Abstract
The infectious bronchitis virus (IBV) is an acute and highly contagious disease, which affects chickens of all ages. Vaccination is the most important way to control this disease. Nevertheless, novel variant strains are constantly reported because of the lack of proofreading capabilities of RNA polymerase and high frequency of homologous RNA recombination. Cross-protection studies has demonstrated that the vaccines could provide great protective effects against viruses of same serotype or genotype. However, the protective effect of different commercial vaccines and vaccine combinations against the prevalent IBV strains in China has rarely been studied. Owing to the multiple genotype or serotype IBV strains prevalence in China, the polyvalent vaccines and their composition were used to expanding the protection spectrum of vaccine in practical application. To evaluate the protection of Chinese commercial IBV polyvalent vaccines against prevalent strains (QX-like and TW I-like), an immune challenge test was conducted. Four polyvalent vaccines, containing 4/91, H120, YX10p90, LDT3-A, and 28/86, were combined to form 8 vaccination strategies, almost all of which could provide more than 70% protection effects against challenge with QX-like strain. Particularly, the best protection rate (93%) was generated by administration the polyvalent vaccine C (H120 + 28/86 + 4/91) at 1 D of age and the polyvalent vaccine B (H120 + 4/91 + YX10p90) at 10 D of age. However, all the vaccination strategies in this study cannot provide great protective effects against TW-like strain, and more vaccines should be included in studies to expand the protection spectrum of vaccine. Therefore, for the newly emerging IBV strains, immunization with polyvalent vaccines via different vaccination strategies could be used to control the prevalence of IBV in a short time, whereas developing the homologous vaccines was not always necessary.
Key words: infectious bronchitis virus, polyvalent vaccine, vaccination strategy, QX-like, TW-like
Introduction
Avian infectious bronchitis (IB) is an acute and highly contagious disease caused by the avian infectious bronchitis virus (IBV) (Cavanagh, 2007). This disease, which affects chickens of all ages, can cause pathologic lesions in the respiratory tract, kidney, and reproductive systems, resulting in severe economic losses (Cook et al., 2012; Jackwood, 2012).
The IBV belongs to the genus Coronavirus, family Coronaviridae, of the order Nidovirales (Mayo and Pringle, 1998). Owing to the discontinuity of RNA replication and the lack of proofreading capabilities of RNA polymerase, the IBV genome is susceptible to recombination and mutation (Duffy et al., 2008). A small change as little as 5% variation in antigenic epitope of the S1 protein composition may contribute to the change of serotype and the emergence of variant strains (Ignjatovic and Galli, 1994).
Currently, vaccination with both live and inactivated vaccines is the most effective measure for prevention and control of IBV (Zhao et al., 2015). However, a rapid evolution rate of Chinese IBV isolates and the lack of cross protection between different serotypes pose significant challenges to IBV infection control (Zhao et al., 2016).
The QX-like IBV strain, which is highly pathogenic and mainly causes nephritis, was previously detected in Shandong Province, China in 1996, then quickly spread across Asia and was reported in many European countries (Cook et al., 2012). At present, the control of QX-like IBV infection is still a problem in many countries (Franzo et al., 2017). The research of epidemiology of the IBV in China during 2004 to 2015 showed that at least 7 genotypes of the IBV circulating in China, and QX-like genotype strains that account for about 60% of the total isolates had become predominant (Feng et al., 2014, 2017; Yan et al., 2019). Existing commercial vaccines have poor protection against QX-like IBV (Feng et al., 2018), for example, H120 belonged to the Massachusetts serotype strain and was one of the most widely used vaccine strains, whereas QX-like IBV was found circulating in most of the H120-vaccinated flocks (Han et al., 2011).
The TW-like IBV strain, which was first isolated in Taiwan, can be divided into 2 subtypes TW I and TW II, causing nephritis and respiratory symptoms, respectively (Wang and Tsai, 1996). Before 2009, the TW-like IBV was rarely detected in mainland China (Luo et al., 2012). In the past 10 yr, the results of the IBV epidemiology in mainland China had shown that the proportion of TW-like IBV isolates had increased gradually, becoming the second most common IBV strains identified in southern China (Xu et al., 2016, 2018; Zhang et al., 2016; Feng et al., 2017). Some study indicated that the TW I-like strains were more prevalent than the QX-like viruses in Guangdong (33.3 vs. 12.2%) and Guangxi (54.5 vs. 28.9%) (Feng et al., 2017). Compared with the common vaccine strains (H120, H5,4/91, W93, Ma5, and LDT3-A), the nucleotide and amino acid identity of TW I-like IBV was less than 82 and 82.3%, respectively (Feng et al., 2017). These observations may suggest the low protection between vaccine strains and TW I-like strains. Moreover, the homologous vaccines for TW-like IBV have not yet been developed, which increases the difficulty for prevention and control of TW-like IBV.
Some studies had showed that a single vaccine with different serotype affords less protection against QX-like and TW-like IBV strains (Gao et al., 2016; Karimi et al., 2018). Despite the development of homologous vaccines was often impractical, immunization with multiple serotype vaccines had been proven to be beneficial and broadened the protective spectrum (i.e., “protectotype concept”) (Cook et al., 1999; Zhao et al., 2015). The aim of this study is to evaluate the efficacy of commercial polyvalent vaccines against QX-like and TW-like IBV strains via different vaccination strategies.
Materials and methods
Ethics Statement
All study procedures and animal care activities were conducted in accordance with the national and institutional guidelines for the care and use of laboratory animals, and the experimental protocols were approved by the Committee for Animal Experiments (approval ID: SYXK2019-0136) of South China Agricultural University.
Chickens
Healthy 1-day-old yellow dwarf chickens were obtained from Wens Foodstuff Group Co., Ltd., China. The study was performed in positive-pressure high-efficiency particulate air–filtered stainless-steel isolators with an enclosed and ventilated environment, and feed and water were provided ad libitum.
Viruses and Vaccines
The challenge strains CK/CH/GX/NN17-5 and CK/CH/GD/GZ14, which were all nephropathogenic strains and isolated from commercial broiler farms in China during 2016 to 2017, belong to the QX-like genotype and TW-like genotype, respectively. The complete genome of the IBV strains (CK/CH/GX/NN17-5 and CK/CH/GD/GZ14) was sequenced and deposited in GenBank under accession number MF447731 and KT946798, respectively.
Four Chinese commercial polyvalent vaccines were used to evaluate the efficacy in this study, containing 4/91 (Dahuanong Animal Health Products Co., Ltd., Guangdong, China), H120 (Dahuanong Animal Health Products Co., Ltd.), YX10p90 (saved by our laboratory), LDT3-A (Weike Biotechnology Development Co., Ltd., Harbin, China), and 28/86 (China Institute of Veterinary Drug Control). All challenge and vaccine strains are listed in Table 1.
Table 1.
Backgrounds of the IBV strains used in this study.
| Strain | Abbreviation | Country (province) | Usage | Genotype | Tissue tropism | Accession no. |
|---|---|---|---|---|---|---|
| 4/91 | UK | Vaccine strain | 793/B | AF093794 | ||
| H120 | Holland | Vaccine strain | Massachusetts | M21970 | ||
| 28/86 | Italy | Vaccine strain | Massachusetts | AY846750 | ||
| LDT3-A | China | Vaccine strain | tl/CH/LDT3/03 | AY702975 | ||
| YX10p90 | China (Zhejiang) | Vaccine strain | QX | MF508703 | ||
| CK/CH/GX/NN17-5 | NN17-5 | China (Guangxi) | Challenge strain | QX | Kidney | MF447731 |
| CK/CH/GD/GZ14 | GZ14 | China (Guangdong) | Challenge strain | TW I | Kidney | KT946798 |
Abbreviation: IBV, infectious bronchitis virus.
Evaluation Efficacy of Vaccines Composition Against QX-Like IBV Challenge
A total of 150 healthy 1-day-old yellow dwarf chickens of similar weight (39 g–42 g) were randomly divided into 10 groups of 15 chickens each, and each group was housed in one isolation unit. Each chicken in the experimental group was individually vaccinated with polyvalent vaccines at 1 and 10 D of age with dosage at 104.5 EID50/0.1 mL via the nasal routes. The control group was inoculated with PBS. Two weeks after second immunization, chickens in experimental groups were challenged intranasally with QX-like IBV NN17-5 strain (106.0 EID50) at 25 D of age.
Evaluation Efficacy of Vaccines Composition Against TW-Like IBV Challenge
A total of 135 1-day-old yellow dwarf chickens were divided into 9 groups of 15 chickens. The chickens were provided food and water ad libitum. At 1 and 10 D age, the chickens in the experimental groups were inoculated intranasally with polyvalent vaccines at 104.5 EID50/0.1 mL. The chickens in the control group were inoculated with PBS. At 25 D of age, chickens in all experimental groups were challenged with TW-like IBV GZ14 strain at 105.0 EID50/0.1 mL (Table 2).
Table 2.
Protective effects in chickens immunized with commercial polyvalent vaccines against the QX-like and TW-like IBV strains.
| Vaccination1 |
Virus recovery5 |
Positive rate of antibody7 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group | 12 | 10 | Challenge3 | Morbidity | Mortality | Clinical protection4 | 56 | 9 | 15 | |
| 1 | Polyvalent vaccine A | Polyvalent vaccine B | NN17-5 | 4/15 | 2/15 | 73% | 15/15 | 11/13 | 5/13 | 40% |
| (H120 + 4/91) | (H120 + 4/91 + YX10p90) | GZ14 | 9/15 | 5/15 | 40% | 15/15 | 7/12 | 5/10 | ||
| 2 | Polyvalent vaccine B | Polyvalent vaccine A | NN17-5 | 4/15 | 2/15 | 73% | 15/15 | 8/14 | 6/13 | 50% |
| (H120 + 4/91 + YX10p90) | (H120 + 4/91) | GZ14 | 8/15 | 5/15 | 47% | 15/15 | 7/11 | 6/10 | ||
| 3 | Polyvalent vaccine B | Polyvalent vaccine C | NN17-5 | 3/15 | 1/15 | 80% | 12/14 | 7/14 | 3/14 | 70% |
| (H120 + 4/91 + YX10p90) | (H120 + 28/86 + 4/91) | GZ14 | 8/15 | 5/15 | 47% | 12/13 | 6/10 | 6/10 | ||
| 4 | Polyvalent vaccine C | Polyvalent vaccine B | NN17-5 | 1/15 | 0/15 | 93% | 13/15 | 3/15 | 1/15 | 80% |
| (H120 + 28/86 + 4/91) | (H120 + 4/91 + YX10p90) | GZ14 | 7/15 | 4/15 | 53% | 13/15 | 7/11 | 5/11 | ||
| 5 | Polyvalent vaccine A | Polyvalent vaccine A | NN17-5 | 5/15 | 2/15 | 67% | 14/15 | 11/13 | 5/13 | 40% |
| (H120 + 4/91) | (H120 + 4/91) | GZ14 | 6/15 | 4/15 | 60% | 12/12 | 11/11 | 6/11 | ||
| 6 | Polyvalent vaccine B | Polyvalent vaccine B | NN17-5 | 4/15 | 3/15 | 73% | 15/15 | 12/12 | 4/12 | 50% |
| (H120 + 4/91 + YX10p90) | (H120 + 4/91 + YX10p90) | GZ14 | 6/15 | 3/15 | 60% | 15/15 | 11/12 | 7/12 | ||
| 7 | Polyvalent vaccine C | Polyvalent vaccine C | NN17-5 | 2/15 | 1/15 | 87% | 15/15 | 8/14 | 3/14 | 70% |
| (H120 + 28/86 + 4/91) | (H120 + 28/86 + 4/91) | GZ14 | 6/15 | 2/15 | 60% | 15/15 | 12/13 | 7/13 | ||
| 8 | Polyvalent vaccine D | Polyvalent vaccine D | NN17-5 | 4/15 | 1/15 | 73% | 15/15 | 13/14 | 6/14 | 30% |
| (H120 + LDT3-A) | (H120 + LDT3-A) | GZ14 | 7/15 | 5/15 | 53% | 14/14 | 10/11 | 7/10 | ||
| 9 | PBS | PBS | NN17-5 | 15/15 | 10/15 | 0 | 12/12 | 7/7 | 3/5 | 10% |
| PBS | PBS | GZ14 | 10/15 | 6/15 | 33% | 14/14 | 9/10 | 7/9 | ||
| 10 | PBS | PBS | None | 0/15 | 0/15 | 100% | 0/15 | 0/15 | 0/15 | 0 |
Abbreviation: IBV, infectious bronchitis virus
Yellow dwarf chickens were vaccinated with polyvalent vaccines via the nasal routes at 1 and 10 D of age.
Age of yellow dwarf chickens.
At 25-day-old, chickens were challenged with NN17-5 and GZ14 via the nasal routes, respectively.
The number of chickens without clinical symptom/the total number of chickens challenged in each group.
Chicken embryo lethality test and RT-PCR identification were used for the detection of virus recovery.
Days post challenge.
At 25 D of age, 10 serums from 10 chickens in each group were collected for detection of positive rate of antibody via ELISA.
Clinical Observations and Sampling
To determine the pathology produced by the challenge strains, all chickens were observed daily for 3 wk after challenge for clinical signs, including tracheal rales, mouth breathing, depression, head twitches, ruffled feathers, and slight watery diarrhea. Blood samples were collected just before revaccination and 0, 5, 9, and 15 D postchallenge (dpc) to collect sera for detection of antibodies using an ELISA Test Kit (IDEXX Laboratories, Westbrook, ME) as per the manufacturer's instructions. Moreover, oral swabs from all chickens were collected at 5, 9, and 15 dpc for the detection of virus shedding by reverse transcription PCR (RT-PCR).
Virus Recovery and RT-PCR Identification
Oral swabs from each group were collected at 5, 9, and 15 dpc for the detection of virus recovery. Each oral swab was eluted in 1 mL of PBS, and the supernatant was filtered through 0.22-μm membrane filters (Millipore Products Division) before inoculation into the allantoic cavity of 9-day-old specific pathogen–free chicken embryos. A total of volume of 200 μL of the previously treated samples were inoculated into specific pathogen–free chicken embryos in triplicate per group and then incubated at 37°C. Allantoic fluid from 2 inoculated embryos of each group was collected at 48 h after inoculation using disposable syringes for the detection of virus recovery by RT-PCR, whereas the remaining embryos were examined 1 wk after inoculation for characteristic lesions of IB, such as dwarfing, stunting, or curling of embryos. Dead chicken embryos and chicken embryos showing characteristic lesions of IB were recorded as positive for virus recovery.
Total viral RNA was extracted from the inoculated allantoic fluid by using the AxyPrep Body Fluid Viral DNA/RNA Miniprep Kit (Axygen, China) as per the manufacturer's instructions. The genomic RNA was reverse transcribed into cDNA using the PrimeScript II First Strand cDNA Synthesis Kit (Takara, Japan) in accordance with the manufacturer's protocol. For PCR, 10 μL of PrimeSTAR Max Premix (2×) (Takara, Japan) and 10 pmol of each primer were added to 2 μL of cDNA as template in a total of 20-μL reaction volume. PCR was performed at 94°C for 5 min, followed by 35 cycles of denaturation (98°C, 10 s), annealing (55°C, 5 s), and polymerization (72°C, 20 s), and a final DNA extension step was performed at 72°C for 10 min. The primers amplifying the S1 gene of IBV were forward: 5′–AAGACTGAACAAAAGACCGACT–3′ and reverse: 5′–CAAAACCTGCCATAACTAACATA–3′, and the expected size of the PCR product was 1,721 bp (Feng et al., 2018). The PCR products were analyzed by 1.0% agarose gel electrophoresis.
Statistical Analysis
Statistical analyses were conducted using SPSS 20.0 software. Independent sample t tests were performed to determine statistically significant differences. The significance was considered as ∗P < 0.05 and ∗∗P < 0.01.
Results
Vaccine Safety
After the chickens were individually vaccinated with commercial polyvalent vaccine A, B, C, and D at 1 and 10 D of age, neither death nor obvious clinical signs of the IBV was observed.
Efficacy of Polyvalent Vaccines Against QX-Like IBV Challenge
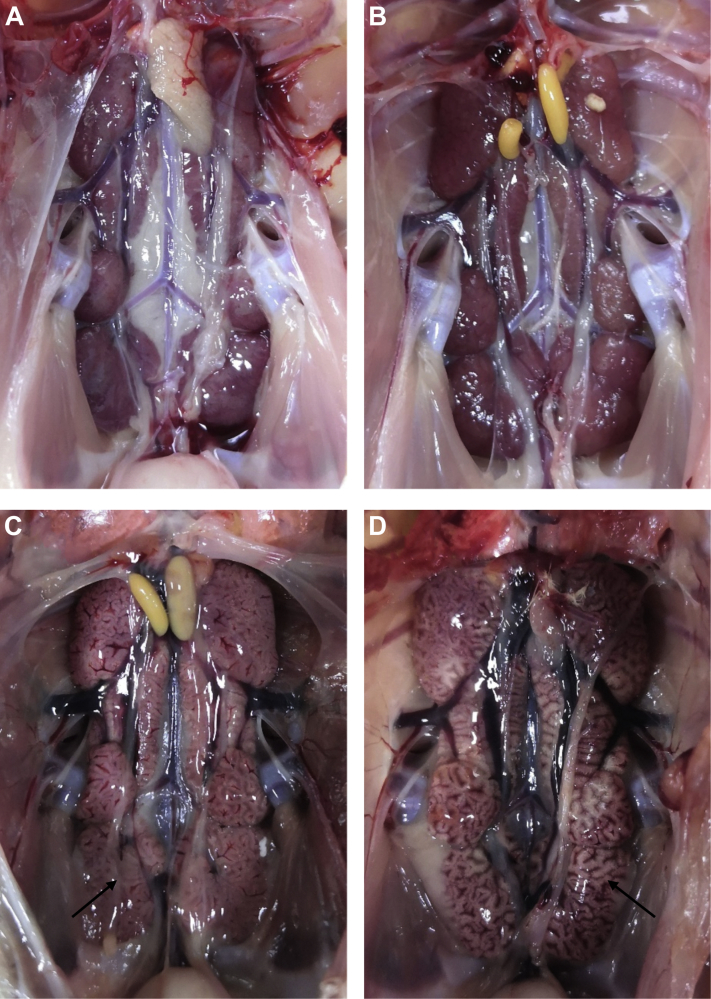
The 25-day-old chickens immunized with polyvalent vaccines were challenged with QX-like strain NN17-5. As shown in Table 2, as per the clinical protection in the chickens that were immunized with the same polyvalent vaccine at 1 and 10 D of age, the protective effects of the polyvalent vaccine C against the QX-like strain were higher than the other polyvalent vaccines. However, the polyvalent vaccine C only provided 87% of clinical protection, and some of the vaccinated chickens still showed clinical signs and death after challenge with the QX-like strain, showing typical kidney lesions that were characterized by enlarged, pale, and marbled kidneys and urate deposition in the tubules and ureters (Figure 1), which suggested that 4 polyvalent vaccines in this study cannot provide full clinical protection against challenge with the QX-like strain.
Figure 1.
Representative images of the kidneys from vaccinated chickens challenged with the QX-like IBV NN17-5 strain. (A) Representative image of the kidney from an uninfected control chicken. (B) No lesions were observed in a vaccinated/challenged chicken in group 4. (C) Representative image of the kidney tissue from a vaccinated/challenged chicken in group 3. (D) Obvious kidney enlargement and marbled kidney from an unvaccinated/challenged chicken. The lesions were indicated with arrows.
The clinical protective effects against challenge with the QX-like strain had been changed by immunizing with polyvalent vaccines via different vaccination strategies. In group 1, the protection rate (73%) was generated by following vaccination with the polyvalent vaccine A at 1 D of age and the polyvalent vaccine B at 10 D of age, and the vaccination strategy of group 2 provided the same protective effect as group 1. In group 4, the protection rate (93%) was generated by administration the polyvalent vaccine C at 1 D of age and the polyvalent vaccine B at 10 D of age, whereas group 3 combined the polyvalent vaccine B at 1 D of age and the polyvalent vaccine C at 10 D of age, which was less protective, that is, 80%. Interestingly, the composition of the polyvalent vaccines in group 3 was the same as that of group 4, whereas the protective effects provided by the 2 vaccination strategies were different.
Efficacy of Polyvalent Vaccines Against TW-Like IBV Challenge
As shown in Table 2, the vaccination challenge test showed that the polyvalent vaccines in this study provided the level of clinical protection against challenge with the TW-like IBV strain less than 60%. Compared with the single vaccination strategy, the efficacy of different vaccination strategies was not obvious. In groups 5, 6, 7, and 8, the clinical protection rate (53–60%) was generated by administrating the same polyvalent vaccine at 1 and 10 D of age, whereas only 40 to 53% of clinical protection rate was generated by different vaccination strategies in groups 1, 2, 3, and 4.
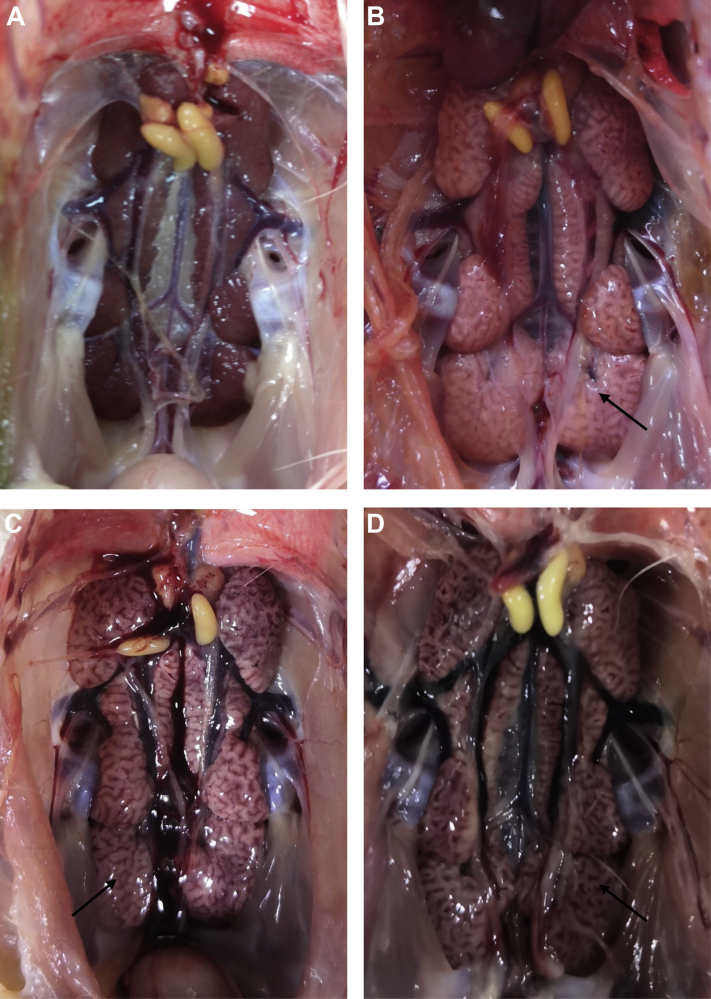
Chickens in experimental groups that were challenged with the TW-like IBV strain showed clinical signs and death at 5 dpc. Diseased chickens showed signs of listlessness, coughing, decreased appetite, increased water intake, and ruffled feather with rapid BW loss. At necropsy, all euthanized chickens showed typical kidney lesions, characterized by enlarged, pale, and marbled kidneys (Figure 2). Meanwhile, no lesions were observed in the negative control group.
Figure 2.
Representative gross lesions in the kidneys of vaccinated chickens challenged with the TW-like IBV GZ14 strain. (A) Kidney tissue from an unvaccinated/unchallenged chicken. (B) Lesions in the kidney of an unvaccinated/challenged chicken. (C, D) Representative images of kidneys from vaccinated/challenged chickens. The lesions were indicated with arrows.
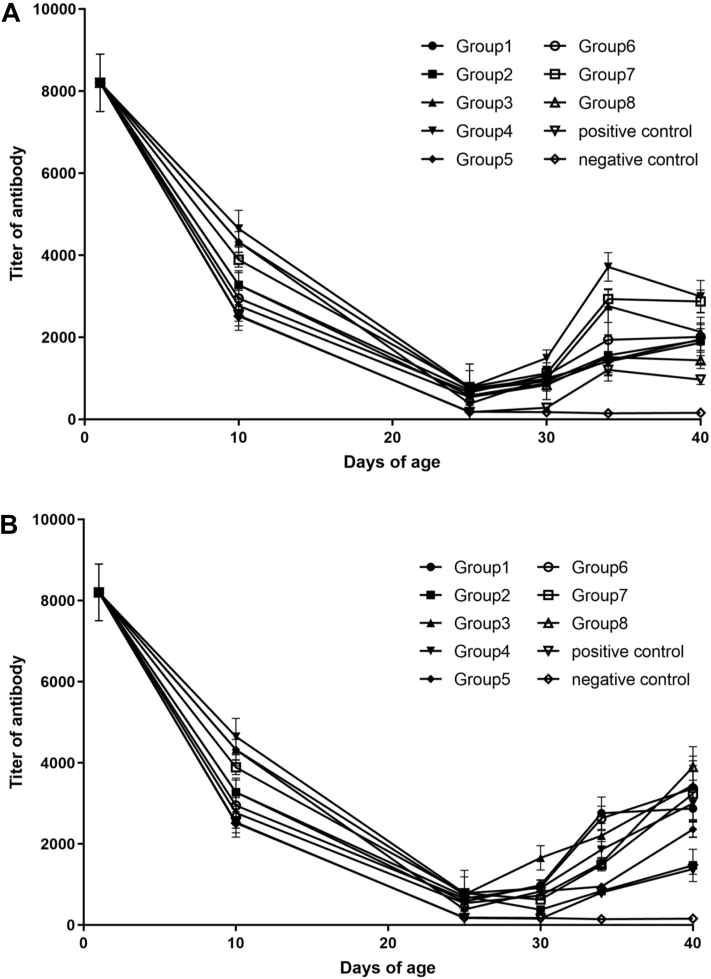
Serology
To evaluate the effects of the polyvalent vaccines on the response of the immune system, the titer of antibody against the IBV was measured using a commercial ELISA kit. Compared with the level of antibody at 25 D of age, the titer of antibody dropped significantly from the level of mean maternal antibody (8203) to the basal level (P < 0.01) (Figure 3). Only 30 to 50% of the chickens showed a positive serum antibody response on the day just before challenge in groups 1, 2, 5, 6, and 8. In contrast, the chickens in groups 3, 4, and 7 showed more than 70% positive serum antibody response. The titer of antibody of chickens challenged with the QX-like strain increased gradually after challenge, reaching the highest titer at 9 dpc and then decreasing. Compared with positive group, the titers of antibody in groups 3, 4, and 7 elevated significantly at 9 dpc (P < 0.01). No significant difference (P > 0.05) was found in titers between the positive group and groups 1, 2, 5, 6, and 8 at 9 dpc (Figure 3A). In addition, the titer of antibody of chickens challenged with the TW-like strain kept increasing after challenge (Figure 3B).
Figure 3.
Change curves of mean titters of antibody against the IBV in different groups. Serum samples were collected from all chickens on days 1, 10, 25, 30, 34, and 40. (A) Mean titers of antibodies against the IBV in the groups challenged with the QX-like IBV NN17-5 strain. (B) Mean titers of antibodies against the IBV in the groups challenged with the TW-like IBV GZ14 strain. Abbreviation: IBV, infectious bronchitis virus.
Virus Recovery
Virus recovery was identified by RT-PCR and chicken embryo lethality test. As shown in Table 2, the challenge viruses, including QX-like and TW-like strains, were reisolated from nearly all of the oral swabs that were collected from the chickens in experimental groups at 5 dpc. In addition to group 4, virus shedding rate was more than 50% in all experimental group at 9 dpc. Fifteen dpc, only 7% of the virus-positive rate was detected in group 4 challenged with the QX-like strain, whereas the rates were 21 to 60% in the other groups challenged with the QX-like strain. In addition, 50 to 78% of the chickens in the groups challenged with the TW-like strain were positive for virus reisolation. This indicates that the vaccination strategies in this study cannot provide protection against tracheal invasion by the TW-like strain.
Discussion
Infectious bronchitis is prevalent in many parts of the world, causing great economic loss to the poultry industry in various countries (Cavanagh et al., 1997; Jackwood, 2012). Although the various live-attenuated and inactivated IBV vaccines have been used in production, outbreaks of IB has occurred sporadically. As some studies have shown, there are lack of cross protection between different IBV serotypes (Cook et al., 2001; Gelb et al., 2005; Zhao et al., 2015). Currently, commercial vaccines cannot provide enough protection for the challenge with heterogeneous strains, and more importantly, an overall higher selective pressure strength was proven after homologous vaccination was introduced, potentially leading to the emergence of new vaccine-escape variants (Franzo et al., 2017, 2019). Therefore, the development of a polyvalent vaccine with multiple genotype composition is urgently required as an important measure to prevent and control IBV in a short time.
Immunization with 1 serotype vaccine cannot provide sufficient protection against heterologous virus, whereas multiple immunization with a combination of different serotypes can provide a vast protection immunity (Cook et al., 1999). A clinical study has revealed that 51% protection rate was generated by administration the Massachusetts serotype vaccine at 1 D of age and the 793/B vaccine at 14 D of age in ciliostasis test on 5 dpc with D388 (QX-like genotype), whereas 74% protection generated at 7 dpc (de Wit et al., 2011). In this study, almost all of vaccination strategies could provide more than 70% protection effects against challenge with the QX-like strain; Moreover, with the increase of the number of different serotypes in vaccination strategies, the protection was greater (Table 2).
Individually, changing the boost of vaccination in the vaccination strategies for inoculating 2 different polyvalent vaccines may result in a change of protective effect. It was indicated in a clinical study that 80 and 60% of the ciliostasis protection effects against Middle East 885 and 1494 IBV strains were provided by administration H120 vaccine at 1 D of age and CR88 at 14 D of age, but more than 80% of the protection effects against tracheal ciliostasis was provided when changed the boost of vaccination (Awad et al., 2015). In this study, 93% clinical protection effects against the QX-like strain was generated by administration of the polyvalent vaccine C at 1 D of age and the polyvalent vaccine B at 10 D of age, whereas the boost of vaccination was changed in group 3, and only 80% protection rate was provided (Table 2). At present, no study has demonstrated the mechanism about the change of protection effect that was based on changing the boost of vaccination (Karimi et al., 2018).
ELISA has been demonstrated to be a reliable and sensitive method for the rapid detection of initial increase in antibodies against the IBV (Chen et al., 2011). Specific pathogen–free chickens used in this study were detected to have higher levels of maternally derived antibodies. It had been reported that maternally derived antibodies had no adverse effect on the efficacy for 1-day-old chickens to be immunized with live IBV vaccines (Bru et al., 2017). The results showed that antibody titer of the vaccinated chickens reached the highest value at 9 dpc with the QX-like strain and then decreased until end of the experiment. Moreover, antibody titer of the vaccinated chickens kept rising after challenging with the TW-like strain, which was not consistent with the actual clinical protective effects. Although low-to-medium humoral response is expected after the administration of live IBV vaccines (Terregino et al., 2008), antibodies against the IBV was not always consistent with actual clinical protection (Cavanagh, 2003).
Among the tissues examined by histopathology, the main target organs of the IBV, trachea and kidney, were most severely damaged (Zhao et al., 2015). In this study, although the clinical protection against QX-like IBV was relatively improved via different vaccination strategies, the circulating virus cannot be cleared effectively, which indicated that both systemic (IgM and IgG) and mucosal (IgA) antibodies produced after immunization were insufficient (Cook et al., 2012).
In summary, the data from this study and the literature clearly demonstrate that a combination of commercial polyvalent vaccines can provide great protection against heterologous viruses. Although all the vaccination strategies in this study cannot provide great protective effects against the TW-like strain, cross protection against heterologous viruses generated by combination of polyvalent vaccines is available (Bande et al., 2015), and more vaccines should be included in studies to expand the protection spectrum of vaccine. The vaccination strategies in this study were all directed against the QX-like and TW-like strains prevalent in southern China. It is important to identify the circulating and prevalent of IBV strains in particular areas, as only then more appropriate and effective vaccination strategies can be formulated. In the short term, immunization with polyvalent vaccines via different vaccination strategies may also control the prevalence of IBV. In the long term, developing new type and efficient IBV vaccines such as viral vector–based vaccines, subunit and peptide-based vaccines, and reverse genetic vaccines is essential.
Acknowledgments
This study was supported by the Key Research and Development Program of Guangdong Province (2019B020218004), the Construction Project of Modern Agricultural Science and Technology Innovation Alliance in Guangdong Province (2019KJ128) and the creation of a triple chimeric vaccine (rIBV-ND-H9) using avian infectious bronchitis attenuated YX10p90 as a vector (2017KZDM008).
Conflict of Interest: The authors declare that there are no conflicts of interest.
References
- Awad F., Forrester A., Baylis M., Lemiere S., Ganapathy K., Hussien H.A., Capua I. Protection conferred by live infectious bronchitis vaccine viruses against variant Middle East IS/885/00-like and IS/1494/06-like isolates in commercial broiler chicks. Vet. Rec. Open. 2015;2:e111. doi: 10.1136/vetreco-2014-000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bande F., Arshad S.S., Bejo M.H., Moeini H., Omar A.R. Progress and challenges toward the development of vaccines against avian infectious bronchitis. J. Immunol. Res. 2015;2015:424860. doi: 10.1155/2015/424860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bru T., Vila R., Cabana M., Geerligs H.J. Protection of chickens vaccinated with combinations of commercial live infectious bronchitis vaccines containing Massachusetts, Dutch and QX-like serotypes against challenge with virulent infectious bronchitis viruses 793B and IS/1494/06 Israel variant 2. Avian Pathol. 2017;46:52–58. doi: 10.1080/03079457.2016.1203393. [DOI] [PubMed] [Google Scholar]
- Cavanagh D., Elus M.M., Cook J.K. Relationship between sequence variation in the S1 spike protein of infectious bronchitis virus and the extent of cross-protection in vivo. Avian Pathol. 1997;26:63–74. doi: 10.1080/03079459708419194. [DOI] [PubMed] [Google Scholar]
- Cavanagh D. Severe acute respiratory syndrome vaccine development: experiences of vaccination against avian infectious bronchitis coronavirus. Avian Pathol. 2003;32:567–582. doi: 10.1080/03079450310001621198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh D. Coronavirus avian infectious bronchitis virus. Vet. Res. 2007;38:281–297. doi: 10.1051/vetres:2006055. [DOI] [PubMed] [Google Scholar]
- Chen H.W., Wang C.H., Cheng I.C. A type-specific blocking ELISA for the detection of infectious bronchitis virus antibody. J. Virol. Methods. 2011;173:7–12. doi: 10.1016/j.jviromet.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook J.K., Chesher J., Baxendale W., Greenwood N., Huggins M.B., Orbell S.J. Protection of chickens against renal damage caused by a nephropathogenic infectious bronchitis virus. Avian Pathol. 2001;30:423–426. doi: 10.1080/03079450120066421. [DOI] [PubMed] [Google Scholar]
- Cook J.K., Jackwood M., Jones R.C. The long view: 40 years of infectious bronchitis research. Avian Pathol. 2012;41:239–250. doi: 10.1080/03079457.2012.680432. [DOI] [PubMed] [Google Scholar]
- Cook J.K., Orbell S.J., Woods M.A., Huggins M.B. Breadth of protection of the respiratory tract provided by different live-attenuated infectious bronchitis vaccines against challenge with infectious bronchitis viruses of heterologous serotypes. Avian Pathol. 1999;28:477–485. doi: 10.1080/03079459994506. [DOI] [PubMed] [Google Scholar]
- de Wit J.J., Nieuwenhuisen-van W.J., Hoogkamer A., van de Sande H., Zuidam G.J., Fabri T.H. Induction of cystic oviducts and protection against early challenge with infectious bronchitis virus serotype D388 (genotype QX) by maternally derived antibodies and by early vaccination. Avian Pathol. 2011;40:463–471. doi: 10.1080/03079457.2011.599060. [DOI] [PubMed] [Google Scholar]
- Duffy S., Shackelton L.A., Holmes E.C. Rates of evolutionary change in viruses: patterns and determinants. Nat. Rev. Genet. 2008;9:267–276. doi: 10.1038/nrg2323. [DOI] [PubMed] [Google Scholar]
- Feng K.Y., Chen T., Zhang X., Shao G.M., Cao Y., Chen D.K., Lin W.C., Chen F., Xie Q.M. Molecular characteristic and pathogenicity analysis of a virulent recombinant avain infectious bronchitis virus isolated in China. Poult. Sci. 2018;97:3519–3531. doi: 10.3382/ps/pey237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng K., Wang F., Xue Y., Zhou Q., Chen F., Bi Y., Xie Q. Epidemiology and characterization of avian infectious bronchitis virus strains circulating in southern China during the period from 2013-2015. Sci. Rep. 2017;7:6576. doi: 10.1038/s41598-017-06987-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng K., Xue Y., Wang F., Chen F., Shu D., Xie Q. Analysis of S1 gene of avian infectious bronchitis virus isolated in southern China during 2011-2012. Virus Genes. 2014;49:292–303. doi: 10.1007/s11262-014-1097-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzo G., Legnardi M., Tucciarone C.M., Drigo M., Martini M., Cecchinato M. Evolution of infectious bronchitis virus in the field after homologous vaccination introduction. Vet. Res. 2019;50:92. doi: 10.1186/s13567-019-0713-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzo G., Massi P., Tucciarone C.M., Barbieri I., Tosi G., Fiorentini L., Ciccozzi M., Lavazza A., Cecchinato M., Moreno A. Think globally, act locally: phylodynamic reconstruction of infectious bronchitis virus (IBV) QX genotype (GI-19 lineage) reveals different population dynamics and spreading patterns when evaluated on different epidemiological scales. PLoS One. 2017;12:e184401. doi: 10.1371/journal.pone.0184401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao M., Wang Q., Zhao W., Chen Y., Zhang T., Han Z., Xu Q., Kong X., Liu S. Serotype, antigenicity, and pathogenicity of a naturally recombinant TW I genotype infectious bronchitis coronavirus in China. Vet. Microbiol. 2016;191:1–8. doi: 10.1016/j.vetmic.2016.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelb J.J., Weisman Y., Ladman B.S., Meir R. S1 gene characteristics and efficacy of vaccination against infectious bronchitis virus field isolates from the United States and Israel (1996 to 2000) Avian Pathol. 2005;34:194–203. doi: 10.1080/03079450500096539. [DOI] [PubMed] [Google Scholar]
- Han Z., Sun C., Yan B., Zhang X., Wang Y., Li C., Zhang Q., Ma Y., Shao Y., Liu Q., Kong X., Liu S. A 15-year analysis of molecular epidemiology of avian infectious bronchitis coronavirus in China. Infect. Genet. Evol. 2011;11:190–200. doi: 10.1016/j.meegid.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignjatovic J., Galli L. The S1 glycoprotein but not the N or M proteins of avian infectious bronchitis virus induces protection in vaccinated chickens. Arch. Virol. 1994;138:117–134. doi: 10.1007/BF01310043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackwood M.W. Review of infectious bronchitis virus around the world. Avian Dis. 2012;56:634–641. doi: 10.1637/10227-043012-Review.1. [DOI] [PubMed] [Google Scholar]
- Karimi V., Ghalyanchilangeroudi A., Hashemzadeh M., Rahimi F., Zabihi P.M., Farahani R.K., Maghsoudloo H., Abdollahi H. Efficacy of H120 and Ma5 avian infectious bronchitis vaccines in early challenge against QX strain. Virusdisease. 2018;29:123–126. doi: 10.1007/s13337-017-0414-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo H., Qin J., Chen F., Xie Q., Bi Y., Cao Y., Xue C. Phylogenetic analysis of the S1 glycoprotein gene of infectious bronchitis viruses isolated in China during 2009-2010. Virus Genes. 2012;44:19–23. doi: 10.1007/s11262-011-0657-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo M.A., Pringle C.R. Virus taxonomy--1997. J. Gen. Virol. 1998;79(Pt 4):649–657. doi: 10.1099/0022-1317-79-4-649. [DOI] [PubMed] [Google Scholar]
- Terregino C., Toffan A., Beato M.S., De Nardi R., Vascellari M., Meini A., Ortali G., Mancin M., Capua I. Pathogenicity of a QX strain of infectious bronchitis virus in specific pathogen free and commercial broiler chickens, and evaluation of protection induced by a vaccination programme based on the Ma5 and 4/91 serotypes. Avian Pathol. 2008;37:487–493. doi: 10.1080/03079450802356938. [DOI] [PubMed] [Google Scholar]
- Wang C.H., Tsai C.T. Genetic grouping for the isolates of avian infectious bronchitis virus in Taiwan. Arch. Virol. 1996;141:1677–1688. doi: 10.1007/BF01718291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu G., Cheng J., Ma S., Jia W., Yan S., Zhang G. Pathogenicity differences between a newly emerged TW-like strain and a prevalent QX-like strain of infectious bronchitis virus. Vet. Microbiol. 2018;227:20–28. doi: 10.1016/j.vetmic.2018.10.019. [DOI] [PubMed] [Google Scholar]
- Xu Q., Han Z., Wang Q., Zhang T., Gao M., Zhao Y., Shao Y., Li H., Kong X., Liu S. Emergence of novel nephropathogenic infectious bronchitis viruses currently circulating in Chinese chicken flocks. Avian Pathol. 2016;45:54–65. doi: 10.1080/03079457.2015.1118435. [DOI] [PubMed] [Google Scholar]
- Yan S., Sun Y., Huang X., Jia W., Xie D., Zhang G. Molecular characteristics and pathogenicity analysis of QX-like avian infectious bronchitis virus isolated in China in 2017 and 2018. Poult. Sci. 2019;98:5336–5341. doi: 10.3382/ps/pez351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Zhou Y., Wang H., Zeng F., Yang X., Zhang Y., Zhang A. Molecular detection and Smoothing spline clustering of the IBV strains detected in China during 2011-2012. Virus Res. 2016;211:145–150. doi: 10.1016/j.virusres.2015.10.015. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Cheng J.L., Liu X.Y., Zhao J., Hu Y.X., Zhang G.Z. Safety and efficacy of an attenuated Chinese QX-like infectious bronchitis virus strain as a candidate vaccine. Vet. Microbiol. 2015;180:49–58. doi: 10.1016/j.vetmic.2015.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Zhang H., Zhao J., Zhong Q., Jin J.H., Zhang G.Z. Evolution of infectious bronchitis virus in China over the past two decades. J. Gen. Virol. 2016;97:1566–1574. doi: 10.1099/jgv.0.000464. [DOI] [PMC free article] [PubMed] [Google Scholar]