Abstract
Coronavirus disease 2019 (COVID-19) is a major public health concern currently. To date, there are no approved antiviral drugs or vaccines against this transmissible disease. This report sheds light on available information for a better understanding of clinical trials and pharmacotherapy related to COVID-19. MEDLINE, PubMed, EMBASE, Scopus databases, Web of Science, WHO, and EU clinical trial sites were used to perform comparative analysis. Information was collected on the use of therapeutic agents for human therapy in patients with COVID-19 up to May 2020. We have extracted data from 60 clinical trials. Amongst these trials, 34 were from the European Union database of clinical trials and 26 from the National Institute of Health. The data selection procedure includes active, completed, and recruitment in progress status. Most of the clinical trials are ongoing and hence, there is a lack of precise results for the treatment.There is a lack of high-quality clinical evidence. The protocol to be developed requires large randomized clinical trials with a combination of available drugs and prospective therapies. We propose the usage of a large number of cases and different statistical analyses to conduct systematic clinical trials. This could provide comprehensive information about the clinical trial and potential therapeutic progress.
Keywords: COVID-19, Corona pandemic, Vaccination, Clinical trials, Therapeutic
COVID-19 is a pneumonia-like disease, which is caused by a novel coronavirus [1]. Coronavirus belongs to Orthocoronavirinae subfamily of the Coronaviridae family within the order Nidovirales. COVID-19 is the defining global health crisis that has spread over 205 countries including USA, Italy, Russia, Spain, Japan, Korea, Iran, and Germany [2,3]. By the end of December 2019, a serious pneumonia like cluster of cases with unknown source expanded globally from Wuhan, China [4]. Various reports suggest that the novel coronavirus is 96.2% identical to a bat CoV RaTG13 [5,6]. Evolutionary analysis of virus genome has suspected bat as a natural host of the virus origin that could have been transmitted from an unknown intermediate host to the humans [7]. The infection poses a significant risk to patients with COVID-19 due to the high frequency of pneumonia, fever, and dry cough. Additionally, patients suffer from the potential damage of vital organs, especially the lungs, heart, liver, and kidneys [[8], [9], [10]]. As per the latest reports from China, the mortality rate of COVID-19 disease is approximately 3–4% [11]. The latest data obtained on 30 June 2020 describes that COVID-19 has infected more than Ten million people worldwide. Amongst these cases, 5.6 million have been successfully treated and 5,08,422 has died. The number of infected people is increasing every day by one thousand worldwide.
Recently, severity model based analysis has showed that the fatality ratio for China is 0·66%, on the Diamond Princess ship is 2.3%, and the large meta-analysis of 36 European countries showed the case-fatality rate of COVID-19 range of 4%–4.5% with an increasing profile with age [[12], [13], [14]]. Furthermore, in current scenario, India is ranked as the fourth highest country with positive cases and a case-fatality rate of 1.9–3.6% [15]. In the current situation, a second wave of coronavirus has hit the major cities of European countries including Sao Paulo and Rio de Janeiro, which is the centre for pandemic. Till date, more than 1.3 million positive cases have been identified in Brazil [16]. In Russia, more than 687,862 cases and 10,296 deaths due to COVID-19 infection has been reported [17].
In order to treat COVID-19, the only available treatment currently available is the retroviral therapy. Further, it was proven that convalescent plasma transfusion (CPT) is useful against COVID-19 [18,19]. At this crucial moment, in-depth research is essential to treat and prevent the disease. Several researchers have promptly carried out clinical research aimed towards the diagnosis, treatment, and prevention of COVID-19 [20]. However, globally there is limited information available to analyze and summarize the registered clinical trials. The purpose of this review is to summaries existing COVID-19 clinical trial data that would aid in selecting the most appropriate COVID-19 treatment.
Search Strategy And Selection Criteria
Using various keywords related to COVID-19 including comorbidities, clinical characteristics, epidemiological, immunotherapy, vaccine, and SARS CoV-2 clinical trial data were obtained from different electronic databases. Some of these databases were European union clinical trials database, Clinical Trial Registry, Clinicatrial.gov, International Clinical Trials Registry Platform (WHO ICTRP), Chinese Biomedical Literature Database, and the Wanfang Database. Pubmed, the National Library of Medicine (NLM), and EMBASE database were also used to identify ongoing trials.
Literature Inclusion And Exclusion Criteria
All investigators have selected only the most appropriate and suitable studies. Inclusion criteria encompasses the following: (1) studies of COVID-19 patients’ clinical trials, (2) detailed protocol of clinical trials, (3) data mining, (4) the original design type (Interventional or observational), (5) reports that involved the treatment of COVID-19. The exclusion criteria includes the following: (1) studies having duplicate data and (2) vague theoretical research and unregistered clinical trials.
Quality Assessment
Quality of clinical data and extracted data from the literature were assessed by rigorous information cross-check. Discrepancy between the investigators was resolved and the final decisions were decided without any conflicts. Relevant data was summarized in a narrative manner and the treatment strategy was grouped. Each table was categorized according to the drug usage. Results were classified based on the type of study, country, dose, duration of administration, an indication of medication, and the number of patients included in the study.
Results
Trial search outcomes
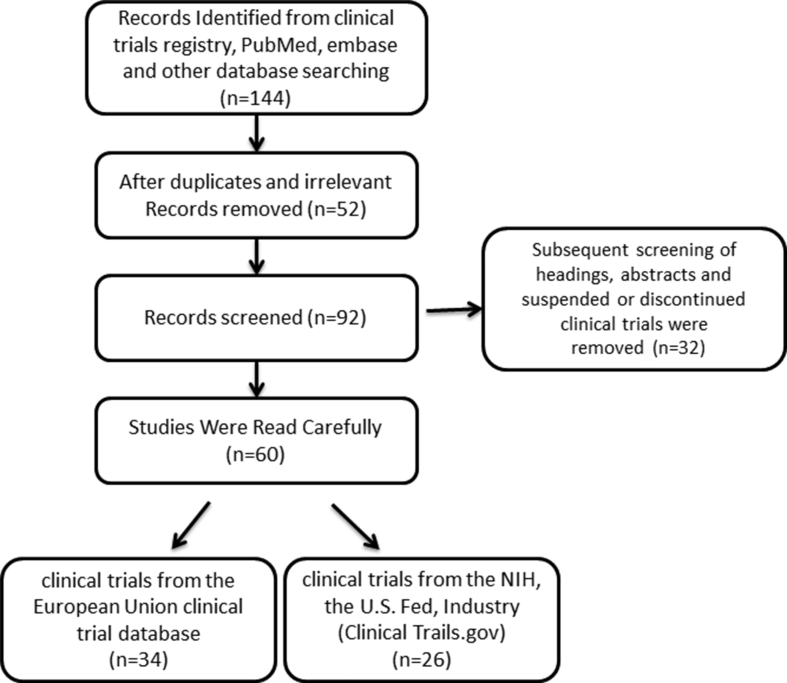
34 clinical trials from the European Union clinical trial database and 26 clinical trials from the National Institute of Health (NIH) clinical trial database were retrieved and presented in the flow chart [Fig. 1]. A total of 60 clinical trials of COVID-19 were classified as either active, completed, or recruiting. 8 patients used hydroxyquinone alone or in combination with other drugs, 6 used remdesivir, 5 used Tocilizumab, Lopinavir/ritonavir either single or combined, 4 used Interferon alpha and beta, and 4 patients used Plaquenil. All other remaining cases used different types of molecules or interventions [Table 1 and Table 2]. Above all, most of the trials have cleared the ethical approval. Some of the case studies are still in the recruitment phase, whereas 20 trials have begun recruiting patients. Amongst them, only 4 trials are in active phase and in the next few days, patient recruitment will begin. 2 clinical trials are still incomplete.
Fig. 1.
Flowchart of study selection for the present study.
Table 1.
List of various combination strategies used in current WHO clinical trials in the treatment of COVID-19.
| NOs | NCT Number | Title | Status | Interventions | Sponsor | Age | Phases | Enrollment | Study Type | Start Date | Completion |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | NCT04333420 | Open label, randomized phase ii/iii study of ifx-1 in patients with severe covid-19 pneumonia | Recruiting | Best supportive Care (BSC) + IFX-1| |
InflaRx GmbH | 18 Years and older | Phase 2 Phase 3 | 130 | Interventional | 31-Mar-20 | 31-Dec-20 |
| 2. | NCT04306497 | Clinical Trial on Regularity of TCM Syndrome and Differentiation Treatment of COVID-19. | Recruiting | TCM prescriptions | Jiangsu Famous Medical Technology Co., Ltd. | 18 Years–75 Years | NA | 340 | Observational | 02-Mar-20 | May-20 |
| 3. | NCT04292899 | Study to Evaluate the Safety and Antiviral Activity of Remdesivir (GS-5734„¢) in Participants With Severe Coronavirus Disease (COVID-19) | Recruiting | Remdesivir | Gilead Sciences | 18 Years and older | Phase 3 | 400 | Interventional | 06-Mar-20 | May-20 |
| 4. | NCT04292730 | Study to Evaluate the Safety and Antiviral Activity of Remdesivir (GS-5734„¢) in Participants With Moderate Coronavirus Disease (COVID-19) Compared to Standard of Care Treatment | Recruiting | Remdesivir | Gilead Sciences | 18 Years and older | Phase 3 | 600 | Interventional | 15-Mar-20 | May-20 |
| 5. | NCT04324489 | DAS181 for Severe COVID-19: Compassionate Use | Recruiting | DAS181 | Renmin Hospital of Wuhan University|Ansun Biopharma, Inc. | 18 Years–70 Years | NA | 4 | Interventional | 06-Mar-20 | 30-Apr-20 |
| 6. | NCT04330690 | Treatments for COVID-19: Canadian Arm of the SOLIDARITY Trial | Active, not recruiting | Lopinavir/ritonavir | Sunnybrook Health Sciences Centre|AbbVie | 6 Months and older | Phase 2 | 440 | Interventional | 18-Mar-20 | NA |
| 7. | NCT04324021 | Efficacy and Safety of Emapalumab and Anakinra in Reducing Hyperinflammation and Respiratory Distress in Patients With COVID-19 Infection. | Recruiting | Emapalumab, Anakinra | Swedish Orphan Biovitrum | 30 Years–79 Years | Phase 2 Phase 3 | 54 | Interventional | Apr-20 | Sep-20 |
| 8. | NCT04327388 | Sarilumab COVID-19 | Recruiting | Sarilumab | Sanofi|Regeneron Pharmaceuticals | 18 Years and older | Phase 2|Phase 3 | 300 | Interventional | 28-Mar-20 | Jun-21 |
| 9. | NCT04334954 | SARS-COV2 Pandemic Serosurvey and Blood Sampling | Recruiting | NA | National Institute of Allergy and Infectious Diseases (NIAID) | 18 Years and older | NA | 1000 | Observational | 09-Apr-20 | 31-Mar-22 |
| 10. | NCT04325646 | Sero-epidemiological Study of the SARS-CoV-2 Virus in France: Constitution of a Collection of Human Biological Samples | Recruiting | Human Biological samples | Institut Pasteur | 5 Years and older | NA | 1000 | Observational | 13-Mar-20 | 28-Feb-23 |
| 11. | NCT04328129 | Household Transmission Investigation Study for COVID-19 in French Guiana | Recruiting | Human biological samples | Institut Pasteur | 5 Years and older | NA | 450 | Interventional | 23-Mar-20 | 23-Mar-22 |
| 12. | NCT04280705 | Adaptive COVID-19 Treatment Trial (ACTT) | Recruiting | Remdesivir | National Institute of Allergy and Infectious Diseases (NIAID) | 18 Years–99 Years | Phase 3 | 440 | Interventional | 21-Feb-20 | 01-Apr-23 |
| 13. | NCT04313127 | A Phase I Clinical Trial in 18–60 Adults | Active, not recruiting | Adenovirus Type 5 Vector |
CanSino Biologics Inc.|Institute of Biotechnology, Academy of Military Medical Sciences. PLA of China|Jiangsu Province Centers for Disease Control and Prevention|Hubei Provincial Center for Disease Control and Prevention|Tongji Hospital | 18 Years–60 Years | Phase 1 | 108 | Interventional | 16-Mar-20 | 20-Dec-22 |
| 14. | NCT04321811 | Behavior, Environment And Treatments for Covid-19 | Recruiting | Human Biological samples | xCures|Genetic Alliance|LunaDNA|Cancer Commons|REDCap Cloud | 18 Years and older | NA | 100000 | Observational | 21-Mar-20 | 20-Mar-22 |
| 15. | NCT04333654 | Hydroxychloroquine in Outpatient, Adults With COVID-19 | Recruiting | Hydroxychloroquine | Sanofi | 18 Years and older | Phase 1 | 210 | Interventional | 31-Mar-20 | May-20 |
| 16. | NCT03808922 | Phase III DAS181 Lower Tract PIV Infection in Immunocompromised Subjects (Substudy: DAS181 for COVID-19): RCT Study | Recruiting | DAS181 | Ansun Biopharma, Inc. | 5 Years and older | Phase 3 | 250 | Interventional | 23-May-19 | 28-Dec-21 |
| 17. | NCT04291729 | Evaluation of GanovôDanoprevir Combined With Ritonavir in the Treatment of Novel Coronavirus Infection | Completed | Ganovo+ritonavir ± Interferon nebulization | The Ninth Hospital of Nanchang|Ascletis Pharmaceuticals Co., Ltd. | 18 Years–75 Years | Phase 4 | 11 | Interventional | 17-Feb-20 | 19-Mar-20 |
| 18. | NCT04334928 | Clinical Trial for the Prevention of SARS-CoV-2Infection in Healthcare Personnel (EPICOS) | Active, not recruiting | Emtricitabine/tenofovir disoproxil|Hydroxychloroquine | Plan Nacional sobre el Sida (PNS)|Effice Servicios Para la Investigation S.L. | 18 Years–65 Years | Phase 3 | 4000 | Interventional | 01-Apr-20 | 31-Jul-20 |
| 19. | NCT04321369 | Impact of Swab Site and Sample Collector on Testing Sensitivity for SARS-CoV-2 Virus in Symptomatic Individuals | Completed | Diagnostic tests | Dr. Deneen Vojta|Quest Diagnostics|Bill and Melinda Gates Foundation|UnitedHealth Group | 5 Years and older | NA | 533 | Observational | 09-Mar-20 | 23-Mar-20 |
| 20. | NCT04327804 | A Longitudinal Study of SARS-CoV-2 Positive Patients Testing Nasal Swabs and Collecting Blood Samples for Research | Recruiting | Diagnostic Test | Dr. Deneen Vojta|PATH|Mayo Clinic|Bill and Melinda Gates Foundation | 5 Years and older | NA | 120 | Observational | 25-Mar-20 | 10-Apr-20 |
| 21. | NCT04283461 | Safety and Immunogenicity Study of 2019-nCoV Vaccine (mRNA-1273) for Prophylaxis SARS CoV-2 Infection | Recruiting | mRNA-1273 | National Institute of Allergy and Infectious Diseases (NIAID) | 18 Years–55 Years | Phase 1 | 45 | Interventional | 03-Mar-20 | 01-Jun-21 |
| 22. | NCT03331445 | Inhaled Gaseous Nitric Oxide (gNO) Antimicrobial Treatment of Difficult Bacterial and Viral Lung (COVID-19) Infections | Active, not recruiting | Nitric Oxide 0.5%/Nitrogen 99.5% Gas for Inhalation | University of British Columbia|Mallinckrodt | 14 Years and older | Phase 2 | 20 | Interventional | 24-Oct-17 | 31-Mar-21 |
| 23. | NCT04331171 | Epidemiological Observation From a Smartphone Self-monitoring Application for Suspected COVID-19 Patients' Triage | Recruiting | Device | Weprom|Institut Pasteur|Assistance Publique - HÃ'pitaux de Paris|DOCAPOST|Direction Générale de l'Offre de Soins | 18 Years and older | NA | 3000000 | Observational | 17-Mar-20 | 31-Jul-20 |
| 24. | NCT04321278 | Safety and Efficacy of Hydroxychloroquine Associated With Azithromycin in SARS-CoV2 Virus (Coalition Covid-19 Brasil II) | Recruiting | Hydroxychloroquine + azithromycin | Hospital Israelita Albert Einstein|EMS|Hospital do Coracao|Hospital Sirio-Libanes|Brazilian Research In Intensive Care Network|Cristália Produtos QuÃmicos FarmacÃauticos Ltda. | 18 Years and older | Phase 3 | 440 | Interventional | 28-Mar-20 | 30-Aug-20 |
| 25. | NCT04326309 | Audio Data Collection for Identification and Classification of Coughing | Recruiting | NA | HealthMode Inc. | 18 Years and older | NA | 1000 | Observational | 25-Mar-20 | 25-Sep-22 |
Table 2.
Characteristics of ongoing European Union Clinical Trials studying the efficacy and safety of Chloroquine, Tocilizumab, Lopinavir/ritonaviror other related formulation for patients with novel coronavirus pneumonia (COVID-19).
| S.No | Sponsor Name | Protocol No | Study Title | Start Date | Ongoing/Completed | Population Age | No. of Subject | Medical Condition | Active Substances | Level | Rout | Country/National Competent Authority |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Akershus University Hospital | Ahus–NO–COVID-19 | Norwegian coronavirus disease 2019 (no covid-19) study: an open labeled randomized controlled pragmatic trial to evaluate the antiviral effect of chloroquine in adult patients with sars-cov-2 infection | 2020-03-23 | Ongoing | Adults, Elderly (18–64) | 200 | SARS-COV-2 infection | hydroxychloroquine sulfate 200 mg |
LLT | Oral | Norway |
| 2 | University of Oxford/Clinical Trials and Research Governance | PRINCIPLE | Platform Randomised trial of interventions against COVID-19 In older people | 2020-03-26 | Ongoing | Adults, Elderly (18–64) | 3000 | Suspected COVID-19 | Hydroxychloroquine Sulfate 200 mg |
PT | Oral | UK - MHRA |
| 3 | Department of Infectious Diseases, Aarhus University Hospital | CamoCO-19-001 | The Impact of Camostat Mesilate on COVID-19 Infection: An investigator-initiated randomized, placebo-controlled, phase iia trial | 2020-03-30 | Ongoing | Adults, Elderly (18–64) | 180 | 2019-nCoV acute respiratory disease | Camostat mesilate 100 mg |
LLT | Oral | Denmark - DHMA |
| 4 | University Hospital Ghent | SARPAC | A prospective, randomized, open-label, interventional study to investigate the efficacy of sargramostim (Leukine®) in improving oxygenation and short- and long-term outcome of COVID-19 patients wit … | 2020-03-24 | Ongoing | Adults, Elderly (18–64) | 80 | Acute hypoxic respiratory failure of COVID-19 patients | Sargramostim 250ug |
LLT | Intravenous | Belgium - FPS Health-DGM |
| 5 | Hellenic Society of Rhythmology | GRECCO-19 | The Greek study in the Effects of Colchicine in Covid-19 complications prevention | 2020-04-01 | Ongoing | Adults, Elderly (18–64) | 180 | myocardial necrosis and pneumonia development in the context of COVID-19 | Colchicine | PT | Intravenous | Greece - EOF |
| 6 | Azienda Unità Sanitaria Locale-IRCCS di Reggio Emilia | RCT-TCZ-COVID-19 | Uno studio randomizzato multicentrico in aperto per valutare l'efficacia della somministrazione precoce del Tocilizumab (TCZ) in pazienti affetti da polmonite da COVID-19 | 2020-03-27 | Ongoing | Adults, Elderly (18–64) | 398 | COVID-19 infection | Tocilizumab 20 mg/ml |
PT | Intravenous | Italy - Italian Medicines Agency |
| 7 | CHU Angers | 49RC20_0071 | HYCOVID - Hydroxychloroquine versus placebo chez les patients ayant une infection COVID-19 à risque d'aggravation secondaire: étude prospective multicentrique randomisée en double aveugle | 2020-03-31 | Ongoing | Adults, Elderly (18–64) | 1300 | Covid-19 | Hydroxychloroquine 200 mg |
LLT | Oral | France - ANSM |
| 8 | F. Hoffmann-La Roche Ltd | WA42380 | A randomized, double-blind, placebo-controlled, multicenter study to evaluate the safety and efficacy of tocilizumab in patients with severe covid-19 pneumonia. | 31-03-20 | Ongoing | Adults, Elderly (18–64) | 50 | COVID-19 pneumonia | Tocilizumab 20 mg/ml |
PT | Intravenous | France - ANSM |
| 9 | Regents of the University of Minnesota | 10 | A Multicenter, Adaptive, Randomised, Blinded Controlled Trial of the Safety and Efficacy of Investigational Therapeutics for the Treatment of COVID-19 in Hospitalized Adults - Version for European U … | 25-03-20 | Ongoing | Adults, Elderly (18–64) | 100, 50, 40 |
Influenza COVID-19 | Remdesivir 100/200 mg |
HLT | Intravenous | Denmark - DHMA & UK - MHRA |
| 10 | UZLeuven | S63874 | Covid-19: A randomized, open-label, adaptive, proof-of- concept clinical trial of new antiviral drug candidates against SARS-cov-2. | 26-03-20 | Ongoing | Adults, Elderly (18–64) | 200 | COVID-19 | itraconazole | LLT | Intravenous | Belgium - FPS Health-DGM |
| 11 | Sanofi-aventis recherche & développement | EFC16858 | An adaptive Phase 2/3, randomized, open-label study assessing efficacy and safety of hydroxychloroquine for hospitalized patients with moderate to severe COVID-19 | 2020-04-02 | Ongoing | Adults, Elderly (18–64) | 40, 50 | Coronavirus infection | Plaquenil 200 mg | PT | Oral | UK – MHRA, France - ANSM |
| 12 | InflaRx GmbH | IFX-1-P.2.9 | A pragmatic adaptive open label, randomized Phase II/III multicenter study of IFX-1 in Patients with severe COVID-19 Pneumonia - “PANAMO” | 2020-03-29 | Ongoing | Adults, Elderly (18–64) | 47 | Severe pneumonia in context of COVID-19 | IFX-1 | PT | Intravenous | Netherlands - Competent Authority |
| 13 | GUSTAVE ROUSSY | 2020/3078 | COVID-19 - Epidemiology of SARS-CoV-2 and Mortality to Covid19 Disease upon Hydroxychloroquine and Azithromycin Therapy in French Cancer patients | 2020-04-03 | Ongoing | Adults, Elderly (18–64) | 1000 | Patients eligible for, or under, or recently treated by chemotherapy (CT) and/or immune-checkpoint blockade (ICB) for the treatment of solid tumors or hematological malignancies. | hydroxychloroquine | LTT | Oral | France - ANSM |
| 14 | Amsterdam UMC | COVID-19 | COUNTER-COVID - Oral imatinib to prevent pulmonary vascular leak in COVID-19 – a randomized, single-blind, placebo controlled, clinical trial in patients with severe COVID-19 disease | 2020-03-31 | Ongoing | Adults, Elderly (18–64) | 304 | Covid19 is characterized by hypoxemic respiratory failure, caused by extensive vascular leak and pulmonary edema early in the course of disease. | Imatinib mesilate | NA | Oral | Netherlands - Competent Authority |
| 15 | ISTITUTO NAZIONALE PER LO STUDIO E LA CURA DEI TUMORI - FONDAZIONE “G. PASCALE” | TOCIVID-19 | Multicenter study on the efficacy and tolerability of tocilizumab in the treatment of patients with COVID-19 pneumonia | 2020-03-18 | Ongoing | Adults, Elderly (18–64) | 330 | COVID-19 | Tocilizumab 20 mg/ml |
PT | Intravenous | Italy - Italian Medicines Agency |
| 16 | Synairgen Research Limited | SG016 | A randomised double-blind placebo-controlled trial to determine the safety and efficacy of inhaled SNG001 (ifnβ-1a for nebulisation) for the treatment of patients with confirmed SARS-cov-2 infectio … | 2020-03-17 | Ongoing | Adults, Elderly (18–64) | 200 | COVID-19 | Interferon beat-1a (IFN-β1a) | LLT | Intravenous | UK - MHRA |
| 17 | Assistance Publique - Hôpitaux de Paris | APHP200375 | Cohort Multiple randomized controlled trials open-label of immune modulatory drugs and other treatments in COVID-19 patients | 2020-03-25 | Ongoing | Adults, Elderly (18–64) | 1000 | COVID-19 | Kevzara | LLT, | Intravenous | France - ANSM |
| 18 | DRCI APHP | APHP200394 | Protective role of inhaled steroids for COVID-19 infection | 2020-04-05 | Ongoing | Adults, Elderly (18–64) | Not announced | COVID-19 | Budesonide | NA | Inhalation | France - ANSM |
| 19 | Gilead Sciences, Inc. | GS-US-540-5774 | A Phase 3 Randomized Study to Evaluate the Safety and Antiviral Activity of Remdesivir (GS-5734™) in Participants with Moderate COVID-19 Compared to Standard of Care Treatment | 2020-03-18 | Ongoing | Adults, Elderly (18–64) | 35, 35, 35,40,50,100 | COVID-19 | Remdesivir 100 mg |
LLT | Intravenous | Germany - BfArM, Spain - AEMPS, France - ANSM, France - ANSM, Netherlands - Authority, UK - MHRA, Sweden - MPA |
| 20 | Gilead Sciences, Inc. | GS-US-540-5773 | A Phase 3 Randomized Study to Evaluate the Safety and Antiviral Activity of Remdesivir (GS-5734™) in Participants with Severe COVID-19 | 2020-03-18 | Ongoing | Adolescents, Under 18, Adults, Elderly | 200, 20, 20, 45, 40, 100, 100 | Coronavirus disease 2019 (COVID-19) | Remdesivir 100 mg |
LLT | Intravenous | Germany - BfArM, Spain - AEMPS, France - ANSM, France - ANSM, Netherlands - Competent Authority, UK - MHRA, Sweden - MPA |
| 21 | APEIRON Respiratory Therapies GmbH | APN01-01-COVID19 | Recombinant human angiotensin-converting enzyme 2 (rhACE2) as a treat-ment for patients with COVID-19 | 2020-04-03 | Ongoing | Adults, Elderly (18–64) | 50 | Severe COVID-19 POSITIVE hospitalized male or female, between 35 and ≤80 years of age | Recombinant human angiotensin-converting enzyme 2 | NA | Intravenous | Denmark - DHMA |
| 22 | Oslo University Hospital | WHO-NOR-COVID-19 | The NOR Solidarity multicenter trial on the efficacy of different anti-viral drugs in SARS-cov-2 infected patients (COVID-19) | 2020-03-26 | Ongoing | Adults, Elderly (18–64) | 443 | SARS-COV-2 infection | Plaquenil | LLT | Oral | Norway - NOMA |
| 23 | CHU de Saint Etienne | 20CH065 | Evaluation of the concentration/viral effect relationship of hydroxychloroquine in COVID-19 patients in the intensive care unit. | 2020-03-30 | Ongoing | Adults, Elderly (18–64) | 50 | covid-19 | Hydroxychloroquine sulfate 400–800 mg |
LLT, PT | Oral | France - ANSM |
| 24 | University of Oxford | NDPHRECOVERY | Randomised Evaluation of COVID-19 Therapy (RECOVERY) | 2020-03-17 | Ongoing | Adults, Elderly (18–64) | Not announced | COVID-19 (infection with SARS-CoV-2 virus) | Lopinavir/ritonavir 200 mg |
PT, PT | Oral | UK - MHRA |
| 25 | Fondation Méditerranée Infection (FMI) - IHU Méditerranée Infection | 202002102 | Treatment of Coronavirus SARS-Cov2 Respiratory Infections with Hydroxychloroquine | 2020-03-05 | Ongoing | Adolescents, (12–17), Adults, Elderly (18–64) |
25 | Patients with documented respiratory infection with coronavirus SARS COV 2 | Plaquenil 200 mg | LLT, | Oral | France - ANSM |
| 26 | University Hospital Ghent | COV-AID | A prospective, randomized, factorial design, interventional study to compare the safety and efficacy of combinations of blockade of interleukin-6 pathway and interleukin-1 pathway to best standard ... | 2020-04-03 | Ongoing | Adults, Elderly (18–64) | 342 | COVID-19 patients with acute hypoxic respiratory failure and systemic cytokine release syndrome. | RoActemra | Intravenous | Belgium - FPS Health-DGM | |
| 27 | INSERM | C20-15 | Multi-centre, adaptive, randomized trial of the safety and efficacy of treatments of COVID-19 in hospitalized adults | 2020-03-09 | Ongoing | Adults, Elderly (18–64) | 1000 | COVID-19 - | Plaquenil 200 mg | LTT | Oral | France - ANSM |
| 28 | Universitätsklinikum Tübingen | COV-HCQ | Randomized controlled trial of hydroxychloroquine versus placebo for the treatment of adult patients with acute coronavirus disease 2019 – COVID-19 | 2020-03-25 | Ongoing | Elderly (>65) | 220 | Acute coronavirus disease 2019 | Chloroquine phosphate 200 mg |
PT | Oral | Germany - BfArM |
| 29 | Uni-Pharma Kleon Tsetis Pharmaceutical Laboratories S.A. | UNIKINON-01/HOPE | CHROLOQUINE PHOSPHATE AGAINST INFECTION BY THE NOVEL CORONAVIRUS SARS-cov-2 (COVID-19): THE HOPE OPEN-LABEL, NON-RANDOMIZED CLINICAL TRIAL | 2020-04-02 | Ongoing | Adults, Elderly (18–64) | 60 | pneumonia from SARS-CoV-2 in patients staying home and improving symptoms of SARS-CoV-2 pneumonia in patients treated in hospital | Tocilizumab | LLT | Intravenous | Greece - EOF |
| 30 | Sanofi-aventis Recherche et Développement | EFC16844 | An adaptive phase 2/3, randomized, double-blind, placebo-controlled, study assessing efficacy and safety of sarilumab for hospitalized patients with COVID-19 | 2020-03-26 | Ongoing | Adults, Elderly (18–64) | 25, 40, 25 | Corona virus infection | Hydrocortisone | PT | Intravenous | Germany - BfArM, France - ANSM, Italy - Italian Medicines Agency |
| 31 | University Medical Center | 73249 | Reducing health care workers absenteeism in SARS-cov-2 pandemic by enhanced trained immune responses through Bacillus Calmette-Guérin vaccination, a randomized controlled trial (COVID-19). | 2020-03-17 | Ongoing | Adults, Elderly (18–64) | 1000 | SARS-CoV-2 infection | BCG-CORONA | HLT | Intravenous | Netherlands - Competent Authority |
| 32 | Hellenic institute for the study of sepsis | ESCAPE | Efficiency in management of organ dysfunction associated with infection by the novel sars-cov-2 virus (covid-19) through a personalized immunotherapy approach: the escape clinical trial | 2020-04-01 | Ongoing | Adults, Elderly (18–64) | 20 | Organ dysfunction by the novel SARS-Cov-2 virus | Tocilizumab 400 mg |
LLT | Intravenous | Greece - EOF |
| 33 | The Parker Institute, Bispebjerg and Frederiksberg Hospital, | APPI2-CV-2020-01 | Effectiveness of Interleukin-6 Receptor Inhibitors in the Management of Patients with Severe SARS-CoV-2 Pneumonia: An Open-Label Multicenter Sequential Randomized Controlled Trial | 2020-04-3 | Ongoing | Adults, Elderly (18–64) | 200 | SARS-CoV-2 infection | Tocilizumab 400 mg |
LLT | Intravenous | Denmark - DHMA |
| 34 | University Medical Center Utrecht | REMAP-CAP | Randomized, Embedded, Multifactorial, Adaptive Platform trial for Community-Acquired Pneumonia (REMAP-CAP) | 2015-09-16 | Ongoing | Adults, Elderly (18–64) | 600,600, 40, 800, 200, 270, 600,30, 60, 152 | Severe Community Acquired Pneumonia | Levofloxacin | LLT | Intravenous | Netherlands, Ireland, Portugal, UK- MHRA, Hungary, Belgium, Germany, Croatia, Spain, FRance |
LLT: Lowest Level Terms, PT: Preferred Terms, HLT: High-Level Terms, NA: Not Available.
The first randomized controlled clinical pathways were sponsored by Dongzhimen Hospital of the Beijing University of Traditional Chinese Medicine (medical aspects of traditional knowledge that developed over generations). Those drugs were registered as “Chinese Severe Pneumonia Medicine with Severe Coronavirus Pneumonia” on 3 January 2020. In this review, we have included 60 trials in terms of clinical trial phases. Amongst them, 43 trials are in the preliminary experimental phase, 7 are in the middle phase, 8 are in phase 3 and extended for validation. 1 trial is completed with Ganovo+ritonavir ± Interferon nebulization drugs and ready for sale as the same diagnostic kit (Quest Diagnostics, Bill and Melinda).
Intervention and evaluation
The leading intervention strategy of registered clinical trials consists of traditional Chinese medicine, western medicine, and conventional integrated treatment. Especially, the outcome of therapy includes treatment time, patient immunity, frequency of use of ventilation, mortality, number of complications, and virological detection indicators. Current duration of the medication is more than 10 days. Medicinal approaches include oral, injection, and inhalation. The control subject was treated regularly with a placebo. At present, 24 western medicinal treatments are registered in clinical trials. On the other hand, single or combination of biological agents such as hydroxychloroquine, camostat mesilate, sargramostim, colchicine, tocilizumab, remdesivir, Itraconazole, IFX-1, Imatinib mesilate, Interferon beta-1a (IFN-β1a), Sarilumab, Budesonide, and Nitric Oxide 0.5%/Nitrogen 99.5% Gas for Inhalation, Recombinant human angiotensin-converting enzyme 2, Plaquenil, lopinavir/ritonavir, RoActemra, Chloroquine phosphate, Hydrocortisone, Levofloxacin, Emapalumab, Anakinra, Sarilumab, Danoprevir+ritonavir ± Interferon, Emtricitabine/tenofovir are listed as intervention methods.
Two clinical trials include biological agents product mRNA, blood stem cells, cord blood mononuclear cells, mesenchymal stem cell (MSC), recombinant cytokine gene-derived vector, and immunoglobulin (IgM, IgG).
Discussion
Since the impact of COVID-19 is extremely severe, the development of an effective treatment strategy against the infection is very critical and concerning throughout the world. Although the molecular diagnosis, treatment, and international public health has improved after experiencing the 2003 SARS (CoV-I) epidemic, due to the new mutant form of the COVID virus, it is difficult to diagnose and treat the infection. Moreover, there is no proven or recognized licensed therapy against COVID-19. This sudden event has caused a high mortality rate in China and other countries around the world, mainly the European regions. The current situation leads us to carry out a systematic review to summarize the on-going clinical trials and possible therapeutic options against COVID-19 [1,11,21]. Intensive clinical trials are being conducted by many researchers to eradicate this disease.
Most of the reports demonstrate the intervention of western medicine and a combination of traditional medicine to treat COVID-19 infection. Most of the studies are still in the preliminary experimental phase of the clinical trail. There are 43 extended (Phase 1) trial studies that show the utilization of drugs (Hydroxychloroquine, Camostat mesilate, Sargramostim, Colchicine, Tocilizumab, Remdesivir, Itraconazole, IFX-1, Imatinib mesilate, Interferon beta-1a (IFN-β1a), Kevzara, Budesonide, Lopinavir/ritonavir, RoActemra, Chloroquine phosphate, Hydrocortisone, Levofloxacin, Emapalumab, Anakinra, Sarilumab). According to the population size used in the clinical trial, 18 interventional clinical trials were carried out, which included 4 to 4000 candidates/trials and 8 observational studies, that included around 1000 to 3000000 candidates. Most of the trials are on-going; thus, the confirmation level and the clinical significance is limited [Table 1].
The alternative strategy to fight against COVID-19 is mainly based on boosting immune responses and to prevent the disease complications. This strategy improves patient immunity by predominating self-immune damage to the cytokine storm (modulating the post-infection immune response) and symptomatic treatment [22]. Although some Chinese herbs have shown both antiviral and high immune effects, current situations prove that the antiviral effect of western drugs is superior and tolerable in comparison to the traditional ayurvedic, Unani, or homeopathic medicines [23,24]. The combination of Qingfeipaidutang and hydroxychloroquine phosphate might be a potential therapeutic strategy for the treatment and management of COVID-19 [25,26]. Recently, an open-label, randomized clinical trial of standard medical treatment or colchicine (1.5-mg loading dose by 0.5 mg after 60 min and maintenance doses of 0.5 mg twice daily) in 105 patients showed that event-free survival time was 20.7 days in the colchicine group and 18.6 days in the control group and a significantly improved time to clinical deterioration [27]. Another multicenter, open-label randomized controlled trial in 160 patients with Shenhuang granule (50 g of Panax ginseng C. A. Mey, 40 g of Rheum palmatum L. stem, 30 g of Sargentodoxa cuneata stem, 30 g of Taraxacum mongolicum, 50 g of Aconiti Lateralis Radix Praeparata, and 6 g of Whitmania pigra Whitman) twice a day for 14 days is underway and the trail results are awaiting [28].
Still, there is a lack of high-grade substantiation evidence that demands further clinical clarification and verification. Several clinical researchers have used biological products for the treatment of COVID-19. In order to treat COVID-19 infection, Steroid-based therapy has been implemented and the result availability is limited for trials [13,29]. The most commonly used drug is chloroquine (anti-malaria) and ribavirin with a broad-spectrum antiviral combination of Remdesivir and IFN-α2b. An interventional study based in Spain was registered at ClinicalTrials.gov on 1 April 2020 (ID: NCT04334928). Currently, this is the only clinical trial underway with Emtricitabine/tenofovir disoproxil/hydroxychloroquine against COVID-19 infection. Due to minimal interaction, the combination of baricitinib and lopinavir/ritonavir/remdeviate antivirals was used to treat the infection COVID-19 during the pandemic. The combination of baricitinib and above-mentioned drugs may reduce viral replication, infectivity, and aberrant host inflammatory response [30].
Future perspective
In most trials, inclusion and exclusion criteria has eliminated children under 18 years, pregnant women, and comorbidities like liver, heart, and kidney disease. This may result in a lack of substantial clinical evidence. The quality of clinical research needs to improve drastically. Registered clinical trials must follow Observatory/Interventional Clinical Trial Guidelines. Clinical trials must not be registered without accurate drug testing. Safety guidelines for in vitro experiments during clinical trials are a major concern. Thus, The National Science Research Administration (NASR) should consider improving the health risks assessment, good research practice, and coordination of fewer promising drugs i.e.; Remdesivir. Moreover, a major limitation of the registered clinical studies is that most of them follow a traditional conservative approach without considering the timeline.
Conclusion
Due to the lack of intensive and high-quality clinical evidence, there is no final consent to the ideal therapy for COVID-19. It is difficult to obtain reliable data even with a small sample size and prolonged study periods. There is a undoubted need for protocol development that can be used for large randomized clinical trials. In order to establish such clinical trials, prospective therapies must be designed. The NASR must improve the good clinical practice in research and coordination along as well as aid in improving the efficacy and quality of the study that could deal with current health emergencies. Besides, during clinical trials, the implementation of a variety of study designs with a large number of cases and different statistical analyses is crucial. Further; we must recognize the ancient history report of previous infectious diseases in order to implement novel conceptual health-related policies.
Conflicts of Interest
The authors have declared that there are no conflicts of interest.
Acknowledgment
We thank Deepak Kumar Verma, IIT Kharagpur, India for providing a factual review and assistance throughout our study and for their help in editing the manuscript.
Footnotes
Peer review under responsibility of Chang Gung University.
References
- 1.Li X., Wang L., Yan S., Yang F., Xiang L., Zhu J. Clinical characteristics of 25 death cases with COVID-19: a retrospective review of medical records in a single medical center, Wuhan, China. Int J Infect Dis. 2020;94:128–132. doi: 10.1016/j.ijid.2020.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cucinotta D., Vanelli M. WHO Declares COVID-19 a pandemic. Acta Biomed : Atenei Parmensis. 2020;91:157–160. doi: 10.23750/abm.v91i1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mahase E. Covid-19: death rate is 0.66% and increases with age, study estimates. BMJ. 2020;369:m1327. doi: 10.1136/bmj.m1327. [DOI] [PubMed] [Google Scholar]
- 4.Jiang F., Deng L., Zhang L., Cai Y., Cheung C.W., Xia Z. Review of the clinical characteristics of coronavirus disease 2019 (COVID-19) J Gen Intern Med. 2020;35:1545–1549. doi: 10.1007/s11606-020-05762-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ribeiro J., Bingre P., Strubbe D., Reino L. Coronavirus: why a permanent ban on wildlife trade might not work in China. Nature. 2020;578:217. doi: 10.1038/d41586-020-00377-x. [DOI] [PubMed] [Google Scholar]
- 7.Ye Z.W., Yuan S., Yuen K.S., Fung S.Y., Chan C.P., Jin D.Y. Zoonotic origins of human coronaviruses. Int J Biol Sci. 2020;16:1686–1697. doi: 10.7150/ijbs.45472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rajgor D.D., Lee M.H., Archuleta S., Bagdasarian N., Quek S.C. The many estimates of the COVID-19 case fatality rate. Lancet Infect Dis. 2020;20:776–777. doi: 10.1016/S1473-3099(20)30244-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhaskar L., Roshan B., Nasri H. The fuzzy connection between SARS-CoV-2 infection and loss of renal function. Am J Nephrol. 2020;51:572–573. doi: 10.1159/000508087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Valizadeh R., Baradaran A., Mirzazadeh A., Bhaskar L.V. Coronavirus-nephropathy; renal involvement in COVID-19. J Ren Inj Prev. 2020;9:18. [Google Scholar]
- 11.Ingravallo F. Death in the era of the COVID-19 pandemic. Lancet Publ Health. 2020;5 doi: 10.1016/S2468-2667(20)30079-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verity R., Okell L.C., Dorigatti I., Winskill P., Whittaker C., Imai N. Estimates of the severity of coronavirus disease 2019: a model-based analysis. Lancet Infect Dis. 2020;20:669–677. doi: 10.1016/S1473-3099(20)30243-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Russell T.W., Hellewell J., Jarvis C.I., van-Zandvoort K., Abbott S., Ratnayake R. Estimating the infection and case fatality ratio for COVID-19 using age-adjusted data from the outbreak on the Diamond Princess cruise ship, February 2020. Euro Surveill. 2020;25:2000256. doi: 10.2807/1560-7917.ES.2020.25.12.2000256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karadag E. Increase in COVID-19 cases and case-fatality and case-recovery rates in Europe: a cross-temporal meta-analysis. J Med Virol. 2020;92:1511–1517. doi: 10.1002/jmv.26035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Preeti Dhillon S.K., Shekhar C., Ram U., Kant Dwivedi L., Yadav S., Unisa S. International Institute for Population Sciences; Mumbai: 2020. Case-fatality ratio and recovery rate of COVID-19: scenario of most affected countries and Indian states. [Google Scholar]
- 16.Coelho F.C., Lana R.M., Cruz O.G., Codeco C.T., Villela D., Bastos L.S. Assessing the potential impact of COVID-19 in Brazil: mobility, morbidity and the burden on the health care system. medRxiv. 2020 doi: 10.1101/2020.03.19.20039131. 2020.03.19.20039131. [preprint]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.https://www.ecdc.europa.eu/en/geographical-distribution-2019-ncov-cases [accessed on 07 July 2020].
- 18.Marano G., Vaglio S., Pupella S., Facco G., Catalano L., Liumbruno G.M. Convalescent plasma: new evidence for an old therapeutic tool? Blood Transfus. 2016;14:152–157. doi: 10.2450/2015.0131-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Verma H.K., Farran B., Bhaskar L.V.K.S. Convalescent plasma transfusion a promising therapy for coronavirus diseases 2019 (COVID-19): current updates. Antibody Therapeut. 2020;3:115–125. doi: 10.1093/abt/tbaa010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Di Gennaro F., Pizzol D., Marotta C., Antunes M., Racalbuto V., Veronese N. Coronavirus diseases (COVID-19) current status and future perspectives: a narrative review. Int J Environ Res Publ Health. 2020;17:2690. doi: 10.3390/ijerph17082690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mahase E. Covid-19: UK records first death, as world's cases exceed 100 000. BMJ. 2020;368:m943. doi: 10.1136/bmj.m943. [DOI] [PubMed] [Google Scholar]
- 22.Felsenstein S., Herbert J.A., McNamara P.S., Hedrich C.M. COVID-19: immunology and treatment options. Clin Immunol. 2020;215:108448. doi: 10.1016/j.clim.2020.108448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vellingiri B., Jayaramayya K., Iyer M., Narayanasamy A., Govindasamy V., Giridharan B. COVID-19: a promising cure for the global panic. Sci Total Environ. 2020;725:138277. doi: 10.1016/j.scitotenv.2020.138277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ni L., Zhou L., Zhou M., Zhao J., Wang D.W. Combination of western medicine and Chinese traditional patent medicine in treating a family case of COVID-19. Front Med. 2020;14:210–214. doi: 10.1007/s11684-020-0757-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang K. Is traditional Chinese medicine useful in the treatment of COVID-19? Am J Emerg Med. 2020 doi: 10.1016/j.ajem.2020.03.046. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ren J.L., Zhang A.H., Wang X.J. Traditional Chinese medicine for COVID-19 treatment. Pharmacol Res. 2020;155:104743. doi: 10.1016/j.phrs.2020.104743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deftereos S.G., Giannopoulos G., Vrachatis D.A., Siasos G.D., Giotaki S.G., Gargalianos P. Effect of colchicine vs standard care on cardiac and inflammatory biomarkers and clinical outcomes in patients hospitalized with coronavirus disease 2019: the GRECCO-19 randomized clinical trial. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.13136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fang B., Zhang W., Wu X., Huang T., Li H., Zheng Y. Shenhuang granule in the treatment of severe coronavirus disease 2019 (COVID-19): study protocol for an open-label randomized controlled clinical trial. Trials. 2020;21:568. doi: 10.1186/s13063-020-04498-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Capuano A., Scavone C., Racagni G., Scaglione F., Italian Society of P. NSAIDs in patients with viral infections, including Covid-19: victims or perpetrators? Pharmacol Res. 2020;157:104849. doi: 10.1016/j.phrs.2020.104849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stebbing J., Phelan A., Griffin I., Tucker C., Oechsle O., Smith D. COVID-19: combining antiviral and anti-inflammatory treatments. Lancet Infect Dis. 2020;20:400–402. doi: 10.1016/S1473-3099(20)30132-8. [DOI] [PMC free article] [PubMed] [Google Scholar]