Abstract
Small heat-shock proteins (sHSPs) are ubiquitously expressed molecular chaperones that inhibit amyloid fibril formation; however, their mechanisms of action remain poorly understood. sHSPs comprise a conserved α-crystallin domain flanked by variable N- and C-terminal regions. To investigate the functional contributions of these three regions, we compared the chaperone activities of various constructs of human αB-crystallin (HSPB5) and heat-shock 27-kDa protein (Hsp27, HSPB1) during amyloid formation by α-synuclein and apolipoprotein C-II. Using an array of approaches, including thioflavin T fluorescence assays and sedimentation analysis, we found that the N-terminal region of Hsp27 and the terminal regions of αB-crystallin are important for delaying amyloid fibril nucleation and for disaggregating mature apolipoprotein C-II fibrils. We further show that the terminal regions are required for stable fibril binding by both sHSPs and for mediating lateral fibril–fibril association, which sequesters preformed fibrils into large aggregates and is believed to have a cytoprotective function. We conclude that although the isolated α-crystallin domain retains some chaperone activity against amyloid formation, the flanking domains contribute additional and important chaperone activities, both in delaying amyloid formation and in mediating interactions of sHSPs with amyloid aggregates. Both these chaperone activities have significant implications for the pathogenesis and progression of diseases associated with amyloid deposition, such as Parkinson's and Alzheimer's diseases.
Keywords: small heat shock protein (sHsp), chaperone, structure–function, amyloid, protein misfolding, αB-crystallin, Hsp27, alpha-synuclein (α-synuclein), apolipoprotein C-II, neurodegenerative disease
Small heat-shock proteins (sHSPs) are a major family of heat-shock proteins, present in all kingdoms of life, that have a low monomeric molecular mass, ranging from 15 to 40 kDa. sHSPs generally comprise a central, ∼90-residue α-crystallin domain (ACD) and variable-length N- and C-terminal regions (NTRs and CTRs, respectively). Despite their small monomeric size, metazoan sHSPs are structurally diverse, often existing as a heterogenous array of oligomeric states. The isolated ACD typically forms a stable dimer (1–3), suggesting that the NTR and CTR regulate higher order oligomerization (4). As a result, relatively few high-resolution structures of sHSPs have been solved. The majority of solved structures are of the isolated ACD (1–3, 5) or of monodisperse sHSP oligomers formed from plant and prokaryotic sHSPs (6, 7).
The human genome encodes 10 sHSPs, with gene names HSPB1–10. Among the best studied of these are HSPB1 (heat-shock 27 kDa protein, Hsp27) and HSPB5 (αB-crystallin, αB-C), which are ubiquitously expressed (8). The sHSPs are involved in a diverse range of cellular processes that include regulating cell growth, differentiation, and motility (9, 10), apoptosis (11), autophagy (12), cytoskeletal dynamics (13, 14), and regulation of the intracellular redox state (15). The unifying feature of sHSPs in these processes is that they act as ATP-independent molecular chaperones. The sHSPs can work cooperatively with ATP-dependent chaperones to refold substrate proteins (16) or participate in shuttling destabilized or misfolded proteins for proteolytic degradation (17). As a result, sHSPs are a key component of protein quality control as part of cellular proteostasis. Mutations in genes encoding sHSPs are associated with the pathology of a variety of diseases such as neuropathies (18), myopathies (19), and cataracts (20).
There is growing evidence that Hsp27 and αB-C are involved in the cellular response to protein aggregation in a variety of neurodegenerative diseases. A pathological hallmark of neurodegenerative diseases, such as Parkinson's disease (PD) and Alzheimer's disease, is the intra- or extracellular aggregation of specific proteins to form amyloid fibrils. α-Synuclein (α-syn) and apolipoprotein C-II (apoC-II) are two examples of proteins that form amyloid fibrils both in vitro and in vivo. apoC-II is a serum protein found associated with very low-density lipoprotein particles where it regulates blood triglycerides. Very low-density lipoprotein–bound apoC-II is comprised of amphipathic α helices (21); however, lipid-free apoC-II is unstable and prone to aggregation. Amyloid formation by apoC-II in vitro is well-characterized (22–24), and mutations in apoC-II cause renal amyloidosis (25, 26). The biological roles of α-syn are not well-understood; however, it is highly expressed in multiple regions of the brain and is thought to play a role in vesicular trafficking and neurotransmitter release (27). Like apoC-II, the N-terminal region of α-syn adopts an amphipathic α-helical conformation upon binding to membranes or lipid-containing vesicles (28). However, α-syn aggregates to form amyloid fibrils that are the principle proteinaceous components of Lewy bodies found in dopaminergic neurons in the substantia nigra of PD patients (29, 30). The expression levels of sHSPs such as Hsp27 and αB-C correlate with the degree of dementia in the brains of Alzheimer's disease and PD patients (31–33). In intracellular models of PD, Hsp27 and αB-C reduce amyloid formation by α-syn (34). In vitro, sHSPs have been shown to inhibit amyloid formation by Aβ (35), β2-microglobulin (35), α-syn (34), tau (36), superoxide dismutase 1 (37), and apoC-II (38). Furthermore, sHSPs colocalize with deposits of aggregated proteins associated with a variety of protein misfolding diseases (39, 40), demonstrating their role in the cellular response to amyloid formation.
The precise mechanisms by which sHSPs bind proteins to inhibit amyloid fibril formation remain elusive. This is in part because Hsp27 and αB-C form polydisperse oligomers (41, 42) that exist in equilibrium with dimeric forms (4, 43). Furthermore, serine phosphorylation in the N-terminal region of Hsp27 and αB-C has the dual effect of regulating both the extent to which the sHSP oligomers dissociate into smaller species, including dimers, and their chaperone activity (44, 45), suggesting that the exchange of suboligomeric species may be important for chaperone activity. Interestingly, the dimeric, isolated ACD of αB-C was found to inhibit both amorphous and fibrillar protein aggregation (5); however, potential substrate-binding sites on αB-C have also been identified in the N- and C-terminal regions (46–48).
To determine the importance of the regions outside the conserved ACD for the ability of Hsp27 and αB-C to inhibit amyloid fibril formation, we directly compared the chaperone activities of WT Hsp27 and αB-C with their ACD-only counterparts against the amyloid formation of two well-characterized proteins: α-syn and apoC-II. Additionally, we investigated the chaperone activities of a full-length Hsp27 construct with three serine-to-aspartate mutations designed to mimic the triply phosphorylated, dimeric form of Hsp27 (Hsp27-3D), as well as a truncated form of this construct lacking the CTR (Hsp27-3DΔCTR). Using in vitro aggregation assays, we show that constructs containing both the NTR and ACD more effectively delay amyloid fibril nucleation and/or reduce the rate of fibril elongation than ACD-only constructs. Additionally, the binding of Hsp27 to preformed fibrils and the disaggregation of apoC-II fibrils were found to be conferred by primarily the NTR. Similarly, the ACD-only construct of αB-C was insufficient for mediating fibril binding and apoC-II fibril disaggregation. Full-length sHSPs also induced the tangling of preformed fibrils into larger aggregates. ACD-only constructs did not display this activity, and a construct containing only the NTR and ACD displayed a reduced ability to tangle preformed fibrils relative to its full-length counterpart. Thus, although the ACD retains some chaperone activity in vitro, the N- and C-terminal regions confer distinct and important chaperone functions against amyloid fibril formation.
Results
The effects of full-length and truncated constructs of Hsp27 and αB-C on α-syn amyloid formation in vitro
The chaperone activities of full-length and truncated sHSPs were investigated using well-established in vitro apoC-II and α-syn amyloid formation assays. Spontaneous fibril formation by α-syn was induced by incubating the protein at 200 μm, 37 °C, with constant agitation. All sHSP variants delayed amyloid formation of α-syn by various degrees, with the exception of Hsp27 ACD (Fig. 1, A–F, and Fig. S1). We did not observe a time-dependent increase in ThT fluorescence when sHSPs were incubated alone, and BSA did not inhibit the amyloid formation of unseeded α-syn fibril formation (Fig. S2). By fitting the raw data to a four-parameter Boltzmann curve (Fig. S1), we derived the duration of the lag phase (Tlag) and the time to half-maximal ThT fluorescence (T50) (Fig. 1, G and H, respectively). Differences in the Tlag and T50 observed across the samples were analyzed using one-way ANOVA with post hoc Tukey test. The full-length constructs (WT αB-C, WT Hsp27, and Hsp27-3D) and Hsp27-3DΔCTR significantly increased the Tlag relative to α-syn incubated alone (p < 0.01), suggesting that these constructs delay fibril nucleation. Conversely, ACD-only constructs of αB-C and Hsp27 did not significantly increase the Tlag. All sHSP constructs except Hsp27 ACD significantly increased the T50 (Fig. 1H), suggesting that the rate of α-syn fibril elongation was reduced relative to α-syn incubated alone. Table S1 shows statistical analysis of differences in Tlag and T50 between the different sHSP constructs. This analysis shows that the chaperone activity of Hsp27-3DΔCTR was more similar to that of WT Hsp27 and Hsp27-3D than to Hsp27 ACD. These findings demonstrate that the NTR of Hsp27 and the terminal regions of αB-C are important for delaying amyloid fibril formation by α-syn in vitro during both the nucleation phase and the elongation phase.
Figure 1.

Kinetics of unseeded α-syn amyloid formation in the presence of sHSPs. A–F, ThT-monitored amyloid formation of α-syn (200 μm) alone (black) or in the presence of the indicated sHSP (10 μm), WT αB-C (red, A), αB-C ACD (green, B), WT Hsp27 (orange, C), Hsp27 ACD (blue, D), Hsp27-3D (magenta, E), and Hsp27-3DΔCTR (cyan, F). The samples were assayed in quadruplicate in two independent experiments. The data are presented as means ± S.E., where n = 8. G and H, Tlag (G) and T50 (H) values are displayed as means ± S.D. overlaid with individual data points (n = 8). Significance values were determined using a one-way ANOVA test with a post hoc Tukey's multiple comparisons test. *, p < 0.05; **, p < 0.01; ***, p < 0.001; ****, p < 0.0001; ns, not significant. I, unseeded α-syn fibrils were formed alone (200 μm) or in the presence of 10 μm of the indicated sHSP construct for 48 h, 37 °C and then pelleted as described under “Experimental procedures.” The supernatant (Sup), sucrose (Suc), and pellet (P) layers were collected and analyzed by SDS-PAGE alongside the total (T) uncentrifuged samples. Also shown are controls of each sHSP construct incubated alone under identical conditions.
Across all samples, large differences in the final ThT plateaus were observed (Fig. 1, A–F). ThT fluorescence can be influenced by nonfibrillar structures, changes in sample viscosity, and the morphology of amyloid fibrils (49, 50). To determine whether the final ThT fluorescence readings correlated with the fraction of aggregated α-syn, we used pelleting assays (38). In these assays amyloid fibrils were pelleted through a 20% (w/v) sucrose layer such that small, soluble proteins remained in the supernatant fraction, whereas large oligomers such as those formed by WT Hsp27 and αB-C entered and remained in the sucrose layer. Thus, we were able to separate fibrils, large oligomers, and small species (e.g. dimers and monomers) in these assays. The supernatant, sucrose, and pellet layers as well as a sample prior to centrifugation (total) were analyzed by SDS-PAGE. We observed that the portion of soluble α-syn remaining at the end of the assays appeared to be unaffected by the presence of sHSPs (Fig. 1I). Thus, the observed differences between the total ThT fluorescence yields for α-syn fibrils formed alone and those formed in the presence of the sHSPs (Fig. 1) were not due to a difference in the fraction of aggregated α-syn. Furthermore, we used transmission electron microscopy (TEM) to confirm that the ThT-reactive species formed in the presence of all sHSP constructs were fibrillar (Fig. S3). Therefore, although the sHSP constructs reduced the rate of α-syn amyloid formation, under these conditions the sHSPs did not halt amyloid assembly at the nucleation stage or significantly alter the total fraction of aggregated α-syn after 42 h, once all samples had reached a ThT fluorescence plateau.
We also confirmed that the quaternary structures of our sHSP constructs were consistent with those observed previously (5, 45, 51) using sedimentation velocity analytical ultracentrifugation (SV-AUC) (Fig. S4 and Table S2). WT αB-C and WT Hsp27 both predominantly form oligomers as indicated by broad sedimentation coefficient distributions with weight-average sedimentation coefficients (s20,w) of ∼12–13 S (Fig. S4, A, C, G, and H, and Table S2). αB-C ACD and Hsp27 ACD form dimers with s20,w values of 2.07 and 2.31 S, respectively (Fig. S4, B, D, G, and H, and Table S2). Hsp27-3D predominantly assembles into dimers as indicated by a major peak in the sedimentation coefficient distribution at ∼2.5 S; however, a minor peak is observed at ∼4 S, indicating the formation of small oligomers for this sHSP construct (Fig. S4, E and H, and Table S2). Deletion of the CTR from Hsp27-3D prevented the formation of these small oligomers, because a single peak was observed for Hsp27-3DΔCTR with a s20,w of 2.58 S (Fig. S4F, S4H and Table S2).
The effects of full-length and truncated constructs of Hsp27 and αB-C on α-syn and apoC-II fibril elongation
To specifically assess the effects of sHSPs on the elongation of α-syn fibrils, we used a seeded assay (52). In these experiments, α-syn began to aggregate immediately, and a ThT fluorescence plateau was reached after ∼10 h (Fig. 2A and Fig. S5A). The non-chaperone control protein, BSA, did not inhibit seeded α-syn fibril formation, and no time-dependent changes in ThT fluorescence were observed for sHSPs incubated alone (Fig. S6). Estimates of the T50 were obtained by fitting the data to a one-phase association model (Fig. 2B and Fig. S5). All sHSP constructs except Hsp27 ACD significantly increased T50 (Fig. 2B). The ACD-only constructs were relatively less effective at delaying seeded α-syn fibril formation than the constructs containing the NTR (Fig. 2B). Pelleting assays indicated that the portion of α-syn remaining soluble at the end of the assay was likely increased by all of the sHSP constructs relative to α-syn seeded alone (Fig. 2C). However, the enhancement of α-syn in the supernatant and its depletion from the pellet were particularly evident for WT αB-C (Fig. 2C). These findings are consistent with the results of the seeded ThT fluorescence assay (Fig. 2A), and the relative efficacy of the sHSP constructs was comparable with the results of the unseeded assay (Fig. 1).
Figure 2.
Kinetics of seeded α-syn amyloid formation and unseeded apoC-II amyloid formation in the presence of sHSPs. A, α-syn amyloid formation (200 μm, 5% fibril seeds, 45 °C) alone (black) or in the presence of the indicated sHSP (50 μm): αB-C WT (red), αB-C ACD (green), WT Hsp27 (orange), Hsp27 ACD (blue), Hsp27-3D (magenta), and Hsp27-3DΔCTR (cyan). The data are presented as means ± S.E., where n = 3. Fits to the data are overlaid. The S.E. is small, and the error bars are generally not visible. B, T50 values derived from fits to the raw data and plotted as means ± S.D., with the individual data points shown and n = 3. Significance values were determined using a one-way ANOVA test with a post hoc Tukey's multiple comparisons test. ***, p < 0.001; ****, p < 0.0001; ns, not significant. C, α-syn fibrils were formed for 24 h (200 μm, 5% fibril seeds, 45 °C) alone or in the presence of the indicated sHSP (50 μm). These samples were then centrifuged, and the supernatant (Sup), sucrose (Suc), and pellet (P) layers were collected and analyzed by SDS-PAGE alongside a sample prior to centrifugation (total, T). Also shown are control samples of each sHSP incubated alone under identical conditions. D, apoC-II (56 μm) unseeded amyloid fibril formation alone at 30 °C (black) or in the presence of the indicated sHSP (12.8 μm): αB-C WT (red), αB-C ACD (green), WT Hsp27 (orange), Hsp27 ACD (blue), Hsp27-3D (magenta), and Hsp27-3DΔCTR (cyan). The data are presented as means ± S.E. where n = 2 with fits to the data overlaid. The S.E. is small, and the error bars are generally not visible. E, T50 values derived from fits to the raw data and plotted as means ± S.D. with the individual data points shown and n = 2. Significance values were determined using a one-way ANOVA test with a post hoc Tukey's multiple comparisons test. ***, p < 0.001; ****, p < 0.0001. F, apoC-II fibrils were formed at 30 °C for 72 h alone (56 μm) or in the presence of 12.8 μm of the indicated sHSP construct. These samples were then centrifuged as described above, and the supernatant (Sup), sucrose (Suc), and pellet (P) layers were collected and analyzed by SDS-PAGE alongside a sample prior to centrifugation (total, T). Also shown are control samples of each sHSP incubated alone under identical conditions.
ApoC-II aggregates readily at room temperature without agitation or seeding (22). Consequently, a lag phase is not typically observed for apoC-II aggregation (Fig. 2D and Fig. S7A). These data were fit to a one-phase association model to obtain estimates of the T50 (Fig. S7). BSA did not inhibit apoC-II fibril formation, and no time-dependent changes in ThT fluorescence were observed for sHSPs incubated alone (Fig. S8). All sHSP constructs significantly increased the T50; thus, these constructs delayed fibril elongation by apoC-II (Fig. 2E). WT αB-C was the most effective chaperone against fibril elongation, and Hsp27 ACD was the least effective (Fig. 2, D and E). ACD-only constructs were again less effective than constructs containing the NTR with respect to increasing the T50 (Fig. 2E). TEM was also used to confirm fibril formation by apoC-II in the absence of sHSPs and in the presence of a subset of the sHSP constructs (Fig. S9).
The sucrose pellet assay demonstrated that all sHSP constructs resulted in a portion of apoC-II remaining soluble at the end of the aggregation assay, in contrast to apoC-II incubated alone, in which we did not observe a band corresponding to apoC-II in the supernatant (Fig. 2F). Qualitative analysis suggests that the full-length WT constructs induced the highest proportion of soluble apoC-II remaining, followed by Hsp27-3D, Hsp27-3DΔCTR, αB-C ACD, and Hsp27 ACD (Fig. 2F). Taken together, these experiments indicate that constructs containing the NTR are relatively more effective than constructs containing only the ACD against both amyloid-forming proteins tested.
The association of sHSPs with amyloid fibrils
Our pelleting assays allow us to determine to what extent each sHSP construct stably cosediments with apoC-II and α-syn fibrils. Previous studies employed fluorescence and EM to establish that the cosedimentation behavior of αB-C and Hsp27 with apoC-II and α-syn fibrils observed in pelleting assays is due to spatial colocalization of these sHSPs with the fibrils (38, 40, 53). We have therefore used pelleting assays as a high-throughput approach to monitor fibril colocalization by sHSPs. WT Hsp27, Hsp27-3D, and WT αB-C were all found primarily in the pellet fraction when present during the aggregation of apoC-II or α-syn (Figs. 1I and 2, C and F). Interestingly, Hsp27-3DΔCTR cosedimented with both apoC-II and α-syn fibrils to a lesser extent than WT Hsp27, suggesting that the CTR of Hsp27 contributes to fibril binding. When incubated with elongating apoC-II fibrils, ACD-only constructs remained primarily in the soluble fraction, suggesting that they did not form stable associations with the fibrils at levels detectable by SDS-PAGE (Fig. 2F). Similarly, when incubated in the presence of elongating α-syn fibrils, Hsp27 ACD remained primarily in the soluble fraction. However, αB-C ACD was found in the pellet fraction when present during both unseeded and seeded α-syn fibril elongation (Figs. 1I and 2C).
To further investigate the cosedimentation of each sHSP construct with amyloid fibrils, mature preformed apoC-II and α-syn amyloid fibrils were incubated with each sHSP construct for 72 h. The fibrils were then pelleted and analyzed as described above. All full-length constructs and Hsp27-3DΔCTR were located primarily in the pellet fractions, suggesting that these constructs stably associate and cosediment with preformed apoC-II and α-syn fibrils (Fig. 3). Neither Hsp27 ACD nor αB-C ACD cosedimented with preformed fibrils of apoC-II or α-syn at levels detectable by SDS-PAGE. This indicates that αB-C ACD can only associate with α-syn fibrils when it is present during amyloid fibril elongation but not once the mature fibrils have been formed. Taken together, these data suggest that the ACD of αB-C is insufficient for fibril binding and that both the NTR and CTR of Hsp27 have roles in fibril binding.
Figure 3.
Fibril pelleting assay of preformed fibrils incubated with sHSP constructs. α-Syn fibrils (200 μm) were formed for 48 h at 37 °C, and apoC-II fibrils (56 μm) were formed for 72 h at 30 °C. These preformed α-syn fibrils (A) and apoC-II fibrils (B) were then incubated alone or with the indicated sHSP at 10 μm (for α-syn) or 12.8 μm (for apoC-II) for a further 72 h. Following this incubation, each sample was pelleted as described above, and the supernatant (Sup), sucrose (Suc), and pellet (P) layers were collected and analyzed by SDS-PAGE alongside the total (T) uncentrifuged samples. Also shown are controls of each sHSP construct incubated alone for 72 h and centrifuged as described above.
The time course of amyloid fibril binding by full-length and NTR + ACD sHSP constructs
We next determined the time frame over which the full-length sHSP constructs and Hsp27-3DΔCTR associate with both apoC-II and α-syn amyloid fibrils. Initially, preformed apoC-II or α-syn fibrils were incubated with each construct for 0, 0.5, 2, or 6 h. We observed a gradual decrease in the amount of WT αB-C detected in the sucrose layer over the incubation period with both apoC-II and α-syn fibrils, coupled with a concomitant increase in the amount detected in the pellet fraction (Fig. 4, A and B). Some WT αB-C remained in the sucrose fraction even after incubation for 6 h with apoC-II fibrils (Fig. 4B). This suggests that the interaction of WT αB-C with amyloid fibrils occurs slowly over the hour time scale. In contrast, the majority of WT Hsp27 cosedimented with both fibril types at 0 h (Fig. 4, C and D), with only a small fraction remaining in the sucrose fraction at 0 h in the presence of α-syn fibrils (Fig. 4C). Hsp27-3D was identified only in the pellet fraction at 0 h and remained in this fraction throughout the time course, suggesting that Hsp27-3D associated completely with apoC-II and α-syn fibrils within the dead time of our assay (Fig. 4, E and F). For Hsp27-3DΔCTR, the majority of the protein was observed in the pellet fraction at all incubation time points with both fibril types (Fig. 4, G and H); however, we always observed a faint band corresponding to Hsp27-3DΔCTR in the supernatant, even after 72 h (Fig. 3, A and B). These results indicate that WT Hsp27, Hsp27-3D, and Hsp27-3DΔCTR rapidly associate with amyloid fibrils, whereas WT αB-C associates with preformed fibrils slowly. Hsp27-3D may bind to α-syn fibrils faster than WT Hsp27. The presence of a population of unbound Hsp27-3DΔCTR throughout the time course suggests that its affinity for fibrils is reduced relative to the full-length constructs.
Figure 4.
Time course pelleting assay of preformed amyloid fibrils incubated with full-length sHSP constructs. α-Syn fibrils (200 μm) were formed for 48 h at 37 °C, and apoC-II fibrils (56 μm) were formed for 72 h at 30 °C. Fibrils were then incubated with the indicated sHSP construct at 10 μm (for α-syn) or 12.8 μm (for apoC-II) for 0–6 h. Following this incubation, each sample was centrifuged as described above. The supernatant (Sup), sucrose (Suc), and pellet (P) layers were collected and analyzed by SDS-PAGE alongside the total (T) uncentrifuged samples. A–H, preformed α-syn fibrils incubated with αB-C WT (A), WT Hsp27 (C), Hsp27-3D (E), and Hsp27-3DΔCTR (G) and preformed apoC-II fibrils incubated with αB-C WT (B), WT Hsp27 (D), Hsp27-3D (F), and Hsp27-3DΔCTR (H). I and J, preformed α-syn or apoC-II fibrils and sHSP constructs incubated alone for 6 h at identical concentrations to those described above.
The dissociation of apoC-II amyloid fibrils induced by sHSPs
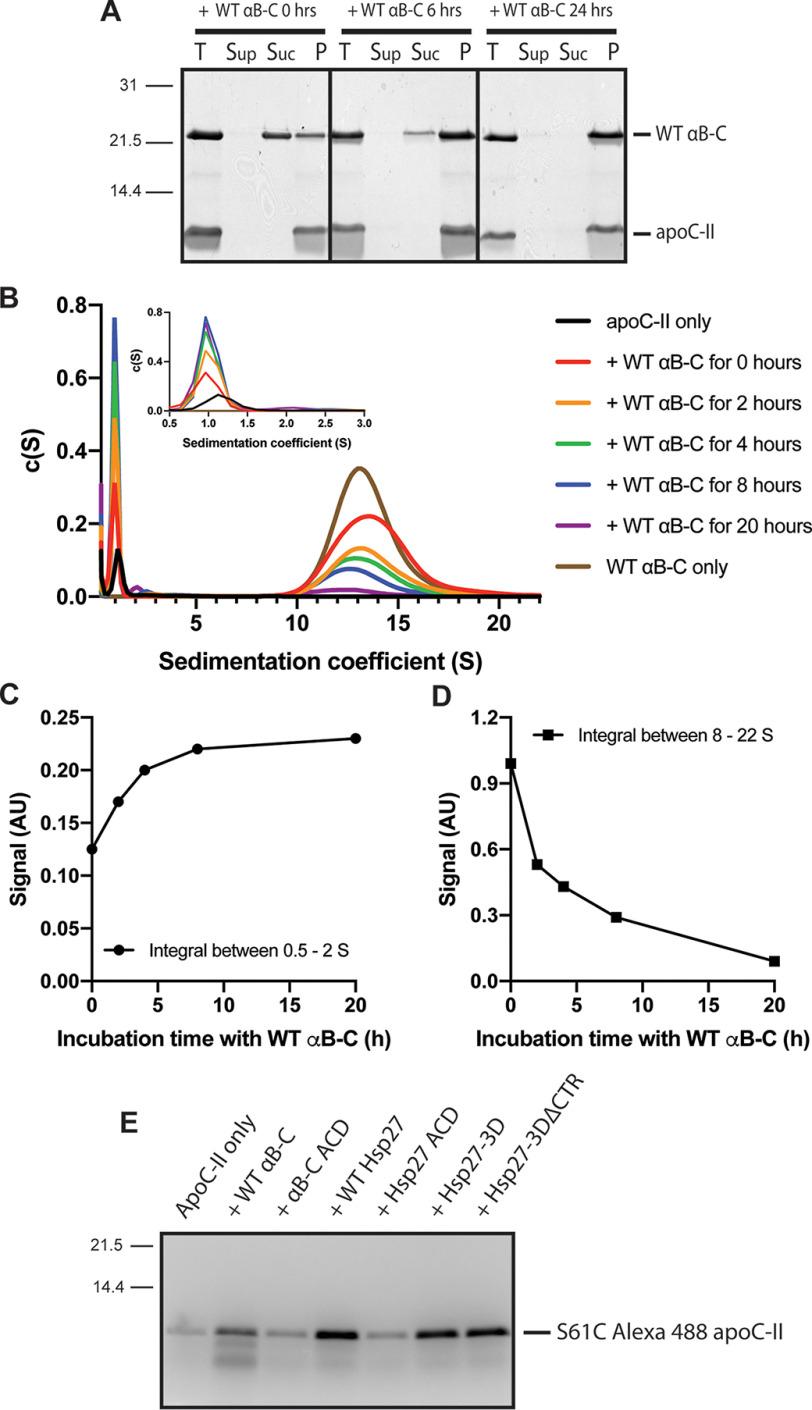
Previous studies have shown that, at equilibrium, a free pool of soluble protein exists in dynamic exchange with amyloid fibrils (54). For apoC-II fibrils, this free pool accounts for ∼5–10% of the total protein concentration (55). Close inspection of the fibril-binding time-course data (Fig. 4) showed a minor band corresponding to apoC-II in the supernatant fraction in the presence of the sHSPs (Fig. 4, B, D, F, and H) that was not observed for apoC-II incubated alone (Fig. 4J). These findings warranted a more quantitative study of the process of apoC-II fibril dissociation and whether the presence of sHSPs affects this process. We analyzed apoC-II fibrils incubated in the presence of WT αB-C for 0–20 h using SV-AUC (Fig. 5, B–D, and Fig. S10). In this experiment, a rotor speed of 40,000 rpm (129,000 × g) was used because this centrifugal force causes the fibrils to pellet within minutes, whereas the oligomers formed by WT αB-C and any soluble, monomeric apoC-II sediment over a period of hours. This regime enables us to monitor the relative proportions of both oligomeric WT αB-C and soluble, monomeric apoC-II. The peak at ∼1 S corresponds to monomeric apoC-II and is visible when fibrils are incubated alone (Fig. 5B). However, with increasing incubation time in the presence of WT αB-C, the proportion of soluble apoC-II increases (Fig. 5C). The peak at ∼13 S, corresponding to oligomeric WT αB-C, decreases with increasing incubation time (Fig. 5D). This observation is consistent with an increasing proportion of WT αB-C associating with the apoC-II fibrils over time (Figs. 4 and 5A) because fibrils sediment too quickly to be observed in this AUC experiment. Pelleting assays also demonstrated that WT Hsp27 and Hsp27-3D disaggregated apoC-II fibrils in a concentration-dependent manner (Fig. S11). To further assess apoC-II fibril dissociation, we formed fluorescently labeled apoC-II fibrils using 10% S61C Alexa 488–labeled apoC-II. These fibrils were incubated alone or in the presence of each sHSP construct for 24 h. The fibrils were pelleted, and the supernatant was analyzed by SDS-PAGE without Coomassie Blue staining. Imaging for Alexa 488–labeled apoC-II indicated that all sHSP constructs containing the NTR enhanced the proportion of soluble apoC-II (Fig. 5E), whereas the ACD-only constructs did not. Therefore, the NTR of Hsp27 and the terminal regions of αB-C are important for apoC-II fibril dissociation. These results extend our previous findings that WT αB-C enhances the dilution-induced dissociation of apoC-II fibrils (38).
Figure 5.
Analysis of the time course of apoC-II fibril dissociation by WT αB-C. ApoC-II fibrils (56 μm) were formed for 72 h at 30 °C. A, fibrils were incubated with WT αB-C (12.8 μm) for 0–24 h. Each sample was centrifuged as described above. The supernatant (Sup), sucrose (Suc), and pellet (P) layers were collected and analyzed by SDS-PAGE alongside the total (T) uncentrifuged samples. B, SV-AUC analysis of preformed apoC-II fibrils (56 μm) incubated with WT αB-C (12.8 μm) for 0 (red), 2 (orange), 4 (green), 8 (blue), or 20 (purple) h prior to SV-AUC. ApoC-II fibrils (black) and WT αB-C (brown) incubated alone for 20 h are also shown. C and D, plots of the integral of the peak between 0.5 and 2 S (C) and between 8 and 22 S (D) in the c(s) distribution shown in B as a function of incubation time with WT αB-C. E, apoC-II fibrils (56 μm) formed with 10% S61C Alexa 488–labeled apoC-II were incubated with the indicated sHSP construct (12.8 μm) for 24 h and centrifuged as described above. The supernatant fractions were analyzed by SDS-PAGE and imaged using a filter set for Alexa 488 as described under “Experimental procedures.”
In contrast to our observations for apoC-II, pelleting assays did not indicate that the proportion of soluble α-syn in equilibrium with the fibrils was increased by the presence of the sHSP constructs (Fig. 4, A, C, E, G, and I). Nevertheless, we tested the ability of WT αB-C to induce dissociation of preformed α-syn fibrils using a pelleting assay (Fig. S12). This experiment shows no observable dissociation of α-syn fibrils by WT αB-C under the conditions and time scales that we tested in this work. Longer incubation times or additional factors such as dilution or ATP-dependent HSPs may be required for the dissociation of α-syn fibrils, as has previously been observed (56, 57).
The effects of fibril binding by sHSPs on the size distributions of preformed amyloid fibrils
We next determined the effect of sHSPs on the size distributions of preformed apoC-II fibrils using SV-AUC (Fig. 6, Figs. S13–S17, and Tables S3–S7). The interaction of WT αB-C with apoC-II fibrils resulted in an increase in the weight-average fibril sedimentation coefficient as indicated by a shift in the sedimentation coefficient distribution toward higher S values (Fig. 6A). We interpret this as an increase in the lateral association of mature fibrils with one another mediated by WT αB-C. In contrast, αB-C ACD, which did not stably colocalize with apoC-II fibrils (Fig. 3B), did not increase the weight-average sedimentation coefficient of the fibrils. Instead we observed a small but reproducible shift in the sedimentation coefficient distributions toward smaller S values (Fig. 6B). Similarly, WT Hsp27 increased, and Hsp27 ACD decreased, the average size of the fibrils (Fig. 6, C and D). The binding of Hsp27-3D to apoC-II fibrils resulted in immediate and visible precipitation such that, when present at the same concentration as the other sHSP constructs (12.8 μm), the fibrils sediment too rapidly to accurately determine the sedimentation coefficient distributions (Fig. 6E and Fig. S14D). At a lower concentration (6.4 μm), the binding of Hsp27-3D to the fibrils also results in a large increase in the fibril size distribution (Fig. 6F, Fig. S15B, and Table S5). Given the observation that full-length Hsp27 constructs appear to bind fibrils faster than our methodologies allow us to detect (Fig. 4, D and F) and that Hsp27-3D resulted in the visible precipitation of apoC-II fibrils within a matter of minutes, we also tested the effect of WT Hsp27 on the size distribution of preformed apoC-II fibrils after a shorter incubation period, immediately after the addition of Hsp27 (Fig. 6G, Fig. S16, and Table S6). The resulting fibril size distribution closely matched that observed after 72 h. The duration of the AUC experiment is ∼5–6 h; therefore, this result indicates that the lateral fibril–fibril association caused by WT Hsp27 primarily occurs within the first few hours after the addition of the sHSP to the fibrils. Interestingly, despite the stable interaction of Hsp27-3DΔCTR with preformed fibrils (Fig. 3B), this construct did not increase the size distributions of the apoC-II fibrils to the same extent as the full-length Hsp27 constructs (Fig. 6, C, F, and H, Fig. S17, and Table S7). This result suggests that the CTR region of Hsp27 plays an important role in promoting lateral association and/or tangling of apoC-II fibrils. Incubation of BSA, a non-chaperone control, with preformed apoC-II fibrils did not result in a change to the weight-average fibril sedimentation coefficient (Fig. S18 and Table S8). Thus, full-length Hsp27 constructs, WT αB-C, and to a lesser extent Hsp27-3DΔCTR increase the size distributions of apoC-II fibrils. This apparent increase in size is likely due to fibril cross-linking mediated by the sHSP, as has been previously observed using WT αB-C (38). The ACD constructs do not exert this effect, which is consistent with our observation that the ACD constructs do not bind stably to apoC-II fibrils (Fig. 3B). The lateral fibril–fibril association observed in the presence of full-length sHSPs using AUC was further confirmed using TEM (Fig. S19). Preformed fibrils incubated alone or in the presence of the ACD-only constructs appeared relatively dispersed on the EM grids, whereas those incubated in the presence of WT αB-C, WT Hsp27, or Hsp27-3D appeared to form denser fibril tangles (Fig. S19).
Figure 6.
SV-AUC analysis of preformed apoC-II fibrils (56 μm) incubated with various sHSP constructs. apoC-II fibrils (56 μm) were formed for 72 h, 30 °C. Subsequently, these fibrils were incubated either alone or with the indicated sHSP for a further 72 h, unless otherwise specified. In each panel, apoC-II fibrils incubated alone is shown in black. ApoC-II fibrils were incubated with 12.8 μm WT αB-C (A), 12.8 μm αB-C ACD (B), 12.8 μm WT Hsp27 (C), 12.8 μm Hsp27 ACD (D), 12.8 μm Hsp27-3D (E), 6.4 μm Hsp27-3D (F), 12.8 μm WT Hsp27 for 0 h (G), and 12.8 μm Hsp27-3DΔCTR (H).
Because α-syn fibrils are too large for SV-AUC analysis, even at the lowest available rotor speed, we monitored the assembly of α-syn fibrils into larger aggregates mediated by the various sHSP constructs by light scattering over a period of up to 6 h. Using this approach, a time-dependent increase in the light scattering of α-syn fibrils incubated in the presence of all full-length sHSPs was detected but not with Hsp27-3DΔCTR or the ACD-only sHSPs (Fig. 7A). Full-length Hsp27 and Hsp27-3D induced an increase in light scattering within 30 min, whereas the increase in light scattering induced by WT αB-C occurred over 2 h. These observations are consistent with the rate of α-syn fibril binding by these full-length sHSPs observed in our pelleting assays (Fig. 4), suggesting that fibril binding and the formation of larger fibrillar aggregates are related processes. That we could not detect an increase in turbidity of α-syn fibrils incubated with Hsp27-3DΔCTR is consistent with the observation that this construct did not cause lateral association of apoC-II fibrils to the same extent as the full-length Hsp27 constructs, providing further evidence that the CTR of Hsp27 is important for this chaperone function. We subsequently used a continuous assay to more accurately track the increase in light scattering mediated by the presence of sHSPs. WT αB-C did not induce a significant increase in light scattering over the first 20 min of incubation (Fig. 7B), consistent with its slower rate of fibril binding. The turbidity of α-syn fibrils incubated with Hsp27-3D increased more rapidly than that of fibrils incubated with WT Hsp27 (Fig. 7, B and C, Fig. S20, B–G, and Table S9). These results are also consistent with our observation that Hsp27-3D may bind α-syn fibrils more rapidly than WT Hsp27 (Fig. 4, C and E). Overall, these light-scattering experiments support our observations that by binding to fibrils, full-length sHSPs mediate their association into larger aggregates. To further confirm that the full-length sHSP constructs induced lateral fibril–fibril association of α-syn fibrils, we used TEM (Fig. S21). α-syn fibrils incubated alone or in the presence of ACD-only sHSPs appeared dispersed on the grids, whereas those incubated in the presence of WT αB-C, WT Hsp27, or Hsp27-3D appeared as larger networks of tangled fibrils (Fig. S21). Fibrils incubated in the presence of Hsp27-3DΔCTR appeared to form slightly larger fibril tangles than those observed for α-syn fibrils incubated alone.
Figure 7.
Analysis of light scattering by α-syn amyloid fibrils in the presence of sHSPs. α-Syn fibrils (200 μm) were formed for 48 h at 37 °C. A, absorbance measurements at 360 nm of preformed α-syn fibrils incubated alone (black) or in the presence of 10 μm WT αB-C (red), αB-C ACD (green), WT Hsp27 (orange), Hsp27 ACD (blue), Hsp27-3D (magenta), or Hsp27-3DΔCTR (cyan) for up to 6 h. B, turbidity measurements for buffer alone (gray), for preformed α-syn fibrils incubated alone (black), and for preformed α-syn fibrils in the presence of 10 μm WT αB-C (red), 10 μm WT Hsp27 (orange), and 10 μm Hsp27-3D (magenta). The data are presented as means ± S.E. where n = 3. C, analysis of the time taken for half-maximal change in turbidity (t1/2) of α-syn fibrils incubated with WT Hsp27 (orange) or Hsp27-3D (magenta) derived from fits to the raw data in B using a four-parameter sigmoidal Hill plot. The data are presented as the means ± S.D. with the individual data points shown and n = 3. A paired t test was used to compare the t1/2 for WT Hsp27 versus Hsp27-3D. **, p < 0.01.
Discussion
It is well-established that the human sHSPs Hsp27 and αB-C are molecular chaperones that inhibit the aggregation of a wide range of client proteins (8, 35, 37, 40). Over the past decade, significant progress has been made in understanding the hierarchical interactions that regulate the oligomerization of αB-C and Hsp27. An interchain β-sheet formed between two ACDs mediates dimerization (2), the CTR interacts with the ACD of adjacent dimers to facilitate the formation of small oligomers (4, 58), and varied interactions involving the NTR regulate higher-order oligomerization (4). Recent work suggested that the isolated ACD of αB-C, which forms a stable dimer, retains chaperone activity comparable with that of the full-length protein (5). Although there is not yet a clear consensus regarding how Hsp27 or αB-C interact with client proteins to inhibit or delay their aggregation, potential substrate-binding sites have been identified within the N- and C-terminal regions, as well as in the ACD (46, 48, 59). To determine the roles that the NTR, ACD, and CTR play in inhibiting amyloid fibril formation, we compared the relative chaperone activities of an array of full-length and truncated constructs of Hsp27 and αB-C against amyloid fibril formation by two disease-associated proteins: apoC-II and α-syn. Importantly, although the ACD-only constructs retain some ability to delay amyloid fibril formation, they lack several specific chaperone properties that we show are conferred by the NTR and CTR. We found that the NTR of Hsp27 and the terminal regions of αB-C are important for delaying the early stages of amyloid formation, for allowing sHSPs to associate with amyloid fibrils, and for inducing apoC-II amyloid fibril disaggregation. The CTR of Hsp27 and either or both of the terminal regions of αB-C were implicated in the lateral association of fibrils that comes about through sHSPs binding stably to fibrils.
We used ThT assays and fibril pelleting assays to assess the relative chaperone abilities of several constructs of αB-C and Hsp27. In the unseeded amyloid fibril formation assays, we observed several differences between the final ThT yields for apoC-II or α-syn fibrils formed in the presence of the αB-C constructs compared with the Hsp27 constructs. However, the α-syn pelleting assay showed no difference in the fraction of aggregated α-syn in the presence of these two sHSP constructs. Furthermore, the pelleting assay performed at the end of unseeded apoC-II fibril formation also indicated that despite differences in the final ThT fluorescence yields, the presence of WT αB-C, WT Hsp27, or Hsp27-3D all resulted in a substantial amount of apoC-II remaining soluble. TEM confirmed the formation of α-syn fibrils in the presence of all of the sHSP constructs and of apoC-II fibrils in the presence of WT αB-C and Hsp27-3D. We hypothesize that the differences in ThT fluorescence could be due to differences in the colocalization of the sHSPs with the fibrils, which may differentially affect the binding of ThT to the fibrils and therefore ThT fluorescence. Alternatively, the various sHSP constructs may induce changes in fibril morphology. It is known that α-syn fibrils exist in various polymorphs (60). It is plausible that these polymorphs bind ThT to differing extents. Another explanation is that the differences in ThT fluorescence we observe are due to differences in the lateral association of the fibrils, induced by the sHSPs, as the fibrils form. This work shows that this lateral fibril–fibril association occurs more slowly in the presence of WT αB-C than for WT Hsp27. The fluorescence yields observed in ThT assays should therefore be interpreted cautiously and verified using orthogonal methodologies, particularly for investigations of molecules that may bind or alter the morphology of the aggregates. Thus, for this work we used ThT fluorescence as a reporter of the duration of the lag phase and the T50 in combination with pelleting assays and TEM.
Across all of our fibril formation assays, the full-length and NTR + ACD constructs were superior chaperones compared with the ACD-only constructs. Only constructs containing the NTR significantly increased the Tlag for α-syn fibril formation. ACD-only constructs were also less effective at delaying α-syn and apoC-II fibril elongation than those with the NTR. WT Hsp27, Hsp27-3D, and Hsp27-3DΔCTR delayed fibril nucleation and elongation by both α-syn and apoC-II to similar extents, indicating that neither the formation of high-molecular-weight Hsp27 oligomers nor the CTR of Hsp27 are required for delaying amyloid formation. Taken together, our results indicate that the NTR of Hsp27 and the terminal regions of αB-C play essential roles in delaying the nucleation phase of amyloid formation and contribute to reducing the rate of fibril elongation.
Pelleting assays with preformed fibrils demonstrated that full-length and NTR + ACD constructs disaggregate apoC-II amyloid fibrils; in contrast, we did not observe apoC-II fibril dissociation induced by the ACD-only constructs. It is likely that by interacting with a soluble form of apoC-II that exists in equilibrium with the fibrils, these sHSP constructs shift the equilibrium in favor of fibril dissociation. This mechanism agrees with previous work investigating the inhibition of apoC-II and α-syn amyloid formation by WT αB-C (38, 40). Interestingly, AUC data showed that the release of soluble apoC-II monomers from apoC-II fibrils occurred at a similar rate to the binding of WT αB-C to fibrils, suggesting a possible correlation between fibril binding by sHSPs and the process of fibril dissociation. Our data cannot rule out promotion of fibril disaggregation, at least in part, resulting from destabilization of apoC-II fibrils through direct interaction of the sHSPs with the fibrils.
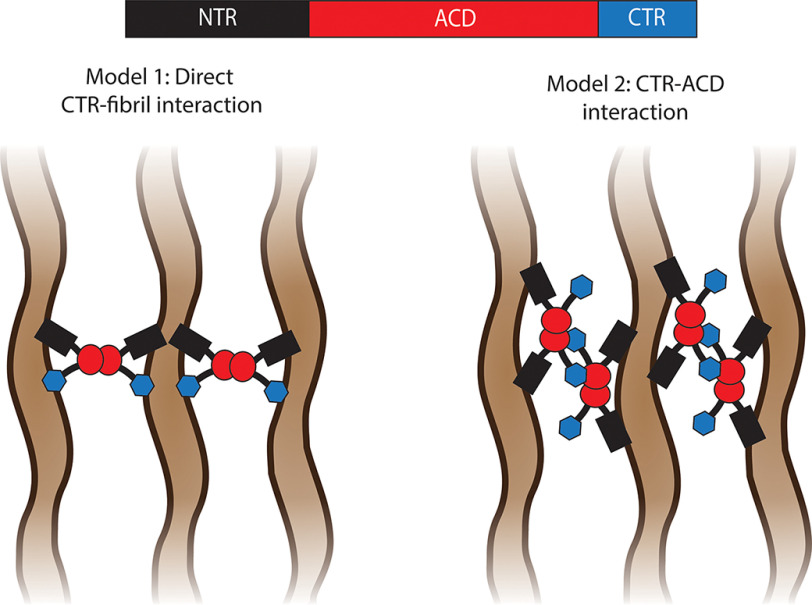
The interaction of sHSPs with preformed protein aggregates has been proposed not only to inhibit fibril elongation but also to reduce the cytotoxicity of fibrils, either by coating the aggregates and reducing their reactive surface area (61) or by sequestering them into larger inclusion body–like structures (38). The assay conditions used in this study do not favor secondary nucleation processes (52); thus, we can only speculate as to how the interactions of sHSPs with preformed fibrils might affect this process. It is likely that the stable binding of fibrils by sHSPs and the lateral fibril–fibril associations induced by the sHSPs observed in this study would reduce the surface area of the fibrils available for secondary nucleation processes. The stable interaction of WT αB-C with apoC-II and Aβ fibrils has been observed previously (35, 38), as has an interaction between WT αB-C, WT Hsp27, and Hsp27-3D with α-syn fibrils (40, 53). Here, we have observed that the stable interaction of sHSPs with preformed amyloid fibrils depends on sequences within the NTR and CTR. Our pelleting assays showed that only the full-length and NTR + ACD constructs interacted with both elongating and mature apoC-II and α-syn fibrils. In these assays, the NTR + ACD construct appeared to have a reduced capacity to cosediment with both types of fibrils, indicating that the CTR must also contribute to fibril binding directly (i.e. through a direct CTR-fibril interaction) or indirectly (i.e. via a CTR-sHSP interaction) (Fig. 8). Although we were unable to detect any stable interaction between the ACD-only constructs and apoC-II fibrils, we did observe an interaction between αB-C ACD and elongating α-syn fibrils. However, when αB-C ACD was added to preformed α-syn fibrils, this interaction was not observed. This suggests that αB-C ACD can interact with some element present when α-syn fibrils are elongating, but not once they are formed. The fact that Hsp27 ACD did not form stable interactions with elongating α-syn fibrils suggests differences in the interaction between these two ACD-only constructs and α-syn.
Figure 8.
Two models for the mechanism of fibril binding and lateral tangling induced by sHSPs. The domains of sHSPs are colored as follows: NTR (black), ACD (red), and CTR (blue). Amyloid fibrils are depicted in brown. In the first model, both the NTR and CTR contribute directly to fibril binding. In the absence of the CTR, an individual sHSP dimer has a reduced fibril-binding affinity and a reduced ability to cause lateral fibril association. In the second model, the CTR of one sHSP that is bound to fibrils via its NTR docks onto the ACD of another fibril-bound sHSP. This CTR-mediated oligomerization results in tangling of the associated fibrils. In the absence of the CTR, CTR–ACD interactions cannot form, and this type of sHSP oligomerization is not possible.
We found that WT Hsp27 binds fibrils more rapidly than WT αB-C, and this capacity is further enhanced in Hsp27-3D. Assuming that dissociated sHSP species (e.g. dimers) are the predominant fibril-binding species, the difference in the rate of fibril binding observed between WT αB-C and WT Hsp27 may be due to differences in the rate of dissociation of sHSP dimers from their oligomeric form and/or the rate of fibril binding by a sHSP dimer. WT Hsp27 binds to fibrils over a period of minutes, whereas WT αB-C binds over a period of hours, meaning that the rate of fibril binding by a Hsp27 dimer is greater than that of αB-C, that the rate of Hsp27 dimer dissociation from a Hsp27 oligomer is faster, or a combination of both of these effects. Hsp27-3D and Hsp27-3DΔCTR both appeared to interact with fibrils faster than WT Hsp27, and this difference is most likely due to differences in the quaternary structure of these constructs. Hsp27-3D and Hsp27-3DΔCTR primarily exist as dimers in solution (51), which we confirmed here by AUC (Fig. S4). Thus, for Hsp27-3D and Hsp27-3DΔCTR, fibril binding can be considered a one-step process, the rate of which depends only on the rate at which dimers bind to fibrils, independent of sHSP oligomer dissociation.
Our fibril-binding studies revealed that the full-length and NTR + ACD constructs increased the weight-average sedimentation coefficient of apoC-II fibrils, suggesting formation of large fibril aggregates through lateral fibril–fibril association. Full-length Hsp27 constructs, particularly Hsp27-3D, were more effective at binding and lateral association of apoC-II fibrils than WT αB-C and Hsp27-3DΔCTR. Both full-length Hsp27 constructs rapidly enhanced the turbidity of the α-syn fibril sample, whereas WT αB-C enhanced the turbidity of the fibril sample over a longer time frame. Additionally, both WT Hsp27 and Hsp27-3D increased the weight average sedimentation coefficient of apoC-II fibrils to a far greater extent than WT αB-C. Under the assumption that sHSPs interact reversibly with preformed fibrils, each cycle of fibril binding has the potential to bring two or more fibrils in close proximity. Therefore, the capacity of each sHSP construct to induce lateral fibril–fibril association could be dependent upon the rate at which they bind, dissociate from, and reassociate with fibrils. The slower rate at which WT αB-C enhanced the turbidity of the α-syn fibrils and the reduced extent to which it enhanced the size distribution of apoC-II fibrils are likely due to its substantially slower rate of fibril association relative to the Hsp27 constructs. Because Hsp27-3DΔCTR was also less effective than the full-length Hsp27 constructs at lateral association of apoC-II fibrils and increasing the light scattering of α-syn fibrils, we conclude that the CTR of Hsp27 is important for this component of the interaction between sHSPs and amyloid fibrils. Two distinct mechanisms may account for these observations (Fig. 8). It is possible that the two NTRs present on full-length sHSP dimers each interact simultaneously with two separate fibrils, resulting in the lateral association of these fibrils. In this model, the CTR may also be directly involved in binding to additional fibrils, and therefore when this domain is absent, the fibril-binding affinity is reduced, reducing the extent to which the sHSPs can induce lateral association of the fibrils. An alternative explanation is that the CTR does not make direct contact with fibrils but instead engages another fibril-bound sHSP dimer via a CTR–ACD interaction (Fig. 8), indirectly resulting in lateral association of the bound fibrils. The CTR is important for the hierarchical assembly of sHSP oligomers via its docking interaction with the ACD of neighboring subunits (58). In this case, because Hsp27-3DΔCTR cannot form these CTR-mediated dimer–dimer interactions, it has a reduced ability to tangle the fibrils.
Conclusion
A variety of classes of HSPs have been shown to form stable associations with amyloid fibrils (61), suggesting that this phenomenon may play a cytoprotective role. The expression levels of sHSPs are increased in the mammalian brain during aging (62), and sHSPs are known to associate with senile plaques and Lewy bodies (63–65). These factors necessitate a detailed understanding of how sHSPs interact with amyloid fibrils and what the consequences of these interactions are in vitro. Using two distinct disease-associated proteins that form fibrils, we observed that the ACDs of αB-C and Hsp27 were less effective at delaying amyloid fibril nucleation and elongation than full-length and NTR + ACD constructs. Only the full-length and NTR + ACD constructs were able to significantly delay amyloid fibril nucleation and disaggregate preformed apoC-II fibrils. Importantly, we show that sequences within the NTR of Hsp27 and the terminal regions of αB-C are required for the association of these sHSPs with preformed amyloid fibrils and their ability to induce disaggregation. The CTR of Hsp27 appears to play an important role in mediating the association of fibrils into larger aggregates. There is growing evidence that the sequestration of small protein aggregates into much larger aggregates, called aggresomes, is cytoprotective (66). The formation of an aggresome limits the cytotoxicity of aggregates spatially while also reducing their reactive surface area. There is also evidence that aggresome formation can enable asymmetric inheritance of protein aggregates in dividing cells (66). Our work demonstrates that the terminal regions of sHSPs are important for this potentially cytoprotective role in vivo.
Overall, the results of this study show that sHSPs interact with various species formed during the aggregation of a protein into amyloid fibrils and that they do so via multiple distinct binding sites involving the N- and C-terminal regions, as well as the ACD. Thus, the ACD alone is not sufficient for all of the chaperone activities exhibited by the full-length protein. This work expands our understanding of the roles of the N- and C-terminal regions of human sHSPs in interacting with proteins that form amyloid. This increased understanding of the mechanisms and consequences of sHSP interactions with amyloid fibrils has important implications in the pathogenesis and progression of diseases associated with amyloid deposition.
Experimental procedures
Protein expression and purification
A pET11a vector encoding for apoC-II expression, pET24a vectors encoding human α-syn, αB-C ACD (residues 68–153), Hsp27 ACD (residues 84–175), or αB-C and pET3a vectors encoding human Hsp27, Hsp27-3D (with S15D, S78D, and S82D mutations), and Hsp27-3DΔCTR (residues 1–175 with S15D, S78D, and S82D mutations) were used for expression of these proteins. Tobacco etch virus protease with L56V and S135G stabilizing mutations was expressed and purified according to the methods developed by Cabrita et al. (67). The expression and purification of WT apoC-II, WT α-syn, WT αB-C, αB-C ACD, WT Hsp27, Hsp27 ACD, Hsp27-3D, and Hsp27-3DΔCTR were all performed using modified protocols from previously published work (2, 22, 68–70).
All liquid cultures used for expression were incubated at 37 °C with shaking at 200 rpm. The expression of recombinant proteins was initiated by the addition of isopropyl-β-d-thiogalactopyranoside to a final concentration of 1 mm (apoC-II) or 0.5 mm (α-syn and all sHSPs) when the A600 of cultures reached 0.8. The cultures were then incubated at 37 °C for 2.5 h (apoC-II) or 4 h (α-syn and all sHSPs). The cultures were harvested by centrifugation and resuspended in 400 ml (apoC-II) or 40 ml (α-syn, WT αB-C, Hsp27 WT, Hsp27-3D) of TN buffer (50 mm Tris, 100 mm NaCl, pH 8.0) or 40 ml (αB-C ACD, Hsp27 ACD) TNI buffer (20 mm Tris, 40 mm imidazole, 500 mm NaCl, pH 8.0) and stored at −20 °C until use.
ApoC-II purification was performed as previously described (22); however, anion-exchange chromatography was performed in 6 m urea, 10 mm Tris, pH 8.0 using DEAE Sephacel™ resin prior to size-exclusion chromatography (SEC). Purification of α-syn was performed according to published methods (69) with an additional SEC chromatography step performed in 6 m urea, 10 mm Tris, pH 8.0, using a 500-ml XK26/60 chromatography column packed with Sephacryl® S200-HR resin. Purified α-syn was lyophilized and resuspended in 6 m guanidine hydrochloride. The purification of Hsp27 WT and αB-C WT was performed as previously described (68). The purification of Hsp27-3D was performed in an identical manner, with the addition of a hydrophobic interaction chromatography step using a HiLoad 26/10 Phenyl-Sepharose High Performance hydrophobic interaction chromatography column prior to SEC. The protein was loaded using 50 mm sodium phosphate, pH 7.4, 750 mm ammonium sulfate and eluted using a gradient from 50 mm sodium phosphate, pH 7.4, to distilled H2O over 15 column volumes. The purification of αB-C ACD and Hsp27 ACD was performed as previously described (2). Hsp27-3DΔCTR was purified according published methods used to purify WT Hsp27 (70). The purity of all protein stocks was determined to be ≥90% using SDS-PAGE and MS. Guanidine hydrochloride stocks of apoC-II and α-syn were stored at −20 °C at ∼30–50 mg/ml (apoC-II) and 100–150 mg/ml (α-syn). Stocks of all sHSPs (100–300 μm) were stored for long-term use at −80 °C or at 4 °C for short-term use (<4 weeks).
apoC-II and α-syn amyloid fibril formation using ThT fluorescence assays
ApoC-II and α-syn were rapidly diluted from guanidine hydrochloride stocks into fibrillation buffer (0.1 m sodium phosphate, pH 7.4, 0.1% sodium azide) to generate monomeric stocks immediately prior to each experiment. These monomeric stocks were aliquoted into wells of a clear, flat-bottomed 96-well tissue culture plate to a final concentration of 56 μm (apoC-II) or 200 μm (α-syn). For ThT assays, 10 μl of a 100 μm ThT stock (prepared in 0.1 m sodium phosphate, pH 7.4, 0.1% sodium azide) was also added along with the required volume of the relevant sHSP or a matching volume of buffer. The total assay volume in each case was 100 μl. The final concentration of guanidine hydrochloride was ∼66 mm in the apoC-II assays and 115 mm in the α-syn assays. The plates were incubated in a FLUOstar® Omega microplate reader (BMG Labtech, Melbourne, Australia) for up to 200 h. For unseeded apoC-II fibril formation, the plates were incubated at 30 °C without shaking. For unseeded α-syn fibril formation, a 2-mm diameter glass bead (Sigma–Aldrich) was added to each well to ensure mixing. The plates were incubated at 37 °C with orbital mixing at 700 rpm during the incubation. For seeded α-syn fibril formation, fibrils formed using the unseeded method were sonicated on ice using a Q500 probe sonicator (Qsonica, Newtown, CT) for 14 rounds of 30-s on/off pulses at an amplitude of 20%. This seed fibril stock was added to fresh, monomeric α-syn to a concentration of 5% of the total α-syn concentration. The mixture was incubated in the microplate reader at 45 °C for up to 30 h without shaking. ThT fluorescence was measured using excitation and emission filters of 440 and 480 nm, respectively.
At the end of unseeded α-syn fibril formation, the data for the change in ThT fluorescence over time were fitted with Boltzmann sigmoidal curves using GraphPad Prism version 8 (GraphPad Software Inc., San Diego, CA). Parameters from these fits were used to estimate the length of the lag phase using equations previously derived (71). The equation of the Boltzmann curve is,
| (Eq. 1) |
where F is the fluorescence intensity at a given time point, T (in units of hours), Fi is the initial fluorescence intensity, Ff is the final fluorescence intensity, T50 is the time to half-maximal change in ThT fluorescence, and k is a factor relating to the steepness of the change in ThT fluorescence. The equation used to derive estimates of the lag time (Tlag) is as follows.
| (Eq. 2) |
At the end of seeded α-syn fibril formation and unseeded apoC-II fibril formation, the data for the change in ThT fluorescence over time were fit with one-phase association curves using GraphPad Prism version 8. The equation for the one-phase association model is,
| (Eq. 3) |
where F is the fluorescence intensity at a given time point (T, in units of hours), Fi is the initial fluorescence intensity, Ff is the plateau in fluorescence intensity, and k is a factor relating to the steepness of the change in ThT fluorescence. The equation used to derive estimates of the T50 is as follows.
| (Eq. 4) |
Fits to the raw data were only used for downstream analysis when the goodness of fit as estimated by R2 values was >0.97. To analyze the statistical significance of the difference in the mean T50 and Tlag values derived from fits to the raw data, one-way ANOVA tests with post hoc Tukey's multiple comparisons tests were performed using GraphPad in Prism version 8.
The time course of fibril formation by α-syn and apoC-II differ substantially across these three assays. For each of these assays, we selected the sHSP concentrations at which chaperone activity was observed, but complete inhibition was not observed. The sHSP concentrations were therefore consistent within a given assay but differed between the three amyloid formation assays.
Fibril pelleting assays using a sucrose cushion
Fibril formation for pelleting assays was performed in a manner identical to that described for the ThT assays with the exception that ThT was omitted from the buffers. apoC-II and α-syn fibrils were separated from monomeric apoC-II, monomeric α-syn, oligomeric sHSPs, and dimeric sHSPs using centrifugal force according to an established protocol (38). Briefly, 100-μl aliquots of each sample were layered on top of 100 μl of 20% (w/v) sucrose dissolved in fibrillation buffer. The samples were centrifuged at 385,000 × g at 20 °C for 30 min (TLA-100 rotor OptimaMax centrifuge, Beckman Coulter, Brea, CA). The top 80 μl of the supernatant and the bottom 80 μl of the sucrose layers were collected, and the pellets were resuspended in 100 μl of fibrillation buffer. The total noncentrifuged, supernatant, sucrose, and pellet fractions were analyzed by SDS-PAGE.
Fluorescent apoC-II fibril pelleting assay
Separate guanidine hydrochloride stocks of WT unlabeled apoC-II and S61C Alexa 488–labeled apoC-II prepared as described previously (72) were rapidly diluted into fibrillation buffer at a ratio of 9:1 WT:S61C Alexa 488 to a total apoC-II concentration of 0.5 mg/ml. This mixture was incubated at 30°C for 1 week to allow fibrils to form. Preformed fibrils were aliquoted and incubated alone or in the presence of each sHSP construct at 30°C for a further 24 h. 80-μl aliquots of each incubation were centrifuged as described above without a sucrose layer. 20 μl was kept as the total uncentrifuged sample. The supernatant and pellet layers were collected. The supernatant fractions were analyzed by SDS-PAGE without Coomassie Blue staining, and the gels were imaged immediately using a ChemiDoc MP imaging system (Bio-Rad) with the Pro-Q Emerald 488 application (530/28 filter set). The total and pellet layers were analyzed in the same way but on separate gels.
Sedimentation velocity analysis
For all AUC experiments, samples of 380 μl were loaded into one sector of a 12-mm double-sector epon-filled centerpiece, and 400 μl of the relevant reference solution was loaded into the other sector of the cell. Radial absorbance scans at appropriate wavelengths were taken using an XL-1 analytical ultracentrifuge (Beckman Coulter) equipped with a Ti50 rotor at 20 °C, collecting scans at 4-min intervals with a radial step size of 0.003 cm. SV-AUC of apoC-II fibrils incubated with WT αB-C for 0–20 h was analyzed at a rotor speed of 40,000 rpm (129,000 × g) with a detection wavelength of 232 nm. The continuous sedimentation coefficient distribution c(s) model using regularization by maximum entropy was used to fit SV-AUC data for oligomeric sHSPs and monomeric apoC-II (73). The area under peaks in the c(s) distributions were obtained by integrating the distributions over the specified range using the SEDFIT software (73). SV-AUC data for Hsp27 ACD, Hsp27-3D, and Hsp27-3DΔCTR were acquired using a rotor speed of 50,000 rpm (201,600 × g) with detection wavelengths of 233 or 280 nm. SV-AUC data for Hsp27 WT were acquired using a rotor speed of 40,000 rpm (129,000 × g) and a detection wavelength of 237 nm. The buffer density (ρ), buffer viscosity (η), and partial specific volume () used for the analysis of Hsp27 WT, Hsp27 ACD, Hsp27-3D, and Hsp27-3DΔCTR were calculated using the SEDNTERP software (version 14.6e) (74). SV-AUC of apoC-II fibrils was analyzed at 8,000 rpm (5,161 × g) using detection wavelengths between 280 and 290 nm. The c(s) distributions were obtained by fitting the data using SEDFIT and a worm-like chain model described previously (75) using Tikhonov–Phillips regularization by second derivative (73, 76).
Turbidity assays
For assays conducted in a FLUOstar® Omega microplate reader, aliquots of 100 μl of α-syn fibrils were incubated at 37 °C without shaking in the presence or absence of the various sHSP constructs. Optical density was recorded using a 355-nm filter for up to 25 min at 20-s intervals. The time to half-maximal change in fibril optical density was determined by fitting the raw data to a four-parameter sigmoidal Hill equation,
| (Eq. 5) |
where OD is the optical density and a given time (t, in min), OD0 is the optical density at time 0, a is the difference between the final and initial optical density, c is the time to half-maximal change in optical density, and b is a scaling factor relating the steepness of the curve. For assays conducted using a DU800 spectrophotometer (Beckman Coulter), absorbance measurements of 100 μl of α-syn fibrils incubated in the presence or absence of the various sHSP constructs were recorded at 360 nm at the indicated time point after the addition of the sHSP. The path length was 10 mm, and at each time point a reference sample was used to obtain absorbance in arbitrary units.
Transmission electron microscopy
Carbon-coated Formvar 300 mesh copper grids were glow-discharged under reduced atmospheric pressure for 30 s at 25 mA. ApoC-II samples were diluted to ∼11 μm in distilled H2O. α-syn samples were diluted to ∼20 μm in distilled H2O. 4-μl aliquots of diluted samples were adsorbed onto the grid for 60 s. The samples were blotted using filter paper, and the grids were stained twice with 4 μl of 2% potassium phosphotungstate, pH 6.8, and air-dried. The grids were observed at the Bio21 Institute Advanced Microscopy Facility under a Talos L120C TEM (Thermo Fisher Scientific) operating at 120 kV. The images were captured digitally using a Ceta 16M digital camera (Thermo Fisher Scientific).
Data availability
All data are contained within the manuscript and supporting information.
Supplementary Material
Acknowledgments
We thank Dr. Amanda Clouser and Prof. Rachel E. Klevit for guidance with expression and purification of Hsp27-3DΔCTR. We also thank the Advanced Microscopy Facility and the Mass Spectrometry and Proteomics Facility at the Bio21 Molecular Science and Biotechnology Institute for technical support.
This article contains supporting information.
Author contributions—E. E. S., D. C., H. E., and M. D. W. G. conceptualization; E. E. S., C. O. Z., P. R. G., H. E., and M. D. W. G. formal analysis; E. E. S. and M. D. W. G. validation; E. E. S., C. O. Z., Y.-F. M., and M. D. W. G. investigation; E. E. S. visualization; E. E. S., D. C., Y.-F. M., P. R. G., H. E., and M. D. W. G. methodology; E. E. S. and M. D. W. G. writing-original draft; E. E. S. and M. D. W. G. project administration; E. E. S., C. O. Z., D. C., Y.-F. M., P. R. G., H. E., and M. D. W. G. writing-review and editing; P. R. G., H. E., and M. D. W. G. supervision; M. D. W. G. funding acquisition.
Funding and additional information—E. E. S. is the recipient of an Australian Government Research Training Program Scholarship. M. D. W. G. is the recipient of Australian Research Council Future Fellowship FT140100544.
Conflict of interest—The authors declare that they have no conflicts of interest with the contents of this article.
- sHSP
- small heat-shock protein
- ACD
- α-crystallin domain
- NTR
- N-terminal region
- CTR
- C-terminal region
- αB-C
- αB-crystallin
- apo
- apolipoprotein
- PD
- Parkinson's disease
- α-syn
- α-synuclein
- ANOVA
- analysis of variance
- Tlag
- duration of the lag phase
- T50
- time to halfmaximal ThT fluorescence
- TEM
- transmission electron microscopy
- SV
- sedimentation velocity
- AUC
- analytical ultracentrifugation
- SEC
- size-exclusion chromatography
- ThT
- thioflavin T.
References
- 1. Baranova E. V., Beelen S., Gusev N. B., and Strelkov S. V. (2009) The taming of small heat-shock proteins: crystallization of the α-crystallin domain from human Hsp27. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 65, 1277–1281 10.1107/S1744309109044571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Laganowsky A., Benesch J. L. P., Landau M., Ding L., Sawaya M. R., Cascio D., Huang Q., Robinson C. V., Horwitz J., and Eisenberg D. (2010) Crystal structures of truncated αA and αB crystallins reveal structural mechanisms of polydispersity important for eye lens function. Protein Sci. 19, 1031–1043 10.1002/pro.380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bagnéris C., Bateman O. A., Naylor C. E., Cronin N., Boelens W. C., Keep N. H., and Slingsby C. (2009) Crystal structures of α-crystallin domain dimers of αB-crystallin and Hsp20. J. Mol. Biol. 392, 1242–1252 10.1016/j.jmb.2009.07.069 [DOI] [PubMed] [Google Scholar]
- 4. Jehle S., Vollmar B. S., Bardiaux B., Dove K. K., Rajagopal P., Gonen T., Oschkinat H., and Klevit R. E. (2011) N-terminal domain of αB-crystallin provides a conformational switch for multimerization and structural heterogeneity. Proc. Natl. Acad. Sci. U.S.A. 108, 6409–6414 10.1073/pnas.1014656108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hochberg G. K. A., Ecroyd H., Liu C., Cox D., Cascio D., Sawaya M. R., Collier M. P., Stroud J., Carver J. A., Baldwin A. J., Robinson C. V., Eisenberg D. S., Benesch J. L. P., and Laganowsky A. (2014) The structured core domain of B-crystallin can prevent amyloid fibrillation and associated toxicity. Proc. Natl. Acad. Sci. U.S.A. 111, E1562–E1570 10.1073/pnas.1322673111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kim K. K., Kim R., and Kim S.-H. (1998) Crystal structure of a small heat-shock protein. Nature 394, 595–599 10.1038/29106 [DOI] [PubMed] [Google Scholar]
- 7. van Montfort R. L., Basha E., Friedrich K. L., Slingsby C., and Vierling E. (2001) Crystal structure and assembly of a eukaryotic small heat shock protein. Nat. Struct. Biol. 8, 1025–1030 10.1038/nsb722 [DOI] [PubMed] [Google Scholar]
- 8. Mymrikov E. V., Daake M., Richter B., Haslbeck M., and Buchner J. (2017) The chaperone activity and substrate spectrum of human small heat shock proteins. J. Biol. Chem. 292, 672–684 10.1074/jbc.M116.760413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Oesterreich S., Weng C.-N., Qiu M., Hilsenbeck S. G., Osborne C. K., and Fuqua S. A. W. (1993) The small heat shock protein hsp27 is correlated with growth and drug resistance in human breast cancer cell lines. Cancer Res. 53, 4443–4448 [PubMed] [Google Scholar]
- 10. Ikeda R., Yoshida K., Ushiyama M., Yamaguchi T., Iwashita K., Futagawa T., Shibayama Y., Oiso S., Takeda Y., Kariyazono H., Furukawa T., Nakamura K., Akiyama S., Inoue I., and Yamada K. (2006) The small heat shock protein αB-crystallin inhibits differentiation-induced caspase 3 activation and myogenic differentiation. Biol. Pharm. Bull. 29, 1815–1819 10.1248/bpb.29.1815 [DOI] [PubMed] [Google Scholar]
- 11. Kamradt M. C., Chen F., Sam S., and Cryns V. L. (2002) The small heat shock protein αB-crystallin negatively regulates apoptosis during myogenic differentiation by inhibiting caspase-3 activation. J. Biol. Chem. 277, 38731–38736 10.1074/jbc.M201770200 [DOI] [PubMed] [Google Scholar]
- 12. Crippa V., Sau D., Rusmini P., Boncoraglio A., Onesto E., Bolzoni E., Galbiati M., Fontana E., Marino M., Carra S., Bendotti C., De Biasi S., and Poletti A. (2010) The small heat shock protein B8 (HspB8) promotes autophagic removal of misfolded proteins involved in amyotrophic lateral sclerosis (ALS). Hum. Mol. Genet. 19, 3440–3456 10.1093/hmg/ddq257 [DOI] [PubMed] [Google Scholar]
- 13. Arai H., and Atomi Y. (1997) Chaperone activity of αB-crystallin suppresses tubulin aggregation through complex formation. Cell Struct. Funct. 22, 539–544 10.1247/csf.22.539 [DOI] [PubMed] [Google Scholar]
- 14. Hino M., Kurogi K., Okubo M. A., Murata-Hori M., and Hosoya H. (2000) Small heat shock protein 27 (HSP27) associates with tubulin/microtubules in HeLa cells. Biochem. Biophys. Res. Commun. 271, 164–169 10.1006/bbrc.2000.2553 [DOI] [PubMed] [Google Scholar]
- 15. Arrigo A. P. (2001) Hsp27: novel regulator of intracellular redox state. IUBMB Life 52, 303–307 10.1080/152165401317291156 [DOI] [PubMed] [Google Scholar]
- 16. Lee G. J., and Vierling E. (2000) A small heat shock protein cooperates with heat shock protein 70 systems to reactivate a heat-denatured protein. Plant Physiol. 122, 189–198 10.1104/pp.122.1.189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ahner A., Gong X., Schmidt B. Z., Peters K. W., Rabeh W. M., Thibodeau P. H., Lukacs G. L., and Frizzell R. A. (2013) Small heat shock proteins target mutant cystic fibrosis transmembrane conductance regulator for degradation via a small ubiquitin-like modifier-dependent pathway. Mol. Biol. Cell 24, 74–84 10.1091/mbc.E12-09-0678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Evgrafov O. V., Mersiyanova I., Irobi J., Van Den Bosch L., Dierick I., Leung C. L., Schagina O., Verpoorten N., Van Impe K., Fedotov V., Dadali E., Auer-Grumbach M., Windpassinger C., Wagner K., Mitrovic Z., et al. (2004) Mutant small heat-shock protein 27 causes axonal Charcot–Marie–Tooth disease and distal hereditary motor neuropathy. Nat. Genet. 36, 602–606 10.1038/ng1354 [DOI] [PubMed] [Google Scholar]
- 19. Vicart P., Caron A., Guicheney P., Li Z., Prévost M. C., Faure A., Chateau D., Chapon F., Tomé F., Dupret J. M., Paulin D., and Fardeau M. (1998) A missense mutation in the αB-crystallin chaperone gene causes a desmin-related myopathy. Nat. Genet. 20, 92–95 10.1038/1765 [DOI] [PubMed] [Google Scholar]
- 20. Clark A. R., Lubsen N. H., and Slingsby C. (2012) sHSP in the eye lens: crystallin mutations, cataract and proteostasis. Int. J. Biochem. Cell Biol. 44, 1687–1697 10.1016/j.biocel.2012.02.015 [DOI] [PubMed] [Google Scholar]
- 21. MacRaild C. A., Howlett G. J., and Gooley P. R. (2004) The structure and interactions of human apolipoprotein C-II in dodecyl phosphocholine. Biochemistry 43, 8084–8093 10.1021/bi049817l [DOI] [PubMed] [Google Scholar]
- 22. Hatters D. M., MacPhee C. E., Lawrence L. J., Sawyer W. H., and Howlett G. J. (2000) Human apolipoprotein C-II forms twisted amyloid ribbons and closed loops. Biochemistry 39, 8276–8283 10.1021/bi000002w [DOI] [PubMed] [Google Scholar]
- 23. Wilson L. M., Mok Y.-F., Binger K. J., Griffin M. D., Mertens H. D., Lin F., Wade J. D., Gooley P. R., and Howlett G. J. (2007) A structural core within apolipoprotein C-II amyloid fibrils identified using hydrogen exchange and proteolysis. J. Mol. Biol. 366, 1639–1651 10.1016/j.jmb.2006.12.040 [DOI] [PubMed] [Google Scholar]
- 24. Binger K. J., Pham C. L., Wilson L. M., Bailey M. F., Lawrence L. J., Schuck P., and Howlett G. J. (2008) Apolipoprotein C-II amyloid fibrils assemble via a reversible pathway that includes fibril breaking and rejoining. J. Mol. Biol. 376, 1116–1129 10.1016/j.jmb.2007.12.055 [DOI] [PubMed] [Google Scholar]
- 25. Lohani S., Schuiteman E., Garg L., Yadav D., and Zarouk S. (2016) Apolipoprotein C-II deposition amyloidosis: a potential misdiagnosis as light chain amyloidosis. Case Reports Nephrol. 2016, 8690642 10.1155/2016/8690642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nasr S. H., Dasari S., Hasadsri L., Theis J. D., Vrana J. A., Gertz M. A., Muppa P., Zimmermann M. T., Grogg K. L., Dispenzieri A., Sethi S., Highsmith W. E., Merlini G., Leung N., and Kurtin P. J. (2017) Novel type of renal amyloidosis derived from apolipoprotein-CII. J.Am. Soc. Nephrol. 28, 439–445 10.1681/ASN.2015111228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Burre J. (2015) The synaptic function of α-synuclein. J. Parkinsons Dis. 5, 699–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Davidson W. S., Jonas A., Clayton D. F., and George J. M. (1998) Stabilization of α-synuclein secondary structure upon binding to synthetic membranes. J. Biol. Chem. 273, 9443–9449 10.1074/jbc.273.16.9443 [DOI] [PubMed] [Google Scholar]
- 29. Baba M., Nakajo S., Tu P.-H., Tomita T., Nakaya K., Lee V. M., Trojanowski J. Q., and Iwatsubo T. (1998) Aggregation of α-synuclein in Lewy bodies of sporadic Parkinson's disease and dementia with Lewy bodies. Am. J. Pathol. 152, 879–884 [PMC free article] [PubMed] [Google Scholar]
- 30. Spillantini M. G., Crowther R. A., Jakes R., Hasegawa M., and Goedert M. (1998) α-Synuclein in filamentous inclusions of Lewy bodies from Parkinson's disease and dementia with Lewy bodies. Proc. Natl. Acad. Sci. U.S.A. 95, 6469–6473 10.1073/pnas.95.11.6469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shinohara H., Inaguma Y., Goto S., Inagaki T., and Kato K. (1993) αB crystallin and HSP28 are enhanced in the cerebral cortex of patients with Alzheimer's disease. J. Neurol. Sci. 119, 203–208 10.1016/0022-510X(93)90135-L [DOI] [PubMed] [Google Scholar]
- 32. Renkawek K., Stege G. J., and Bosman G. J. (1999) Dementia, gliosis and expression of the small heat shock proteins hsp27 and αB-crystallin in Parkinson's disease. Neuroreport 10, 2273–2276 10.1097/00001756-199908020-00009 [DOI] [PubMed] [Google Scholar]
- 33. Renkawek K., Bosman G. J., and de Jong W. W. (1994) Expression of small heat-shock protein hsp 27 in reactive gliosis in Alzheimer disease and other types of dementia. Acta Neuropathol. 87, 511–519 10.1007/BF00294178 [DOI] [PubMed] [Google Scholar]
- 34. Cox D., and Ecroyd H. (2017) The small heat shock proteins αB-crystallin (HSPB5) and Hsp27 (HSPB1) inhibit the intracellular aggregation of α-synuclein. Cell Stress Chaperones 22, 589–600 10.1007/s12192-017-0785-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Raman B., Ban T., Sakai M., Pasta S. Y., Ramakrishna T., Naiki H., Goto Y., and Rao C. M. (2005) αB-crystallin, a small heat-shock protein, prevents the amyloid fibril growth of an amyloid β-peptide and β2-microglobulin. Biochem. J. 392, 573–581 10.1042/BJ20050339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Baughman H. E. R., Clouser A. F., Klevit R. E., and Nath A. (2018) HspB1 and Hsc70 chaperones engage distinct Tau species and have different inhibitory effects on amyloid formation. J. Biol. Chem. 293, 2687–2700 10.1074/jbc.M117.803411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yerbury J. J., Gower D., Vanags L., Roberts K., Lee J. A., and Ecroyd H. (2013) The small heat shock proteins αB-crystallin and Hsp27 suppress SOD1 aggregation in vitro. Cell Stress Chaperones 18, 251–257 10.1007/s12192-012-0371-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Binger K. J., Ecroyd H., Yang S., Carver J. A., Howlett G. J., and Griffin M. D. (2013) Avoiding the oligomeric state: αB-crystallin inhibits fragmentation and induces dissociation of apolipoprotein C-II amyloid fibrils. FASEB J. 27, 1214–1222 10.1096/fj.12-220657 [DOI] [PubMed] [Google Scholar]
- 39. Der Perng M., Su M., Wen S. F., Li R., Gibbon T., Prescott A. R., Brenner M., and Quinlan R. A. (2006) The Alexander disease-causing glial fibrillary acidic protein mutant, R416W, accumulates into Rosenthal fibers by a pathway that involves filament aggregation and the association of αB-crystallin and HSP27. Am. J. Human Genet. 79, 197–213 10.1086/504411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cox D., Whiten D. R., Brown J. W. P., Horrocks M. H., Gil R. S., Dobson C. M., Klenerman D., Oijen A. M. V., and Ecroyd H. (2018) The small heat shock protein Hsp27 binds α-synuclein fibrils, preventing elongation and cytotoxicity. J. Biol. Chem. 293, 4486–4497 10.1074/jbc.M117.813865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lambert H., Charette S. J., Bernier A. F., Guimond A., and Landry J. (1999) HSP27 multimerization mediated by phosphorylation-sensitive intermolecular interactions at the amino terminus. J. Biol. Chem. 274, 9378–9385 10.1074/jbc.274.14.9378 [DOI] [PubMed] [Google Scholar]
- 42. Aquilina J. A., Benesch J. L., Bateman O. A., Slingsby C., and Robinson C. V. (2003) Polydispersity of a mammalian chaperone: mass spectrometry reveals the population of oligomers in B-crystallin. Proc. Natl. Acad. Sci. U.S.A. 100, 10611–10616 10.1073/pnas.1932958100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Baldwin A. J., Hilton G. R., Lioe H., Bagnéris C., Benesch J. L., and Kay L. E. (2011) Quaternary dynamics of αB-crystallin as a direct consequence of localised tertiary fluctuations in the C-terminus. J. Mol. Biol. 413, 310–320 10.1016/j.jmb.2011.07.017 [DOI] [PubMed] [Google Scholar]
- 44. Aquilina J. A., Benesch J. L. P., Ding L. L., Yaron O., Horwitz J., and Robinson C. V. (2004) Phosphorylation of αB-crystallin alters chaperone function through loss of dimeric substructure. J. Biol. Chem. 279, 28675–28680 10.1074/jbc.M403348200 [DOI] [PubMed] [Google Scholar]
- 45. Jovcevski B., Kelly M. A., Rote A. P., Berg T., Gastall H. Y., Benesch J. L., Aquilina J. A., and Ecroyd H. (2015) Phosphomimics destabilize Hsp27 oligomeric assemblies and enhance chaperone activity. Chem. Biol. 22, 186–195 10.1016/j.chembiol.2015.01.001 [DOI] [PubMed] [Google Scholar]
- 46. Pasta S. Y., Raman B., Ramakrishna T., and Rao C. M. (2002) Role of the C-terminal extensions of α-crystallins: swapping the C-terminal extension of α-crystallin to αB-crystallin results in enhanced chaperone activity. J. Biol. Chem. 277, 45821–45828 10.1074/jbc.M206499200 [DOI] [PubMed] [Google Scholar]
- 47. Ghosh J. G., Shenoy A. K. Jr., and Clark J. I. (2006) N- and C-terminal motifs in human αB crystallin play an important role in the recognition, selection, and solubilization of substrates. Biochemistry 45, 13847–13854 10.1021/bi061471m [DOI] [PubMed] [Google Scholar]
- 48. Aquilina J. A., and Watt S. J. (2007) The N-terminal domain of αB-crystallin is protected from proteolysis by bound substrate. Biochem. Biophys. Res. Commun. 353, 1115–1120 10.1016/j.bbrc.2006.12.176 [DOI] [PubMed] [Google Scholar]
- 49. Biancalana M., and Koide S. (2010) Molecular mechanism of thioflavin-T binding to amyloid fibrils. Biochim. Biophys. Acta 1804, 1405–1412 10.1016/j.bbapap.2010.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gade Malmos K., Blancas-Mejia L. M., Weber B., Buchner J., Ramirez-Alvarado M., Naiki H., and Otzen D. (2017) ThT 101: a primer on the use of thioflavin T to investigate amyloid formation. Amyloid 24, 1–16 10.1080/13506129.2017.1304905 [DOI] [PubMed] [Google Scholar]
- 51. Hayes D., Napoli V., Mazurkie A., Stafford W. F., and Graceffa P. (2009) Phosphorylation dependence of Hsp27 multimeric size and molecular chaperone function. J. Biol. Chem. 284, 18801–18807 10.1074/jbc.M109.011353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Buell A. K., Galvagnion C., Gaspar R., Sparr E., Vendruscolo M., Knowles T. P. J., Linse S., and Dobson C. M. (2014) Solution conditions determine the relative importance of nucleation and growth processes in α-synuclein aggregation. Proc. Natl. Acad. Sci. U.S.A. 111, 7671–7676 10.1073/pnas.1315346111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Waudby C. A., Knowles T. P. J., Devlin G. L., Skepper J. N., Ecroyd H., Carver J. A., Welland M. E., Christodoulou J., Dobson C. M., and Meehan S. (2010) The interaction of αB-crystallin with mature α-synuclein amyloid fibrils inhibits their elongation. Biophys. J. 98, 843–851 10.1016/j.bpj.2009.10.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Carulla N., Caddy G. L., Hall D. R., Zurdo J., Gairí M., Feliz M., Giralt E., Robinson C. V., and Dobson C. M. (2005) Molecular recycling within amyloid fibrils. Nature 436, 554–558 10.1038/nature03986 [DOI] [PubMed] [Google Scholar]
- 55. Binger K. J., Griffin M. D., and Howlett G. J. (2008) Methionine oxidation inhibits assembly and promotes disassembly of apolipoprotein C-II amyloid fibrils. Biochemistry 47, 10208–10217 10.1021/bi8009339 [DOI] [PubMed] [Google Scholar]
- 56. Duennwald M. L., Echeverria A., and Shorter J. (2012) Small heat shock proteins potentiate amyloid dissolution by protein disaggregases from yeast and humans. PLoS Biol. 10, e1001346 10.1371/journal.pbio.1001346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Gao X., Carroni M., Nussbaum-Krammer C., Mogk A., Nillegoda N. B., Szlachcic A., Guilbride D. L., Saibil H. R., Mayer M. P., and Bukau B. (2015) Human Hsp70 disaggregase reverses Parkinson's-linked α-synuclein amyloid fibrils. Mol. Cell 59, 781–793 10.1016/j.molcel.2015.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Delbecq S. P., Rosenbaum J. C., and Klevit R. E. (2015) A mechanism of subunit recruitment in human small heat shock protein oligomers. Biochemistry 54, 4276–4284 10.1021/acs.biochem.5b00490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mainz A., Peschek J., Stavropoulou M., Back K. C., Bardiaux B., Asami S., Prade E., Peters C., Weinkauf S., Buchner J., and Reif B. (2015) The chaperone αB-crystallin uses different interfaces to capture an amorphous and an amyloid client. Nat. Struct. Mol. Biol. 22, 898–905 10.1038/nsmb.3108 [DOI] [PubMed] [Google Scholar]
- 60. Li B., Ge P., Murray K. A., Sheth P., Zhang M., Nair G., Sawaya M. R., Shin W. S., Boyer D. R., Ye S., Eisenberg D. S., Zhou Z. H., and Jiang L. (2018) Cryo-EM of full-length α-synuclein reveals fibril polymorphs with a common structural kernel. Nat. Commun. 9, 3609 10.1038/s41467-018-05971-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mannini B., Cascella R., Zampagni M., Waarde-Verhagen M., van, Meehan S., Roodveldt C., Campioni S., Boninsegna M., Penco A., Relini A., Kampinga H. H., Dobson C. M., Wilson M. R., Cecchi C., and Chiti F. (2012) Molecular mechanisms used by chaperones to reduce the toxicity of aberrant protein oligomers. Proc. Natl. Acad. Sci. U.S.A. 109, 12479–12484 10.1073/pnas.1117799109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ori A., Toyama B. H., Harris M. S., Bock T., Iskar M., Bork P., Ingolia N. T., Hetzer M. W., and Beck M. (2015) Integrated transcriptome and proteome analyses reveal organ-specific proteome deterioration in old rats. Cell Systems 1, 224–237 10.1016/j.cels.2015.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lowe J., Landon M., Pike I., Spendlove I., Mcdermott H., and John Mayer R. (1990) Dementia with β-amyloid deposition: involvement of αB-crystallin supports two main diseases. Lancet 336, 515–516 10.1016/0140-6736(90)92075-S [DOI] [PubMed] [Google Scholar]
- 64. Outeiro T. F., Klucken J., Strathearn K. E., Liu F., Nguyen P., Rochet J.-C., Hyman B. T., and McLean P. J. (2006) Small heat shock proteins protect against α-synuclein-induced toxicity and aggregation. Biochem. Biophys. Res. Commun. 351, 631–638 10.1016/j.bbrc.2006.10.085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wilhelmus M. M., Otte-Höller I., Wesseling P., de Waal R. M., Boelens W. C., and Verbeek M. M. (2006) Specific association of small heat shock proteins with the pathological hallmarks of Alzheimer's disease brains. Neuropathol. Appl. Neurobiol. 32, 119–130 10.1111/j.1365-2990.2006.00689.x [DOI] [PubMed] [Google Scholar]
- 66. Rujano M. A., Bosveld F., Salomons F. A., Dijk F., van Waarde M. A., van der Want J. J., de Vos R. A., Brunt E. R., Sibon O. C., and Kampinga H. H. (2006) Polarised asymmetric inheritance of accumulated protein damage in higher eukaryotes. PLoS Biol. 4, e417 10.1371/journal.pbio.0040417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Cabrita L. D., Gilis D., Robertson A. L., Dehouck Y., Rooman M., and Bottomley S. P. (2007) Enhancing the stability and solubility of TEV protease using in silico design. Protein Sci. 16, 2360–2367 10.1110/ps.072822507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Horwitz J., Bova M., Huang Q. L., Ding L., Yaron O., and Lowman S. (1998) Mutation of αB-crystallin: effects on chaperone-like activity. Int. J. Biol. Macromol. 22, 263–269 10.1016/S0141-8130(98)00024-5 [DOI] [PubMed] [Google Scholar]
- 69. Narhi L., Wood S. J., Steavenson S., Jiang Y., Wu G. M., Anafi D., Kaufman S. A., Martin F., Sitney K., Denis P., Louis J.-C., Wypych J., Biere A. L., and Citron M. (1999) Both familial Parkinson's disease mutations accelerate α-synuclein aggregation. J. Biol. Chem. 274, 9843–9846 10.1074/jbc.274.14.9843 [DOI] [PubMed] [Google Scholar]
- 70. Clouser A. F., and Klevit R. E. (2017) pH-dependent structural modulation is conserved in the human small heat shock protein HSBP1. Cell Stress Chaperones 22, 569–575 10.1007/s12192-017-0783-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Cox D., Selig E., Griffin M. D., Carver J. A., and Ecroyd H. (2016) Small heat-shock proteins prevent α-synuclein aggregation via transient interactions and their efficacy is affected by the rate of aggregation. J. Biol. Chem. 291, 22618–22629 10.1074/jbc.M116.739250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ryan T. M., Howlett G. J., and Bailey M. F. (2008) Fluorescence detection of a lipid-induced tetrameric intermediate in amyloid fibril formation by apolipoprotein C-II. J. Biol. Chem. 283, 35118–35128 10.1074/jbc.M804004200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Schuck P. (2000) Size-distribution analysis of macromolecules by sedimentation velocity ultracentrifugation and lamm equation modeling. Biophys. J. 78, 1606–1619 10.1016/S0006-3495(00)76713-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Harding S. E., Rowe A. J., and Horton J. C. (1992) Analytical Ultracentrifugation in Biochemistry and Polymer Science, Royal Society of Chemistry, Cambridge, UK [Google Scholar]
- 75. MacRaild C. A., Hatters D. M., Lawrence L. J., and Howlett G. J. (2003) Sedimentation velocity analysis of flexible macromolecules: self-association and tangling of amyloid fibrils. Biophys. J. 84, 2562–2569 10.1016/S0006-3495(03)75061-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Schuck P., and Rossmanith P. (2000) Determination of the sedimentation coefficient distribution by least-squares boundary modeling. Biopolymers 54, 328–341 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are contained within the manuscript and supporting information.