Abstract
Euglycemic diabetic ketoacidosis is a rare but serious adverse effect of sodium-glucose cotransporter 2 (SGLT2) inhibitors. We present a case of a woman in her 40s with type 2 diabetes mellitus hospitalized for revascularization for moyamoya disease who developed empagliflozin-associated euglycemic diabetic ketoacidosis despite having stopped the medication before admission. Surgical stress, acute postoperative illness, and decreased carbohydrate intake are postulated to be contributing factors to the development of ketosis in this patient, while near-normal glucose levels initially suggested nondiabetic ketoacidosis physiology and led to delayed diagnosis and treatment. Patients with type 2 diabetes mellitus may develop diabetic ketoacidosis during states of relative insulinopenia, most frequently from inadequate medication or intercurrent illness. During periods of carbohydrate deficiency, volume depletion, and upregulation of counter-regulatory stress hormones, SGLT2 inhibitor therapy can promote lipolysis and ketogenesis while maintaining euglycemia. Clinical considerations to ensure safe SGLT2 inhibitor therapy include appropriate holding parameters, timely diagnosis of euglycemic diabetic ketoacidosis, and recognition that the pharmacologic effects of SGLT2 inhibitor treatment may persist beyond several half-lives of elimination.
Index Words: Euglycemic diabetic ketoacidosis, SGLT2 inhibitor, empagliflozin, diabetes mellitus
Introduction
Sodium-glucose cotransporter 2 (SGLT2) inhibitors represent the newest class of oral diabetes medications. With mounting evidence demonstrating improved cardiovascular and kidney outcomes independent of glycemic control, the use of these agents is anticipated to grow rapidly. However, rare but serious adverse drug effects, including euglycemic diabetic ketoacidosis, remain a concern. Nephrologists should be well versed in the unique characteristics of these medications, including rare but real risks for euglycemic diabetic ketoacidosis, to ensure their effective and safe use.
Case Report
A woman in her 40s with type 2 diabetes mellitus presented for scheduled cerebral revascularization for moyamoya disease. Home medications included atorvastatin, levothyroxine, metformin, pioglitazone, and empagliflozin. Treatment with antihyperglycemics was withheld on admission. Blood glucose levels during the early hospitalization period were managed using insulin lispro. Her immediate perioperative course was favorable. She was extubated and transferred to the intensive care unit for monitoring.
A few hours postoperatively, the patient developed slurred speech. Magnetic resonance imaging of the brain revealed an acute left anterior cerebral infarct. Isotonic crystalloid fluids, phenylephrine, and fludrocortisone were administered to sustain perfusion pressure goals. During the next 24 hours, she developed progressive metabolic acidosis, with an arterial blood gas showing a nadir pH of 7.01, with an associated Pco2 level of 11 mm Hg (Table 1). The anion gap increased to a peak level of 27. A serum lactate level was normal. A bicarbonate drip was started to correct severe acidemia. During the first 48 hours postoperatively, nutritional support had not yet been started due to concerns for dysphagia and aspiration risk. Nephrology was consulted for evaluation and management of acidosis.
Table 1.
Inpatient Laboratory Values
| Admission | POD0 | POD1 | POD2 | |
|---|---|---|---|---|
| Hematocrit, % | 30.9 | 31.5 | 30.4 | |
| Sodium, mmol/L | 139 | 140 | 138 | 136 |
| Potassium, mmol/L | 4.0 | 4.0 | 3.8 | 4.7 |
| Chloride, mmol/L | 102 | 111 | 107 | 112 |
| Serum CO2, mmol/L | 27 | 14 | <5 | |
| Urea nitrogen, mg/dL | 18 | 8 | 17 | |
| Creatinine, mg/dL | 0.57 | 0.42 | 0.48 | |
| Calcium, mg/dL | 9.1 | 8.1 | 8.2 | |
| Albumin, g/dL | 4.4 | |||
| Glucose, mg/dL | 146 | 133-164 | 149 | 160-167 |
| Anion gap | 10 | 17 | 27 | |
| pH | 7.36 | 7.01 | 7.30 | |
| Pco2, mm Hg | 38.2 | 11.5 | 12.3 | |
| Lactate, mmol/L | 1.0 | |||
| β-hydroxybutyrate, mmol | 7.7 | 3.5 |
Note: Conversion factors for units: creatinine in mg/dL to μmol/L, ×88.4; calcium in mg/dL to mmol/L, ×0.2495; lactate in mmol/L to mg/dL, ×9.01; glucose in mg/dL to mmol/L, ×0.05551; urea nitrogen in mg/dL to mmol/L, ×0.357.
Abbreviations: CO2, carbon dioxide; POD, postoperative day.
Because of near-normal blood glucose concentrations, testing for diabetic ketoacidosis had not initially been performed. Based on chart review, the last dose of empagliflozin was taken at least 18 hours before surgery. Because the patient had been using empagliflozin until the recent preoperative period, we initiated screening for diabetic ketoacidosis. A urinalysis revealed glucose (2+) and ketones (4+). A β-Hydroxybutyrate level was elevated at 7.7 (reference range, 0.2-2.8) mmol/L. A diagnosis of euglycemic diabetic ketoacidosis, presumably related to SGLT2 inhibitor therapy, was made. The patient was started on treatment with dextrose and insulin drips, leading to closure of the anion gap acidosis. Unfortunately, due to the severity of brain injury resulting from her stroke, the patient’s clinical condition worsened. Comfort measures were instituted, after which the patient died.
Discussion
Whereas diabetic ketoacidosis is defined by the triad of hyperglycemia, ketosis, and anion gap metabolic acidosis, current definitions of euglycemic diabetic ketoacidosis include blood glucose level < 250 mg/dL.1 Munro et al2 first described euglycemic diabetic ketoacidosis in 1973. In 211 episodes of diabetic ketoacidosis, 37 episodes in 17 patients presented with a blood glucose level < 300 mg/dL.
Reported rates of euglycemic diabetic ketoacidosis during clinical trials have been rare: 0.52, 0.76, and 0.24 per 1,000 patient-years for canagliflozin, 100 mg and 300 mg, and non-SGLT2 inhibitor therapy, respectively; and 0.2 to 0.6 per 1,000 patient-years for empagliflozin, 10 mg and 25 mg, respectively.1 In March 2015, the US Food and Drug Administration (FDA) issued a warning for SGLT2 inhibitor–associated diabetic ketoacidosis, after 20 cases had been reported.3 In December 2015, the FDA released another statement regarding 73 cases of diabetic ketoacidosis requiring hospitalization or emergency department presentation. The median time to diabetic ketoacidosis after initiation of SGLT2 inhibitor therapy was 2 weeks (ranging from 1-175 days), and 50% of cases were associated with precipitating events, including acute illness (eg, infection and surgery), reduced oral intake, and reduced insulin dose.4 Fralick et al5 reported that patients prescribed SGLT2 inhibitors were twice as likely to develop diabetic ketoacidosis compared with matched patients receiving prescriptions for dipeptidyl peptidase-4 inhibitors, within 180 days of follow-up. Retrospective analyses of patients who developed diabetic ketoacidosis while taking an SGLT2 inhibitor revealed circumstances including off-label use in patients with type 1 diabetes mellitus, missed diagnoses of type 1 diabetes mellitus, or latent autoimmune diabetes of adulthood.6,7
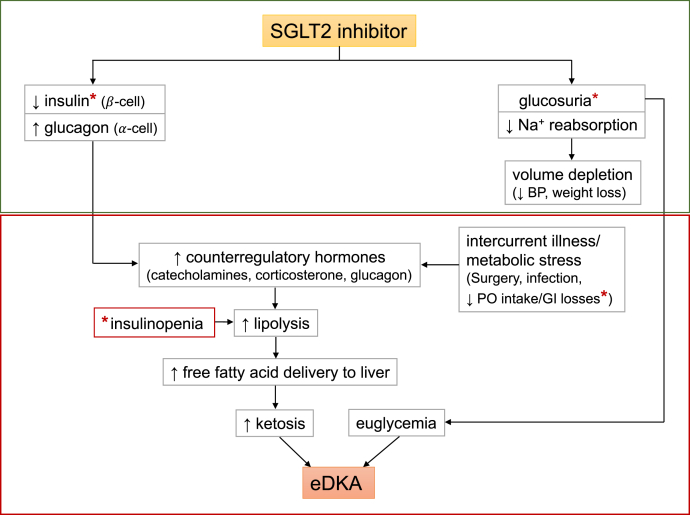
Diabetic ketoacidosis occurs in the setting of insulinopenia, either relative (eg, acute illness and reduced oral intake) or absolute. This leads to reduced glucose use, increased lipolysis, and increased free fatty acid transport to the liver. Glucagon levels increase, leading to free fatty acid oxidation and ketosis.8,9 The proposed mechanism of SGLT2 inhibitor–associated euglycemic diabetic ketoacidosis implicates glucosuria leading to decreased plasma glucose levels and decreased insulin release (Fig 1). Carbohydrate deficit, insulinopenia, and increased glucagon release lead to upregulation of lipolysis and ketogenesis. Decreased carbohydrate intake and/or deficit related to glucosuria contribute to normoglycemia in these patients.10 In diabetic as well as healthy rats administered an SGLT2 inhibitor, after a volume-depleting stress, euglycemic diabetic ketoacidosis occurs in the setting of relative insulinopenia and increased plasma catecholamine and corticosterone levels.11
Figure 1.
Proposed role of sodium-glucose cotransporter 2 (SGLT2) inhibition in euglycemic diabetic ketoacidosis (eDKA). Classic DKA results from insulin deficiency (absolute or relative) and concurrent increase in counter-regulatory hormones leading to ketosis, hyperglycemia, and osmotic diuresis. In contrast, SGLT2 inhibitor therapy in a well-compensated individual at baseline causes glucosuria, mild volume depletion, and lower serum glucose levels, associated with decreased insulin secretion (green box). During times of intercurrent illness and/or metabolic stress (eg, surgery or gastrointestinal illness), decreased carbohydrate intake coupled with lower serum glucose levels can further depress insulin secretion. This can ultimately lead to eDKA (red box). ∗Possible pathways of carbohydrate deficiency and causes of insulinopenia. Abbreviations: BP, blood pressure; PO, oral.
Current data for cardiovascular and kidney outcomes of SGLT2 inhibitor therapy suggest that long-term treatment benefits outweigh short-term risks. The American Diabetes Association recommends SGLT2 inhibitors as 1 of 6 preferred medications in the treatment of type 2 diabetes mellitus, as an adjunct to metformin, or as monotherapy in patients who cannot tolerate metformin.12,13 However, patients and providers need to be aware of treatment risks and strategies to mitigate these risks.
Providers should advise patients about circumstances in which SGLT2 inhibitor treatment should be withheld, such as with anticipated procedures/surgeries, or at the time of acute illness, in which there is decreased oral intake. Hospitalized patients are often more prone to developing risk factors for diabetic ketoacidosis, whether related to surgery, infection, volume depletion, or decreased oral intake, and inpatient providers should remain vigilant for potential evidence of the development of euglycemic diabetic ketoacidosis. In the outpatient setting, clinical scenarios associated with deceased oral intake and volume depletion should prompt providers to advise their patients to temporarily discontinue SGLT2 inhibitor therapy and monitor for ketosis by using available home testing kits (urine test strips or blood ketone meters).
A systematic review reveals multiple case reports of euglycemic diabetic ketoacidosis in the endocrinology, critical care, anesthesia, and surgical literatures. To date, only a few cases of euglycemic diabetic ketoacidosis have been described in the nephrology literature.14, 15, 16 With the advent of CREDENCE (Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation) results, it is anticipated that SGLT2 inhibitors will be more frequently prescribed for patients with type 2 diabetes mellitus who are at risk for progressive nephropathy.17 Moreover, it is likely that nephrologists may increasingly become the primary prescribers of this class of medications due to their renoprotective effects. Depending on results of the DAPA-CKD (Dapagliflozin and Prevention of Adverse Outcomes in Chronic Kidney Disease) study (NCT03036150), which has included enrollment of patients with nondiabetic proteinuric chronic kidney disease, prescription of SGLT2 inhibitors may fall squarely into the hands of nephrologists, although DAPA-CKD excluded from enrollment patients receiving active immunosuppressive therapy, who represent a considerable number of our patients with nondiabetic proteinuric chronic kidney disease. Whether nondiabetic patients receiving SGLT2 inhibitor therapy will be at risk for the development of euglycemic diabetic ketoacidosis is unknown. In the cited animal model by Perry et al,11 even healthy nondiabetic rats, when administered an SGLT2 inhibitor and exposed to a volume-depleting stress, developed euglycemic diabetic ketoacidosis.
Questions remain regarding indications and timing for withholding of SGLT2 inhibitor treatment. Endocrinology clinical practice guidelines recommend stopping treatment with SGLT2 inhibitors at least 24 hours before elective surgery, invasive procedures, and anticipated severe stressful physical activity and/or metabolically challenging activities, such as marathon running.18 However, case reports suggest that the pharmacologic effects of SGLT2 inhibitors persist beyond 5 half-lives of elimination (2-3 days), with glucosuria and ketonemia lasting as long as 9 to 10 days after discontinuation.16,19 In our patient, repeated urinalyses showed persistent glucose (3+) 4 days after the last dose of empagliflozin had been taken. Hence, the optimal timing of discontinuation of SGLT2 inhibitor treatment is unknown, but temporary discontinuation for a period longer than would be suggested by consideration of the half-life of elimination alone is probably warranted.
We describe a case of a woman with type 2 diabetes mellitus maintained on a regimen including empagliflozin who developed severe high-anion-gap euglycemic diabetic ketoacidosis following moyamoya revascularization surgery. Identification of diabetic ketoacidosis and institution of treatment were delayed due to the patient being euglycemic at the time of becoming acidemic, thereby distracting clinicians from initial consideration of diabetic ketoacidosis physiology.
Due to the favorable cardiorenal protective effects of SGLT2 inhibitors, their use is expected to grow significantly. Appropriate counseling and oversight of patients at risk for euglycemic diabetic ketoacidosis due to intercurrent volume-depleting illnesses with diminished oral intake, infection, surgery, or other metabolic stressors will be essential to the safe use of these medications. For hospitalized patients prescribed SGLT2 inhibitors who develop high-anion-gap acidosis, despite euglycemic status, clinicians should maintain a high index of suspicion for euglycemic diabetic ketoacidosis so that appropriate therapy can be provided in a timely manner.
Article Information
Authors’ Full Names and Academic Degrees
Katherine M. Wang, MD, and Robert T. Isom, MD.
Support
None.
Financial Disclosure
The authors declare that they have no relevant financial interests.
Patient Consent
The authors declare that they have obtained consent from the patients discussed in the report.
Peer Review
Received July 24, 2019. Evaluated by 1 external peer reviewer, with direct editorial input from an Associate Editor and the Editor-in-Chief. Accepted in revised form June 26, 2019.
Footnotes
Complete author and article information provided before references.
References
- 1.Barski L., Eshkoli T., Brandstaetter E., Jotkowitz A. Euglycemic diabetic ketoacidosis. Eur J Intern Med. 2019;63:9–14. doi: 10.1016/j.ejim.2019.03.014. [DOI] [PubMed] [Google Scholar]
- 2.Munro J.F., Campbell I.W., McCuish A.C., Duncan L.J.P. Euglycemic diabetic ketoacidosis. Br Med J. 1973;2(5866):578–580. doi: 10.1136/bmj.2.5866.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.FDA FDA warns that SGLT2 inhibitors for diabetes may result in a serious condition of too much acid in the blood. 2015. https://wayback.archive-it.org/7993/20170112031553/http://www.fda.gov/Drugs/DrugSafety/ucm446845.htm Accessed October 28, 2019.
- 4.FDA FDA Drug Safety Communication: FDA revises labels of SGLT2 inhibitors for diabetes to include warnings about too much acid in the blood and serious urinary tract infections. 2015. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-revises-labels-sglt2-inhibitors-diabetes-include-warnings-about Accessed October 28, 2019.
- 5.Fralick M., Schneeweiss S., Patorno E. Risk of diabetic ketoacidosis after initiation of an SGLT2 inhibitor. N Engl J Med. 2017;376(23):2300–2302. doi: 10.1056/NEJMc1701990. [DOI] [PubMed] [Google Scholar]
- 6.Meyer E.J., Gabb G., Jesudason D. SGLT2 inhibitor-associated euglycemic diabetic ketoacidosis: a South Australian clinical case series and Australian spontaneous adverse event notifications. Diabetes Care. 2018;41(4):E47–E49. doi: 10.2337/dc17-1721. [DOI] [PubMed] [Google Scholar]
- 7.Dashora U., Gallagher A., Dhatariya K., Winocour P., Gregory R., Abcd C. Association of British Clinical Diabetologists (ABCD) position statement on the risk of diabetic ketoacidosis associated with the use of sodium-glucose cotransporter-2 inhibitors. Br J Diabetes. 2016;16(4) 206-210. [Google Scholar]
- 8.Fayfman M., Pasquel F.J., Umpierrez G.E. Management of hyperglycemic crises diabetic ketoacidosis and hyperglycemic hyperosmolar state. Med Clin North Am. 2017;101(3) doi: 10.1016/j.mcna.2016.12.011. 587-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Felig P. Current concepts - diabetic ketoacidosis. N Engl J Med. 1974;290(24):1360–1363. doi: 10.1056/NEJM197406132902405. [DOI] [PubMed] [Google Scholar]
- 10.Milder D.A., Milder T.Y., Kam P.C.A. Sodium-glucose co-transporter type-2 inhibitors: pharmacology and peri-operative considerations. Anaesthesia. 2018;73(8):1008–1018. doi: 10.1111/anae.14251. [DOI] [PubMed] [Google Scholar]
- 11.Perry R.J., Rabin-Court A., Song J.D. Dehydration and insulinopenia are necessary and sufficient for euglycemic ketoacidosis in SGLT2 inhibitor-treated rats. Nat Commun. 2019;10:548. doi: 10.1038/s41467-019-08466-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donnan K., Segar L. SGLT2 inhibitors and metformin: dual antihyperglycemic therapy and the risk of metabolic acidosis in type 2 diabetes. Eur J Pharmacol. 2019;846:23–29. doi: 10.1016/j.ejphar.2019.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cefalu W.T., Berg E.G., Saraco M. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2019. Diabetes Care. 2019;42(suppl 1):S90–S102. doi: 10.2337/dc19-S009. [DOI] [PubMed] [Google Scholar]
- 14.Chan M.J., Weng C.H., Hsu C.W., Huang W.H., Yen T.H. Dapagliflozin associated ketoacidosis: a must know fact for nephrologists. Nephrology. 2018;23(2):192. doi: 10.1111/nep.13039. [DOI] [PubMed] [Google Scholar]
- 15.D'Elia J.A., Segal A.R., Bayliss G.P., Weinrauch L.A. Sodium-glucose cotransporter-2 inhibition and acidosis in patients with type 2 diabetes: a review of US FDA data and possible conclusions. Int J Nephrol Renovasc Dis. 2017;10:153–158. doi: 10.2147/IJNRD.S135899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kohli J., Goldfarb S. Metabolic acidosis in a patient with type 2 diabetes. Am J Kidney Dis. 2017;69(6):XI–XIII. doi: 10.1053/j.ajkd.2017.02.369. [DOI] [PubMed] [Google Scholar]
- 17.Perkovic V., J M., Neal B. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380:2295–2306. doi: 10.1056/NEJMoa1811744. [DOI] [PubMed] [Google Scholar]
- 18.Handelsman Y., Henry R.R., Bloomgarden Z.T. American Association of Clinical Endocrinologists and American College of Endocrinology position statement on the association of SGLT-2 inhibitors and diabetic ketoacidosis. Endocr Pract. 2016;22(6):753–762. doi: 10.4158/EP161292.PS. [DOI] [PubMed] [Google Scholar]
- 19.Pujara S., Ioachimescu A. Prolonged ketosis in a patient with euglycemic diabetic ketoacidosis secondary to dapagliflozin. J Invest Med High Impact Case Rep. 2017;5(2) doi: 10.1177/2324709617710040. [DOI] [PMC free article] [PubMed] [Google Scholar]