Abstract
Background and aims
Coronavirus disease 2019 (COVID-19) pandemic is ongoing. Except for lung injury, it is possible that COVID-19 patients develop liver injury. Thus, we conducted a systematic review and meta-analysis to explore the incidence, risk factors, and prognosis of abnormal liver biochemical tests in COVID-19 patients.
Methods
PubMed, Embase, Cochrane Library, China National Knowledge Infrastructure (CNKI), VIP, and Wanfang databases were searched. The incidence of abnormal liver biochemical tests, including aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), gamma-glutamyl transpeptidase (GGT), total bilirubin (TBIL), and albumin (ALB), was pooled. Risk ratio (RR) was calculated to explore the association of abnormal liver biochemical tests with severity and prognosis of COVID-19 patients.
Results
Forty-five studies were included. The pooled incidence of any abnormal liver biochemical indicator at admission and during hospitalization was 27.2% and 36%, respectively. Among the abnormal liver biochemical indicators observed at admission, abnormal ALB was the most common, followed by GGT, AST, ALT, TBIL, and ALP (39.8%, 35.8%, 21.8%, 20.4%, 8.8%, and 4.7%). Among the abnormal liver biochemical indicators observed during hospitalization, abnormal ALT was more common than AST and TBIL (38.4%, 28.1%, and 23.2%). Severe and/or critical patients had a significantly higher pooled incidence of abnormal liver biochemical indicators at admission than mild and/or moderate patients. Non-survivors had a significantly higher incidence of abnormal liver biochemical indicators than survivors (RR = 1.34, p = 0.04).
Conclusions
Abnormal liver biochemical tests are common in COVID-19 patients. Liver biochemical indicators are closely related to the severity and prognosis of COVID-19 patients.
Electronic supplementary material
The online version of this article (10.1007/s12072-020-10074-6) contains supplementary material, which is available to authorized users.
Keywords: COVID-19, SARS-CoV-2, Hepatic, Liver, Incidence, Risk
Introduction
Until May 6, 2020, coronavirus disease 2019 (COVID-19) has posed a serious threat to global public health with a total of 3,272,202 confirmed cases and 230,104 deaths documented in 212 countries [1]. Its pathogen is named the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [2]. Except for fever, cough, and dyspnea as the major clinical presentations [3], COVID-19 patients may also develop different degrees of liver injury [4, 5]. The major mechanism of liver injury in COVID-19 patients is thought to be the binding of SARS-CoV-2 to angiotensin converting enzyme 2 (ACE2) receptor [6], which is highly expressed in bile duct cells [7], and then damages bile duct cells, thereby resulting in abnormal liver biochemical tests reflected by elevated alkaline phosphatase (ALP) and gamma-glutamyl transpeptidase (GGT) [8]. In addition, liver injury in SARS-CoV-2 infection may be caused by either systemic inflammation response or drug hepatotoxicity, which is supported by the first autopsy pathological analysis of a COVID-19 patient showing moderate microvesicular steatosis and mild lobular and portal activity in the liver tissue [9]. Multiple organ failure (MOF) is another possible cause of liver injury in COVID-19 patients, as SARS-CoV-2 can cause acute respiratory distress syndrome (ARDS) and MOF, thereby leading to hepatic ischemia, which could be worsened by the use of vasopressor medications, and hypoxia reperfusion injury in critically ill patients [10, 11]. Indeed, evidence also suggested that critically ill patients have a higher proportion of liver enzyme abnormality than patients with mild disease [8].
It is important for physicians, especially hepatologists, to appreciate the epidemiology and potential risk of liver injury in COVID-19 patients [12, 13]. However, the data regarding abnormal liver biochemical tests in COVID-19 patients are often heterogeneous among studies, and assessing the risk of liver injury in such patients remains challenging. In this study, we have systematically collected the current evidence with two major objectives: (1) to achieve more generalizable conclusions regarding the incidence of abnormal liver biochemical tests in COVID-19 patients; and (2) to explore the relationships of abnormal liver biochemical indicators with the severity and prognosis of COVID-19 patients.
Methods
This meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement and the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) guidelines. The MOOSE and PRISMA checklists are shown in the Supplementary Materials.
Search strategy
All published literature which reported liver biochemical tests in COVID-19 patients were identified via the PubMed, Embase, Cochrane Library, China National Knowledge Infrastructure (CNKI), VIP, and Wanfang databases. The search terms were (“2019 novel coronavirus-infected pneumonia” OR “COVID-19” OR “2019 novel coronavirus” OR “2019 novel coronavirus pneumonia” OR “severe acute respiratory syndrome coronavirus 2” OR “SARS-CoV-2” OR “novel coronavirus pneumonia”) AND (“liver” OR “hepatic”). The last retrieval date was April 27, 2020.
Study selection
There was neither publication language nor publication status restriction. All eligible studies reported the incidence and/or risk factors of abnormal liver biochemical tests in COVID-19 patients. Exclusion criteria were as follows: (1) duplicates; (2) case reports, reviews or meta-analyses, guidelines, consensus, experimental or animal studies, comments or letters, notes, and correspondences; (3) irrelevant papers; (4) absence of detailed data; (5) duplicate study population.
Data extraction
Data extraction was performed by 2 investigators. The following data were extracted from the included studies: the first author, publication year, region, source of cases, enrollment period, cases with COVID-19, age, gender, history of pre-existing liver diseases, treatments, clinical outcomes, and liver biochemical tests at admission or during hospitalization. Liver biochemical indicators analyzed included alanine aminotransferase (ALT), aspartate aminotransferase (AST), ALP, GGT, total bilirubin (TBIL), and albumin (ALB).
Study quality
The quality of cohort studies was assessed using the Newcastle–Ottawa Scale (NOS), which includes study selection (four items), comparability (two items), and exposure/outcome (three items). The highest score is 9 points, and a score of more than 6 points is considered high quality.
Definitions
The definition regarding the severity of COVID-19 patients was inconsistent among these included studies. Among them, 36 studies employed the definitions from the Chinese practice guidelines regarding diagnosis and treatment of novel coronavirus pneumonia, in which patients were divided into four subtypes (mild, moderate, severe, and critical); 4 studies employed the definitions from the American Thoracic Society guidelines, in which patients were categorized into severe and non-severe types; and the remaining 5 studies did not clearly report the definition regarding the severity of COVID-19 patients. Therefore, as for the present systematic review, the severity of COVID-19 patients was mainly dependent upon the definitions from each individual study.
The time when liver biochemical indicators were measured during hospitalization was not strictly defined in the present systematic review, because it was not strictly or consistently defined among these included studies. Generally, liver biochemical indicators at admission refer to those measured at admission or within 24 h after admission; and liver biochemical indicators during hospitalization refer to those measured at any time during hospitalization.
Statistical analysis
All statistical analyses were performed using StatsDirect statistical software version 2.8.0 (StatsDirect Ltd., Sale, Cheshire, UK), STATA version 12.0 (Stata Corp., College Station, Texas, USA), and Review Manager software version 5.3 (Cochrane collaboration, the Nordic Cochrane Centre, Copenhagen, Denmark). First, the incidence of abnormal liver biochemical tests from each study were pooled, and the pooled proportion with 95% confidence interval (CI) was calculated. Second, we calculated the risk ratios (RRs) or mean differences (MDs) with 95% CIs. The meta-analyses were performed by using a random-effect model. Heterogeneity among the studies was also assessed. I2 > 50% and/or p < 0.1 were considered to have statistically significant heterogeneity. Publication bias was assessed with Egger test. p < 0.1 was considered as a statistically significant publication bias. Subgroup analyses were performed according to the source of cases (single-center versus multiple-center), sample size (≥ 100 versus < 100), NOS (≥ 7 versus < 7), proportion of male (≥ 50% versus < 50%), and proportion of patients with pre-existing liver disease (≥ 10% versus < 10%). Meta-regression analyses employed the covariates that were the same as the strata of subgroup analyses, including the source of cases, sample size, NOS, proportion of male, and proportion of patients with pre-existing liver disease. Sensitivity analyses were conducted by sequentially excluding one study at a time. These analyses were performed to explore the sources of heterogeneity among studies.
Results
Study selection
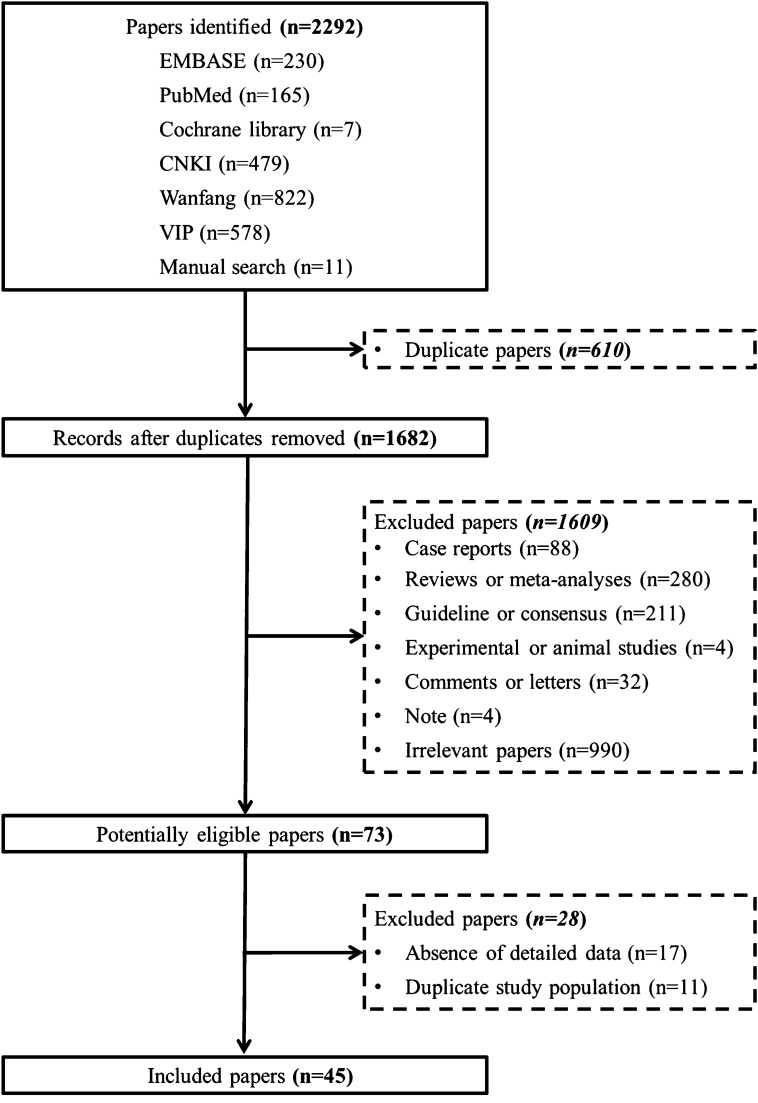
Overall, 2281 papers were identified via the 6 databases, and 11 papers were identified via a manual search. Finally, 45 studies were included in this meta-analysis (Fig. 1) [14–58].
Fig. 1.
Flow chart of study selection
Study characteristics
Characteristics of the included studies are summarized in Table 1. Among them, 30 studies [15, 16, 19, 21, 23, 25–30, 33–37, 39–41,43–46, 49, 50, 52–56] were formally published as full texts, 9 studies [18, 24, 31, 32, 38, 42, 47, 48, 51] were published in press, and the remaining 6 studies [14, 17, 20, 22, 57, 58] were preprinted. The number of COVID-19 patients ranged from 18 to 1099 among these included studies. Nearly all of these included studies (44/45) were conducted in China, and the remaining one in Singapore; 33 [14–16, 18, 20–22, 24–26, 28–35, 38, 39, 41, 43–47, 49–52, 54, 56, 58] and 12 studies [17, 19, 23, 27, 36, 37, 40, 42, 48, 53, 55, 57] were single-center and multi-center studies, respectively. Treatment and clinical outcomes of COVID-19 patients are summarized in Supplementary Table 1.
Table 1.
An overview of included papers regarding COVID-19
| First author (year) | Country | Source of cases | Enrollment period | Sample size (n) | Age, years Median (IQR) | Male No. (%) | History of liver diseases No. (%) | Any abnormal liver biochemical indicator at admission or during hospitalization | Abnormal liver biochemical indicator at admission or during hospitalization No. (%) |
|---|---|---|---|---|---|---|---|---|---|
| Zhang (2020) [14] | China | Renmin Hospital, Wuhan University | 2020.01.11–2020.02.10 | 82 | 72.5 (65–80) | 54 (65.9%) | 2 (2.4%) | 64/82d | Abnormal ALT 22 (30.6%); Abnormal AST 44 (61.1%); Abnormal TBIL 22 (30.6%); Abnormal ALB 56 (77.8%) |
| Li (2020) [15] | China | Guangzhou Eighth People’s Hospital | 2020.01.24–2020.02.25 | 82 | 44.8 ± 15.6b | 48 (58.5%) | NA | 38/82 | Abnormal ALT 12 (14.6%); Abnormal AST 10 (12.1%); Abnormal TBIL 11 (13.4%); Abnormal ALB 36 (43.9%) |
| Li (2020) [16] | China | Zhuzhou Central Hospital | 2020.01.20–2020.02.27 | 80 | 47.5 (3–90)a | 40 (50%) | 3 (3.8%) | NA | NA |
| Qi (2020) [17] | China | Three designated hospitals of Chongqing | 2020.01.19–2020.02.16 | 267 | 48 (35–65) | 149 (55.8%) | NA | NA | Abnormal ALT 20 (7.5%); Abnormal AST 19 (7.1%); Abnormal TBIL 6 (2.2%); Abnormal ALB 63 (23.6%) |
| Sun (2020) [18] | China | Tongji Hospital, Huazhong University of Science and Technology | 2020.02.09–2020.02.27 | 51 | NA | NA | 7 (13.7%) | 29/51 | Abnormal ALT 24 (47.1%); Abnormal AST 24 (47.1%); Abnormal ALB 26 (51%); Abnormal GGT 18 (35.3%); Abnormal ALP 7 (13.7%) |
| Guan (2020) [19] | China | 552 Hospitals | Till Jan. 29, 2020 | 1099 | 47 (35–58) | 637 (58.1%) | 23 (2.1%) | NA | Abnormal AST 168 (22.2%); Abnormal ALT 158 (21.3%); Abnormal TBIL 76 (10.5%) |
| Wang (2020) [20] | China | The Public Health Treatment Center of Changsha | 2020.01.17–2020.02.20 | 242 | 45 (1-84)a | 119 (49.2%) | 12 (5%) | NA | NA |
| Huang (2020) [21] | China | Jinyintan Hospital | 2019.12.16–2020.01.02 | 41 | 49 (41–58) | 30 (73%) | 1 (2.4%) | NA | Abnormal AST 15 (36.6%) |
| Huang (2020) [22] | China | Fifth Hospital of Wuhan | 2020.01.21–2020.02.10 | 36 | 69.22 ± 9.64b | 25 (69.44%) | NA | 22/36 | Abnormal ALT 4 (13.3%); Abnormal AST 18 (58.1%); Abnormal TBIL 4 (12.9%); Abnormal ALB 25 (80.6%) |
| Jin (2020) [23] | China | Designated hospitals in Zhejiang | 2020.01.17–2020.02.08 | 651 | NA | 301 (46.2%) | 25 (3.8%) | 64/651d | NA |
| Wen (2020) [24] | China | Fifth Medical Center of the PLA General Hospital | 2020.01.20–2020.02.08 | 46 | 41.8 ± 16.3b | 27 (58.7%) | NA | 8/46 | Abnormal ALT 8 (17.4%); Abnormal AST 5 (10.9%); Abnormal ALB 1 (2.2%) |
| Yang (2020) [25] | China | Nanjing Public Health Center | 2020.01.01 to date | 57 | 37 (5–97)a | 29 (50.9%) | NA | 9/57 | Abnormal ALT 9 (15.8%); Abnormal AST 4 (7%) |
| Cheng (2020) [26] | China | Jinyintan Hospital | 2020.01.01–2020.02.06 | 463 | 51 (43–60) | 244 (52.7%) | 22 (4.8%) | NA | Abnormal ALT 114 (24.6%); Abnormal TBIL 19 (4.1%); Abnormal ALB 125 (27%) |
| Liu (2020) [27] | China | Seven designated hospitals of China | 2020.01.23–2020.02.03 | 32 | 38.50 (26.25–45.75) | 20 (62.5%) | 1 (3.1%) | NA | Abnormal ALTc 9 (28.1%); Abnormal ASTc 2 (6.25%) |
| Liu (2020) [28] | China | The Affiliated Hospital of Jianghan University | 2020.01.10–2020.01.30 | 30 | 35 ± 8b | 10 (33%) | NA | 7/30 | Abnormal AST and ALT 7 (23.3%) |
| Xu (2020) [29] | China | Fifth People’s Hospital of Xinyang | 2020.01.22–2020.01.29 | 23 | 46 (40.5–52) | 15 (65.2%) | NA | 5/23 | Abnormal ALT 5 (21.7%); Abnormal AST 5 (21.7%) |
| Qian (2020) [30] | China | Shanghai Public Health Clinical Center | 2020.01.20–2020.02.24 | 324 | 51 (36–64) | 167 (51.5%) | 90 (27.8%) | NA | Abnormal ALT 51 (15.7%); Abnormal TBIL 21 (6.5%); Abnormal ALB 136 (42%); Abnormal ALP 4 (1.2%) |
| Zhao (2020) [31] | China | The Fourth People’s Hospital of Nanning | 2020.01.22–2020.02.05 | 28 | 44.5 (11–68)a | 11 (39.3%) | NA | NA | Abnormal AST 3 (10.7%); Abnormal ALT 6 (21.4%) |
| Wang (2020) [32] | China | Tongji Hospital, Huazhong University of Science and Technology | 2020.01.10–2020.02.14 | 333 | NA | NA | 12 (3.6%) |
89/333 43/333d |
Abnormal ALT or AST 89 (26.7%); Abnormal TBILc 13 (3.9%); Abnormal ALB 82 (24.6%) |
| Zhong (2020) [33] | China | Hainan General Hospital | 2020.01.21–2020.02.10 | 62 | (51.8 ± 13.5)b | 40 (64.5%) | NA | 10/52 | Abnormal ALT 6 (11.5%); Abnormal AST 4 (7.7%); Abnormal ALB 8 (15.4%) |
| Xiang (2020) [34] | China | The First Affiliated Hospital of Nanchang University | 2020.01.21–2020.01.27 | 49 | 42.9 (18–78) | 33 (67.3) | 6 (12.2%) | NA | NA |
| Wan (2020) [35] | China | Chongqing University Three Gorges Hospital | 2020.01.23–2020.02.08 | 135 | 47 (36–55) | 72 (53.3%) | 2 (1.5%) | NA | Abnormal AST 30 (22.2%) |
| Xu (2020) [36] | China | Seven designated tertiary hospitals of Zhejiang | 2020.01.10–2020.01.26 | 62 | 41 (32–52) | 35 (56%) | 7 (11.3%) | NA | Abnormal AST 10 (16.1%) |
| Yang (2020) [37] | China | Three tertiary hospitals of Wenzhou | 2020.01.17–2020.02.10 | 149 | 45.11 ± 13.35b | 81(54.4%) | NA | NA | Abnormal AST 27 (18.1%); Abnormal ALT 18 (12.1%); Abnormal TBIL 4 (2.7%); Abnormal ALB 9 (6%) |
| Yao (2020) [38] | China | Tangdu Hospital | 2020.01.21–2020.02.21 | 40 | 53.87 ± 15.84b | 25 (62.5%) | 3 (7.5%) |
0 22/40d |
Abnormal ALTc 21 (52.5%); Abnormal ASTc 16 (40%); Abnormal TBILc 10 (25%) |
| Ma (2020) [39] | China | Wuhan Children’s Hospital | NA | 115 | 51 day–15 year | 74 (63.5%) | NA | NA | Abnormal ALT 11 (9.6%); Abnormal TBIL 3 (2.6%) |
| Young (2020) [40] | Singapore | Four hospitals in Singapore | 2020.01.23–2020.02.03 | 18 | 47 (31–73)b | 9 (50%) | NA | 3/18d | NA |
| Zhou (2020) [41] | China | The First People’s Hospital of Xianning City | 2019.12–2020.02 | 107 | NA | NA | NA | NA | Abnormal ALT 25 (23%); Abnormal AST 23 (21%) |
| Zhao (2020) [42] | China | The Second Affiliated Hospital of Anhui Medical University and Suzhou Municipal Hospital in Anhui | 2020.01.23–2020.02.05 | 19 | 48 (27–56) | 11 (57.9%) | 1 (5.3%) | NA | Abnormal AST 5 (27.8%); Abnormal ALT 5 (27.8%); Abnormal GGT 8 (44.4%) |
| Du (2020) [43] | China | Wuhan Pulmonary Hospital | 2019.12.25–2020.02.07 | 179 | 57.6 ± 13.7b | 97 (54.2%) | NA | 57/179 | Abnormal AST 57 (31.8%) |
| Wang (2020) [44] | China | Renmin Hospital of Wuhan University | 2020.01.01–2020.02.06 | 339 | 69 (65–76) | 166 (49%) | 2 (0.6%) | 96/334 | NA |
| Xie (2020) [45] | China | Jinyintan Hospital | 2020.02.02–2020.02.23 | 79 | 60 (48–66) | 44 (55.7%) | NA | NA | Abnormal ALT 25 (31.6%); Abnormal AST 28 (35.4%); Abnormal TBIL 4 (5.1%) |
| Pan (2020) [46] | China | Wuhan Hanan Hospital, Wuhan Union Hospital, and Huanggang Central Hospital | 2020.01.18–2020.02.28 | 204 | 52.91 ± 15.98b | 107 (52.5%) | 2 (1.9%) | NA | Abnormal ALT 27 (13.2%); Abnormal AST 22 (10.8%) |
| Cai (2020) [47] | China | The Third People’s Hospital of Shenzhen | 2020.01.11–2020.02.21 | 417 | 47 (34–60) | 198 (47.5%) | 21 (5%) |
192/417 318/417d |
Abnormal ALT 54 (12.9%); Abnormal AST 76 (18.2%); Abnormal TBIL 96 (23%); Abnormal ALP 16 (3.8%); Abnormal GGT 68 (16.3%); Abnormal ALT 187 (44.8%)c; Abnormal AST 150 (36%)c; Abnormal TBIL 204 (48.9%)c; Abnormal ALP 32 (7.7%)c; Abnormal GGT 155 (37.2%)c |
| Sun (2020) [48] | China | Designated hospitals in Nanyang City | 2020.01.24–2020.02.16 | 150 | 45 ± 16b | 67 (44.7%) | 1 (0.6%) | NA | Abnormal ALT 24 (16%); Abnormal AST 15 (10%); Abnormal TBIL 3 (2%) |
| Zheng (2020) [49] | China | North Hospital of Changsha first Hospital | 2020.01.17–2020.02.07 | 161 | 45 (33.5–57) | 80 (49.7%) | 4 (2.5%) | NA | Abnormal ALT 13 (8.1%); Abnormal AST 22 (13.7%); Abnormal TBIL 9 (5.6%) |
| Zhang (2020) [50] | China | Wuhan Xinzhou District People’s Hospital | 2020.01.16–2020.02.25 | 95 | 49 (39–58) | 53 (55.8%) | NA | NA | Abnormal ALT 52 (54.7%)c; Abnormal AST 45 (47.4%)c |
| Zhang (2020) [51] | China | Zhongnan Hospital of Wuhan University | 2020.01.18–2020.02.22 | 115 | 49.52 ± 17.06b | 49 (42.60%) | NA | NA | Abnormal ALT 11 (9.6%); Abnormal AST 17 (14.8%); Abnormal TBIL 8 (6.96%); Abnormal ALP 6 (5.21%); Abnormal GGT 15 (13%); Abnormal ALB 63 (54.78%) |
| Fang (2020) [52] | China |
Infectious Hospital of Anhui Provincial Hospital |
2020.01.22–2020.02.19 | 79 | 45. 1 ± 16. 6b | 45 (57%) | 3 (3.8%) | NA | Abnormal ALT 12 (15.2%); Abnormal AST 9 (11.4%); Abnormal TBIL 20 (25.3%) |
| Li (2020) [53] | China | Designated hospitals in Bozhou | 2020.02.01–2020.02.12 | 28 | 42.2 ± 14.9b | 15 (53.6%) | NA | NA | Abnormal ALT 23 (82.1%); Abnormal AST 14 (50%); Abnormal GGT 23 (82.1%); Abnormal TBIL 2 (7.1%) |
| Wang (2020) [54] | China | Xixi Hospital in Hangzhou | 2020.01.23–2020.02.24 | 72 | NA | 32 (44.4%) | NA | 17/72 | NA |
| Yuan (2020) [55] | China | Chongqing Public Health Medical Treatment Center | 2020.01.24–2020.02.23 | 223 | 46.5 ± 16.1b | 106 (47.5%) | 8 (3.6%) | NA | Abnormal ALT 42 (18.8%); Abnormal AST 32 (14.3%) |
| Bai (2020) [56] | China | Wuhan Union Hospital | 2020.01.29–2020.02.26 | 58 | 62.12 ± 12.95b | 28 (48.3%) | NA | NA | Abnormal ALT 32 (55.2%); Abnormal AST 25 (43.1%); Abnormal ALB 43 (74.1%) |
| Huang (2020) [57] | China | Ten designated hospitals in Jiangsu | 2020.01.22–2020.02.10 | 221 | 45 (33.5–56) | 126 (57%) | 6 (2.7%) | NA | NA |
| Li (2020) [58] | China | Beijing You’an Hospital | 2020.01.21–2020.02.29 | 85 | 49 (36–64) | 47 (55.3%) | 6 (7%) |
21/85 12/85d |
Abnormal ALT 21 (24.7%); Abnormal ALT 12 (14.1%)c; Abnormal AST 12 (14.1%)c |
ALT alanine aminotransferase, AST aspartate aminotransferase, ALP alkaline phosphatase, GGT gamma-glutamyl transpeptidase, TBIL total bilirubin, ALB albumin, NA not available
aMedian (range)
bMean ± SD
cLiver biochemical indicator during hospitalization
dProportion of any abnormal liver biochemical indicator during hospitalization
Study quality
The NOS score ranged between 3 and 8 points. Eight studies [15, 16, 21, 23, 29, 36, 40, 50] were considered to be of high quality (Supplementary Table 2).
Incidence
The results of meta-analyses regarding the incidence of abnormal liver biochemical tests in COVID-19 patients are shown in Table 2.
Table 2.
Incidence of abnormal liver biochemical indicator: results of meta-analyses
| Groups | No. studies | Range (%) | Pooled proportion using random-effects model | Heterogeneity | Publication bias | |
|---|---|---|---|---|---|---|
| I2 | p | Egger:bias | ||||
| Any abnormal liver biochemical indicator at admission | 12 | 0–61.1 | 0.272 (95% CI, 0.190–0.363) | 88.6% (95% CI, 82.3–92%) | < 0.0001 | 4.611 (95% CI, – 0.262 to 9.483) |
| p = 0.0612 | ||||||
| Abnormal ALT at admission | 28 | 7.5–55.2 | 0.204 (95% CI, 0.168–0.243) | 88% (95% CI, 84.1–90.5%) | < 0.0001 | 2.650 (95% CI, 0.505–4.795) |
| p = 0.0174 | ||||||
| Abnormal AST at admission | 28 | 7–61.1 | 0.218 (95% CI, 0.176–0.263) | 89.3% (95% CI, 86.2–91.5%) | < 0.0001 | 2.890 (95% CI, 0.740–5.039) |
| p = 0.0104 | ||||||
| Abnormal ALP at admission | 4 | 1.2–13.7 | 0.047 (95% CI, 0.018–0.089) | 81.6% (95% CI, 28.9–91.1%) | 0.001 | 2.7486 (95% CI, – 1.591 to 7.088) |
| p = 0.1124 | ||||||
| Abnormal GGT at admission | 5 | 13–82.1 | 0.358 (95% CI, 0.178–0.561) | 94.2% (95% CI, 90–96.2%) | < 0.0001 | 5.229 (95% CI, – 3.173 to 13.631) |
| p = 0.142 | ||||||
| Abnormal TBIL at admission | 16 | 2.0–30.6 | 0.088 (95% CI, 0.055–0.128) | 92.1% (95% CI, 89.2–93.9%) | < 0.0001 | 3.183 (95% CI, 0.029–6.338) |
| p = 0.0482 | ||||||
| Abnormal ALB on admission | 16 | 2.2–80.6 | 0.398 (95% CI, 0.306–0.495) | 96.1% (95% CI, 95.2–96.8%) | < 0.0001 | 8.819 (95% CI, 2.302–15.335) |
| p = 0.0116 | ||||||
| Any abnormal liver biochemical indicator during hospitalization | 7 | 9.8–78.0 | 0.360 (95% CI, 0.118–0.648) | 99.2% (95% CI, 99–99.3%) | < 0.0001 | 7.738 (95% CI, – 13.782 to 29.258) |
| p = 0.3978 | ||||||
| Abnormal ALT during hospitalization | 5 | 14.1–54.7 | 0.384 (95% CI, 0.242–0.537) | 91.3% (95% CI, 82.5–94.6%) | < 0.0001 | – 0.620 (95% CI, – 14.725 to 13.486) |
| p = 0.8977 | ||||||
| Abnormal AST during hospitalization | 5 | 6.3–47.4 | 0.281 (95% CI, 0.159–0.422) | 90.6% (95% CI, 80.5–94.3%) | < 0.0001 | – 1.705 (95% CI, – 17.062 to 13.651) |
| p = 0.7471 | ||||||
| Abnormal TBIL during hospitalization | 3 | 3.9–48.9 | 0.232 (95% CI, 0.006–0.642) | 99.2% (95% CI, 98.9–99.3%) | < 0.0001 | NA |
| NA | ||||||
ALT alanine aminotransferase, AST aspartate aminotransferase, ALP alkaline phosphatase, GGT gammaglutamyltranspeptidase, TBIL total bilirubin, ALB albumin, NA not available, — the results cannot be calculated
Overall analyses
The pooled incidence of any abnormal liver biochemical indicator at admission was 27.2% (95% CI 19–36.3%). The pooled incidence of abnormal ALT, AST, ALP, GGT, TBIL, and ALB at admission was 20.4% (95% CI 16.8–24.3%), 21.8% (95% CI 17.6–26.3%), 4.7% (95% CI 1.8–8.9%), 35.8% (95% CI 17.8–56.1%), 8.8% (95% CI 5.5–12.8%), and 39.8% (95% CI 30.6–49.5%), respectively.
The pooled incidence of any abnormal liver biochemical indicator during hospitalization was 36% (95% CI 12.3–57.1%). The pooled incidence of abnormal ALT, AST, and TBIL during hospitalization was 38.4% (95% CI 24.2–53.7%), 28.1% (95% CI 15.9–42.2%), and 23.2% (95% CI 6–64.2%), respectively.
Subgroup analyses
The results of subgroup analyses are summarized in Supplementary Table 3. The heterogeneity remained statistically significant in all subgroup analyses.
Meta-regression analyses
The results of meta-regression analyses are summarized in Supplementary Table 4. As for any abnormal liver biochemical indicator observed at admission, meta-regression analyses indicated that the source of cases (p = 0.000) might be a potential source of heterogeneity. As for abnormal ALT observed at admission, meta-regression analyses indicated that the sample size (p = 0.019) might be the potential source of heterogeneity. As for any abnormal liver biochemical indicator observed during hospitalization, meta-regression analyses indicated that the proportion of patients with pre-existing liver disease (p = 0.028) might be a potential source of heterogeneity. However, no source of heterogeneity could be identified for other liver biochemical indicators observed at admission or during hospitalization.
Sensitivity analyses
Sensitivity analyses did not identify any study as the potential source of heterogeneity.
Risk factors
Non-severe versus severe according to the American Thoracic Society guideline
Two studies [17, 19] compared the incidence of abnormal ALT, AST, and TBIL at admission between severe and non-severe patients. Meta-analyses demonstrated that severe patients had a significantly higher incidence of abnormal AST at admission (RR = 2.91, 95% CI 1.36–6.22; p = 0.006). The incidence of abnormal ALT (RR = 2.32, 95% CI 0.78–6.88; p = 0.13) and TBIL (RR = 1.95, 95% CI 0.68–5.58; p = 0.21) at admission were not significantly different between severe and non-severe patients.
Mild and moderate versus severe and critical according to the Chinese practice guideline
Only one study [38] compared the incidence of any abnormal liver biochemical indicator at admission between mild and moderate patients versus severe and critical patients, and demonstrated that severe and critical patients had a significantly higher incidence of any abnormal liver biochemical indicator at admission (RR = 2.78, 95% CI 1.28–6.06; p = 0.01). Three studies [49, 51, 55] compared the incidence of abnormal ALT at admission between mild and moderate patients versus severe and critical patients, and demonstrated that severe and critical patients had a significantly higher incidence of abnormal ALT at admission (RR = 3.03, 95% CI 1.76–5.23; p < 0.0001). Four studies [35, 49, 51, 55] compared the incidence of abnormal AST at admission between mild and moderate patients versus severe and critical patients, and demonstrated that severe and critical patients had a significantly higher incidence of abnormal AST (RR = 3.84, 95% CI 2.53–5.82; p < 0.00001) at admission. Two studies [49, 51] compared the incidence of abnormal TBIL at admission between mild and moderate patients versus severe and critical patients, and demonstrated that severe and critical patients had a significantly higher incidence of abnormal TBIL at admission (RR = 3.16, 95% CI 1.24–8.09; p = 0.02).
Eight studies [16, 20, 30, 35, 49, 51, 55, 57] compared the ALT level at admission between mild and moderate patients versus severe and critical patients. Meta-analyses demonstrated that ALT level was significantly higher in severe and critical patients than in mild and moderate patients (MD = 7.64, 95% CI 1.94–13.35; p = 0.009).
Seven studies [16, 20, 30, 35, 49, 51, 55] compared the AST level at admission between mild and moderate patients versus severe and critical patients. Meta-analyses demonstrated that AST level was significantly higher in severe and critical patients than in mild and moderate patients (MD = 13.20, 95% CI 7.57–18.82; p < 0.00001). Seven studies [20, 30, 35, 49, 51, 55, 57] compared the TBIL level at admission between mild and moderate patients versus severe and critical patients. Meta-analyses demonstrated that TBIL level was lower in severe and critical patients than in mild and moderate patients (MD = − 0.22, 95% CI − 5.08 to 4.63; p = 0.93). Five studies [16, 30, 35, 55, 57] compared the ALB level at admission between mild and moderate patients versus severe and critical patients. Meta-analyses demonstrated that ALB level was significantly lower in severe and critical patients than in mild and moderate patients (MD = − 4.84, 95% CI − 6.68 to − 3.00; p < 0.00001).
Moderate versus severe and critical according to the Chinese practice guideline
Only one study [18] compared the incidence of any abnormal liver biochemical indicator at admission between moderate patients versus severe and critical patients, and demonstrated that severe and critical patients had a significantly higher incidence of any abnormal liver biochemical indicator at admission (RR = 1.91, 95% CI 1.17–3.1; p = 0.009).
Three studies [15, 34, 52] compared the ALT, AST, TBIL and ALB levels at admission between moderate patients and severe and critical patients. Meta-analyses demonstrated that the ALT (MD = 9.76, 95% CI 4.64–14.89; p = 0.0002), AST (MD = 6.32, 95% CI 2.90–9.74; p = 0.0003) and TBIL (MD = 3.14, 95% CI 1.14–5.14; p = 0.002) levels were significantly higher in severe and critical patients than in moderate patients, and the ALB level was significantly lower in severe and critical patients (MD = − 7.30, 95% CI − 8.69 to − 5.90; p < 0.00001).
Moderate versus severe according to the Chinese practice guideline
Five studies [26, 28, 29, 45, 54] compared the ALT and AST levels at admission between moderate and severe patients. Meta-analyses demonstrated that the ALT (MD = 11.99, 95% CI − 3.59 to 27.57; p = 0.13) and AST (MD = 10.30, 95% CI 0.11–20.49; p = 0.05) levels were higher in severe patients than in moderate patients. Three studies [26, 28, 54] compared the ALB level at admission between moderate and severe patients. Meta-analyses demonstrated that ALB level was significantly lower in severe patients than in moderate patients (MD = -4.62, 95% CI − 8.12 to − 1.13; p = 0.01). Two studies [26, 45] compared the TBIL level at admission between moderate and severe patients. Meta-analyses demonstrated that the TBIL level was lower in severe patients than in moderate patients (MD = − 0.12, 95% CI − 0.87 to 0.62; p = 0.75).
At admission versus during hospitalization
Two studies [32, 47] compared the ALT level detected at admission versus during hospitalization, and demonstrated that the ALT level detected during hospitalization was significantly higher than that obtained at admission (MD = 20.30, 95% CI 16.51–24.06; p < 0.00001).
The first week after admission versus the second week after admission
Only one study [38] compared the ALT level at the first week after admission versus the second week after admission, and demonstrated that ALT level detected at the second week after admission was significantly higher than that detected at the first week after admission (MD = 130.75, 95% CI 116.14–145.36; p < 0.00001).
Number of drug products combined
Only one study [38] compared the proportion of drug products ≥ 3 between patients with liver injury and those with normal liver biochemistry, and demonstrated that the proportion of drug products ≥ 3 was significantly higher in patients with liver injury than in those with normal liver biochemistry (RR = 9.00, 95% CI 1.28–63.26; p = 0.03).
Effect of liver biochemical indicators on prognosis of COVID-19 patients
Survivors versus non-survivors
Three studies [43, 44, 50] compared the incidence of any abnormal liver biochemical indicator between survivors versus non-survivors. Meta-analyses demonstrated that non-survivors had a significantly higher incidence of any abnormal liver biochemical indicator (RR = 1.34, 95% CI 1.02–1.77; p = 0.04).
With versus without composite endpoint
Two studies [19, 50] compared the incidence of abnormal ALT and AST between patients who achieved the composite endpoint versus those who did not achieve the composite endpoint. Both of them employed the same composite endpoint defined as admission to the ICU, mechanical ventilation, or death. Meta-analyses demonstrated that patients who achieved the composite endpoint had a significantly higher incidence of abnormal ALT (RR = 1.96, 95% CI 1.54–2.49; p < 0.00001) and AST (RR = 2.30, 95% CI 1.81–2.92; p < 0.00001).
Discussion
Our study suggested that the pooled incidence of any abnormal liver biochemical indicator detected during hospitalization seemed to be higher than that detected at admission (36% versus 27.2%). Except for the disease progression of COVID-19 during hospitalization, this might be partly due to the toxicity of drugs used during hospitalization. Patients are often given empirical antiviral therapy for COVID-19 after admission, of which some can be potentially hepatotoxic. At the time of this writing, recent studies have reported that lopinavir might be potentially effective against SARS-CoV-2 [59], but lopinavir/ritonavir is mainly metabolized by cytochrome P 450 3A4 (CYP3A4) enzymes in the liver, which is likely to cause elevated serum transaminases [60]. In addition, some patients may be treated with antipyretic agents during hospitalization, of which most contain acetaminophen that can be hepatotoxic in high doses and/or combination with other drugs and even cause liver failure [61].
Among the abnormal liver biochemical indicators observed at admission, abnormal ALB (39.8%) was the most frequent, followed by abnormal GGT (35.8%), AST (21.8%), ALT (20.4%), TBIL (8.8%), and ALP (4.7%). Decreased ALB level is usually considered to indicate that the synthetic function of the liver be damaged to some extent. However, a decline of ALB level may be related to the disease severity of COVID-19, as COVID-19 can cause pulmonary exudation, thereby leading to abnormal ALB distribution, and an insufficient intake of nutrients or impairment of normal utilization/metabolism of nutrients may also decrease the ALB level in COVID-19 patients. SARS-CoV-2 is prone to damage bile duct cells where the ACE2 is highly expressed [7]. Thus, abnormal GGT and ALP levels should have been more common. However, in the settings of liver injury, the de-differentiation and proliferation of ACE2-expressing bile duct epithelial cells are involved in liver tissue repair, and some newborn hepatocytes retain the characteristics of ACE2 expression and may be susceptible to SARS-CoV-2 [62], thereby affecting hepatocytes and presenting with abnormal AST and ALT levels. Both ALP and GGT are considered as cholangiocyte-related enzymes, but the pooled incidence of abnormal ALP seems to be remarkably higher than that of abnormal GGT (35.8% versus 4.7%). This counter-intuitive phenomenon may be attributed to a difference in the distribution of ALP and GGT. ALP is present in bile duct, bone, intestine, kidney, and placenta, while GGT is widely distributed in the cell membranes of many tissues, such as bile duct, kidney, pancreas, gallbladder, spleen, heart, brain, and seminal vesicle. Thus, GGT, as an indicator of bile duct injury, may be less sensitive than ALP.
Severe and critical COVID-19 patients and non-survivors are more prone to have abnormal liver biochemistry. This finding might be explained by several points, as follows. First, inflammatory factor storm is suspected to be associated with abnormal liver biochemistry in severe and critical COVID-19 patients. It has been recognized that the occurrence of MOF is mainly associated with the sudden initiation of an inflammatory storm in the critically ill patients [63]. The release of numerous inflammatory cytokines induces ARDS and systemic inflammatory response syndrome (SIRS) and subsequently causes hypoxia in the body, thereby leading to an injury in lung, liver, myocardium, and kidney. This may be aggravated by the use of vasopressor drugs to maintain blood pressure in an ICU setting. Second, hepatic ischemia and hypoxia reperfusion dysfunction may be one of the main mechanisms of liver injury in severe and critical COVID-19 patients. COVID-19 patients often have varied degrees of hypoxemia, of whom more than 40% need to receive oxygen therapy [19]. COVID-19-related complications include ARDS, SIRS, and MOF, which may cause hepatic ischemia and hypoxia reperfusion dysfunction. Both in vivo and in vitro models of liver ischemia and hypoxia suggested that liver cell death and inflammatory cell infiltration could be caused by ischemia and hypoxia [11]. Meanwhile, oxygen reduction and lipid accumulation in liver tissue during shock and hypoxic conditions may further promote the release of multiple inflammatory factors and then lead to liver injury [64]. Third, drug toxicity may contribute to liver damage in severe and critical COVID-19 patients. Compared with patients experiencing a mild or moderate clinical course, severe and critical patients require longer duration of antiviral therapy and multiple drugs combined. Our findings found that the number of drugs products ≥ 3 might be related to liver injury. Additionally, it has been reported that antiviral medications (lopinavir/ritonavir, arbidol, hydroxychloroquine), antipyretics (acetaminophen), antibiotics (macrolides, quinolones), and traditional Chinese medicine can cause liver damage [60, 65–67]. Also, some critical patients would be treated with steroids, which are mainly metabolized in the liver and could cause mild hepatotoxicity. In the setting of steroids combined with HIV protease inhibitors (such as lopinavir), the risk of liver damage could be further increased [68]. Notably, at the beginning of the COVID-19 crisis, in the complete absence of known antiviral/disease modulating medications, many drugs and combinations of drugs, which would have been previously considered for use only in a controlled experimental setting, may have been used empirically in clinical practice. Fourth, our subgroup analysis suggested that the incidence of any abnormal liver biochemical indicator at admission seemed to be higher in the subgroup where the proportion of pre-existing liver disease was ≥ 10% than in the subgroup where the proportion of re-existing liver disease was < 10%. This finding suggests that COVID-19 patients with pre-existing liver diseases may be more prone to have abnormal liver biochemical indicators and that monitoring liver function should be more intensive in such patients. Notably, this may not be a direct consequence of pre-existing liver diseases, but related to the dysfunction of innate immune response against the virus [69].
Our study has several limitations. First, the heterogeneity remained statistically significant in most of our meta-analyses. Despite this, we performed subgroup analyses, meta-regression analyses, and sensitivity analyses, but the potential source of heterogeneity could not be clearly identified. Indeed, among the proportion meta-analyses, a statistically significant heterogeneity is often unavoidable [70, 5]. Second, the follow-up duration was different among these included studies. Third, all but one included study was from China and even that study was also from its neighboring country. Thus, the present findings might be more appropriate for the Chinese population or Asian population and it remains to be seen whether our findings can be generalized to European or American populations. In the future, it will be interesting to conduct a similar systematic review with papers that are likely to be forthcoming from Europe and America. However, at the time of writing this meta-analysis, the papers from Europe and America have yet to appear in the literature. Fourth, most of these included studies were retrospective, which might cause a recall bias, especially about data entry. Fifth, abnormal liver biochemical test, rather than liver injury, was assessed, because the definition of liver injury was unclear or inconsistent among these included studies.
In conclusion, abnormal liver biochemistry, primarily characterized as decreased ALB and elevated GGT, AST, and ALT, is common in COVID-19 patients. Abnormal liver biochemical indicators are closely related to the severity and prognosis of COVID-19 patients. Additionally, a higher incidence of abnormal liver biochemistry in COVID-19 patients during hospitalization warrants that liver biochemical tests should be closely monitored and timely measures should be taken. In future, it is necessary to extrapolate these findings to patients who have advanced chronic liver diseases or liver cirrhosis, because there is a potential chance for co-incidental COVID-19 infection to result in hepatic decompensation in patients with little hepatic reserve.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Abbreviations
- COVID-19
Coronavirus disease 2019
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- ACE2
Angiotensin converting enzyme 2
- MOF
Multiple organ failure
- ARDS
Acute respiratory distress syndrome
- NOS
Newcastle-Ottawa scale
- RR
Risk ratio
- MD
Mean difference
- CI
Confidence interval
- ALT
Alanine aminotransferase
- AST
Aspartate aminotransferase
- ALP
Alkaline phosphatase
- GGT
Gamma-glutamyl transpeptidase
- TBIL
Total bilirubin
- ALB
Albumin
- SIRS
Systemic inflammatory response syndrome
- CYP3A4
Cytochrome P 450 3A4
Authors’ contributions
Conceptualization: XQ; Methodology: YW, HL, XG, and XQ; Validation: XQ, XG, and HL; Formal analysis: YW, HL, XG, and XQ; Investigation: YW, HL, XG, EMY, NM-S, GBLS, RT, FGR, AS, and XQ; Data curation: YW, HL, and XQ; Writing–original draft: YW and XQ; Writing–review and editing: YW, HL, XG, EMY, NM-S, GBLS, RT, FGR, AS, and XQ; Supervision: XQ; Project administration: XQ.
Funding
None.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no competing interest
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yanyan Wu, Hongyu Li, Xiaozhong Guo are Co-first authors.
Contributor Information
Yanyan Wu, Email: 2963013648@qq.com.
Hongyu Li, Email: 13309887041@163.com.
Xiaozhong Guo, Email: guo_xiao_zhong@126.com.
Eric M. Yoshida, Email: emyoshida@shaw.ca
Nahum Mendez-Sanchez, Email: nmendez@medicasur.org.mx.
Giovanni Battista Levi Sandri, Email: gblevisandri@gmail.com.
Rolf Teschke, Email: rolf.teschke@gmx.de.
Fernando Gomes Romeiro, Email: fgromeiro@gmail.com.
Akash Shukla, Email: drakashshukla@yahoo.com.
Xingshun Qi, Email: xingshunqi@126.com.
References
- 1.https://www.who.int/emergencies/diseases/novel-coronavirus-2019. Accessed 6 May 2020
- 2.Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol 2020;5(4):536–544 [DOI] [PMC free article] [PubMed]
- 3.Wu Y, Li H, Xu X, Zheng K, Qi X, Guo X. Clinical characteristics and outcomes of 2019 novel coronavirus pneumonia: a meta-analysis (Article in Chinese) Zhonghua Gan Zang Bing Za Zhi. 2020;28(3):240–246. doi: 10.3760/cma.j.cn501113-20200224-00067. [DOI] [PubMed] [Google Scholar]
- 4.Xu L, Liu J, Lu M, Yang D, Zheng X. Liver injury during highly pathogenic human coronavirus infections. Liver Int. 2020;40(5):998–1004. doi: 10.1111/liv.14435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chinese Digestion Association, Chinese Medical Doctor Association; Chinese Society of Hepatology, Chinese Medical Association The protocol for prevention, diagnosis and treatment of liver injury in coronavirus disease 2019 (Article in Chinese) Zhonghua Gan Zang Bing Za Zhi. 2020;28(3):217–221. doi: 10.3760/cma.j.cn501113-20200309-00095. [DOI] [PubMed] [Google Scholar]
- 6.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS-CoV-2 Cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chai X, Hu L, Zhang Y, et al. Specific ACE2 expression in cholangiocytes may cause liver damage after 2019-nCoV infection. BioRxiv. 2020 doi: 10.1101/2020.02.03.931766. [DOI] [Google Scholar]
- 8.Hu LL, Wang WJ, Zhu QJ, Yang L. Novel coronavirus pneumonia related liver injury: etiological analysis and treatment strategy (Article in Chinese) Zhonghua Gan Zang Bing Za Zhi. 2020;28(2):97–99. doi: 10.3760/cma.j.issn.1007-3418.2020.02.001. [DOI] [PubMed] [Google Scholar]
- 9.Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang L, Wang W, Wang X, et al. Creg in hepatocytes ameliorates liver ischemia/reperfusion injury in a TAK1-dependent manner in mice. Hepatology. 2019;69(1):294–313. doi: 10.1002/hep.30203. [DOI] [PubMed] [Google Scholar]
- 12.Boettler T, Newsome PN, Mondelli MU, et al. Care of patients with liver disease during the COVID-19 pandemic: EASL-ESCMID position paper. JHEP Rep Innov Hepatol. 2020;2(3):100113. doi: 10.1016/j.jhepr.2020.100113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Méndez-Sánchez N, Valencia-Rodríguez A, Qi X, et al. What has the COVID-19 pandemic taught us so far? Addressing the problem from a hepatologist’s perspective. J Clin Transl Hepatol. 2020;8:1–4. doi: 10.14218/JCTH.2020.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang B, Zhou X, Qiu Y, Feng F, Feng J, Jia Y, et al. Clinical characteristics of 82 death cases with COVID-19. medRxiv 2020. 10.1101/2020.02.26.20028191. Accessed 25 Mar 2020
- 15.Li C, Li M, Gan L, et al. Heart and liver damages in coronavirus sisease-2019. Guangdong Med J. 2020;41:1–4. [Google Scholar]
- 16.Li D, Long Y, Huang P, et al. Clinical characteristics of 80 patients with COVID-19 in Zhuzhou City. Chin J Infect Control. 2020;19(3):1–7. [Google Scholar]
- 17.Qi D, Yan X, Tang X, Yan X, Tang X, Peng J, et al. Epidemiological and clinical features of 2019-nCoV acute respiratory disease cases in Chongqing municipality, China: a retrospective, descriptive, multiple-center study. Lancet Respir Med 2020. https://ssrn.com/abstract=3546122. Accessed 6 Apr 2020
- 18.Sun D, Lv G. Related factors and clinical significance of liver function damage in patients with COVID-19 (Article in Chinese). Chin J Dig Sur 2020. http://rs.yiigle.com/yufabiao/1189815.htm. Accessed 6 Apr 2020
- 19.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang G, Wu C, Zhang Q, Zhang Q, Wu F, Yu B, et al. Epidemiological and Clinical Features of Corona Virus Disease 2019 (COVID-19) in Changsha, China. Lancet Infect Dis 2020. https://ssrn.com/abstract=3548770. Accessed 6 Apr 2020
- 21.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang Y, Yang R, Xu Y, Gong P. Clinical characteristics of 36 non-survivors with COVID-19 in Wuhan, China. medRxiv. 2020 doi: 10.1101/2020022720029009. [DOI] [Google Scholar]
- 23.Jin X, Lian JS, Hu JH, et al. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut. 2020;69(6):1002–1009. doi: 10.1136/gutjnl-2020-320926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wen K, Li W, Zhang D, Zhang A, Zhang T, Zhao P, et al. Epidemiological and clinical characteristics of 46 newly-admitted coronavirus disease 2019 cases in Beijing (Article in Chinese). Zhonghua Chuan Ran Bing Za Zhi 2020. http://rs.yiigle.com/yufabiao/1182702.htm. Accessed 6 Apr 2020
- 25.Yang K, Ren M, Xiao L, Liu Y, Shi D, Lu H, et al. Epidemiological and clinical characteristics of 57 cases of new coronavirus pneumonia in non-epidemic areas (Article in Chinese). J Third Milit Med Univ 2020; 1–5
- 26.Cheng K, Wei M, Shen H, et al. Clinical characteristics of 463 patients with common and severe type coronavirus disease. Shanghai Med J. 2019;2020:1–15. [Google Scholar]
- 27.Liu C, Jiang ZC, Shao CX, et al. Preliminary study of the relationship between novel coronavirus pneumonia and liver function damage: a multicenter study. Zhonghua gan zang bing za zhi Zhonghua ganzangbing zazhi Chin J Hepatol. 2020;28(2):148–152. doi: 10.3760/cma.j.issn.1007-3418.2020.02.003. [DOI] [PubMed] [Google Scholar]
- 28.Liu M, He P, Liu HG, et al. Clinical characteristics of 30 medical workers infected with new coronavirus pneumonia. Liver Int. 2020;43(3):209–214. doi: 10.3760/cma.j.issn.1001-0939.2020.03.014. [DOI] [PubMed] [Google Scholar]
- 29.Xu M, Li M, Zhan W, et al. Clinical analysis of 23 cases of 2019 novel coronavirus infection in Xinyang City, Henan Province. Chin Crit Care Med. 2020;32(2):421–425. doi: 10.3760/cma.j.cn121430-20200301-00153. [DOI] [PubMed] [Google Scholar]
- 30.Qian ZP, Mei X, Zhang YY, et al. Analysis of baseline liver biochemical parameters in 324 cases with novel coronavirus pneumonia in Shanghai area. Zhonghua gan zang bing za zhi Zhonghua ganzangbing zazhi Chin J Hepatol. 2020;28:E005. doi: 10.3760/cma.j.cn501113-20200229-00076. [DOI] [PubMed] [Google Scholar]
- 31.Zhao R, Liang Y, Lin Y, Lu L, Li Q, Li Y, et al. Clinical characteristics of 28 patients with novel coronavirus pneumonia (Article in Chinese). Zhonghua Chuan Ran Bing Za Zhi 2020. 10.3760/cma.j.issn.1000-6680.2020.02.000. Accessed 6 Feb 2020
- 32.Wang S, Han P, Xiao F, Huang X, Cao L, Zhou Z, et al. Manifestations of liver injury in 333 hospitalized patients with coronavirus disease 2019 (Article in Chinese). Chin J Dig 2020;40(3). 10.3760/cma.j.issn.0254-1432.2020.03.000
- 33.Zhong S, Lin F, Shi L. The clinical characteristics and outcome of 62 patients with COVID-19 (Article in Chinese) Med J Chin People's Liber Army. 2020;45(4):370–374. [Google Scholar]
- 34.Xiang T, Liu J, et al. Analysis of clinical characteristics of 49 patients with Novel Coronavirus Pneumonia in Jiangxi province. Chin J Respir Crit Care Med. 2020;19(2):1–7. [Google Scholar]
- 35.Wan S, Xiang Y, Fang W, Zheng Y. Clinical features and treatment of COVID-19 patients in Northeast Chongqing. J Med Virol. 2020;92(7):797–806. doi: 10.1002/jmv.25783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu XW, Wu XX, Jiang XG, et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ. 2020;368:m606. doi: 10.1136/bmj.m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang W, Cao Q, Qin L, et al. Clinical characteristics and imaging manifestations of the 2019 novel coronavirus disease (COVID-19): a multi-center study in Wenzhou city, Zhejiang, China. J Infect. 2020;80(4):388–393. doi: 10.1016/j.jinf.2020.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yao N, Wang SN, Lian JQ, et al. Clinical characteristics and influencing factors of patients with novel coronavirus pneumonia combined with liver injury in Shaanxi region. Zhonghua gan zang bing za zhi Zhonghua ganzangbing zazhi Chin J Hepatol. 2020;28:E003. doi: 10.3760/cma.j.cn501113-20200226-00070. [DOI] [PubMed] [Google Scholar]
- 39.Ma Y, Xia S, Wang M, et al. Clinical features of children with SARS-CoV-2 infection: an analysis of 115 cases. Chin J Contemp Pediatr. 2020;22(4):1–4. doi: 10.7499/j.issn.1008-8830.2003016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Young BE, Ong SWX, Kalimuddin S, et al. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. JAMA J Am Med Assoc. 2020;323(15):1488–1494. doi: 10.1001/jama.2020.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou Y, Zhu C, Wan X, Feng C. Clinical characteristics and laboratory results of 3886 patients with fever and cough (Article in Chinese) Lab Med Clin. 2020;17(11):1541–1545. [Google Scholar]
- 42.Zhao D, Yao F, Wang L, Zheng L, Gao Y, Ye J, et al. A comparative study on the clinical features of COVID-19 pneumonia to other pneumonias. Clin Infect Dis 2020;ciaa247. 10.1093/cid/ciaa247 [DOI] [PMC free article] [PubMed]
- 43.Du RH, Liang LR, Yang CQ, et al. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: a prospective cohort study. Eur Respir J. 2020;55(5):2000524. doi: 10.1183/13993003.00524-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang L, He W, Yu X, et al. Coronavirus disease 2019 in elderly patients: characteristics and prognostic factors based on 4-week follow-up. J Infect. 2020;80(6):639–645. doi: 10.1016/j.jinf.2020.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xie H, Zhao J, Lian N, Lin S, Xie Q, Zhuo H. Clinical characteristics of non-ICU hospitalized patients with coronavirus disease 2019 and liver injury: a retrospective study. Liver Int. 2020;40(6):1321–1326. doi: 10.1111/liv.14449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pan L, Mu M, Yang P, Sun Y, Wang R, Yan J, et al. Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, China: a descriptive, cross-sectional, multicenter study. Am J Gastroenterol. 2020;115(5):766–773. doi: 10.14309/ajg.0000000000000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cai Q, Huang D, Yu H, Zhu Z, Xia Z, Su Y, et al. COVID-19: Abnormal liver function tests. J Hepatol. 2020 doi: 10.1016/j.jhep.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sun C, Zhang XB, Dai Y, Xu XZ, Zhao J. Clinical analysis of 150 cases of 2019 novel coronavirus infection in Nanyang City, Henan Province. Zhonghua jie he he hu xi za zhi Zhonghua jiehe he huxi zazhi Chin J Tuberc Respir Dis. 2020;43:E042. doi: 10.3760/cma.j.cn112147-20200224-00168. [DOI] [PubMed] [Google Scholar]
- 49.Zheng F, Tang W, Li H, Huang YX, Xie YL, Zhou ZG. Clinical characteristics of 161 cases of corona virus disease 2019 (COVID-19) in Changsha. Eur Rev Med Pharmacol Sci. 2020;24(6):3404–3410. doi: 10.26355/eurrev_202003_20711. [DOI] [PubMed] [Google Scholar]
- 50.Zhang G, Zhang J, Wang B, Zhu X, Wang Q, Qiu S. Analysis of clinical characteristics and laboratory findings of 95 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a retrospective analysis. Respir Res. 2020;21(1):74. doi: 10.1186/s12931-020-01338-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang Y, Zheng L, Liu L, Zhao M, Xiao J, Zhao Q. Liver impairment in COVID-19 patients: a retrospective analysis of 115 cases from a single center in Wuhan city, China. Liver Int. 2020 doi: 10.1111/liv.14455. [DOI] [PubMed] [Google Scholar]
- 52.Fang XW, Mei Q, Yang TJ, et al. Clinical characteristics and treatment strategies of 79 patients with COVID-19. Chin Pharmacol Bull. 2020;36(04):453–459. [Google Scholar]
- 53.Li GM, Pan X. Features of liver injury in patients with coronavirus disease 2019 in Bozhou, China. J Clin Hepatol. 2020;36(04):772–774. [Google Scholar]
- 54.Wang XF, Zhou ZQ, Yang HH, et al. Extrapulmonary organ damage and clinical significance in patients with coronavirus disease 2019. Zhejiang Med. 2020;42(05):485–488. [Google Scholar]
- 55.Yuan J, Sun YY, Zuo YJ, et al. A retrospective analysis of the clinical characteristica of 233 NCP patients in Chongqng. J Southwest Univ (Natural Science Edition) 2020;42(03):17–24. [Google Scholar]
- 56.Bai P, He W, Zhang X, Liu S, Jin J. Analysis of clinical features of 58 patients with severe or critical 2019 novel coronavirus pneumonia (Article in Chinese) Zhonghua Ji Zhen Yi Xue Za Zhi. 2020;4:483–487. [Google Scholar]
- 57.Huang R, Zhu L, Xue L, Liu L, Yan X, Wang J, et al. Clinical findings of patients with coronavirus disease 2019 in Jiangsu province, China: a retrospective, multi-center study. Lancet Respira Med 2020. https://ssrn.com/abstract=3548785. Accessed 25 Mar 2020
- 58.Li L, Li S, Xu M, Yu P, Zheng S, Duan Z, et al. Risk factors related to hepatic injury in patients with corona virus disease 2019. medRxiv 2020. 10.1101/2020.02.28.20028514
- 59.Jeong H, Min S, Chae H, Kim S, Lee G, Namgoong SK, et al. Chloroquine and lopinavir (COVID-19 Drug Candidates) signal amplification by reversible exchange. ChemRxiv 2020. https://www.researchgate.net/publication/340387695_Chloroquine_and_Lopinavir_COVID-19_Drug_Candidates_Signal_Amplification_by_Reversible_Exchange. Accessed 6 Apr 2020
- 60.Lan NT, Thu NT, Barrail-Tran A. Randomised pharmacokinetic trial of rifabutin with lopinavir/ritonavir-antiretroviral therapy in patients with HIV -associated tuberculosis in Vietnam. PLoS One. 2014;9(1):e84866. doi: 10.1371/journal.pone.0084866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Larson AM. Acetaminophen hepatotoxicity. Clin Liver Dis. 2007;11(3):525–548. doi: 10.1016/j.cld.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 62.Guan G, Gao L, Wang JWEA. Exploring the mechanism of liver enzyme abnormalities in patients with novel coronavirus-infected pneumonia. Chin J Hepatol. 2020;28(2):100–106. doi: 10.3760/cma.j.issn.1007-3418.2020.02.002. [DOI] [PubMed] [Google Scholar]
- 63.Prompetchara E, Ketloy C, Palaga T. Immune responses in COVID-19 and potential vaccines: lessons learned from SARS and MERS epidemic. Asian Pac J Allergy Immunol. 2020;38(1):1–9. doi: 10.12932/AP-200220-0772. [DOI] [PubMed] [Google Scholar]
- 64.Zhang XJ, Cheng X, Yan ZZ, et al. An ALOX12-12-HETE-GPR31 signaling axis is a key mediator of hepatic ischemia-reperfusion injury. Nat Med. 2018;24(1):73–83. doi: 10.1038/nm.4451. [DOI] [PubMed] [Google Scholar]
- 65.Chalasani N, Bonkovsky HL, Fontana R, et al. Features and outcomes of 899 patients with drug-induced liver injury: the DILIN prospective study. Gastroenterology. 2015;148(7):1340–1352e7. doi: 10.1053/j.gastro.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Giner GV, Oltra M, Rueda D. Severe acute hepatitis related to hydroxychloroquine in a woman with mixed connnective tissue disease. Clin Rheumatol. 2007;26:971–972. doi: 10.1007/s10067-006-0218-1. [DOI] [PubMed] [Google Scholar]
- 67.Chen DL, Yang F, LuoZ Y. Current status and bottle-necks of global pharmaceutical developments against COVID-19. Chin Pharmacol Bull. 2020;36(4):1–11. [Google Scholar]
- 68.Cottin JSP, Pizzoglio V. Methylprednisolone-related liver injury: a descriptive study using the French pharmacovigi-lance database. Clin Res Hepatol Gastroenterol. 2019;7401(19):30270. doi: 10.1016/j.clinre.2019.12.008. [DOI] [PubMed] [Google Scholar]
- 69.Mantovani A, Beatrice G, Dalbeni A. Coronavirus disease 2019 and prevalence of chronic liver disease: a meta-analysis. Liver Int. 2020;40(6):1316–1320. doi: 10.1111/liv.14465. [DOI] [PubMed] [Google Scholar]
- 70.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 71.Li Y, De Stefano V, Li H, et al. Epidemiology of Budd-Chiari syndrome: a systematic review and meta-analysis. Clin Res Hepatol Gastroenterol. 2019;43(4):468–474. doi: 10.1016/j.clinre.2018.10.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.