, Schonfeld D, Mather O, Cesura A, Barker J, The structure of mamalian serine racemase: evidence for conformational changes upon inhibitor binding, J. Biol. Chem. 285 (2010) 12873–12881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Lim Y-H, Yokoigawa K, Esaki N, Soda K, A new amino acid racemase with threonine-alpha-epimerase activity from Pseudomonas putida, J. Bacteriol. 175 (1993) 4213–4217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Arias CA, Weisner J, mM J Blackburn PE Reynolds, Serine and alanine racemase activities of VanT: a protein necessary for vancomycin resistance in Enterococcus gallinarum BM4174, Microbiology 146 (2000) 1727–1734. [DOI] [PubMed] [Google Scholar]
- [32].Dowhan W Jr., Snell EE, D-serine dehydratase from Escherichia coli. III. Resolution of pyridoxal 5′-phosphate and coenzymespecificity, J. Biol. Chem. 245 (1970) 4629–4635. [PubMed] [Google Scholar]
- [33].Schnackerz KD, Ehrlich J, Giesemann W, Reed TA, Mechanism of action of D-serine dehydratase. Identification of a transient intermediate, Biochemistry 18 (1979) 3557–3563. [DOI] [PubMed] [Google Scholar]
- [34].Schmitt W, Schiltz E, Sequence of Escherichia coli D-serine dehydratase. Location of the pyridoxal-phosphate binding site, FEBS Lett. 134 (1981) 57–62. [DOI] [PubMed] [Google Scholar]
- [35].Ehrlich JH, Schnackerz KD, Transient intermediates in D-serine dehydratase catalysis, Z. Physiol. Chem. 354 (1972) 1183. [Google Scholar]
- [36].Schnackerz KD, Ehrlich JH, Wipf A, Wirths GG, Fluorescence and CD measurements on D-serine apodehydratase reconstituted with analogs of pyridoxal 5′-phosphate, Z. Physiol. Chem. 354 (1973) 1241. [Google Scholar]
- [37].Federiuk CS, Shafer JA, A reaction pathway for transimination of the pyridoxal 5′-phosphate in D-serine dehydrates by amino acids, J. Biol. Chem. 258 (1983) 5372–5378. [PubMed] [Google Scholar]
- [38].McClure GD, Cook PF, Product binding to the alpha-carboxyl subsite results in a conformational change at the active site of O-acetylserine sulfhydrylase-A: evidence from fluorescence spectroscopy, Biochemistry 33 (1994) 1674–1683. [DOI] [PubMed] [Google Scholar]
- [39].Schnackerz KD, Tai CH, Pötsch RKW, Cook PF, Substitution of pyridoxal 5′-phosphate in D-serine dehydratase from Escherichia coli by cofactor analogues provides information on cofactor binding and catalysis, J. Biol. Chem. 274 (1999) 36935–36943. [DOI] [PubMed] [Google Scholar]
- [40].Bakardjieva A, Schnackerz KD, Modification of tryptophan residues of D-serine dehydratase from E. coli by Koshland reagent, Abstract 04–3-390, International Congr. Biochemistry, Hamburg 1976, 1976. [Google Scholar]
- [41].Toney MD, Hohenester E, Cowan SW, Jansonius JN, Dialkylglycine decarboxylase structure: bifunctional active site and alkali metal sites, Science 261 (1993) 765–759. [DOI] [PubMed] [Google Scholar]
- [42].Cook PF, Hara S, Nalabolu S, Schnackerz KD, pH dependence of the absorbance and 31P NMR spectra of O-acetylserine sulfhydrylase in the absence and presence of O-acetyl-L-serine, Biochemistry 31 (1992) 2298–2303. [DOI] [PubMed] [Google Scholar]
- [43].Hyde CC, Ahmed SA, Padlan EA, Miles EW, Davies DR, Three-dimensional structure of the typtophan synthase alpha 2 beta 2 multienzyme complex from Salmonella typhimurium, J. Biol. Chem. 263 (1988) 17857–17871. [PubMed] [Google Scholar]
- [44].Burkhard P, Rao GSJ, Hohenester E, Schnackerz KD, Cook PF, Jansonius JN, Three-dimenional structure of O-acetylserine sulfhydrylase from Salmonella typhimurium, J. Mol. Biol. 283 (1998) 121–133. [DOI] [PubMed] [Google Scholar]
- [45].Sun L, Bartlam M, Liu Y, Pang H, Rao Z, Crystal structure of the pyridoxal 5′-phopshate-dependent serine dehydratase from human liver, Protein Sci. 14 (2005) 791–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Meier M, Janosik M, Kery V, Kraus JP, Burkhard P, Structure of human cystathionine beta-synthase: a unique pyridoxal 5′-phosphate-dependent heme protein, EMBO J. 20 (2001) 3910–3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Omi R, Goto M, Miyahara I, Mizuguchi H, Hayashi H, Kagamiyama H, Hirotsu K, Crystal structures of threonine synthase from Thermus thermophilus HB8: confornational change, substrate recognition, and mechanism, J. Biol. Chem. 278 (2003) 46035–46045. [DOI] [PubMed] [Google Scholar]
- [48].Marceau M, Lewis SD, Kojiro CL, Mountjoy K, Shafer JA, Disruption of the active site interactions with pyridoxal 5′-phosphate and substrates by conservative replacements in the glycine-rich loop of Escherichia coli D-serine dehydratase, J. Biol. Chem. 265 (1990) 20421–20429. [PubMed] [Google Scholar]
- [49].Hwang BY, Cho BK, Yun H, Koteshwar K, Kim BG, Revisit of aminotransferases in the genomic era and its application to biocatalysis, J. Mol. Catal B, Enzymatic 37 (2005) 47–55. [Google Scholar]
- [50].Palm D, Klein HW, Schinzel R, Bühner M, Helmreich EJM, The role of pyridoxal 5′-phosphate in glycogen phosphorylase catalysis, Biochemistry 29 (1990) 645–661. [DOI] [PubMed] [Google Scholar]
- [51].Major DT, Gao J, A combined quantum mechnicaland molecular mechanical study of reaction mechanism and α-amino acidity in alanine racemase, J. Am. Chem. Soc. 128 (2006) 16345–16357. [DOI] [PubMed] [Google Scholar]
- [52].Griswold WR, Toney MD, Role of the pyridine nitrogen in pyridoxal 5′-phosphate catalysis: activity of three classes of PLP enzymes reconstituted with deazapyridoxal 5′-phosphate, J. Am. Chem. Soc. 133 (2011) 4823–4830. [DOI] [PubMed] [Google Scholar]
- [53].Dupourque D, Newton WA, Snell EE, Purification and properties of D-serine dehydratase from Escherichia coli, J. Biol. Chem. 241 (1966) 1233–1238. [PubMed] [Google Scholar]