Abstract
The current COVID-19 pandemic, caused by SARS-CoV-2, has impacted many facets of hematopoietic cell transplantation (HCT) in both developed and developing countries. Realizing the challenges as a result of this pandemic affecting the daily practice of the HCT centers and the recognition of the variability in practice worldwide, the Worldwide Network for Blood and Marrow Transplantation (WBMT) and the Center for International Blood and Marrow Transplant Research's (CIBMTR) Health Services and International Studies Committee have jointly produced an expert opinion statement as a general guide to deal with certain aspects of HCT, including diagnostics for SARS-CoV-2 in HCT recipient, pre- and post-HCT management, donor issues, medical tourism, and facilities management. During these crucial times, which may last for months or years, the HCT community must reorganize to proceed with transplantation activity in those patients who urgently require it, albeit with extreme caution. This shared knowledge may be of value to the HCT community in the absence of high-quality evidence-based medicine.
© 2020 American Society for Transplantation and Cellular Therapy. Published by Elsevier Inc.
Keywords: Transplantation, COVID-19, Pandemic, Stem cells
INTRODUCTION
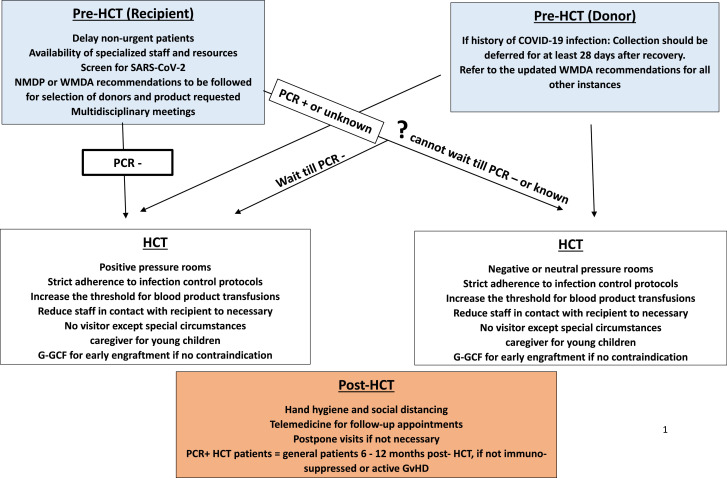
In recent history, several epidemics have affected the care of hematopoietic cell transplantation (HCT) recipients/donors, including severe acute respiratory syndrome (SARS) coronavirus (SARS-CoV-1), influenza A virus subtype H1N1, Zika virus, and Ebola virus. The issues arising from these epidemics were relatively geographically limited compared with the current COVID-19 pandemic, caused by SARS-CoV-2. By July 19, 2020, more that 13 million cases of COVID-19 had been reported worldwide [1]. Numerous aspects of the virus remain unknown, including the immune response and natural course of the disease, the exact incubation period, and the rate of spread among asymptomatic people, among other factors. These uncertainties make it particularly challenging to study the pattern of possible spread and create efficient management and prevention strategies. It is also difficult to predict the disease severity of COVID-19 in HCT recipients. Thus far, limited data have been published on disease severity, clinical course, and duration of infectivity of COVID-19 in HCT recipients [2,3]. Importantly, HCT remains the standard of care and the sole potential curative therapy for many hematologic malignancies, genetic diseases, hemoglobinopathies, autoimmune diseases, and immunodeficiencies [4,5]. The transplantation community is facing many challenges concerning the day-to-day practice of HCT, with disruptions in the entire process of referrals for evaluation, scheduling, admission, chemotherapy/radiation administration, donor procurement, and discharge planning, among other aspects (Figure 1 ).
Figure 1.
General principles for management of HCT patients during the SARS-CoV-2 pandemic.
SARS-CoV-2 is a highly communicable virus that incites a variable immune response, resulting in uncertainties about protective as well as deleterious immune responses to the virus. Although the majority of infected individuals clear the virus without major complications, in a minority of the patients, the infection leads to a life-threatening clinical situation involving any organ (especially the lungs), diffuse intravascular thrombosis, and ultimately acute respiratory distress syndrome (ARDS) and multiorgan failure, which has a complex pathology and mechanism [6].
Whether the incidence of the severe syndrome of COVID-19 infection is diminished or enhanced in patients post-HCT relative to the general population or other cancer populations, and what risk factors are predictive of lower respiratory infection and infection severity, are unclear at present. In addition, many donor issues have arisen as a consequence of global lockdown, travel restrictions, and quarantine procedures in various countries. Finally, many drugs currently used in the HCT arena, such as Bruton's tyrosine kinase inhibitors (eg, ibrutinib), ruxolitinib, tocilizumab, azithromycin, and mesenchymal stromal cells (all used to treat acute or chronic graft-versus-host-disease [GVHD]), are being studied in clinical trials to evaluate their efficacy (or lack thereof) for treating COVID-19 and/or its complications. Therefore, many practice issues regarding the use of these agents in HCT recipients are currently under debate.
The Worldwide Network for Blood and Marrow Transplantation (WBMT) is a nonprofit organization for education, scientific inquiry, and philanthropy serving all aspects of HCT under the laws of Switzerland and as a nongovernmental organization (NGO) in an official relationship with the World Health Organization (WHO) since 2007. The WBMT serves philanthropic purposes concerning aspects of global HCT and represents 22 international societies working in the field and more than 1600 transplantation centers worldwide. The Center for International Blood and Marrow Transplant Research (CIBMTR) is a working group of >380 international transplantation centers (and a founding member of the WBMT) that collects detailed information on autologous and allogeneic HCT recipients as well as on recipients of cellular therapies, such as chimeric antigen receptor (CAR) T cell therapy. The CIBMTR's Health Services and International Studies Committee deals with aspects related to HCT. The WBMT and CIBMTR experts and leadership acknowledge the challenges of the COVID-19 crisis that may differ in developing vs developed countries.
While acknowledging all the aforementioned challenges and taking into account current recommendations and guidelines issued by the American Society for Transplantation and Cellular Therapy and the European Society for Blood and Marrow Transplantation (EBMT) (both of which are WBMT members), here we aim to provide a consensus opinion from the WBMT, the CIBMTR's Health Services and International Studies Committee, and other HCT experts from multiple continents regarding the current worldwide threat to HCT recipients from the COVID-19 pandemic [7,8]. For Recommendations regarding the management of patients being treated with CAR T cell therapy are presented in a recent publication on this topic [9].
ORGANIZATIONAL ISSUES
Creating hospital-wide multidisciplinary COVID-19 teams (including specialists in infectious diseases, critical care medicine, pulmonary medicine, etc) is essential to facilitate the management of patients and make adjustments based on new information as it becomes available. Enabling telephone or video communication for multidisciplinary team meetings is important for the coordinated care of patients. To facilitate reporting, some registries, including the CIBMTR and EBMT, are actively collecting information on HCT and other cellular therapy patients infected with COVID-19. HCT programs require adequate HCT staff resuming transplantations during the COVID-19 pandemic.
SUPPORTIVE CARE
Continuing availability of blood products is a prerequisite for planning HCT, given that blood supply has been a major issue during the pandemic. The possibility of reducing the threshold for blood product transfusion should be discussed at the beginning of HCT. In addition, blood loss prevention strategies (eg, minimizing blood draws or treatment with tranexamic acid if clinically permissible) should be maximized [10]. The use of pediatric tubes and reassessment of scheduled laboratory work may be useful as well.
Holistic management of patients remains as essential as ever, maybe more important than ever. Patients undergoing HCT should have regular visits with social support services, given the uncertainties around returning to work and associated healthcare costs, particularly those in a jurisdiction without universal healthcare, to minimize financial and social toxicity. It is appropriate to vigilantly monitor for the psychological effects of the current COVID-19 pandemic on HCT survivors and the staff taking care of them. At the same time, the risk of infection from these visits must be considered, including such factors as the time after HCT. Additional measures to ensure construction of well-rounded management plans include providing appropriate physiotherapy and nutritional guidance.
Transplantation pharmacists should continuously monitor the potential drug toxicities and interactions of the medications given post-HCT via telehealth. In addition, the drug inventory should be evaluated at routine intervals for the essential drugs used for HCT patients. The use of any over-the-counter drugs or self-treatment with complementary and alternative medicine without discussion with the primary transplantation physician and the clinical pharmacist is not recommended. We refer the readers to the American Society for Transplantation and Cellular Therapy’s pharmacy practice management guideline for COVID-19 patients [11].
COVID-19: CLINICAL SPECTRUM AND DIAGNOSTIC TESTING
Individuals infected with SARS-CoV-2 can present with a variety of symptoms, including fever, rhinorrhea, cough, chest pain, shortness of breath, diarrhea, and skin rash.
Two kinds of tests are available for COVID-19: a viral test using PCR to detect the virus and an antibody-based test to detect the occurrence of previous infection (which can take 1 to 3 weeks after infection to become positive). Notably, PCR-based testing does not distinguish between live and dead SARS-CoV-2. For diagnosing COVID-19 infection in HCT recipients, testing with real-time RT-PCR ideally should be done on nasopharyngeal swabs; however, sputum samples and nasal swabs also can be used [12]. Each center needs to understand the sensitivity, specificity, and predictive values of the specific test being used, which may be affected by the regional prevalence of the infection. Pitfalls in SARS-CoV-2 PCR diagnostics have been reported recently [13]. At present, there is no known role for antibody-based (serologic) testing for SARS-CoV-2 for diagnostic purposes. Exposure (especially to contacts with COVID-19 patients) and symptom history, physical examination, and chest imaging are helpful in evaluating HCT recipients.
In the event that the diagnosis is not clear, bronchoscopy with bronchoalveolar lavage may be pursued in selected cases, albeit with extreme caution because this procedure also puts the pulmonologist at risk of acquiring the SARS-CoV-2 infection. Specific symptoms/findings ascribed to SARS-CoV-2 infection including fever, chills, cough, shortness of breath or difficulty breathing, fatigue, muscle/body aches, headache, new loss of taste or smell, sore throat, congestion or runny nose, nausea or vomiting, and diarrhea. The degree of symptoms may range from asymptomatic (eg, no fever) to mild to severe [14]. Laboratory tests, such as C-reactive protein, D-dimer, and lactate dehydrogenase levels, may help assess the severity of infection and optimally should be performed in every patient with confirmed COVID-19 [15,16]. Suspected patients with classic COVID-19 symptoms in an appropriate medical setting may be cohorted or isolated as soon as possible in special wards or units (temporary or permanent depending on the institutional policies and/or resources). Only when the RT-PCR test comes back negative should these patients be considered standard-risk HCT recipients and managed accordingly. In the absence of appropriate diagnostic tools (ie, RT-PCR), physicians may need to rely on history, physical exam, and chest imaging, in which case a chest computed tomography scan may be helpful, as it is more sensitive than a chest radiograph.
HCT-RELATED TREATMENTS AND COVID-19 THERAPIES
Currently approved therapies for COVID-19 complications include remdesivir in the United States (under emergency use authorization) and Japan and tocilizumab in China. Ongoing clinical trials are examining various treatment protocols. At present, there are no regulatory agency–approved therapies or vaccines for the treatment or prevention of COVID-19. Hydroxychloroquine, alone or in combination with azithromycin, has been widely used based on anecdotal and limited observational evidence.
On June 15, 2020, the US Food and Drug Administration (FDA) revoked chloroquine and hydroxychloroquine's emergency use authorization in response to the publication of new scientific data showing that these drugs are unlikely to be effective in treating COVID-19 [17,18]. In light of ongoing serious cardiac adverse events and other potential serious side effects, the potential benefits of chloroquine and hydroxychloroquine do not outweigh the risks of their use. The US Centers for Disease Control and Prevention has removed both drugs from its guidelines.
Table 1 reviews several of these agents, including their current uses in HCT recipients and in treating GVHD. It is important to consider the possible increased risk of complications in HCT recipients with COVID-19 owing to the status of immune reconstitution (delayed in allogeneic HCT recipients for up to a year), concurrent medications including immunosuppressive drugs, the general state of the patient (ie, performance status and psychosocial condition), existing comorbidities (especially cardiovascular and pulmonary), presence or absence of GVHD, disease status (ie, COVID-19 severity), mucositis (because mucosal barrier injury predisposes to infection), and malnutrition (which itself can suppress the immunologic response to infection) 19, 20, 21. In addition, superimposed bacterial infection, fungal infection, and reactivation of other common viral infections, such as cytomegalovirus or Epstein-Barr virus infection, may occur, and patients should be closely monitored and treated as indicated. There are no data to support the discontinuation of immunosuppressive drugs in HCT recipients infected with COVID-19. At present, little data are available on the effectiveness of various drugs/immunomodulators for treating COVID-19; however, numerous randomized clinical trials are currently underway using a variety of agents for COVID-19 management. Therefore, we recommend that unapproved drug use in HCT recipients should be considered in a clinical trial or approved for exceptional use after hospital Ethics Committee approval.
Table 1.
Agents Considered for the Treatment of COVID-19 Relevant to HCT, and Common Drugs Used in HCT Relevant to COVID-19 Infection
| Drug Name | COVID-19 Studies | Use in HCT | Special Considerations |
|---|---|---|---|
| Agents with the greatest promise | |||
| Remdesivir | Benefit in animal models against the MERS virus and SARS virus; therefore, may have potential activity against COVID-19 [31,32]. On May 2020, the FDA approved remdesivir for severely ill hospitalized patients [33]. A randomized study showed no statistically significant improvement in the primary endpoint [34]. | No definite role specifically in HCT. | Use only in the context of clinical trials. Can cause hepatotoxicity, thereby caution in patients with liver GVHD |
| Tocilizumab | Single-arm trial showing moderate/minimal impact on clinical outcomes [35]. Approved in China for treatment of COVID-19 complications [36]. | Used for the treatment of acute GVHD. | Reactivation of hepatitis B, hepatotoxicity, and serious infections can occur. |
| ARDS is usually associated with IL-6 increase thereby providing the rationale for anti-IL6 or anti-IL6 receptor antibody therapy [28,37]. | Also used for the treatment of cytokine release syndrome after CAR T cell therapy. | Consider avoiding in cases of active liver GVHD. | |
| Tocilizumab also increases the risk of secondary infections [38]. | If supply is an issue, then reserve it for COVID-19 patient clinical trials and use alternate agents for acute GVHD. | ||
| CAR T cell approved therapy may be affected due to the restricted availability of this drug; thus, consider limiting CAR T cell therapy to those with an urgent need. | |||
| Convalescent plasma recovered from COVID-19 patients | Failure of clinical improvement in 2 case series [39,40]. Hundreds of trials currently underway. Exact role in COVID-19 trials undefined. | Not used in GVHD or HCT. | Allergic reactions can occur. |
| Given the weak data on efficacy reported so far, it should be used only in clinical trials. | |||
|
Agents that likely will not work, according to the literature | |||
| Azithromycin | Tested in a French trial and found to reinforce the positive effect of hydroxychloroquine on the COVID-19 viral load [41]. | Used as a treatment for lung GVHD (BOS). | Can cause QTc prolongation, torsades de pointes, ventricular tachycardia, and sudden cardiac death, especially when used together with chloroquine. |
| A significant number of patients with GVHD will also be on “azoles” for antifungal prophylaxis, so the risk of QTc prolongation could be further enhanced if used concurrently with full-dose azithromycin, chloroquine, or both. | |||
| Chloroquine and hydroxychloroquine | Best evidence thus far has failed to demonstrate benefit in hard clinical outcomes, but some trial results have been encouraging, with a suggestion of reduced viral load or reduced PCR positivity of COVID-19 [42]. | Used occasionally to treat chronic GVHD. | Metabolized by cytochrome P450. Significant QTc prolongation. |
| One US retrospective analysis showed no benefit and association with higher mortality in patients receiving hydroxychloroquine [43]. Other studies have shown no benefit and potential harm, such as arrhythmias 44, 45, 46, 47. | Concerns about increased toxicity with cyclosporine and imatinib (used in chronic GVHD), such as myopathies. | ||
| Agents that possibly may work | |||
| Antivirals | |||
| Lopinavir/ritonavir | Recently published trials showed no significant effect on mortality. Very low-level evidence due to risk of bias, such as lack of blinding [48]. | No definite role specifically in HCT. | Severe GI symptoms, QTc prolongation, and multiple drug interactions due to CYP3A inhibition, especially with salmeterol-fluticasone, which, as the FAM protocol, is used frequently used to treat BOS. |
| Favipiravir | Preliminary results of a Japanese clinical trial showed that in COVID-19, compared with arbidol, favipiravir did not significantly improve the clinical recovery rate at day 7 [49]. | No definite role specifically in HCT. | Elevated serum uric acid has been associated with the use of favipiravir. |
| Herbal therapies: Nigella sativa | Potential demonstrated in molecular docking study [50]. | No role in HCT but has the propensity to cause prolonged QTc | Use should be strictly within the context of a clinical trial. |
| Avoid use in patients with lung GVHD receiving azithromycin. | |||
| Cytokine inhibitors | |||
| Eculizumab | Improvement in the COVID-19-associated ARDS/pneumonia in a case series [51]. | Treatment for HCT-associated microangiopathy (TTP). | Given its association with a serious increase in the risk of infection (meningococcus), use only in clinical trials. |
| Siltuximab | An improvement in the clinical condition was observed in 33% (7 of 21) of patients, 43% (9 of 21) of patients stabilized, as evidenced by no clinically relevant change in their condition, and 24% (5 of 21) experienced a worsening of their condition [43]. | Approved by the EMA and FDA for treatment of adults with HHV6-/HIV- multicentric Castleman's disease | Use only in the context of clinical trials. |
| Ruxolitinib | Possible role in hemophagocytic lymphohistiocytosis due to COVID-19 (trials started) | Approved for the treatment of steroid-refractory acute GVHD. | Risk of infection and thrombocytopenia with full-dose ruxolitinib. Thrombocytopenia and an ITP-like syndrome have been described in COVID-19 patients; thus, cautious use in clinical trials only is recommended. |
| Ibrutinib | Reported as perhaps beneficial for COVID-19 in a retrospective study [52]. | Approved therapy for chronic GVHD. | All patients on the reported study were already on ibrutinib. |
| Caution against using ibrutinib solely for COVID-19 infection in HCT recipients until trial data available | |||
| Immunosuppressives | |||
| Corticosteroids | Dexamethasone has been shown to improve mortality in COVID-19 patients [53]. | The primary treatment for both acute and chronic GVHD. | Judicious use of corticosteroids if needed. |
| IDSA recommendation only in cases of MAS or ARDS due to COVID-19 [54,55]. | Risk of osteonecrosis high in HCT recipients. | ||
| Mesenchymal stromal cells | Improved outcome in a single-arm trial of 7 patients [56]. | Approved treatment for acute GVHD in Canada, New Zealand, and Japan | Can be used in the context of clinical trials both autologous and allogeneic HCT recipients. |
| Multiple trials going on in ARDS due to COVID-19 | |||
| Interactions with other drugs | |||
| Voriconazole and posaconazole | No known role in COVID-19 | Commonly used in GVHD. | Azithromycin interaction with the CYP3A4 inducers. |
| CAR T cells (cryopreserved vs. fresh products) | No role in COVID-19 | Approved for post-transplantation relapse of ALL and NHL. | Post-CAR T cell infusion, any drug treatment should strictly be in the context of clinical trials. |
| CAR T cell therapy may be affected owing to the restricted availability of tocilizumab; therefore, consider CAR T cell therapy only for those with an urgent need. | |||
| General guidance from the CAR T cell consortium should be considered [9]. | |||
| ACE inhibitors | Hypothetically could increase the likelihood of acquiring SARS-CoV-2 by increasing ACE2 expression (virus-binding site) [57,58]. | No known role for treatment of any aspect of HCT. | Until data are available, do not stop ACE inhibitors in HCT recipients who are already on treatment. |
| Arbidol | A Chinese randomized controlled open-labeled trial demonstrated arbidol monotherapy had little benefit for mild/moderate COVID-19 infection [59]. | No definite role specifically in HCT. | Adverse events include diarrhea and nausea. |
BOS indicates bronchiolitis obliterans syndrome; MERS, Middle East respiratory syndrome; ACE: angiotensin-converting enzyme; MAS, macrophage activation syndrome; TTP, thrombotic thrombocytopenic purpura; EMA, European Medicines Agency; ALL, acute lymphoblastic leukemia; NHL, non-Hodgkin lymphoma.
PRETRANSPLANTATION PLANNING AND TRANSPLANTATION MANAGEMENT
Successful coordination of an HCT program with the overall strategy of the hospital or health system for coping with the current pandemic is needed. It is important to ensure that the staff is protected so that the center does not experience loss of expert personnel to exposure (necessitating quarantine) or infection. Strict adherence to infection control measures is imperative to minimize the transmission of the virus in HCT inpatient and outpatient units.
All patients, including those without symptoms, should be thoroughly evaluated for clinical symptoms that might suggest incipient COVID-19, and both symptomatic and asymptomatic patients should be tested for SARS-CoV-2 with RT-PCR 1 or 2 days before entering the transplantation ward for conditioning. In patients at high risk of acquiring SARS-CoV-2, such as patients exposed to persons with active upper respiratory tract infection symptoms, then a second RT-PCR analysis is advisable even if the first RT-PCR was negative, owing to the possibility of false-negative results. In addition, a computed tomography scan of the chest can be very helpful in these cases, given its greater sensitivity than chest radiography [22]. Centers should allocate adequate space for symptomatic patients awaiting the results of SARS-CoV-2 testing; ideally, this should be in a negative-pressure room preferably separate from the transplantation unit. Patients scheduled for transplantation should attempt to reduce the risk of exposure by home isolation for at least 14 days before starting conditioning. If the transplantation is postponed owing to concerns about intensive care unit capacity or infection prevalence, it is imperative to complete the proper risk assessments and clearly communicate the reasons for the delay to the patient. Furthermore, it is necessary to document the multidisciplinary meeting discussions in the medical record.
From the WBMT perspective, the decision to proceed to transplantation should be individualized, weighing the risks for serious complications from COVID-19 infection against the risks of disease progression if HCT is delayed.
Urgent Transplantations
Delaying urgent transplantations, such as for acute leukemias, high-risk myelodysplastic syndrome, and certain refractory bone marrow failure syndromes, and autologous HCTs (if being performed for curative intent) for high-risk myelomas, Hodgkin lymphoma, large cell lymphomas, or germ cell tumors in patients at substantial risk of loss of disease control is not recommended.
Nonurgent Transplantations
In general, nonurgent transplants should be deferred as clinically permissible, especially for patients with stable, slowly progressive, nonmalignant disorders, such as hemoglobinopathies, selected immunodeficiencies, and selected genetic conditions.
DONOR ISSUES
In general, using peripheral blood stem cells (PBSCs) with cryopreservation and testing the quality of thawed product before the start of conditioning are strongly encouraged. The use of post-transplantation cyclophosphamide has increased significantly worldwide for both haploidentical and HLA-matched HCTs, and a recent study showed no detrimental effect of cryopreserved products on survival when using post-transplantation cyclophosphamide for GVHD prophylaxis in patients undergoing HCT for hematologic malignancies [10]. Therefore, we consider this a reasonable strategy for HCT for hematologic malignancies; however, for bone marrow failure (especially for severe aplastic anemia), fresh bone marrow products remain the standard of care, as supported by a recent study [11].
In rare cases, SARS-CoV-2 has been detected in blood, but there are no reports of transmission from donor products [23,24]. According to the FDA, no confirmed or suspected cases of transfusion-transmitted COVID-19 have been reported to date. Current American Association of Blood Banks guidelines do not recommend universal screening for SARS-CoV-2 in blood products, and current FDA guidelines recommend considering the donor's infection and exposure history in the 28 days before donation [25,26]. The National Marrow Donor Program/Be The Match has already implemented a donor screening questionnaire [27]. The World Marrow Donor Association has also produced recommendations and we endorse those guidelines (Table 2 ). Within 28 days before donation, donors should avoid any contact with individuals with a syndrome suggestive of COVID-19, crowded places, and large gatherings.
Table 2.
Recommendations for evaluation of HCT donors
| Donor Status | Recommendation |
|---|---|
| History of COVID-19 infection | Collection should be deferred for at least 28 days after recovery.If the patient's need for transplant is urgent, the donor is completely well and there are no suitable alternative donors, earlier collection may be considered if local public health requirements permit |
| Contact with COVID-19 – donors who report contact with a confirmed case | Collection should be deferred for 4 weeks after a donor's last contact with a person with confirmed COVID-19 infection.If the patient's need for transplant is urgent, the donor is completely well and there are no suitable alternative donors, earlier collection may be considered if local public health requirements permit, subject to careful risk assessment. |
| Donors who have travelled internationally or reside in a high risk country | Please refer to the updated WMDA guidelineshttps://share.wmda.info/pages/viewpage.action?pageId=344866320#/ |
Ideally, a backup donor should be available, but this might not be possible in all cases. PBSCs should be used unless there is a strong indication for using bone marrow. The rationale for using PBSCs is less need for blood transfusions (due to earlier engraftment), no need for using inpatient rooms and personal protective equipment for bone marrow harvest, and faster neutrophil engraftment, which may result in earlier discharge of recipients. Coordination with the local and international authorities (especially with the border customs officials in certain countries) may be needed to ensure trouble-free transport of cell therapy products. Donor safety issues must be a top priority during this pandemic, and the World Marrow Donor Association and the local donor stem cell registry recommendations should be followed. In some countries, the use of umbilical cord blood units as a backup strategy may be preferred, depending on the existing resources and current practices.
The role of postconvalescent plasma for prevention and therapy remains undefined, and this should be used only in clinical trials. Many trials are currently running or in evaluation. The standardization of antibody concentration remains an important issue.
MANAGING COVID-19 INFECTION IN THE PERITRANSPLANTATION PERIOD
Given the lack of data on the management of patients infected with COVID-19 who are undergoing HCT, the treatment paradigm should follow the institutional policies. In general, if SARS-CoV-2 is suspected or confirmed before the initiation of a conditioning regimen, then HCT should be delayed until the patient completely recovers from the infection (at least 14 days). Two consecutive negative RT-PCR analyses (at least 1 day apart) are advised for confirmed cases. For scenarios during the conditioning regimen, the following principles may help.
If conditioning has proceeded to a point at which the risk of myelosuppression or myeloablation is low, then subsequent doses may be withheld. If conditioning duration/intensity has passed that point, then conditioning should be completed and the HCT product infused. If sufficient myeloablative conditioning has been delivered to a recipient and SARS-CoV-2 is confirmed, the transplantation process should continue owing to anticipated bone marrow aplasia. The COVID-19 infection should be managed symptomatically or on a clinical trial with a cautious evaluation of drug-drug interactions.
If infection with COVID-19 is diagnosed on the day of transplantation (day 0), then HCT should proceed as intended, with no delays in the PBSC or bone marrow infusion. Convalescent plasma infusions, if indicated for COVID-19 infection on a clinical trial, should be avoided on the day of transplantation to minimize the risk of allergic reactions.
If the COVID-19 infection is diagnosed after the stem cell infusion, then it should be treated symptomatically and/or on a clinical trial, taking the possibility of manageable drug interferences into account (Table 1).
In all other scenarios, the patient should be evaluated by a multidisciplinary team for an individualized decision.
HOSPITALIZATION AND TRANSPLANTATION
Special infection control measures, including strict use of proper personal protective equipment, should be followed as recommended by international, national, and local guidelines. A team/unit dedicated to the care of COVID-19 patients should be separated and isolated from staff caring for HCT recipients. Ideally, there should be a specific site/unit for cohorting these patients. Some hospitals in developing countries may have little capacity for private rooms; nonetheless, given their immunocompromised state, HCT recipients should not be cohorted in shared rooms.
Regarding the selection of the appropriate industrial climate control system for HCT recipients, we recommend the following:
HCT recipients with suspected COVID-19 should not stay in a positive-pressure room. They should be tested in a neutral-pressure (or negative-pressure) room (a private room if available). If RT-PCR is not available and/or suspicion is high, the patient should be kept in a private room (neutral or negative pressure) if possible, or cohorted. It may be advisable to perform these tests immediately before admission and wait for results if possible.
HCT recipients with confirmed COVID-19 infection should not stay in a positive-pressure room and must be transferred to a negative-pressure (or neutral-pressure) room immediately.
HCT recipients with a negative test for SARS-CoV-2 and no symptoms should continue being managed in a high-efficiency particulate air (HEPA)-filtered positive-pressure room in accordance with the institutional policy for immunocompromised patients.
Staff should be screened for symptoms before entering the HCT unit, and surgical face masks and eye protection should be worn at all times during ward rounds, as recommended by the EBMT [8]. Social distancing rules should be strictly enforced. The number of individuals should be minimized during ward rounds and adequate distance from patients maintained to the degree possible. For the protection of the patient and the medical team, visitors should not be allowed during the hospital stay. An exception may be made in extraordinary circumstances (eg, patient dying from COVID-19) if this does not represent a conflict with the hospital's strategy or policies. Video communication may help in such circumstances.
Prophylactic G-CSF therapy for early neutrophil engraftment may be considered for HCT recipients unless contraindicated. There is no published evidence indicating that this practice can improve outcomes; however, there could be an advantage of early hospital discharge to reduce the risk of infection. Theoretically, the inflammatory cascade in ARDS [28] (mediated in part by IL-6) may be enhanced by cytokines; however, at present there are no data to support G-CSF administration. Cytokines may accelerate the risk of ARDS in confirmed cases of COVID-19 [29]. The transfusion thresholds should be minimized in the light of an actual or anticipated shortage of blood products. As a general guideline, a hemoglobin threshold of <70 g/L should be considered as an indication for blood transfusion in both autologous and allogeneic HCT recipients [30]. For platelet transfusion, a threshold of <10 × 109/μL is reasonable in most cases.
POST-TRANSPLANTATION MANAGEMENT
HCT recipients may be at increased risk for both secondary infections and organ damage from COVID-19. The usual recommendations for infection prevention, including maintaining strict hand hygiene, wearing surgical or procedural masks at all times, and practicing social/physical distancing, should be strongly encouraged.
Outpatient visits should be postponed or conducted using telephone and/or televideo conferences where indicated. During the early post-HCT period, especially for allogeneic HCT recipients, a face-to-face visit may be necessary to detect early signs of acute GVHD of the skin and mucosa. Ambulatory transplantations should be encouraged to minimize exposure to hospital visits, if the necessary infrastructure is available.
Prolonged viral shedding, a common occurrence with other respiratory viruses, is likely in HCT recipients. Separate areas in outpatient settings for potential infectious and noninfectious individuals should be available, with checkpoints at the entrance. Patients with confirmed COVID-19 infection should be tested regularly (depending on the institutional policies and/or governmental regulations) until the RT-PCR results turn negative. Until then, these patients should be considered potentially infectious. Other important issues to address include social distancing and mask wearing in the waiting area, direct rooming (ie, no waiting), barring of visitors from the outpatient area, and avoidance of common areas (including cafeteria).
A real-world practice question is when and how to manage an HCT recipient who contracts COVID-19 post-transplantation. This should be an individualized decision. As general guidance, autologous HCT recipients beyond 6 months post-transplantation and allogeneic HCT recipients beyond 1-year post-transplantation without immunosuppression and GVHD may be treated according to general COVID-19 management guidelines. This has practical implications for vaccinations and COVID-19 treatment strategies. If possible, these patients should be cared for in the transplantation center and entered into a clinical trial if available. Of note, the role of convalescent plasma for treatment of COVID-19 has not been proven in the allogeneic HCT setting.
TRAVEL FOR HCT AND MEDICAL TOURISM
Patients requiring HCT in a country that has facilities for HCT should ideally stay in their home country, provided that their clinical condition allows for a delay in the procedure. This may be different for international travel within driving distance. For example, patients with severe aplastic anemia will have particular difficulty if no transplantation center in their home country performs allogeneic HCT for bone marrow failure syndromes, and timely HCT may be required in pediatric patients diagnosed with certain inherited conditions (eg, Hurler’s syndrome).
Patients who underwent HCT in a foreign country should avoid travel back to their respective countries in the immediate post-HCT period, especially if they are currently residing in a country/state/province/city that has an extremely high prevalence of SARS-CoV-2. HCT recipients who will require frequent visits in the initial months following HCT should remain at the original transplantation center until their clinical condition stabilizes (eg, 3 to 4 months after allogeneic HCT).
CONCLUSIONS
The COVID-19 pandemic has impacted all aspects of HCT. We believe that our shared knowledge will be of value to the HCT community in the current absence of high-quality evidence-based medicine. This emergency situation may persist for months or even years until herd immunity is achieved, owing to the availability of an effective vaccine or as a result of natural infection. During these crucial times, the HCT community must reorganize to proceed with transplantation in those who urgently need it and concurrently deal with the factors that accompany a pandemic, such as prevention of nosocomial transmission, protection of HCT staff, and treatment of COVID-19 in HCT recipients. The extreme changeability of the global geopolitical climate due to COVID-19 requires that HCT programs rethink their practices. We hope that this article provides general guidance to clinicians and institutional leadership to deal with the real-world scenarios.
ACKNOWLEDGMENTS
Financial disclosure:
Conflict of interest statement: M.K. has received honoraria from Gilead and Alexion. M.K.D. has served as a consultant for Pharmacyclics and Daiichi Sankyo. M.H. has received research support/funding from Takeda Pharmaceutical, Spectrum Pharmaceuticals, and Astellas Pharma; served as a consultant for Janssen R&D and Incyte; and served on the speaker's bureau for Sanofi Genzyme and AstraZeneca. D.W. reports research support from Incyte and consulting fees from FATE. S.H. reports honoraria and travel funding from Pfizer, Novartis, Gilead, Merck, Sanofi, Mallinckrodt, and Janssen. M.-A.P. has received honoraria from Abbvie, Bellicum, Celgene, Bristol-Myers Squibb, Incyte, Kite/Gilead, Merck, Novartis, Nektar Therapeutics, Omeros, and Takeda; has served on data safety and monitoring boards for Cidara Therapeutics, Servier, and Medigene and scientific advisory boards for MolMed and NexImmune; has received research support for clinical trials from Incyte, Kite/Gilead; and Miltenyi Biotec; and serves in a volunteer capacity as a member of the Board of Directors of Be The Match (National Marrow Donor Program), as well as on the CIBMTR Cellular Immunotherapy Data Resource Executive Committee. C.C. reports honoraria/travel funding from Astellas Pharma, Gilead, Merck, and Pfizer. A.W. has received research support from Ansun, WB Tech, Amazon and Allovir, and is a consultant for Kyorin.
Footnotes
Financial disclosure: See Acknowledgments on page 2188.
REFERENCES
- 1.World Health Organization. Coronavirus disease 2019 (COVID-2019) situation report 181: 19 July 2020. Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports. Accessed 19 July 2020
- 2.Huang J, Lin H, Wu Y, et al COVID‐19 in posttransplantation patients‐-report of two cases. Am J Transplant. 2020;20:1879–1881. doi: 10.1111/ajt.15896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balduzzi A, Brivio E, Rovelli A, et al. Lessons after the early management of the COVID-19 outbreak in a pediatric transplant and hemato-oncology center embedded within a COVID-19 dedicated hospital in Lombardia, Italy [e-pub ahead of print]. Estote parati. Bone Marrow Transplant. doi: 10.1038/s41409-020-0895-4, Accessed 19 July 2020. [DOI] [PMC free article] [PubMed]
- 4.Majhail NS, Farnia SH, Carpenter PA. Indications for autologous and allogeneic hematopoietic cell transplantation: guidelines from the American Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2015;21:1863–1869. doi: 10.1016/j.bbmt.2015.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Niederwieser D, Baldomero H, Szer J. Hematopoietic stem cell transplantation activity worldwide in 2012 and a SWOT analysis of the Worldwide Network for Blood and Marrow Transplantation Group including the global survey. Bone Marrow Transplant. 2016;51:778–785. doi: 10.1038/bmt.2016.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu C, Chen X, Cai Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:1–11. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.American Society for Transplantation and Cellular Therapy. Interim guidelines for covid-19 management in hematopoietic cell transplant and cellular therapy patients. Available through: https://higherlogicdownload.s3.amazonaws.com/ASBMT/a1e2ac9a-36d2-4e23-945c-45118b667268/UploadedImages/COVID-19_Interim_Patient_Guidelines_3_9_20_V2.pdf. Accessed 19 July 2020
- 8.Ljungman P, Mikulska M, de la Camara R, et al. The challenge of COVID-19 and hematopoietic cell transplantation; EBMT recommendations for management of hematopoietic cell transplant recipients, their donors, and patients undergoing CAR T-cell therapy [e-pub ahead of print]. Bone Marrow Transplant. doi: 10.1038/s41409-020-0919-0, Accessed 19 July 2020. [DOI] [PMC free article] [PubMed]
- 9.Bachanova V, Bishop MR, Dahi P. Chimeric antigen receptor T cell therapy during the COVID-19 pandemic. Biol Blood Marrow Transplant. 2020;26:1239–1246. doi: 10.1016/j.bbmt.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stanworth SJ, Estcourt LJ, Powter G. A no-prophylaxis platelet-transfusion strategy for hematologic cancers. N Engl J Med. 2013;368:1771–1780. doi: 10.1056/NEJMoa1212772. [DOI] [PubMed] [Google Scholar]
- 11.Mahmoudjafari Z, Alexander M, Roddy J, et al American Society for Transplantation and Cellular Therapy Pharmacy Special Interest Group position statement on pharmacy practice management and clinical management for COVID-19 in hematopoietic cell transplantation and cellular therapy patients in the United States. Biol Blood Marrow Transplant. 2020;26:1043–1049. doi: 10.1016/j.bbmt.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang W, Xu Y, Gao R. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323:1843–1844. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wernike K, Keller M, Conraths FJ, Mettenleiter TC, Groschup MH, Beer M. Pitfalls in SARS-CoV-2 PCR diagnostics [e-pub ahead of print].Transbound Emerg Dis. doi: 10.1111/tbed.13684, Accessed 19 July 2020. [DOI] [PMC free article] [PubMed]
- 14.Centers for Disease Control and Prevention. Coronavirus disease: symptoms. Available at: https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html. Accessed 25 June 2020.
- 15.Gao Y, Li T, Han M. Diagnostic utility of clinical laboratory data determinations for patients with the severe COVID‐19. J Med Virol. 2020;92:791–796. doi: 10.1002/jmv.25770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lippi G, Plebani M. Laboratory abnormalities in patients with COVID-2019 infection. Clin Chem Lab Med. 2020;58:1131–1134. doi: 10.1515/cclm-2020-0198. [DOI] [PubMed] [Google Scholar]
- 17.Principi N, Esposito S.Chloroquine or hydroxychloroquine for prophylaxis of COVID-19 [e-pub ahead of print]. Lancet Infect Dis. doi: 10.1016/S1473-3099(20)30296-6, Accessed 19 July 2020. [DOI] [PMC free article] [PubMed]
- 18.Cortegiani A, Ingoglia G, Ippolito M, Giarratano A, Einav S. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J Crit Care. 2020;57:279–283. doi: 10.1016/j.jcrc.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang L, Zhu F, Xie L. Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann Oncol. 2020;31:894–901. doi: 10.1016/j.annonc.2020.03.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xia Y, Jin R, Zhao J, Li W, Shen H. Risk of COVID-19 for patients with cancer. Lancet Oncol. 2020;21:e180. doi: 10.1016/S1470-2045(20)30150-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fung M, Babik JM. COVID-19 in immunocompromised hosts: what we know so far. Clin Infect Dis. doi: 10.1093/cid/ciaa863. Accessed 19 July 2020. [e-pub ahead of print]. [DOI] [PMC free article] [PubMed]
- 22.Xu B, Xing Y, Peng J, et al. Chest CT for detecting COVID-19: a systematic review and meta-analysis of diagnostic accuracy. Eur Radiol. doi: 10.1007/s00330-020-06934-2. Accessed 19 July 2020. [e-pub ahead of print]. [DOI] [PMC free article] [PubMed]
- 23.Huang C, Wang Y, Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang L, Zhao L, Gong H, Wang L, Wang L. Severe acute respiratory syndrome coronavirus 2 RNA detected in blood donations. Emerg Infect Dis. 2020;26:1631–1633. doi: 10.3201/eid2607.200839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Food and Drug Administration. Important Information for Human Cell, Tissue, or Cellular or Tissue-based Product (HCT/P) Establishments Regarding the 2019 Novel Coronavirus Outbreak 2/14/2020. Available at:https://www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/important-information-human-cell-tissue-or-cellular-or-tissue-based-product-hctp-establishments. Accessed 19 July 2020.
- 26.American Association of Blood Banks. Update: Impact of 2019 novel coronavirus and blood safety. February 25, 2020. Available at:http://www.aabb.org/advocacy/regulatorygovernment/Documents/Impact-of-2019-Novel-Coronavirus-on-Blood-Donation.pdf. Accessed 19 July 2020.
- 27.Worldwide Network for Blood & Marrow Transplantation (WBMT). Coronavirus and haematopoietic stem cell transplantation [press release]. February 24, 2020. https://www.wbmt.org/wp-content/uploads/2020/03/WBMT_COVID-19-2.pdf. Re-accessed 19 July 2020
- 28.Ranieri VM, Rubenfeld GD, et al Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 29.Nawar T, Morjaria S, Kaltsas A, et al. Granulocyte-colony stimulating factor in COVID-19: is it stimulating more than just the bone marrow? Am J Hematol. doi: 10.1002/ajh.25870. Re-accessed 19 July 2020. [DOI] [PMC free article] [PubMed]
- 30.Tay J, Allan DS, Chatelain E. Liberal versus restrictive red blood cell transfusion thresholds in hematopoietic cell transplantation: a randomized, open label, phase III, noninferiority trial. J Clin Oncol. 2020;38:1463–1473. doi: 10.1200/JCO.19.01836. [DOI] [PubMed] [Google Scholar]
- 31.Sheahan TP, Sims AC, Graham RL. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci Transl Med. 2017;9 doi: 10.1126/scitranslmed.aal3653. eaal3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Wit E, Feldmann F, Cronin J. Prophylactic and therapeutic remdesivir (GS-5734) treatment in the rhesus macaque model of MERS-CoV infection. Proc Natl Acad Sci U S A. 2020;117:6771–6776. doi: 10.1073/pnas.1922083117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.US Food and Drug Administration. Coronavirus (COVID-19) update: FDA issues emergency use authorization for potential COVID-19 treatment. Available at: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-issues-emergency-use-authorization-potential-covid-19-treatment. Accessed 19 July 2020.
- 34.Wang Y, Zhang D, Du G. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu X, Han M, Li T. Effective treatment of severe COVID-19 patients with tocilizumab. ChinaXiv. 2020 doi: 10.1073/pnas.2005615117. 202003.00026v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu R, Miller J. China approves use of Roche drug in battle against coronavirus complications. March 4, 2020. Available at:https://www.reuters.com/article/us-health-coronavirus-china-roche-hldg/china-approves-use-of-roche-drug-in-battle-against-coronavirus-complications-idUSKBN20R0LF. Accessed 18 April 2020.
- 37.McGonagle D, Sharif K, O'Regan A, Bridgewood C. Interleukin-6 use in COVID-19 pneumonia related macrophage activation syndrome. Autoimmun Rev. 2020;19 doi: 10.1016/j.autrev.2020.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kimmig LM, Wu D, Gold M. IL6 inhibition in critically ill COVID-19 patients is associated with increased secondary infections. medRxiv. 2020 doi: 10.3389/fmed.2020.583897. 05.15.20103531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shen C, Wang Z, Zhao F. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020;323:1582–1589. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duan K, Liu B, Li C. The feasibility of convalescent plasma therapy in severe COVID-19 patients: a pilot study. medRxiv. 2020 03.16.20036145. [Google Scholar]
- 41.Gautret P, Lagier JC, Parola P. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020;56 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 42.Chen Z, Hu J, Zhang Z. Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial. medRxiv. 2020 03.22.20040758. [Google Scholar]
- 43.Magagnoli J, Narendran S, Pereira F. Outcomes of hydroxychloroquine usage in United States veterans hospitalized with COVID-19. medRxiv. 2020 doi: 10.1016/j.medj.2020.06.001. 04.16.20065920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Geleris J, Sun Y, Platt J. Observational study of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med. 2020;382:2411–2418. doi: 10.1056/NEJMoa2012410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosenberg ES, Dufort EM, Udo T. Association of treatment with hydroxychloroquine or azithromycin with in-hospital mortality in patients with COVID-19 in New York State. JAMA. 2020;323:2493–2502. doi: 10.1001/jama.2020.8630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tang W, Cao Z, Han M. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open-label, randomised controlled trial. BMJ. 2020;369:m1849. doi: 10.1136/bmj.m1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mehra MR, Desai SS, Ruschitzka F, Patel AN. RETRACTED: Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis. Lancet. 5-22-2020. doi: 10.1016/S0140-6736(20)31180-6. [DOI] [PMC free article] [PubMed] [Retracted]
- 48.Cao B, Wang Y, Wen D. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen C, Zhang Y, Huang J. Favipiravir versus arbidol for COVID-19: a randomized clinical trial. medRxiv. 2020 03.17.20037432. [Google Scholar]
- 50.Bouchentouf S, Missoum N. Identification of compounds from Nigella sativa as new potential inhibitors of 2019 novel coronavirus (COVID-19): molecular docking study. medRxiv. 2020 202004.0079.v1. [Google Scholar]
- 51.Diurno F Numis FG, Porta G Eculizumab treatment in patients with COVID-19: preliminary results from real life ASL Napoli 2 Nord experience. Eur Rev Med Pharmacol Sci. 2020;24:4040–4047. doi: 10.26355/eurrev_202004_20875. [DOI] [PubMed] [Google Scholar]
- 52.Gritti G, Raimondi F, Ripamonti D. Use of siltuximab in patients with COVID-19 pneumonia requiring ventilatory support. medRxiv. 2020;04:1912–1915. doi: 10.1101/2020.04.01.20048561. [DOI] [Google Scholar]
- 53.RECOVERY Collaborative Group; Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with Covid-19: preliminary report [e-pub ahead of print]. N Engl J Med. doi: 10.1056/NEJMoa2021436. Accessed 19 July 2020. [DOI]
- 54.Zhao R, Wang H, Wang X, Feng F. Steroid therapy and the risk of osteonecrosis in SARS patients: a dose-response meta-analysis. Osteoporos Int. 2017;28:1027–1034. doi: 10.1007/s00198-016-3824-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang Y, Jiang W, He Q. Early, low-dose and short-term application of corticosteroid treatment in patients with severe COVID-19 pneumonia: single-center experience from Wuhan, China. medRxiv. 2020 03.06.20032342. [Google Scholar]
- 56.Leng Z, Zhu R, Hou W. Transplantation of ACE2- mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging Dis. 2020;11:216–228. doi: 10.14336/AD.2020.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Voiriot G, Philippot Q, Elabbadi A, Elbim C, Chalumeau M, Fartoukh M. Risks related to the use of non-steroidal anti-inflammatory drugs in community-acquired pneumonia in adult and pediatric patients. J Clin Med. 2019;8:786. doi: 10.3390/jcm8060786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hoffmann M, Kleine-Weber H, Schroeder S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181 doi: 10.1016/j.cell.2020.02.052. 271-280.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li Y, Xie Z, Lin W. An exploratory randomized controlled study on the efficacy and safety of lopinavir/ritonavir or arbidol treating adult patients hospitalized with mild/moderate COVID-19 (ELACOI) medRxiv. 2020 03.19.20038984. [Google Scholar]