Abstract
A patient with renal glucosuria due to a congenital knock-out of the sodium-glucose cotransporter 2 (SGLT-2) protein because of a compound heterozygous mutation in the SLC5A2 gene may provide a natural model mimicking the effects of long-term SGLT-2 inhibitor therapy, which has been shown to exert kidney-protective effects beyond its antidiabetic properties. One possible mechanism for the protective effects of SGLT-2 inhibitor therapy might be the activation of tubuloglomerular feedback by increased outflow of sodium, chloride, and glucose to distal parts of the nephron, including the macula densa. Subsequently, afferent arteriolar vasoconstriction is induced and blood flow, intraglomerular filtration pressure, and glomerular filtration rate (GFR) all decline. However, prolonged tubuloglomerular feedback activation could change the sensitivity of tubuloglomerular feedback and hence decrease the beneficial effects of SGLT-2 inhibition on kidney function. Tubuloglomerular feedback is mediated by the Na+/K+/2Cl− cotransporter. Hence furosemide, which blocks this cotransporter, is a medical option to test tubuloglomerular feedback because GFR should increase after administration of this loop diuretic. In our patient with long-term activated tubuloglomerular feedback due to SGLT-2 mutations, we show that the sensitivity of tubuloglomerular feedback is maintained, demonstrated by an increase in GFR measured using iohexol clearance following furosemide administration. This observation supports the idea that long-term SGLT-2 inhibitor therapy is kidney protective through a functional tubuloglomerular feedback.
Index Words: SGLT-2 Inhibitor, glomerular filtration rate, tubuloglomerular feedback, kidney protection, iohexol clearance
Introduction
Recent trials suggest that some glucose-lowering agents, such as sodium-glucose cotransporter 2 (SGLT-2) inhibitors or glucagon-like peptide 1 agonists, exert protective effects on the kidney beyond their glycated hemoglobin–lowering capacity.1,2 SGLT-2 inhibitors reduce intraglomerular filtration pressure by activating tubuloglomerular feedback as they increase sodium and chloride delivery to macula densa cells. However, prolonged activation might change the sensitivity of tubuloglomerular feedback,3 which could potentially decrease the beneficial effects of SGLT-2 inhibitors on kidney function over the long term. To improve our understanding of these complex interactions, we describe the effects of intravenous furosemide, a loop diuretic that interferes with sodium and chloride uptake of macula densa cells and thus tubuloglomerular feedback activity, on various aspects of glomerular and tubular function in an older woman with renal glucosuria due to a genetic mutation in the SGLT-2 transporter.
Case Report
A 75-year-old woman had diabetes mellitus diagnosed in her late adolescence. Serum glucose, oral glucose loading tests, and glycated hemoglobin values were always in the normal range and therefore she never received antidiabetic therapies. Genetic screening for familial renal glucosuria finally revealed a compound heterozygous mutation in the SLC5A2 gene encoding for SGLT-2 (c.506delC frameshift and c.1021+1G>A exon skipping; the former, leading to a frameshift and premature stop after 17 amino acids, is listed as pathogenic in the Human Gene Mutation Database, whereas the latter presumably leads to exon 8 skipping. This mutation is novel and a pathogenic effect is very likely). Besides mild arterial hypertension, hypothyroidism, and chronic venous insufficiency in both lower extremities, she was in good health. Echocardiography revealed a normal left ventricular ejection fraction without relevant pathologic findings. Kidney function was normal (serum creatinine, 0.85 mg/dL, and estimated glomerular filtration rate [GFR], 67 mL/min/1.73 m2), as was glycated hemoglobin level (5.5%) and other laboratory parameters. Medications consisted of nebivolol, 5 mg, daily; levothyroxine, 50 μg, daily; and furosemide, 40 mg, daily.
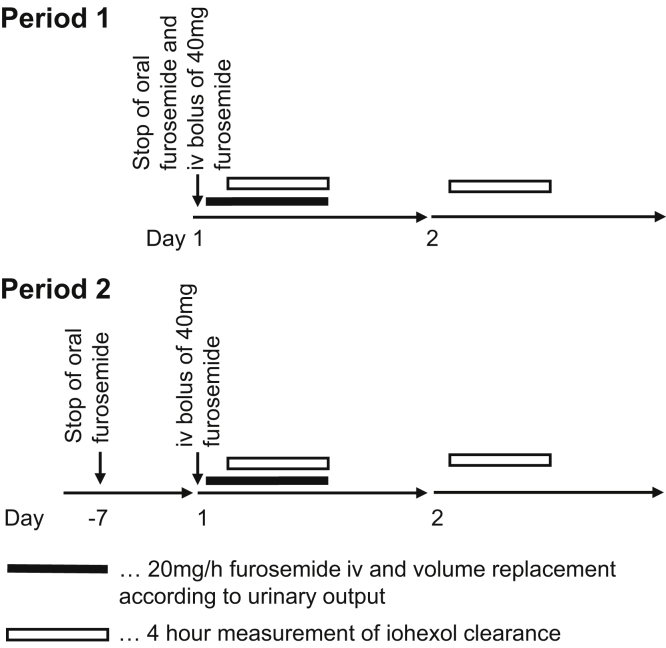
To assess the impact of acute intravenous loop diuretic treatment in the setting of renal glucosuria, the patient underwent repeated iohexol clearance studies on 2 occasions 15 weeks apart (Fig 1). Long-term oral furosemide therapy was discontinued on the first day of the first assessment (period 1) and was withheld for 7 days before the second testing (period 2). On the experimental days, intravenous furosemide was started 15 minutes before the 4-hour iohexol clearance measurements. Other laboratory parameters were determined during period 2 only.
Figure 1.
Scheme of iohexol clearance studies with and without furosemide. Abbreviation: iv, intravenous.
On the first morning of periods 1 and 2, an intravenous bolus of 40 mg of furosemide was administered, followed by a constant infusion of 20 mg/h after urinary bladder catheterization. On the following day, a second 4-hour measurement of iohexol clearance but without administration of the diuretic was performed. During period 2, we also measured serum creatinine at the beginning and end of each of the 2 iohexol clearance experiments with and without furosemide and 4-hour urine was collected to measure the loop diuretic effect on urine osmolality and electrolyte, glucose, creatinine, and urea excretion. On each study day, urinary volume loss was replaced by an intravenous infusion balanced solution (Elo-Mel Isoton, Fresenius Kabi Austria Ldt) to keep intravascular volume constant. As a result, blood pressure and body weight were stable during the experiments. Iohexol was administered as an intravenous bolus and clearance was calculated from plasma disappearance using a 2-compartment model, as proposed by Stevens and Levey,4 with measurement of plasma iohexol using high-performance liquid chromatography with tandem mass spectrometry, as described previously.5
Iohexol clearance was higher during furosemide infusion when compared with sessions without furosemide infusion on both occasions. During period 2, serum creatinine levels increased slightly during both furosemide administration (0.81 to 0.96 mg/dL) and on the nonfurosemide day (0.87 to 0.93 mg/dL). Interestingly, urinary creatinine excretion decreased significantly during furosemide administration (Table 1), as did consequently creatinine clearance (96 to 72 mL/min). Furosemide increased urinary volume from 800 to 5,100 mL. During the control experiment on day 2, the patient lost 10.9 g of glucose in urine, similar to the 10.8 g lost the prior day during furosemide administration. Urinary urea excretion also remained stable (5.5 vs 6.1 g). On the contrary, as anticipated, urinary sodium, potassium, chloride, calcium, and magnesium excretion were significantly higher during loop diuretic administration (Table 1).
Table 1.
Body Weight, Blood Pressure, Kidney Function, and Solute Excretion During the Experimental Periods
| Period 1 Furosemide | Period 1 Control | Period 2 Furosemide | Period 2 Control | |
|---|---|---|---|---|
| Weight, kg | 80.8 | 81.3 | 82.5 | 81.6 |
| Blood pressure, mm Hg | 142/85 | 154/85 | 156/85 | 160/90 |
| Iohexol clearance, mL/min/1.73 m2 | 95 | 80 | 83 | 72 |
| 4-h urinary output, mL | NA | NA | 800 | 5,100 |
| Glucose, mg | NA | NA | 10,944 | 10,812 |
| Urine osmolality, mOsmol | NA | NA | 297 | 315 |
| Urea, g | NA | NA | 5.51 | 6.12 |
| Sodium, mmol | NA | NA | 648 | 31 |
| Potassium, mmol | NA | NA | 56 | 8 |
| Chloride, mmol | NA | NA | 638 | 16 |
| Calcium, mmol | NA | NA | 8.57 | 1.04 |
| Magnesium, mmol | NA | NA | 4.74 | 1.01 |
| Creatinine, mg | NA | NA | 158 | 208 |
Abbreviation: NA, not assessed.
Discussion
In this patient with congenital renal glucosuria, a natural model mimicking the effects of long-term SGLT-2 inhibitor therapy, tubuloglomerular feedback remained functional, supporting the concept that long-term SGLT-2 inhibitor therapy is kidney protective through a functional tubuloglomerular feedback.
The genetic defect of the SGLT-2 transporter, which is located in the proximal tubule, results in increased outflow of sodium, chloride, and glucose to the more distal parts of the nephron, including the macula densa. Sodium and chloride delivery to this structure activates tubuloglomerular feedback, inducing afferent arteriolar vasoconstriction, thereby reducing blood flow, intraglomerular filtration pressure, and GFR. Tubuloglomerular feedback sensing is mediated by the Na+/K+/2Cl− cotransporter and blocking this transporter by loop diuretics therefore should result in afferent glomerular vasodilation and an increase in GFR, exactly as observed in our study.
The effects of acute and long-term pharmacologic blockade of the SGLT-2 receptor by dapagliflozin on glomerular and tubular function was studied by Thomson et al3 in a streptozotocin-induced diabetes model. Dapagliflozin acutely increased distal chloride delivery, activated tubuloglomerular feedback, and reduced GFR. Prolonged drug therapy for 12 days did not alter proximal tubular electrolyte outflow when compared to acute administration. Nonetheless, the effects on tubuloglomerular feedback and GFR were ameliorated to some extent, eventually on the basis of a compensatory increase of electrolyte reabsorption in the loop of Henle proximal to the macula densa. As a result, effects on hyperfiltration were maximal in the setting of acute SGLT-2 blockade but partially attenuated during the prolonged phase. At least in this animal experiment it was not necessary to postulate a desensitization of tubuloglomerular feedback through prolonged activation to explain the somewhat reduced effect of long-term SGLT-2 loss of function on GFR because long-term sodium and chloride exposure was attenuated.
SGLT-2 inhibitors improve both cardiovascular and kidney outcomes in individuals with diabetes.1 In the kidney, it has been postulated that these agents lower intraglomerular pressure, a major driver of kidney disease progression, by inducing vasoconstriction of the afferent glomerular arteriole through activation of tubuloglomerular feedback. Accordingly, it is hypothesized that the protective effect of these drugs on the kidney might diminish over time if either resetting of tubuloglomerular feedback or compensatory increased electrolyte reabsorption proximal to the macula densa occurs. However, in our patient, we demonstrated that even after life-long SGLT-2 loss of function, some degree of tubuloglomerular feedback activation is still present because blocking the entry of sodium and chloride into macula densa cells by furosemide was associated with an increase in GFR as assessed using iohexol clearance. In general, these results are compatible with the results of Thomson et al3 in animal models.
Unfortunately, we cannot conclude in our patient whether tubuloglomerular feedback is completely or only partially maintained. However, in the EMPA-REG OUTCOME trial,6 SGLT-2 inhibition by empagliflozin resulted in an early decrease in estimated GFR by ∼5 mL/min/1.73 m2, a magnitude very similar to that observed in our patient when we reversed the SGLT-2 inhibition effect by furosemide. One therefore could argue that tubuloglomerular feedback activity is maintained even in long-term SGLT-2 blockade, which should preserve long-term protection of the kidney. It is important to note that our results were obtained in an older adult because it could be speculated that aging negatively affects the tubuloglomerular feedback response by hyperfiltration in remnant nephrons as nephron numbers gradually decrease.
Of note, baseline iohexol clearance without furosemide differed between period 1 and period 2 (80 vs 72 mL/min/1.73 m2). However, this variation of ∼10% is within the published intraindividual variation for GFR in patients with chronic kidney disease7 and pediatric kidney transplant recipients8 and distinctly lower than the effect of furosemide in our setting.
In our study, the acute effects of furosemide in a human SGLT-2 knockout on GFR as measured using the gold-standard method of iohexol clearance could not be replicated by measuring creatinine clearance. Urinary creatinine excretion during administration of the loop diuretic decreased by almost 30%, thus massively influencing the validity of creatinine clearance as a measure of GFR. We cannot completely explain this phenomenon, although it has been shown that urinary flow rate influences creatinine secretion.9
In conclusion, in the setting of renal glucosuria, tubuloglomerular feedback activity is maintained. Given the kidney and cardiovascular protective effects of SGLT-2 inhibitors in the setting of diabetes, further work is required to better understand the relationship between the dose and route of administration of furosemide and the impact of pharmacologic SGLT-2 inhibition, especially in patients with heart failure.
Article Information
Authors’ Full Names and Academic Degrees
Hannes Neuwirt, MD, Andrea Burtscher, MD, David Cherney, MD, Gert Mayer, MD, and Christoph Ebenbichler, MD.
Support
None.
Financial Disclosure
Dr Cherney has received consulting fees or speaking honorarium or both from Janssen, Boehringer Ingelheim, Eli-Lilly, AstraZeneca, Merck, Sanofi, Mitsubishi-Tanabe, and Prometic and operating funds from Janssen, Boehringer Ingelheim-Eli, Lilly, AstraZeneca, and Merck. Dr Mayer has received consulting fees or speaking honorarium or both from Jansen, Boehringer Ingelheim, Eli-Lily, and Astra Zeneca. The remaining authors declare that they have no relevant financial interests.
Patient Consent
The authors declare that they have obtained consent from the patient discussed in the report.
Peer Review
Received July 17, 2019. Evaluated by 2 external peer reviewers, with direct editorial input from the Editor-in-Chief. Accepted in revised form September 19, 2019.
Footnotes
Complete author and article information provided before references.
References
- 1.Zelniker T.A., Wiviott S.D., Raz I. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393(10166):31–39. doi: 10.1016/S0140-6736(18)32590-X. [DOI] [PubMed] [Google Scholar]
- 2.Mann J.F.E., Orsted D.D., Brown-Frandsen K. Liraglutide and renal outcomes in type 2 diabetes. N Engl J Med. 2017;377(9):839–848. doi: 10.1056/NEJMoa1616011. [DOI] [PubMed] [Google Scholar]
- 3.Thomson S.C., Rieg T., Miracle C. Acute and chronic effects of SGLT2 blockade on glomerular and tubular function in the early diabetic rat. Am J Physiol Regul Integr Comp Physiol. 2012;302(1):R75–R83. doi: 10.1152/ajpregu.00357.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stevens L.A., Levey A.S. Measured GFR as a confirmatory test for estimated GFR. J Am Soc Nephrol. 2009;20(11):2305–2313. doi: 10.1681/ASN.2009020171. [DOI] [PubMed] [Google Scholar]
- 5.Lee S.Y., Chun M.R., Kim D.J., Kim J.W. Determination of iohexol clearance by high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) J Chromatogr B Analyt Technol Biomed Life Sci. 2006;839(1-2):124–129. doi: 10.1016/j.jchromb.2006.03.057. [DOI] [PubMed] [Google Scholar]
- 6.Mayer G.J., Wanner C., Weir M.R. Analysis from the EMPA-REG OUTCOME((R)) trial indicates empagliflozin may assist in preventing the progression of chronic kidney disease in patients with type 2 diabetes irrespective of medications that alter intrarenal hemodynamics. Kidney Int. 2019;96(2):489–504. doi: 10.1016/j.kint.2019.02.033. [DOI] [PubMed] [Google Scholar]
- 7.Waikar S.S., Rebholz C.M., Zheng Z. Biological variability of estimated GFR and albuminuria in CKD. Am J Kidney Dis. 2018;72(4):538–546. doi: 10.1053/j.ajkd.2018.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsampalieros A., Lepage N., Feber J. Intraindividual variability of the modified Schwartz and novel CKiD GFR equations in pediatric renal transplant patients. Pediatr Transplant. 2011;15(7):760–765. doi: 10.1111/j.1399-3046.2011.01568.x. [DOI] [PubMed] [Google Scholar]
- 9.Andreasen F., Hansen U., Husted S.E., Mogensen C.E., Pedersen E.B. The influence of age on renal and extrarenal effects of frusemide. Br J Clin Pharmacol. 1984;18(1):65–74. doi: 10.1111/j.1365-2125.1984.tb05023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]