1. Introduction
Here, we summarize the current strategies for early detection of pancreatic ductal adenocarcinoma (PDA) including traditional and novel detection methods. PDA remains the deadliest of the common cancers, with little change in patient survival in the past several decades. Although the estimated time line of carcinogenesis is over 20 years, PDA usually is diagnosed at a late, metastatic stage, where it essentially is incurable.
“Ideally, PDA is detected at a “carcinoma in situ” stage where the tumor is local and patients can be more efficiently treated”
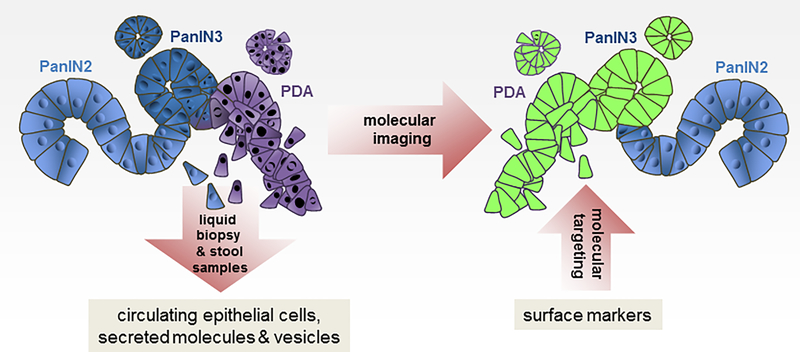
Efforts in early detection and imaging are based on current multistep carcinogenesis models for PDA development and progression. PDA develops from duct-like precursor cells that form low-grade pancreatic intraepithelial neoplasia 1 and 2 (PanIN1, PanIN2), which progress through a multistep carcinogenesis process to carcinoma in situ (PanIN3 lesions) and eventually metastatic disease. Since PDA quickly progresses through T1-T4 stages, early detection requires the identification of PanIN3 lesions or tumors that are at a localized disease stage, with the overall goals to make pancreatic cancer a preventable disease or to provide a survival advantage to the patient. In order to achieve these goals novel detection methods need to be established. These include the identification of circulating biomarkers in liquid biopsy (i.e. blood, pancreatic juice) or stool, as well as molecular imaging of lesion biomarkers (detection in situ) and possible targeting of PDA based on these markers (Figure 1). Moreover, for lipid biopsies, it was shown that combining markers can be superior to single markers and may be the key for successful early detection of pancreatic cancer [1]. A further combination with molecular imaging markers may be needed for additional risk assessment.
Figure 1: Early Detection of Pancreatic Ductal Adenocarcinoma.
While PanIN2 still represent low-grade dysplasia, detection of PanIN3-associated changes is paramount to current efforts in early detection and imaging. In order to discover PDA at an early stage and to make it a preventable disease novel detection methods need to be established. These include the identification of circulating biomarkers in liquid biopsy (i.e. blood, pancreatic juice) or stool, as well as molecular imaging of lesion biomarkers (detection in situ) and possible molecular targeting of PDA based on these markers.
2. Early detection markers
“Early detection methods ideally are non-invasive and work with liquid biopsies”
Liquid biopsy methods (with the exception of pancreatic juice collection) are the least invasive and straight-forward means to test for biomarkers indicative for PDA development. Potential biomarkers that can be detected in liquid biopsies include circulating epithelial/tumor cells, cell free tumor DNA (cftDNA), epigenetically-altered DNA, microRNA, protein or RNA markers in exosomes, secreted molecules, or altered molecule profiles that indicate shifts in metabolism (Figure 1).
Circulating epithelial cells can shed from premalignant lesions such as IPMN and PanIN early in PDA development. While expression of a panel of EMT markers is indicative for circulating PanIN cells, the expression of cytokeratins, EpCAM and novel noncoding RNA (HSATII) is indicative for circulating IPMN cells [2,3]. In established PDA, circulating tumor cells show a decrease in expression of E-cadherin and Muc-1, as well as an upregulation of SPARC, Cadherin11 and Aldh1 expression, and can be immmunomagnetically-enriched using some of these markers[4].
Circulating cell-free pancreatic tumor DNA (ctDNA) in blood plasma, pancreatic juice or stool can be used to detected DNA mutations or methylated and silenced genes such as ADAMTS1 and BNC1 [5,6]. A challenge is that the percentage of ctDNA fragments is relatively low as compared to all DNA in the fluid; and DNA mutations are difficult to detect in early stages of PDA development [7]. Another challenge of detection of tumor markers in liquid biopsy-based assays is that they often cannot be specifically linked to pancreatic cancer. One solution is the combined detection of different markers, which can increase the specificity and sensitivity of assays [1,8]. As an example, a combination of ctDNA and protein biomarkers is capable of detecting nearly two-thirds of pancreatic cancers that had no evidence of distant metastasis at the time of surgical resection [1]. This could be further complemented with the inclusion of additional markers that indicate altered metabolism. For example, PDA causes new onset diabetes [9], and diabetes-induced metabolic changes such as altered glucose homeostasis and altered serum lipid profiles can be other markers for early onset [10,11]. Another example is the detection of shifts in circulating amino-acids which also occur early in the development of PDA [12].
3. Imaging and molecular imaging as means for early detection and prevention
Conventional standard imaging technologies to detect and stage progressed PDA with high accuracy and to evaluate its resectability include high-quality contrast-enhanced computed tomography (CT) and magnetic resonance (MR) cholangiopancreatography [1,13]. Additional 3-dimensional re-construction of existing CT scan data by cinematic rendering improves the visualization of tumors and determination of tumor resectability [14]. Magnetic resonance imaging (MRI) and endoscopic ultrasound (EUS) also allow detecting small pancreatic cysts [15].
While above methods are optimized to detect progressed tumors, early diagnosis requires the detection of malignant precursors [16]. Radiomics, the generation of minable high-dimensional data through conversion of digital images, allows obtaining addition insight into pancreatic tissue organization. Retrospective reviews of CT scans using this method suggests pancreatic parenchymal inhomogeneity and loss of fatty marbling in the pancreas as events that suggest PDA up to 34 months before actual diagnosis [16,17]. Radiomics, alone or in combination with biomarkers, also allows distinguishing low-grade from high-grade IPMNs, and therefore decisions on which patients benefit from surgery [18,19].
Molecular imaging to detect early changes i.e. between low grade and high grade dysplasia will allow early diagnosis in individuals with no obvious symptoms (Figure 1). Thus, current approaches with molecular imaging of radiotracer-targeted indicative molecules hold most promise for early disease detection [20,21]. Examples are vascular epithelial growth factor receptor 2 (VEGFR2), which is overexpressed in the neovasculature of PDA [22], or stromal targets such as insulin-like growth factor 1 (IGF1) [23], PD-L1 [24] and SPARC [25]. Other markers more specific for malignant transformation or early stage PDA are membrane glycoproteins such as carbohydrate antigen 19–9 (CA 19–9) and thymocyte differentiation antigen 1 (Thy1) [21]. Moreover, plectin 1, which stains positive in 60% of PanIN3, but not in PanIN1/2 has been identified as a marker for progression of preneoplastic lesions to carcinoma in situ [20].
Disadvantage of molecular imaging is that it is not feasible for early detection on a population-wide basis [13]. However, it could be used on individuals that are positive in tests based on liquid biopsies or with genetic predisposition. Another exciting aspect is that once imaging markers are identified, these surface molecules may also be used for targeted treatment strategies (Figure 1).
4. Expert opinion
“Ideal early detection tests would combine multiple markers to ensure specificity for developing PDA”
Early detection and tumor resection or its targeted therapy at an early stage is the greatest hope for patient survival. Therefore a combined approach of different strategies may be needed. Ideally, a panel of different early detection markers (protein DNA, RNA, or exosomes) in body fluids, liquid biopsy or stool will allow non-invasive prescreening of the general public. A challenge with developing such methods is that they have to be able to detect developing cancer, ideally at a stage where the tumor is local. They also need to be specific in detecting pancreatic cancer, exclude false positives and distinguish from other cancers or other diseases such as pancreatitis. A second strategy then needs to focus on more precise in situ detection and targeting of actual cancerous lesions. This approach needs the identification of molecular markers specific for PanIN3 and tumor lesions, ideally on the cell surface. These markers then can be used to detect lesion, but also to specifically target cancerous cells with a tailored delivery system for chemotherapeutic or pathway targeting drugs. Moreover, these markers can be used as monitoring system or theragnostic markers for efficient treatment, tumor regression or recurrence. A challenge with such markers is that they ideally should be membrane proteins that are specific for cancerous lesions over low-grade lesions. Unfortunately, currently there are only very few suitable biomarkers available for both of above strategies.
5. Five-year view
While genetic counseling is a commonly-used tool for individuals with a family history for pancreatic cancer, a test for early detection for average-risk individuals is lacking. Recent efforts not only identified a multitude of potential early detection markers, but also showed that their combination will increase specificity of detection. We predict that within the next five years, based on such combination of markers, pancreas cancer-specific tests will be developed that eventually will allow screening of a broad public. We also predict that next the field will increasingly focus on the identification of lesion molecule markers that not only allow early detection in situ, but also allow specific targeting of early lesions. Long-term, both blood or body fluid-based screening and lesion-marker based detection and targeting will be combined for early detection, early treatment and monitoring of tumor response.
Acknowledgments
Funding
This work was supported by the NIH grants CA200572, CA229560 and U01CA224145; as well as the Chartrand Foundation, the Champions for Hope program of the Funk-Zitiello Foundation, and the Sky Foundation.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Footnotes
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Cohen JD, Javed AA, Thoburn C et al. Combined circulating tumor DNA and protein biomarker-based liquid biopsy for the earlier detection of pancreatic cancers. Proc Natl Acad Sci U S A, 114(38), 10202–10207 (2017).* Provides a rationale for combination of circulating proteins and mutations in cell-free DNA for early detection of pancreatic cancer.
- 2.Franses JW, Basar O, Kadayifci A et al. Improved Detection of Circulating Epithelial Cells in Patients with Intraductal Papillary Mucinous Neoplasms. Oncologist, 23(1), 121–127 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rhim AD, Mirek ET, Aiello NM et al. EMT and dissemination precede pancreatic tumor formation. Cell, 148(1–2), 349–361 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Albuquerque A, Kubisch I, Breier G et al. Multimarker gene analysis of circulating tumor cells in pancreatic cancer patients: a feasibility study. Oncology, 82(1), 3–10 (2012). [DOI] [PubMed] [Google Scholar]
- 5.Suenaga M, Dudley B, Karloski E et al. The Effect of Pancreatic Juice Collection Time on the Detection of KRAS Mutations. Pancreas, 47(1), 35–39 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yi JM, Guzzetta AA, Bailey VJ et al. Novel methylation biomarker panel for the early detection of pancreatic cancer. Clin Cancer Res, 19(23), 6544–6555 (2013).* Identifies a panel of methylation biomarkers for early detection of panceratic cancer.
- 7.Yu J, Sadakari Y, Shindo K et al. Digital next-generation sequencing identifies low-abundance mutations in pancreatic juice samples collected from the duodenum of patients with pancreatic cancer and intraductal papillary mucinous neoplasms. Gut, 66(9), 1677–1687 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lennon AM, Wolfgang CL, Canto MI et al. The early detection of pancreatic cancer: what will it take to diagnose and treat curable pancreatic neoplasia? Cancer Res, 74(13), 3381–3389 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chari ST, Leibson CL, Rabe KG et al. Pancreatic cancer-associated diabetes mellitus: prevalence and temporal association with diagnosis of cancer. Gastroenterology, 134(1), 95–101 (2008).* Concludes that identification of biomarkers for pancreatic cancer-induced diabetes may allow early detection of panceratic cancer in patients with new-onset diabetes.
- 10.Maitra A, Sharma A, Brand RE et al. A Prospective Study to Establish a New-Onset Diabetes Cohort: From the Consortium for the Study of Chronic Pancreatitis, Diabetes, and Pancreatic Cancer. Pancreas, 47(10), 1244–1248 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mantyselka P, Kautiainen H, Saltevo J et al. Weight change and lipoprotein particle concentration and particle size: a cohort study with 6.5-year follow-up. Atherosclerosis, 223(1), 239–243 (2012). [DOI] [PubMed] [Google Scholar]
- 12.Mayers JR, Wu C, Clish CB et al. Elevation of circulating branched-chain amino acids is an early event in human pancreatic adenocarcinoma development. Nat Med, 20(10), 1193–1198 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dimastromatteo J, Brentnall T, Kelly KA. Imaging in pancreatic disease. Nat Rev Gastroenterol Hepatol, 14(2), 97–109 (2017). [DOI] [PubMed] [Google Scholar]
- 14.Chu LC, Johnson PT, Fishman EK. Cinematic rendering of pancreatic neoplasms: preliminary observations and opportunities. Abdom Radiol (NY), (2018). [DOI] [PubMed] [Google Scholar]
- 15.Brune K, Abe T, Canto M et al. Multifocal neoplastic precursor lesions associated with lobular atrophy of the pancreas in patients having a strong family history of pancreatic cancer. Am J Surg Pathol, 30(9), 1067–1076 (2006). [PMC free article] [PubMed] [Google Scholar]
- 16.Chu LC, Goggins MG, Fishman EK. Diagnosis and Detection of Pancreatic Cancer. Cancer J, 23(6), 333–342 (2017). [DOI] [PubMed] [Google Scholar]
- 17.Gonoi W, Hayashi TY, Okuma H et al. Development of pancreatic cancer is predictable well in advance using contrast-enhanced CT: a case-cohort study. Eur Radiol, 27(12), 4941–4950 (2017). [DOI] [PubMed] [Google Scholar]
- 18.Hanania AN, Bantis LE, Feng Z et al. Quantitative imaging to evaluate malignant potential of IPMNs. Oncotarget, 7(52), 85776–85784 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Permuth JB, Choi J, Balarunathan Y et al. Combining radiomic features with a miRNA classifier may improve prediction of malignant pathology for pancreatic intraductal papillary mucinous neoplasms. Oncotarget, 7(52), 85785–85797 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bausch D, Thomas S, Mino-Kenudson M et al. Plectin-1 as a novel biomarker for pancreatic cancer. Clin Cancer Res, 17(2), 302–309 (2011).* Identifies Plectin-1 as a novel biomarker upregulated in 60% of PanIN3. Provides the groundwork for lesion-specific detection and targeting strategies.
- 21.Foygel K, Wang H, Machtaler S et al. Detection of pancreatic ductal adenocarcinoma in mice by ultrasound imaging of thymocyte differentiation antigen 1. Gastroenterology, 145(4), 885–894 e883 (2013).* Describes thymocyte differentiation antigen 1 (Thy1) as as marker for PDA neovasculature that (in mice) can be detected by ultrasound molecular imaging.
- 22.Itakura J, Ishiwata T, Friess H et al. Enhanced expression of vascular endothelial growth factor in human pancreatic cancer correlates with local disease progression. Clin Cancer Res, 3(8), 1309–1316 (1997). [PubMed] [Google Scholar]
- 23.Ouban A, Muraca P, Yeatman T, Coppola D. Expression and distribution of insulin-like growth factor-1 receptor in human carcinomas. Hum Pathol, 34(8), 803–808 (2003). [DOI] [PubMed] [Google Scholar]
- 24.Heskamp S, Hobo W, Molkenboer-Kuenen JD et al. Noninvasive Imaging of Tumor PD-L1 Expression Using Radiolabeled Anti-PD-L1 Antibodies. Cancer Res, 75(14), 2928–2936 (2015). [DOI] [PubMed] [Google Scholar]
- 25.Neuzillet C, Tijeras-Raballand A, Cros J, Faivre S, Hammel P, Raymond E. Stromal expression of SPARC in pancreatic adenocarcinoma. Cancer Metastasis Rev, 32(3–4), 585–602 (2013). [DOI] [PubMed] [Google Scholar]