Abstract
Rationale & Objective
The number of glomeruli is often used to determine the adequacy of a kidney biopsy (eg, at least 10 glomeruli). It is often assumed that biopsy specimens with limited amounts of cortex are too imprecise for detection of focal pathology.
Study Design
Clinical-pathologic correlation (cross-sectional).
Setting & Participants
Living kidney donors who underwent a needle core biopsy of their kidney at the time of donation.
Exposure
The amount of cortex biopsied as determined by either the number of glomeruli or area of cortex on histology.
Outcome
The percentage of globally sclerotic glomeruli, density of interstitial fibrosis foci, and severity of arteriosclerosis were determined.
Analytical approach
A beta-binomial model assessed how the mean percentage of globally sclerotic glomeruli and patient variability in percentage of globally sclerotic glomeruli differed with the number of glomeruli on the biopsy specimen. Additional models assessed the association of interstitial fibrosis and arteriosclerosis with number of glomeruli.
Results
There were 2,915 kidney donors studied. Fewer glomeruli on the biopsy specimen associated with higher mean percentage of globally sclerotic glomeruli and higher patient variability in percentage of globally sclerotic glomeruli. Smaller cortical volume on imaging correlated with both less cortex on biopsy and higher percentage of globally sclerotic glomeruli. Based on a statistical simulation, the probability of patient percentage of globally sclerotic glomeruli ≥ 10% if the biopsy percentage of globally sclerotic glomeruli is ≥10% (positive predictive value) was 45% with 1 to 9 glomeruli versus 31% with 10 or more glomeruli; the negative predictive value was 91% versus 98%. Fewer glomeruli also associated with more interstitial fibrosis and arteriosclerosis.
Limitations
The study was limited to living kidney donors. Patient variability in percentage of globally sclerotic glomeruli was based on a statistical model because multiple biopsy specimens per patient were not available.
Conclusions
The amount of cortex on a needle core biopsy is not completely random. Chronic changes from loss of cortex contribute to low amounts of cortex on a kidney biopsy specimen.
Index Words: Renal pathology, renal morphometry, renal cortex, glomeruli, glomerulosclerosis, kidney biopsy
With a kidney biopsy, the intention is to obtain an adequate amount of cortex for diagnostic purposes without damaging the kidney. The use of needle core biopsies helps ensure minimal damage and allows for a less invasive percutaneous approach. However, small amounts of cortex are sometimes obtained with needle core biopsies due to the tissue specimen being too deep (sampling medulla) or too superficial (sampling perirenal fat). An assessment is often made as to whether enough cortex was sampled for meaningful interpretation of the biopsy findings. The adequacy of a kidney biopsy is often determined by the number of glomeruli (per section or overall) rather than area of cortex because the number of glomeruli is simpler to measure. However, there can be 7-fold variation in glomerular density on a biopsy section1 such that number of glomeruli is an imperfect surrogate for the amount of cortex. Further, pathologists tend to underreport the number of glomeruli on a biopsy specimen.2
The amount of cortex needed to have an adequate tissue biopsy is unclear. For truly diffuse diseases, any amount of cortex biopsied should be adequate for detecting the pathology. However, many kidney diseases have focal pathology, especially during the early stages of the disease. Thus, having an adequate amount of cortex biopsied to detect focal pathology is of significant concern. The Banff criteria for classifying biopsy findings in kidney transplant recipients required at least 7 glomeruli for a specimen to be “adequate” in 19933 and was updated to require 10 glomeruli in 1997,4 in part due to concerns for missing a potential rejection. Standardized criteria for chronic changes on biopsy, including arteriosclerosis, glomerulosclerosis, and interstitial fibrosis/tubular atrophy, were recently proposed for kidney biopsies, and 10 glomeruli were also required for the biopsy sample to be adequate.5 Although a repeat biopsy can be performed if the number of glomeruli is inadequate, this exposes the patient to the risks and costs of another invasive procedure. It is often assumed that the amount of cortex obtained from a needle core biopsy specimen is influenced by biopsy technique but is otherwise random. However, it is important to know whether there is a relationship between the amount of cortex biopsied and the presence of pathology on the biopsy specimen.
The objective of our study was to determine whether inferences regarding chronic changes can be made from biopsy specimens with small amounts of cortex. To do this, we accounted for: (1) the increase in sampling variability inherent with a small tissue biopsy and (2) a possible relationship between the amount of cortex on the biopsy specimen and underlying chronic changes. We performed this study using a large number of kidney biopsies from living kidney donors, in which mild chronic changes are generally attributed to nephrosclerosis.6, 7
Methods
Study Population
The Aging Kidney Anatomy study6 was performed under institutional review board (IRB #10-004644) approval with a waiver of informed consent (analyses only used data available from routine clinical care). Living kidney donors underwent a core-needle biopsy of the donated kidney cortex at the time of transplantation surgery at Mayo Clinic Minnesota, Mayo Clinic Arizona, or Cleveland Clinic Ohio between 2000 and 2015. These implantation biopsies are obtained as part of routine clinical care in transplantation programs that monitor the allograft with kidney biopsies at regular intervals.
All kidney donors underwent a thorough evaluation before donation with a prescheduled battery of tests. The inclusion and exclusion criteria for kidney donation vary by site and era but generally included 24-hour urine albumin excretion < 30 mg and measured glomerular filtration rate normal for age.8, 9 Mild hypertension in older donors and moderate obesity (body mass index of 30-35 kg/m2) were allowed. Patients with diabetes or cardiovascular disease were excluded from donation. Hypertension was defined as blood pressure > 140/90 mm Hg or use of hypertensive medication to lower blood pressure.
Amount of Cortex Biopsied
An intraoperative needle core biopsy of the renal cortex was performed at the time of transplantation surgery. The tissue specimen was fixed in formalin and embedded in paraffin. Unstained sections from the tissue block were sent to the Mayo Clinic in Minnesota from the other sites for staining. Two consecutive sections (2- to 3-μm thickness) from the biopsy core were stained, one with periodic acid–Schiff and one with Masson trichrome, and then scanned into high-resolution digital images (Aperio XT digital scanner; Leica Biosystems).
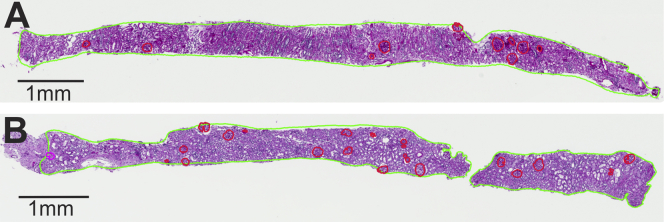
The amount of cortex on these biopsy sections was quantified using 2 different approaches: area of cortex and number of glomeruli. Area of cortex was delineated as the region of the tissue section that contained glomeruli. The number of glomeruli was counted as the total number of glomerular profiles (nonsclerotic and globally sclerotic glomeruli [GSGs]). Nonsclerotic glomeruli on the section edge (ie, those bisected by the biopsy needle) were counted as half a glomerulus.10 Figure 1 shows examples of how the amount of cortex can vary substantially when determined by area of cortex versus by number of glomeruli. The area of medulla and presence of capsule were further delineated on each biopsy image.
Figure 1.
An example of disagreement between number of glomeruli and area of cortex in quantifying the amount of cortex biopsied. Biopsy specimens with: (A) 10 glomerular profiles in 4 mm2 of cortex and (B) 20 glomerular profiles in 4 mm2 of cortex. Cortical area outlined in green, nonsclerotic glomeruli outlined in red, and a globally sclerotic glomerulus outlined in magenta.
Chronic Changes on the Biopsy Specimen
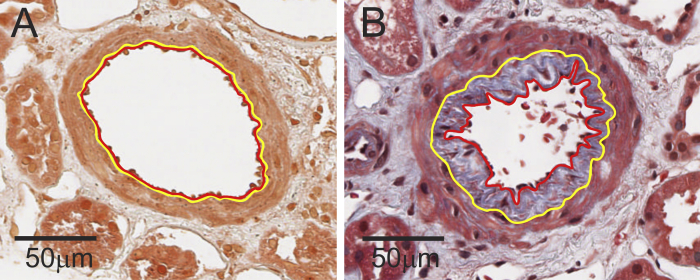
The chronic changes studied on the biopsy specimen were limited to those based on completely objective measurements.11 This included percentage of GSGs (%GSGs), interstitial fibrosis/tubular atrophy foci density, presence of any arteriosclerosis, and severity of arteriosclerosis. The %GSGs was calculated from the number of GSG profiles divided by the number of glomeruli profiles (averaged across both sections). The number of interstitial fibrosis/tubular atrophy foci in the cortex was counted from the number of distinct contiguous regions of interstitial fibrosis/tubular atrophy surrounded by normal parenchyma. The number of interstitial fibrosis/tubular atrophy foci in a given area of cortex also aligns well with the study goal of focal pathology detection, as described previously.6 The number of interstitial fibrosis/tubular atrophy foci was chosen because the percentage of interstitial fibrosis/tubular atrophy is minimal and difficult to meaningfully estimate by visual inspection in a healthy population (97% have <5% interstitial fibrosis/tubular atrophy).6 The number of interstitial fibrosis/tubular atrophy foci per area of cortex has been shown to correlate with older age, hypertension, reduced cortical volume, and kidney surface roughness.6 In biopsy specimens with an artery (most orthogonal artery used), we traced the luminal boundary and intimal media boundary in the Masson trichrome–stained image. The severity of arteriosclerosis was determined by the percentage of luminal stenosis (area of intima divided by the total area of intima and area of lumen), as previously reported (Fig 2).6, 10
Figure 2.
Arteriosclerosis was assessed by percentage of luminal stenosis of the most orthogonal artery if present: (A) 0% and (B) 54% luminal stenosis.
Potential Determinants of the Amount of Cortex Biopsied
Clinical, biopsy, and imaging characteristics were also evaluated for a possible association with the amount of cortex biopsied. Clinical characteristics studied were age and hypertension because these are risk factors for chronic changes in living kidney donors.6, 7 The biopsy characteristics studied were presence of capsule (reflecting a superficial biopsy) and medullary area (reflecting a deeper biopsy). The volume of cortex of the donated kidney was calculated from angiographic-phase computed tomography images obtained as part of the predonation evaluation and converted into 3-dimensional object maps.12 Kidney surface roughness was scored based on the involved proportion of the 3-dimensional object map of the donated kidney: 0, no roughness; 1, roughness up to 25%; 2, roughness of 26% to 50%; and 3, roughness >50%.6
Statistical Analysis
The relationship between %GSGs and amount of cortex biopsied was investigated using a statistical model based on the beta-binomial distribution. A linear regression model would fit the data poorly because the distribution of biopsy %GSGs is distinctly non-normal, with a mean that potentially has a nonlinear relationship with amount of cortex (due to being bounded by 0% and 100%) and a variance that strongly depends on the amount of cortex. A logistic regression model with number of GSGs as the outcome variable would address those problems but would assume that the probability for a glomerulus to be globally sclerotic is the same for all patients who have the same predictor values, when we expect the probability to vary from patient to patient. The beta-binomial model allows for such patient variability (Item S1).
The beta-binomial model describes the number of GSGs on biopsy as following a binomial distribution according to the individual patient’s probability for a glomerulus to be globally sclerotic, which we call “patient %GSGs,” and the number of glomeruli on biopsy. The model assumes that individual patient %GSGs follows a beta distribution with parameters for the mean (μ) and variability (φ). For our analysis, we allow both μ and φ to depend on measured variables (eg, number of glomeruli and patient age) using a vector generalized linear model.13 Standard statistical theory allows us to fit the model based on the observed counts of GSGs in the biopsy specimens in a manner that accounts for the greater sampling variability among biopsy specimens with fewer glomeruli. Both the mean and variability of the distribution are reported on a scale of 0% to 100%. By the nature of the beta-binomial model, the mean of the biopsy %GSGs is equal to μ, the mean of the patient %GSGs. The parameter φ accounts for variance between patients; it takes the value 0% if all patients have the same patient %GSG of μ and takes the value 100% if all patients have patient %GSGs equal to either 0% or 100%. To identify potential mechanisms linking %GSGs to amount of cortex biopsied, we assessed the Spearman rank correlation of clinical factors, imaging factors, and biopsy factors with amount of cortex.
Further analyses used our beta-binomial model to investigate how detection of long-term changes compares between biopsy specimens with fewer versus more glomeruli. Scoring systems have used biopsy %GSGs ≥ 10% to distinguish at least mild glomerulosclerosis from none or minimal.4, 5 Thus, we calculated the probability of patient %GSGs ≥ 10% if the biopsy %GSGs was ≥10% (positive predictive value), based on the fitted beta-binomial model (ie, based on a statistical simulation because the gold standard of sectioning the entire kidney is not possible). We also calculated the probability of patient %GSGs < 10% if the biopsy %GSGs were <10% (negative predictive value), the probability that biopsy %GSGs was ≥10% if the patient %GSGs was ≥10% (sensitivity), and the probability that biopsy %GSGs was <10% if the patient %GSGs was <10% (specificity; see Item S1).
The density of interstitial fibrosis/tubular atrophy foci and its dependence on the amount of cortex was analyzed using a generalized linear model based on the negative binomial distribution. The probability of having any arteriosclerosis (provided that an artery was present in the biopsy) and its dependence on the amount of cortex was analyzed using logistic regression. Severity of arteriosclerosis and its dependence on the amount of cortex were analyzed using linear regression.
Results
Study Sample
There were 2,915 kidney donors with implantation biopsies studied (1,917, Mayo Clinic Rochester; 578, Mayo Clinic Arizona; and 420, Cleveland Clinic Ohio). Clinical, imaging, and biopsy characteristics are shown in Table 1. Overall, only 35% of biopsies had at least 1 GSG. The mean and 95th percentile for biopsy %GSGs were 3.7% and 17.2%.
Table 1.
Clinical, Imaging, and Biopsy Characteristics of 2,915 Living Kidney Donors by %GSGs on Biopsy
| Characteristics by %GSG Group | 0%-<10% (n = 2,527) | 10%-<25% (n = 310) | ≥25% (n = 78) |
|---|---|---|---|
| Clinical | |||
| Age, y | 43 ± 12 | 50 ± 11 | 55 ± 11 |
| Male sex | 1,042 (41%) | 117 (38%) | 28 (36%) |
| Race | |||
| White | 2,140 (85%) | 261 (84%) | 66 (85%) |
| Black | 80 (3.2%) | 7 (2.3%) | 5 (6.4%) |
| Other | 79 (3.1%) | 10 (3.2%) | 0 (0.0%) |
| Unknown | 228 (9.1%) | 32 (10%) | 7 (9.0%) |
| Hypertension | 212 (8.0%) | 45 (15%) | 17 (22%) |
| Measured GFR | 105 ± 21 | 100 ± 21 | 100 ± 21 |
| CT of biopsied kidney | |||
| Cortical volume, cm3 | 103 ± 21 | 100 ± 22 | 97 ± 19 |
| Surface roughness score | 1.1 ± 1.1 | 1.4 ± 1.1 | 1.4 ± 1.1 |
| Biopsy | |||
| Cortical area, mm2 | 6.4 ± 3.3 | 5.5 ± 2.4 | 3.8 ± 2.1 |
| No. of glomeruli | 18 ± 11 | 15 ± 7.7 | 9.5 ± 7.5 |
| <10 glomeruli | 516 (20%) | 93 (30%) | 52 (67%) |
| Capsule present | 209 (8.3%) | 24 (7.7%) | 4 (5.1%) |
| Medulla area, mm2 | 0.79 ± 1.70 | 0.95 ± 2.10 | 1.38 ± 2.41 |
| IF/TA present | 552 (22%) | 153 (49%) | 49 (63%) |
| No. of IF/TA foci | 0.37 ± 0.90 | 0.97 ± 1.35 | 1.55 ± 1.86 |
| Artery present | 2,191 (87%) | 257 (83%) | 58 (74%) |
| Percentage luminal stenosis, % | 33% ± 21% | 39% ± 21% | 44% ± 21% |
Note: Values given as mean ± standard deviation or number (percent).
Abbreviations: CT, computed tomography; GFR, glomerular filtration rate; GSGs, globally sclerotic glomeruli; IF/TA, interstitial fibrosis/tubular atrophy.
Glomerulosclerosis Associated With Less Cortex Biopsied
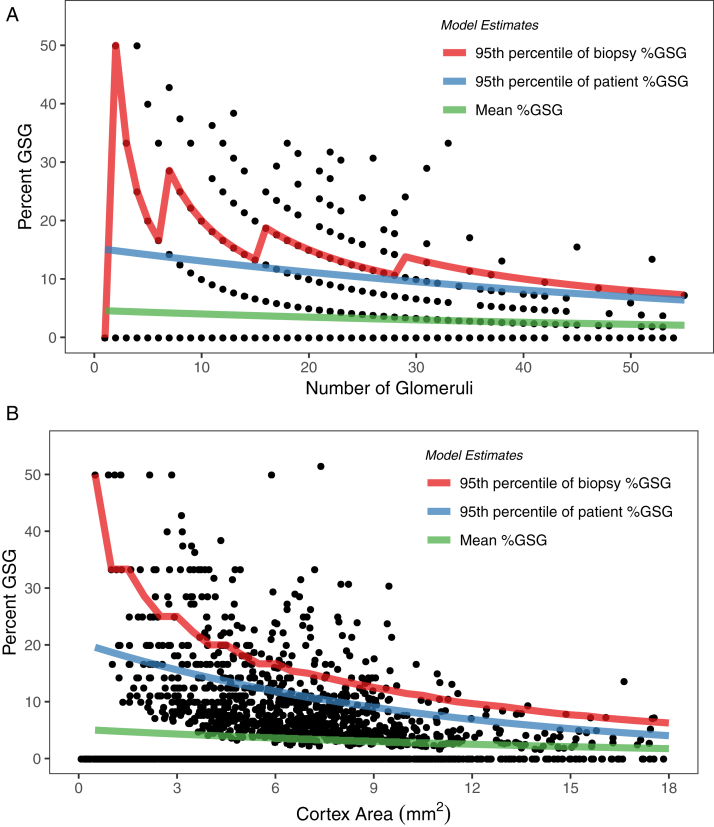
There was an increase in mean %GSGs with less cortex as determined either by number of glomeruli or area of cortex (Table 2). These associations remained significant in subgroup analysis requiring at least 10 glomeruli (or 4 mm2 or cortex) to be present and in analysis limited to biopsy specimens that were only cortex or to specimens that contained both cortex and medulla. An increase in variability of patient %GSGs was evident with less cortex by either number of glomeruli or area of cortex (Table 2). In subgroup analyses, the association between variability of patient %GSGs and area of cortex remained significant but was no longer significant between %GSGs and number of glomeruli. Table 3 and Figure 3 show that mean %GSGs and 95th percentiles for both biopsy %GSGs and patient %GSGs increased with less cortex on biopsy.
Table 2.
Estimation of Mean %GSG and Patient Variability in %GSGs by Amount of Cortex Biopsied (per SD)
| Mean %GSGs | P | Variability in Patient %GSGs | P | |
|---|---|---|---|---|
| By No. of Glomeruli | ||||
| Percentage change per 11 more glomeruli | ||||
| Overall (n = 2,915) | −15% (−20% to −10%) | <0.001 | −19% (−31% to −4.3%) | 0.01 |
| ≥10 glomeruli (n = 2,254) | −11% (−17% to −4.6%) | 0.001 | −12% (−27% to 4.3%) | 0.1 |
| Only cortex (n = 2,101) | −14% (−20% to −7.2%) | <0.001 | −14% (−29% to 4.3%) | 0.1 |
| Cortex and medulla (n = 814) | −17% (−26% to −6.1%) | 0.003 | −25% (−45% to 2.1%) | 0.07 |
| By Area of Cortex | ||||
| Percentage change per 3.2 mm2 more cortex area | ||||
| Overall (n = 2,915) | −18% (−23% to −13%) | <0.001 | −38% (−49% to −25%) | <0.001 |
| ≥ 4 mm2 cortex (n = 2,200) | −15% (−21% to −8.8%) | <0.001 | −38% (−51% to −21%) | <0.001 |
| Only cortex (n = 2,101) | −18% (−24% to −11%) | <0.001 | −37% (−51% to −20%) | <0.001 |
| Cortex and medulla (n = 814) | −17% (−26% to −8.1%) | <0.001 | −38% (−56% to −13%) | 0.006 |
Note: Values given as percentage (95% confidence interval).
Abbreviations: GSGs, globally sclerotic glomeruli; SD, standard deviation.
Table 3.
Mean, Patient Variability, and 95th Percentiles for %GSGs by Different Amounts of Cortex Biopsied
| Amount of Cortex Biopsied | n | Mean %GSGs | Variability in Patient %GSGs | Patient 95th Percentile | Biopsied 95th Percentile |
|---|---|---|---|---|---|
| No. of glomeruli | |||||
| 1-4 | 198 | 5.1% | 11.5% | 20.9% | 33.3% |
| 5-9 | 463 | 5.1% | 7.6% | 17.7% | 25.0% |
| 10-14 | 592 | 3.7% | 4.1% | 11.4% | 18.2% |
| 15-19 | 570 | 3.5% | 3.9% | 10.8% | 15.8% |
| 20-24 | 462 | 3.1% | 3.2% | 9.4% | 13.6% |
| 25-29 | 267 | 3.1% | 4.8% | 10.8% | 13.8% |
| ≥30 | 363 | 2.6% | 3.2% | 8.5% | 10.3% |
| Area of cortex, mm2 | |||||
| <2 | 199 | 5.6% | 11.5% | 22.1% | 37.5% |
| ≥2-<4 | 516 | 4.7% | 6.8% | 16.1% | 25.0% |
| ≥4-<6 | 766 | 3.6% | 4.7% | 11.8% | 16.7% |
| ≥6-<8 | 747 | 3.3% | 4.5% | 11.0% | 15.0% |
| ≥8-<10 | 420 | 3.2% | 3.4% | 9.7% | 13.3% |
| ≥10-<12 | 138 | 2.3% | 1.6% | 6.0% | 9.1% |
| ≥12 | 129 | 2.2% | 1.1% | 5.2% | 7.8% |
Note: P values for trends shown in Table 2.
Abbreviations: GSGs, globally sclerotic glomeruli; SD, standard deviation.
Figure 3.
Mean percentage of globally sclerotic glomeruli (%GSGs) and 95th percentiles for patient %GSGs and biopsy %GSGs by: (A) number of glomeruli (continuous) and (B) area of cortex (continuous). The mean and 95th percentiles for %GSGs increased with less cortex. Measurement error (as reflected in the difference between biopsy and patient 95th percentiles) also increased with less cortex.
Other Chronic Changes Associated With Amount of Cortex Biopsied
Similar to findings with %GSGs, the density of interstitial fibrosis/tubular atrophy, presence of arteriosclerosis (any luminal stenosis), and severity of arteriosclerosis (percentage of luminal stenosis) increased with less cortex biopsied (Table 4). The association with interstitial fibrosis/tubular atrophy was stronger with less area of cortex than with fewer number of glomeruli (similar to the finding seen with %GSGs). The presence of arteriosclerosis was more strongly associated with less cortex on biopsy, whereas severity of arteriosclerosis was more strongly associated with fewer glomeruli on biopsy.
Table 4.
Estimation of Density of IF/TA Foci, Presence of Arteriosclerosis (any luminal stenosis by intimal thickening), and Severity of Arteriosclerosis (percentage luminal stenosis) by Amount of Cortex Biopsied (per SD)
| Nephrosclerosis Finding | Per Increase SD in Amount of Cortex | P |
|---|---|---|
| By No. of Glomeruli | ||
| Change per 11 more glomeruli | ||
| IF/TA foci density | −12% (−18% to −4.5%) | 0.003 |
| Any luminal stenosis | 0.95 (0.85 to 1.06) | 0.3 |
| Percentage luminal stenosis | −2.4 (−3.1 to −1.6) | <0.001 |
| By Area of Cortex | ||
| Change per 3.2 mm2 more cortex | ||
| IF/TA foci density | −25% (−31% to −19%) | <0.001 |
| Any luminal stenosis | 0.85 (0.77 to 0.95) | 0.003 |
| Percentage luminal stenosis | −0.73 (−1.5 to −0.01) | 0.05 |
Note: Values given as percentage (95% CI) or odds ratio (95% CI).
Abbreviations: CI, confidence interval; IF/TA, interstitial fibrosis/tubular atrophy; SD, standard deviation.
Accuracy of Detecting Glomerulosclerosis From a Tissue Biopsy
Based on the statistical model, the probability of patient %GSGs ≥ 10% if the biopsy %GSGs was ≥10% (positive predictive value) in persons with 1 to 9 glomeruli was 45% compared to 31% in persons with 10 or more glomeruli. Likewise, when comparing biopsy %GSGs ≥ 10% with 1 to 9 glomeruli with those with 10 or more glomeruli, negative predictive value was 91% compared to 98%, sensitivity was 59% compared to 63%, and specificity was 86% compared to 92%, respectively.
Potential Pathways for Nephrosclerosis to Associate With Less Cortex on Biopsy
We assessed clinical, biopsy, and imaging characteristics for their correlation with biopsy %GSGs and area of cortex (Table 5). Older age and less cortical volume on computed tomography were characteristics that correlated with higher biopsy %GSGs and less area of cortex on biopsy. Other clinical and imaging factors (hypertension and kidney surface roughness) correlated with higher biopsy %GSGs but did not correlate with less area of cortex. Lower glomerular filtration rate correlated with higher biopsy %GSGs, but not after age adjustment as previously reported.6 Biopsy depth factors (absence of capsule and presence of medulla) correlated with less area of cortex but did not correlate with biopsy %GSGs.
Table 5.
Spearman’s Correlation of Clinical, Imaging, and Biopsy Characteristics With %GSGs and Area of Cortex on Biopsy
| Characteristic | Correlation With %GSGs on Biopsy |
Correlation With Area of Cortex on Biopsy |
||
|---|---|---|---|---|
| rs (95% CI) | P | rs (95% CI) | P | |
| Clinical | ||||
| Age | 0.28 (0.25 to 0.32) | <0.001 | −0.05 (−0.08 to −0.01) | 0.01 |
| GFRa | −0.14 (−0.18 to −0.10) | <0.001 | −0.03 (−0.06 to 0.01) | 0.2 |
| Hypertension | 0.15 (0.12 to 0.19) | <0.001 | 0.01 (−0.03 to 0.04) | 0.7 |
| CT scan object map of the biopsied kidney | ||||
| Volume of cortex | −0.08 (−0.12 to −0.04) | <0.001 | 0.06 (0.02 to 0.10) | <0.001 |
| Surface roughness | 0.10 (0.06 to 0.13) | <0.001 | −0.02 (−0.05 to 0.02) | 0.4 |
| Kidney biopsy depth | ||||
| Presence of capsule | 0.03 (−0.01 to 0.06) | 0.16 | 0.15 (0.11 to 0.19) | <0.001 |
| Area of medulla | −0.03 (−0.07 to 0.01) | 0.1 | −0.34 (−0.38 to −0.31) | <0.001 |
Abbreviations: CI, confidence interval; CT, computed toography; GFR, glomerular filtration rate; GSGs, globally sclerotic glomeruli.
GFR did not correlate with %GSGs after age adjustment: rs = −0.03 (95% CI, −0.06 to 0.005; P = 0.11).
Discussion
Less cortex on biopsy was associated with the presence of more chronic changes. This included more globally sclerotic glomeruli, more interstitial fibrosis/tubular atrophy, and more arteriosclerosis. This was unexpected because it is generally assumed that biopsies with limited cortex should still have on average the same amount of chronic changes as biopsies with more cortex. In this healthy population, chronic changes occur primarily with older age and hypertension.7 These chronic changes result in loss of nephrons and loss of cortical volume.6, 10 Loss of cortical volume from chronic changes may increase the propensity to undersample cortex with a needle biopsy. Thus, disregarding biopsy specimens for an inadequate amount of sampled cortex fails to take into account that underlying pathology (specifically chronic changes) may contribute to the presence of limited cortex on the biopsy specimen.
Whereas measurement error will increase with less cortex sampled, we found that both mean %GSGs and variability in patient %GSGs also increased with less cortex biopsied. The reason why is not entirely clear. Lower cortical volume on computed tomography weakly correlated with both greater mean %GSGs and less cortex on biopsy. Thus, loss of kidney cortex from chronic changes may lead to undersampling of the cortex by the biopsy needle. However, because these correlations were weak, other factors likely link more %GSGs to less cortex on biopsy. Needle core biopsy specimens are known to be somewhat elastic and it is possible that biopsy specimens with chronic changes are stretched when the needle tip hits a “scarred region,” resulting in undersampling cortex. Specimens with chronic changes may also be more fragile, resulting in tissue fragments being lost during the processing. The increase in variability in patient %GSGs (rather than just an increase in mean %GSGs) suggests that there is more inherent biological heterogeneity when less cortex is biopsied. This is consistent with some biopsy specimens having limited amounts of cortex due to technique (such as going too deep and biopsying medulla), whereas other biopsy specimens have limited amounts of cortex due to the glomerulosclerosis itself and associated pathology (interstitial fibrosis/tubular atrophy and arteriosclerosis).
Using a statistical model to characterize the relationship between biopsy %GSGs and patient %GSGs, we evaluated the validity of the 10-glomeruli threshold that has been advocated for the minimum number of glomeruli needed for assessing chronic changes in an allograft or native kidney biopsy specimen.4, 5 Biopsy specimens with fewer glomeruli are expected to be less precise for estimating kidney pathology. However, we found that biopsies with fewer than 10 glomeruli had an even higher positive predictive value for detecting patient %GSGs ≥ 10% than biopsies with 10 or more glomeruli.
There are 2 reasons that can explain this finding. First, biopsy specimens with less cortex were more likely to occur in patients who have higher %GSGs. Second, numerically, having fewer glomeruli requires the minimum threshold that is ≥10% to be much higher. For example, with 5 glomeruli, 20% (1/5) is the minimum abnormal threshold, whereas with 35 glomeruli, 11% (4/35) is the minimum abnormal threshold. A biopsy with fewer than 10 glomeruli had a lower negative predictive value for patient %GSGs ≥ 10%. This finding is consistent with biopsy specimens having less cortex to be more likely to come from kidneys with underlying chronic changes, even if the chronic changes were not evident on the limited biopsy specimen.
In clinical practice, there is often uncertainty with the interpretation of findings on biopsy specimens with limited cortex. Pathologists are less likely to agree on biopsy findings in biopsy specimens with limited cortex.14 Prior reports have assessed how often a biopsy specimen is 'nondiagnostic' in various settings.15, 16, 17, 18, 19, 20 However these studies did not specifically assess the detection of focal chronic changes with amount of cortex. Even if a biopsy specimen with limited cortex is nondiagnostic for a specific disease, it can still be informative regarding the presence of chronic changes. Chronic changes are prognostically important for the risk for progressive chronic kidney disease,21 even in biopsy specimens with fewer than 10 glomeruli.22
In light of these findings, chronic changes in a biopsy specimen with limited amounts of cortex should not be dismissed as being spurious or attributed to an inadequate sample that just happened to be a region of an isolated focal scar. Rather, chronic changes detected on the limited biopsy specimen are similarly reflective of chronic changes in the entire kidney, as would chronic changes on a biopsy specimen with more cortex. However, if chronic changes are not seen in a biopsy specimen with limited amounts of cortex, there should be less certainty that this is representative of the entire kidney than a biopsy specimen with more cortex.23
A limitation of these findings is that the study population was living kidney donors, which is a fairly homogenous population with limited chronic changes on biopsy. Patients who undergo kidney biopsies for clinical indications are much more heterogeneous with more severe chronic changes on biopsy specimens. This current study provides “proof of principal” that underlying chronic pathology correlates with “inadequate amounts of cortex,” and further study is warranted in more diseased populations. Another limitation is that the patient %GSGs was statistically modeled with only a single biopsy per patient rather than multiple within-patient kidney biopsies.
A strength of our approach is that the beta-binomial model accounts for the impact of biopsy size on sample variability. Biopsy specimens obtained during surgery were advantageous in this study for ensuring that the amount of tissue obtained was not biased by patient size factors such as obesity, but this may also limit the generalizability of the findings to percutaneous biopsies.
What is an adequate amount of cortex on kidney biopsy specimens to detect chronic changes? The answer appears to be that focal chronic changes detected on a limited tissue sample (such as <10 glomeruli) are just as relevant as those detected on larger tissue samples of cortex, and biopsies with limited cortex should not be ignored or discarded. This is in part because chronic changes are more common when tissue biopsy samples yield smaller amounts of cortex. However, when chronic changes are not detected on a limited tissue sample, there should be a higher level of suspicion that focal chronic changes were missed.
Article Information
Authors’ Full Names and Academic Degrees
Robert S. Niznik, MB BCh, Camden L. Lopez, MS, Walter K. Kremers, PhD, Aleksandar Denic, MD, Sanjeev Sethi, MD, Mark D. Stegall, MD, Joshua J. Augustine, MD, and Andrew D. Rule, MD
Authors’ Contributions
Study design: ADR, CLL, WKK; provided the data, RSN, MDS, ADR, AD, JJA; statistical analysis: CLL, WKK; data interpretation: CLL, WKK, SS, MDS, ADR. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Support
The study was supported with funding from the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases (R01 DK090358); and they had no role in study design; collection, analysis, and interpretation of data; writing the report; or the decision to submit the report for publication.
Financial Disclosure
The authors declare that they have no relevant financial interests.
Peer Review
Received March 16, 2019. Evaluated by 2 external peer reviewers, with direct editorial input from the Statistical Editor and the Editor-in-Chief. Accepted in revised form May 16, 2019.
Footnotes
Complete author and article information provided before references.
Item S1: Supplementary methods.
Supplementary Material
Item S1.
References
- 1.Tsuboi N., Kawamura T., Koike K. Glomerular density in renal biopsy specimens predicts the long-term prognosis of IgA nephropathy. Clin J Am Soc Nephrol. 2010;5(1):39–44. doi: 10.2215/CJN.04680709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosenberg A.Z., Palmer M., Merlino L. The application of digital pathology to improve accuracy in glomerular enumeration in renal biopsies. PLoS One. 2016;11(6) doi: 10.1371/journal.pone.0156441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Solez K., Axelsen R.A., Benediktsson H. International standardization of criteria for the histologic diagnosis of renal allograft rejection: the Banff working classification of kidney transplant pathology. Kidney Int. 1993;44(2):411–422. doi: 10.1038/ki.1993.259. [DOI] [PubMed] [Google Scholar]
- 4.Racusen L.C., Solez K., Colvin R.B. The Banff 97 working classification of renal allograft pathology. Kidney Int. 1999;55(2):713–723. doi: 10.1046/j.1523-1755.1999.00299.x. [DOI] [PubMed] [Google Scholar]
- 5.Sethi S., D'Agati V.D., Nast C.C. A proposal for standardized grading of chronic changes in native kidney biopsy specimens. Kidney Int. 2017;91(4):787–789. doi: 10.1016/j.kint.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Denic A., Alexander M.P., Kaushik V. Detection and clinical patterns of nephron hypertrophy and nephrosclerosis among apparently healthy adults. Am J Kidney Dis. 2016;68(1):58–67. doi: 10.1053/j.ajkd.2015.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rule A.D., Amer H., Cornell L.D. THe association between age and nephrosclerosis on renal biopsy among healthy adults. Ann Intern Med. 2010;152(9):561–567. doi: 10.1059/0003-4819-152-9-201005040-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poggio E.D., Rule A.D., Tanchanco R. Demographic and clinical characteristics associated with glomerular filtration rates in living kidney donors. Kidney Int. 2009;75(10):1079–1087. doi: 10.1038/ki.2009.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tong A., Chapman J.R., Wong G., de Bruijn J., Craig J.C. Screening and follow-up of living kidney donors: a systematic review of clinical practice guidelines. Transplantation. 2011;92(9):962–972. doi: 10.1097/TP.0b013e3182328276. [DOI] [PubMed] [Google Scholar]
- 10.Denic A., Lieske J.C., Chakkera H.A. The substantial loss of nephrons in healthy human kidneys with aging. J Am Soc Nephrol. 2017;28(1):313–320. doi: 10.1681/ASN.2016020154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liapis H., Gaut J.P., Klein C. Banff histopathological consensus criteria for preimplantation kidney biopsies. Am J Transplant. 2017;17(1):140–150. doi: 10.1111/ajt.13929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang X., Vrtiska T.J., Avula R.T. Age, kidney function, and risk factors associate differently with cortical and medullary volumes of the kidney. Kidney Int. 2014;85(3):677–685. doi: 10.1038/ki.2013.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yee T.W. Vector Generalized Linear and Additive Models: With an Implementation in R. 2015. https://CRAN.R-project.org/package=VGAM Accessed March 15, 2019.
- 14.Cimen S., Geldenhuys L., Guler S., Imamoglu A., Molinari M. Impact of specimen adequacy on the assessment of renal allograft biopsy specimens. Braz J Med Biol Res. 2016;49(4):e5301. doi: 10.1590/1414-431X20165301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scheckner B., Peyser A., Rube J. Diagnostic yield of renal biopsies: a retrospective single center review. BMC Nephrol. 2009;10:11. doi: 10.1186/1471-2369-10-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Draman C.R., Seman M.R., Mohd Noor F.S., Kelsom W.M. Diagnostic yield of kidney biopsies performed in a suburban, satellite hospital. Saudi J Kidney Dis Transplant. 2013;24(1):178–183. doi: 10.4103/1319-2442.106365. [DOI] [PubMed] [Google Scholar]
- 17.Carrington C.P., Williams A., Griffiths D.F., Riley S.G., Donovan K.L. Adult day-case renal biopsy: a single-centre experience. Nephrol Dial Transplant. 2011;26(5):1559–1563. doi: 10.1093/ndt/gfq571. [DOI] [PubMed] [Google Scholar]
- 18.Hussain F., Mallik M., Marks S.D., Watson A.R. Renal biopsies in children: current practice and audit of outcomes. Nephrol Dial Transplant. 2010;25(2):485–489. doi: 10.1093/ndt/gfp434. [DOI] [PubMed] [Google Scholar]
- 19.Misra S., Gyamlani G., Swaminathan S. Safety and diagnostic yield of transjugular renal biopsy. J Vasc Interv Radiol. 2008;19(4):546–551. doi: 10.1016/j.jvir.2007.12.447. [DOI] [PubMed] [Google Scholar]
- 20.Maya I.D., Maddela P., Barker J., Allon M. Percutaneous renal biopsy: comparison of blind and real-time ultrasound-guided technique. Semin Dial. 2007;20(4):355–358. doi: 10.1111/j.1525-139X.2007.00295.x. [DOI] [PubMed] [Google Scholar]
- 21.Srivastava A., Palsson R., Kaze A.D. The prognostic value of histopathologic lesions in native kidney biopsy specimens: results from the Boston Kidney Biopsy Cohort Study. J Am Soc Nephrol. 2018;29(8):2213–2224. doi: 10.1681/ASN.2017121260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hommos M.S., Zeng C., Liu Z. Global glomerulosclerosis with nephrotic syndrome; the clinical importance of age adjustment. Kidney Int. 2018;93(5):1175–1182. doi: 10.1016/j.kint.2017.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hommos M.S., Glassock R.J., Rule A.D. Structural and functional changes in human kidneys with healthy aging. J Am Soc Nephrol. 2017;28(10):2838–2844. doi: 10.1681/ASN.2017040421. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Item S1.