Abstract
Rationale & Objective
Observational studies have suggested that periodontal disease may be a modifiable risk factor for chronic kidney disease (CKD). The Kidney and Periodontal Disease (KAPD) Study was designed to determine the feasibility of conducting a periodontal disease treatment trial among a high-risk (mostly poor and racial/ethnic minority) population and estimate the magnitude and variability of kidney and inflammatory biomarker levels in response to intensive periodontal treatment.
Study Design
Single-center, unmasked, intention-to-treat, randomized, controlled, pilot trial with 2:1 allocation to the treatment and comparison groups.
Setting & Participants
English- and Spanish-speaking individuals aged 20 to 75 years receiving primary care within the San Francisco Community Health Network with evidence of both moderate to severe periodontal disease and CKD.
Intervention
Immediate intensive nonsurgical periodontal treatment versus rescue treatment for progressive disease at baseline and 4, 8, and 12 months.
Outcomes
Feasibility and process outcomes. Levels of biomarkers of kidney function, kidney injury, and systemic inflammation obtained at baseline and 4 and 12 months.
Results
KAPD randomly assigned 51 participants to the immediate (34 participants) or rescue (17 participants) groups. 14% dropped out of the study (4 immediate, 3 rescue) and 80% completed all 4 visits of the 12-month protocol (28 immediate, 13 rescue). Fewer than half the teeth recommended for extraction were extracted and 40% of immediate group visits were outside the protocol window. Bleeding on probing and probing depth improved more in the immediate group than in the rescue group; there was no significant separation in periodontal status. Levels of markers of vascular endothelial and systemic injury declined in both groups.
Limitations
No true control group.
Conclusions
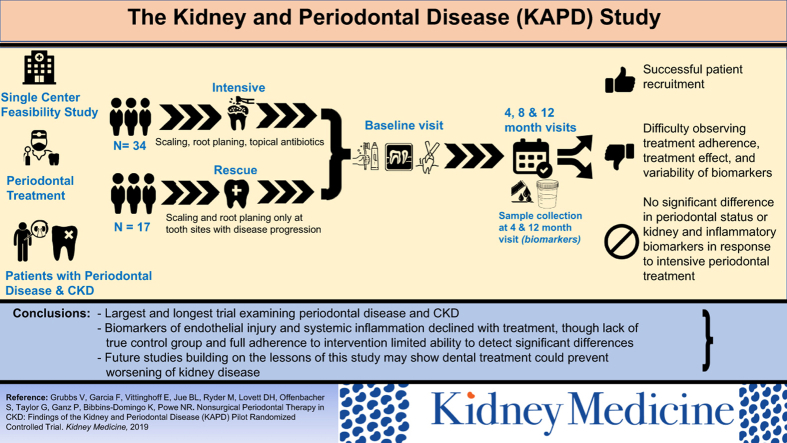
This 12-month, pilot, randomized, controlled trial successfully recruited and retained a high-risk population but was less successful observing treatment adherence, treatment effect, and variability of biomarker levels. Although KAPD did not meet all of its goals, important lessons learned can be applied to future studies.
Funding
National Institute of Diabetes and Digestive and Kidney Disease (Bethesda, MD; grant number 1K23DK093710-01A1) and Harold Amos Medical Faculty Development Program of the Robert Wood Johnson Foundation, Princeton, NJ. Funders had no role in study design; collection, analysis, or interpretation of data; writing the report; or the decision to submit the report for publication.
Trial Registration
Index Words: Chronic kidney disease, periodontal disease, periodontitis, non-surgical periodontal disease treatment
Graphical abstract
Chronic kidney disease (CKD) and periodontal disease are public health problems that disproportionately affect poor and minority populations. Severe periodontal disease has been repeatedly associated with faster kidney function decline in observational studies. We found that severe periodontal diseases was common in cohorts of middle-aged African Americans and older men,1,2 and was associated with a 4.2- and 2-fold greater rate of incident CKD over approximately 5 years, respectively. However, to date, there are no published randomized clinical trial data on the potential effects of periodontal treatment on kidney function.
We developed the Kidney and Periodontal Disease (KAPD) Study to fill this knowledge gap. The study goals were to test the feasibility of conducting the trial among a high-risk (mostly poor and racial/ethnic minority) population and obtain preliminary estimates of the magnitude and variability of change in levels of kidney and inflammatory biomarkers in response to intensive periodontal treatment during a 12-month period among participants with both CKD and periodontal disease. We hypothesized that the KAPD Study was feasible and that intensive periodontal intervention would slow the progression of CKD as measured by kidney biomarkers compared with the non–intensive treatment group.
Methods
Study Design
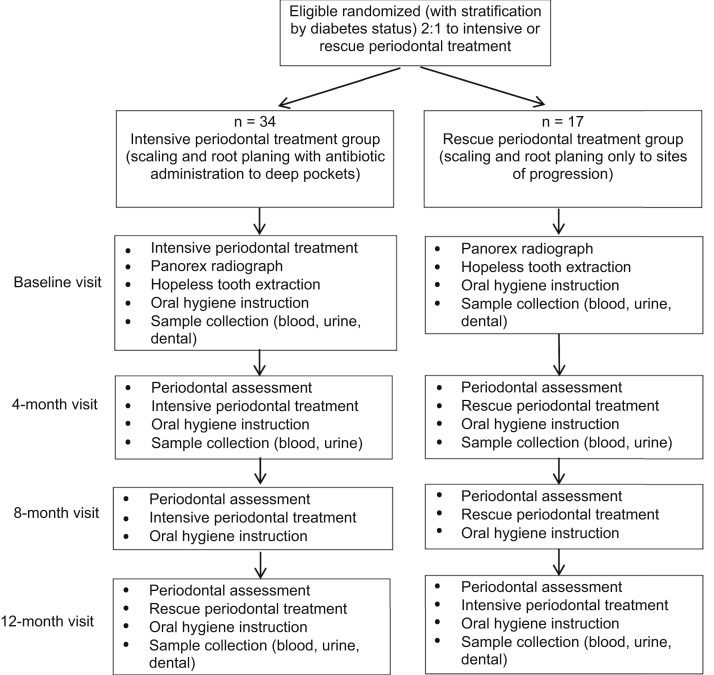
The KAPD Study was a single-center, unmasked, randomized, controlled pilot trial with 2 intent-to-treat groups: immediate intensive nonsurgical periodontal treatment (immediate group) or rescue treatment only to worsening tooth sites with intensive periodontal treatment at the end of the study (rescue group; Fig 1). The protocol was approved by the University of California, San Francisco Institutional Review Board (IRB# 12-09801) and registered with clinicaltrials.gov (NCT01802216). Details of the study protocol are described elsewhere.3
Figure 1.
Kidney and Periodontal Disease Study design. Reproduced from Grubbs et al3 with permission from Elsevier.
Participant Selection and Eligibility
Briefly, KAPD invited English- and Spanish-speaking individuals aged 20 to 75 years who were receiving primary care within the San Francisco Community Health Network and had evidence of both moderate to severe periodontal disease and CKD to participate. Under a waiver for screening of health records, potentially eligible participants were identified by language, age, and estimated glomerular filtration rate (eGFR) criteria through electronic medical record (EMR) extraction. Study staff reviewed EMRs of identified patients, confirmed that these initial screening criteria were accurate, and excluded those who were receiving dialysis, had evidence of recent acute kidney injury (eGFR increase by >50% in prior 6 months), were clinically inappropriate, had an allergy to tetracycline or minocycline, had a history of either infective endocarditis or a heart valve replaced or repaired with prosthetic material, or the medication list included anticoagulants (except aspirin, dipyridamole, or clopidogrel) or long-term immunosuppressive medications. Patients with recent acute kidney injury were excluded to avoid falsely attributing any improvements in kidney biomarker levels to the periodontal intervention. Patients were considered clinically inappropriate if the EMR had a condition that may have precluded them from being able to participate in a 12-month study protocol (eg, deceased, active illicit drug use, untreated psychosis, assaultive behavior, or metastatic malignancy). Patients with a history of either infective endocarditis or heart valve replacement or repair with prosthetic material were excluded because they would have required antibiotic prophylaxis for tooth extractions, a treatment that was not part of the study protocol.
Moderate to severe CKD was defined at screening by at least 2 eGFR measurements (using the Modification of Diet in Renal Disease [MDRD] Study equation as reported by the San Francisco Community Health Network clinical laboratory) in the past 12 months between 15 and 60 mL/min/1.73 m2. Periodontal disease was defined using the Centers for Disease Control and Prevention/American Academy of Periodontology (CDC/AAP) 2003 consensus definition4 and by extent of bleeding on probing. The CDC/AAP criteria define moderate periodontal disease as 2 or more interproximal sites with a ≥4-mm clinical attachment loss (not on the same tooth) or 2 or more interproximal sites with probing depth ≥ 5 mm, also not on the same tooth. The criteria define severe periodontal disease as the presence of 2 or more interproximal sites with ≥6-mm clinical attachment loss (not on the same tooth) and 1 or more interproximal site(s) with ≥5-mm probing depth. In addition to meeting CDC/AAP criteria, participants had to have bleeding on probing on at least 30% of examined sites.
All participants who were found to be ineligible for study participation were given a handout of local dentists and recommendation for care as needed. Written informed consent was obtained before in-person medical review and oral examination.
Randomization
Participants were assigned using a random number generator in a 2:1 ratio to the immediate or rescue treatment group. After randomization, participants were evaluated at baseline and at 4, 8, and 12 months for repeat periodontal assessment and measurements of various kidney and inflammatory biomarkers. Randomization was stratified by the presence of diabetes mellitus to ensure that the groups were balanced for this strong risk factor for causing/accelerating both CKD and periodontal disease. Participants were informed of their randomization assignment at the baseline visit.
Measures and Data Collection
Blood and urine samples were collected at the baseline and 4- and 12-month study visits. Primary outcome measures included a traditional marker of kidney function (serum creatinine) and markers of kidney structure (as glomerular injury [albuminuria and serum neutrophil gelatinase-associated lipocalin (NGAL) and tubular injury (urine NGAL/creatinine)], vascular endothelial injury (asymmetrical dimethylarginine [ADMA]), and systemic inflammation (interleukin 6 and C-reactive protein [CRP]) to predict the effect of treatment and identify the specific mechanisms through which the periodontal pathogen may exert its effects. Creatinine values were used to estimate GFR using the CKD Epidemiology Collaboration (CKD-EPI) equation.5 Study data were collected and managed using REDCap (Research Electronic Data Capture) hosted at University of California, San Francisco.6,7
Interventions
All participants received instruction in oral hygiene at the baseline and 4-, 8-, and 12-month study visits. Participants in the intensive group received full-mouth scaling and root planing (below the gumline deep cleaning treatment), local controlled-release antibiotic administration (minocycline hydrochloride [HCl]; Arestin microspheres [Valeant Pharmaceuticals]) in deeper gum pockets ≥ 5 mm, and recommendation for extraction of teeth that could not be saved (hopeless teeth) at the baseline study visit. Hopeless teeth were defined as those with 2 or more of the following: (1) loss of >75% of the supporting bone, (2) probing depths > 8 mm, (3) class III furcation involvement, (4) class III mobility with tooth movement in lateral (bucco-lingual or mesio-distal) and vertical directions, (5) poor crown to root ratios, and (6) root proximity with minimal interproximal bone and evidence of horizontal bone loss. Hopeless teeth were determined by one of the calibrated study dentists and confirmed based on panorex x-rays at the baseline visit and, if clinically necessary, at the subsequent 4-, 8-, and/or 12-month study visits.
Additional deep cleaning and antibiotic administration occurred at study months 4 and 8 as needed for sites with persistent pockets ≥ 5 mm. This antibiotic formulation was chosen because it is active against a broad spectrum of Gram-negative and Gram-positive anaerobes, including those implicated in adult periodontal diseases. Further, its controlled delivery platform (microspheres) gives up to 21 days of high concentrations of minocycline in the periodontal pockets without detectable systemic exposure.8 Deep cleaning only at sites with progressive disease occurred at study month 12.
Given the relatively long 12-month duration of the protocol, the IRB deemed it unethical to have a true control group that would receive no periodontal treatment until the end of the study. Therefore, participants in the rescue treatment group also had extraction of hopeless teeth at the baseline visit and scaling and root planing without antibiotic administration at study months 4 and 8 only to sites with progressive periodontal disease (pockets that had increased by ≥3 mm in probing depth relative to prior examination). Full-mouth scaling and root planing with antibiotic administration to deeper gum pockets was performed at the 12-month study visit.
Sample Size and Data Analysis
Our accrual target of 51 participants was assigned 2:1 to intensive (n = 34) or rescue treatment (n = 17). This target was deemed typical for a pilot study and feasible given available resources.
Baseline characteristics of the immediate and rescue groups were summarized using mean and standard deviation (SD) or proportion as appropriate and compared using t and Fisher exact tests. Intervention effects on continuous, binary, and ordinal outcomes were estimated using generalized estimating equations linear, logistic, and proportional odds models with independence working correlation, robust standard errors, and terms for treatment, month, and their interaction; models for continuous outcomes were adjusted for the baseline value. Biomarker levels including serum and urinary NGAL, interleukin 6, and CRP were log-transformed for analysis. Between-group differences were modeled as constant or varying arbitrarily across the follow-up visits and also as linearly increasing. Between-group differences in linear trend were assessed based on the P value for the interaction between group and months since baseline. Because this was a small pilot study, P values were not penalized for multiple comparisons. All analyses were implemented using Stata, version 15.1 (Stata Corp).
Results
Participant Enrollment and Follow-up
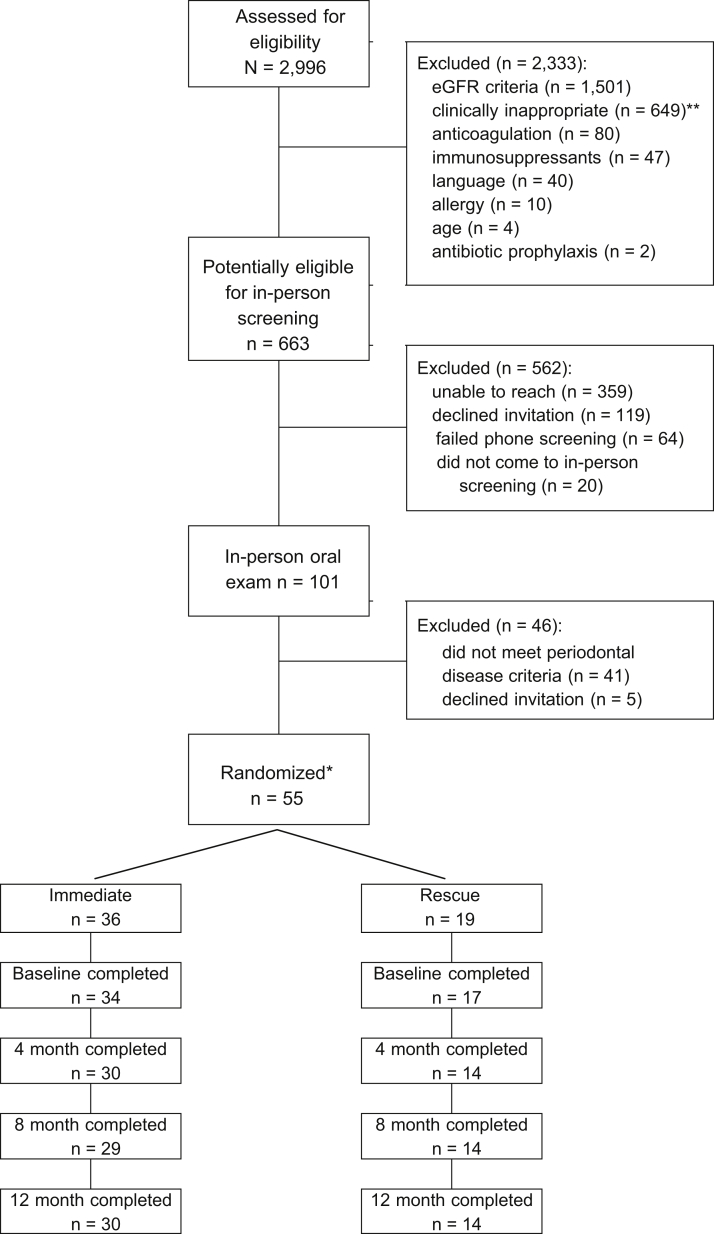
KAPD enrolled participants between February 2014 and September 2016. We screened 2,999 EMR records to identify 101 potentially eligible participants who were invited to in-person screening. Of the 2,333 participants who were not eligible after EMR screening, 1,503 (64%) did not meet eGFR criteria (did not have CKD or were receiving dialysis) and 649 (28%) were deemed clinically inappropriate. Of the 663 potentially eligible participants, we were unable to reach 359 (54%), 119 (18%) declined in-person invitation, and 101 (15%) attended the in-person screening. More than half (n = 55; 54%) of those who attended in-person screening were eligible for participation. Of the 46 ineligible participants, 41 (89%) were excluded for not meeting periodontal disease severity criteria. Additional details are shown in Figure 2.
Figure 2.
Kidney and Periodontal Disease Study consort diagram. Abbreviation: eGFR, estimate glomerular filtration rate. *Not considered enrolled until attended baseline visit. **Clinically inappropriate: deceased, n = 143; residential facility, n = 106; active drug/alcohol abuse, n = 101; homeless/no telephone, n = 85; limited life expectancy, n = 80; unstable mental disorder, n = 25; transferred care, n = 20; assaultive behavior, n = 14; edentulous, n = 4; and other unable to participate in study, n = 71.
Fifty-five participants were randomly assigned (36 to immediate and 19 to rescue), but 4 were lost to follow-up before the baseline visit (2 immediate and 2 rescue). We considered participants enrolled if they attended the baseline visit (n = 51). Seven participants dropped out of the study after enrollment (4 immediate and 3 rescue), leaving 44 (86.2%) who completed the study. Eighty percent of participants completed all 4 study visits (28 immediate and 13 rescue), 6% completed 3 visits (2 immediate and 1 rescue), 2% completed 2 visits (0 immediate and 1 rescue), and 12% completed only the baseline visit (4 immediate and 2 rescue). Most follow-up study visits were completed within the protocol (120 ± 14 days): 54 of 90 immediate-group visits (60%) and 29 of 41 (71%) rescue-group visits, P = 0.2. Immediate- and rescue-group participants had on average 143 (SD, 61) and 137 (SD, 48) days between study visits, respectively, P = 0.5.
Participant Baseline Characteristics
Of 51 enrolled participants, 34 (67.7%) were men, 24 (47.0%) were African American, 10 (19.6%) were Hispanic, 24 (47.0%) had diabetes, and 5 (10%) were current smokers (Table 1). Mean age of study participants was 59 (range, 34-73) years and 71% had CKD stage 3 (mean eGFR, 43 [SD, 15] and 46 [SD, 11] mL/min/1.73 m2 in the immediate and rescue groups, respectively].
Table 1.
Baseline Participant Characteristics by KAPD Study Treatment Group
| Characteristic | Immediate (n = 34) | Rescue (n = 17) | P |
|---|---|---|---|
| eGFR, mL/min/1.73 m2 | 43 (15) | 46 (11) | 0.5 |
| Age, y | 59.2 (7.7) | 58.9 (8.5) | 0.9 |
| Men | 21 (62%) | 13 (76%) | 0.4 |
| Race/ethnicity | 0.3 | ||
| White | 5 (15%) | 4 (24%) | |
| African American | 19 (56%) | 5 (29%) | |
| Hispanic | 5 (15%) | 5 (29%) | |
| Asian | 4 (12%) | 3 (18%) | |
| Other | 1 (3%) | 0 (0%) | |
| Diabetes | 16 (47%) | 8 (47%) | 1.0 |
| Hemoglobin A1c, mg/dL among yes | 7.2 (1.5) | 6.9 (1.9) | 0.7 |
| Current smoker | 3 (9%) | 2 (12%) | 0.8 |
| Last time went to dentist | 0.2 | ||
| Within last 6 mo | 10 (29%) | 6 (35%) | |
| 6-12 mo | 1 (3%) | 1 (6%) | |
| 1-3 y | 7 (21%) | 5 (30%) | |
| 3-5 y | 4 (12%) | 3 (18%) | |
| >5 y | 12 (35%) | 1 (6%) | |
| Don’t know | 0 (0%) | 1 (6%) | |
| Self-rating teeth and gums | 0.6 | ||
| Excellent/very good | 2 (6%) | 0 (0%) | |
| Good/fair | 17 (50%) | 11 (64%) | |
| Poor | 13 (38%) | 4 (24%) | |
| Don’t know | 2 (6%) | 2 (12%) | |
| Periodontal status by CDC criteria | 0.4 | ||
| Mild | 0 (0%) | 1 (6%) | |
| Moderate | 12 (35%) | 7 (41%) | |
| Severe | 22 (65%) | 9 (53%) | |
| % sites examined with BOP (SD) | 51 (17) | 58 (23) | 0.3 |
| Nonmissing teeth, n (SD) | 23 (5) | 24 (5) | 0.8 |
| Hopeless teeth, mean (max)a | 1.5 (9) | 1.1 (7) | 0.2 |
| Pocket probing depth, mm | 3.0 (0.8) | 3.0 (0.7) | 0.9 |
| Prevalence of examined sites with pocket-probing depth | |||
| ≥4 mm, % (SD) | 39 (25) | 36 (23) | 0.7 |
| ≥5 mm, % (SD) | 22 (20) | 18 (19) | 0.5 |
| Clinical attachment loss, mm | 2.8 (1.1) | 2.5 (1.3) | 0.4 |
| Prevalence of examined sites with clinical attachment loss | |||
| ≥3 mm, % (SD) | 54 (30) | 41 (30) | 0.2 |
| ≥4 mm, % (SD) | 35 (27) | 25 (27) | 0.2 |
| ≥6 mm, % (SD) | 13 (15) | 9 (16) | 0.4 |
Note: Unless otherwise noted, values for categorical variables are given as n (percentage of column); values for continuous variables are given as mean (SD).
Abbreviations: BOP, bleeding on probing; CDC, Centers for Disease Control and Prevention; eGFR, estimated glomerular filtration rate; KAPD, Kidney and Periodontal Disease; SD, standard deviation.
Hopeless teeth were defined as those with 2 or more of the following: (1) loss of >75% of supporting bone, (2) probing depths > 8 mm, (3) class III furcation involvement, (4) class III mobility with tooth movement in lateral (bucco-lingual or mesio-distal) and vertical directions, (5) poor crown to root ratios, and (6) root proximity with minimal interproximal bone and evidence of horizontal bone loss.
About a quarter (27%) of all participants reported that they had seen a dentist within the year before study enrollment. Nearly two-thirds (65%) of the immediate group had severe periodontal disease, while about half (53%) of rescue-group participants had severe periodontal disease. The remaining participants had moderate periodontal disease, except for 1 participant with mild periodontal disease who was enrolled and randomly assigned to the rescue treatment group due to an error in software programming.
Interventions Recommended and Performed
At the baseline visit, dental providers determined that 132 teeth were hopeless and recommended extraction (0-9 and 0-7 teeth among immediate- and rescue-group participants, respectively; P = 0.2), but only 52 teeth were extracted. The number of tooth extractions averaged 0.8 at the baseline in both groups, then declined substantially and nondifferentially at follow-up study visits (P = 0.34). No additional teeth were determined hopeless at subsequent visits.
The median minocycline HCl doses applied for the immediate group was 17 (interquartile range [IQR], 6, 28) at baseline, 5 (IQR, 1, 17) at 4 months, and 4 (IQR, 0, 13) at 8 months. The rescue group received 8 (IQR, 0, 23) minocycline HCl doses at the 12-month visit. There was only 1 adverse event reported after the baseline visit; “brown staining on tongue” in an immediate-group participant that resolved without intervention. Although tongue discoloration is not a published adverse effect of minocycline HCl, the participant was allowed to decline further minocycline HCl application.
Five rescue-group participants required rescue treatment for progressive periodontal disease. A total of 16 tooth sites at the 4-month visit and 11 sites at the 8-month visit received rescue treatment.
Eleven immediate- and 9 rescue-group participants reported going to a dentist, dental surgeon, or hygienist outside the KAPD Study between study visits. Two rescue-group participants and 5 immediate-group participants reported seeing an outside provider for a cleaning. One rescue participant reported an outside cleaning at the 8-month visit and another at the 12-month visit. One immediate-group participant reported an outside cleaning at the 4-month visit, 2 at the 8-month visit, and 2 at the 12-month visit. Other reasons provided for seeing an outside dental provider were for consultations (n = 3), x-ray (n = 2), and repairs to crowns (n = 2).
Outcomes
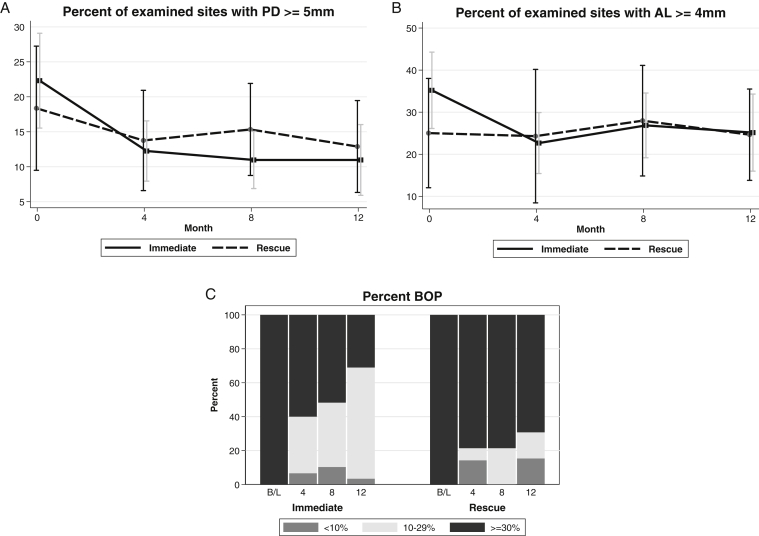
Periodontal status by probing depth and bleeding on probing appeared to improve for the immediate group more than the rescue group, but there was no statistically significant separation between groups for either criterion; P-trend = 0.9 and 0.1, respectively (Fig 3). After the baseline visit, attachment loss improved for the immediate group and was relatively unchanged for the rescue group. Attachment loss remained about the same for both groups for the rest of the study (P-trend = 0.8).
Figure 3.
Change in periodontal status defined by (A) pocket probing depth (PD), (B) attachment loss (AL), or (C) bleeding on probing (BOP) by Kidney and Periodontal Disease (KAPD) Study visit and treatment group. Percent examined sites with (A) pocket PD ≥ 5 mm, (B) AL ≥ 4 mm, and (C) BOP.
At baseline, about half the participants reported brushing their teeth at least twice a day (immediate, 56%, and rescue, 41%), but only 21% and 12% of immediate- and rescue-group participants, respectively, reported flossing at least twice a day. These oral hygiene behaviors did not change over time despite oral hygiene instruction at each study visit for all participants; P > 0.2 (Table 2).
Table 2.
Oral Hygiene Behaviors by KAPD Study Treatment Group and Study Visit
| Behavior | Baseline | 4 mo | 8-mo | 12 mo | P-Trend |
|---|---|---|---|---|---|
| Brush teeth some days | 0.3 | ||||
| Immediate | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Rescue | 3 (18%) | 3 (20%) | 3 (21%) | 3 (21%) | |
| Brush teeth 1×/d | |||||
| Immediate | 15 (44%) | 14 (45%) | 12 (41%) | 13 (43%) | |
| Rescue | 7 (41%) | 6 (43%) | 6 (43%) | 6 (43%) | |
| Brush teeth ≥2×/d | |||||
| Immediate | 19 (56%) | 17 (55%) | 17 (59%) | 17 (57%) | |
| Rescue | 7 (41%) | 5 (36%) | 5 (36%) | 5 (36%) | |
| Floss not at all | 0.9 | ||||
| Immediate | 12 (36%) | 11 (37%) | 10 (36%) | 10 (34%) | |
| Rescue | 5 (29%) | 4 (29%) | 4 (29%) | 4 (29%) | |
| Floss some days | |||||
| Immediate | 9 (27%) | 9 (30%) | 9 (32%) | 9 (31%) | |
| Rescue | 7 (41%) | 6 (43%) | 6 (43%) | 6 (43%) | |
| Floss 1×/d | |||||
| Immediate | 5 (15%) | 4 (13%) | 4 (14%) | 4 (14%) | |
| Rescue | 3 (18%) | 2 (14%) | 2 (14%) | 2 (14%) | |
| Floss ≥2×/d | |||||
| Immediate | 7 (21%) | 6 (20%) | 5 (18%) | 6 (21%) | |
| Rescue | 2 (12%) | 2 (14%) | 2 (14%) | 2 (14%) |
Note: Values expressed as number (percentage).
Abbreviation: KAPD, Kidney and Periodontal Disease.
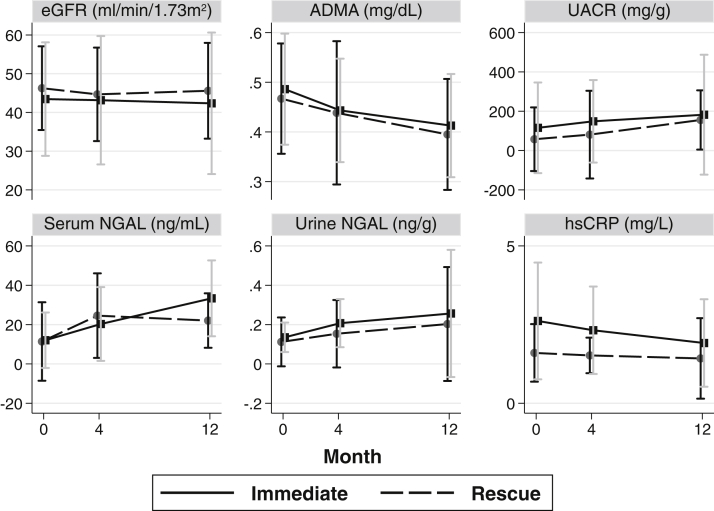
Biomarker results by study visit and treatment group are shown in Table 3 and Figure 4. Mean eGFR was lower at baseline in the immediate treatment group compared with the rescue group and decreased similarly in both groups. Urinary albumin-creatinine ratio was higher at each measurement for the immediate treatment group compared with the rescue group and increased similarly in both groups. Median serum NGAL level increased steadily from baseline at the 4- and 12-month visits in the immediate group. It was higher at the 4-month visit in the rescue group, then decreased slightly at 12 months. Median urine NGAL excretion was lower at baseline in the immediate group than in the rescue group. The 12-month urine NGAL excretion was about the same as baseline for the immediate group but significantly lower in the rescue group. Baseline mean ADMA level was similar in both groups and decreased similarly with each visit. Median CRP level was considerably higher at baseline in the immediate group than in the rescue group, but values decreased similarly in both groups at subsequent visits. Median interleukin 6 level at baseline was nearly twice that in the immediate group than in the rescue group. Values slightly decreased in the immediate group but increased in the rescue group.
Table 3.
Biomarkers by KAPD Study Visit and Treatment Group
| Baseline Visit | 4-mo Visit | 12-mo Visit | Difference in Trends P | |
|---|---|---|---|---|
| eGFR, mL/min/1.73 m2 | 0.8 | |||
| Immediate | 43.4 (14.7) | 43.2 (16.6) | 42.4 (18.3) | |
| Rescue | 46.3 (10.8) | 44.7 (12.1) | 45.6 (12.4) | |
| UACR, mg/g | 0.6 | |||
| Immediate | 116 [11, 472] | 149 [17, 437] | 182 [17, 626] | |
| Rescue | 58 [3, 327] | 81 [17, 463] | 156 [7, 309] | |
| Serum NGAL, ng/mL | 0.9 | |||
| Immediate | 12.1 [5.8, 34.0] | 20.3 [9.1, 46.6] | 33.4 [10.1, 48.7] | |
| Rescue | 11.4 [4.8, 44.7] | 24.6 [6.0, 49.0] | 22.0 [13.1, 40.8] | |
| Urine NGAL/creatinine, ng/g | 0.4 | |||
| Immediate | 142 [105, 204] | 128 [97, 154] | 140 [109, 203] | |
| Rescue | 150 [95, 182] | 126 [108, 164] | 132 [96, 168] | |
| ADMA, mg/dL | 0.6 | |||
| Immediate | 0.49 (0.11) | 0.44 (0.10) | 0.41 (0.10) | |
| Rescue | 0.47 (0.11) | 0.44 (0.14) | 0.40 (0.11) | |
| IL-6, pg/mL | 0.7 | |||
| Immediate | 4.01 [2.11, 6.73] | 3.31 [2.17, 5.94] | 3.41 [1.94, 4.92] | |
| Rescue | 2.10 [1.60, 3.44] | 2.61 [2.30, 3.88] | 2.65 [1.83, 4.85] | |
| CRP, mg/L | 0.1 | |||
| Immediate | 2.62 [1.38, 5.09] | 2.32 [1.13, 3.91] | 1.92 [0.77, 3.55] | |
| Rescue | 1.60 [0.71, 2.54] | 1.52 [0.87, 2.00] | 1.42 [0.63, 3.19] |
Note: Values expressed a median [interquartile range] (analysis done on log-scale) or mean (standard deviation). Number of participants at each stage: baseline visit: immediate group, n = 34; rescue group, n = 17; 4- and 12-month visits: immediate group, n = 30; rescue group, n = 14.
Abbreviations: ADMA, asymmetrical dimethylarginine; CRP, C-reactive protein; eGFR, estimated glomerular filtration rate; IL-6, interleukin 6; KAPD, Kidney and Periodontal Disease; NGAL, neutrophil gelatinase-associated lipocalin; UACR, urinary albumin-creatinine ratio.
Figure 4.
Biomarker levels by Kidney and Periodontal Disease (KAPD) Study visit and treatment group show mean and standard deviation for estimated glomerular filtration rate (eGFR) and asymmetrical dimethylarginine (ADMA) values, median and interquartile range for urinary albumin-creatinine ratio (UACR), serum neutrophil gelatinase-associated lipocalin (NGAL), urinary NGAL excretion, and high-sensitivity C-reactive protein (hsCRP) levels.
Discussion
Though the KAPD Study was a pilot randomized controlled trial, to our knowledge, it is the largest and longest-running randomized trial examining periodontal disease treatment among participants with CKD completed to date. The study was intended to show the feasibility of conducting the trial among a high-risk (mostly poor and racial/ethnic minority) population and to obtain preliminary estimates of the magnitude and variability of change in kidney and inflammatory biomarker levels in response to intensive periodontal treatment over a 12-month period. With a drop-out rate of 14% and 80% of participants completing all 4 study visits, the trial was feasible in terms of recruitment and retention but was less successful with completion of treatment recommendations (ie, visits every 4 months and tooth extractions), treatment effect, and variability of biomarker levels. Bleeding on probing and probing depth improved with intensive treatment but was not significantly different from improvement observed in the rescue group. Although we observed a pattern of decline for ADMA and CRP levels, we were unable to establish preliminary estimates for the magnitude of the effect of measured biomarkers in response to intensive periodontal treatment.
Many factors contributed to our successful recruitment and retention of participants with CKD enrolled in a dental intervention study. We employed a bilingual research staff, updated participant contact information at every study visit, communicated with participants on a regular basis to ensure that contact information was correct, conducted a study raffle, and paid participants. Participants invited for in-person screening were paid US $10 cash regardless of screening outcome. Enrolled participants received Visa gift cards in the amounts of US $50 for baseline and 12-month study visits, US $25 for 4- and 8-month study visits, additional Visa gift cards of US $25 if extractions were required, and US $50 if all 4 study visits were completed. Each participant also received free periodontal treatment, panorex radiograph(s), and extractions, as well as a sample bag of oral hygiene items at each study visit.
It is particularly interesting that levels of ADMA (a marker of endothelial dysfunction) and CRP (a marker of systemic inflammation) decreased with each study visit because this pattern would be the expected result of periodontal disease treatment given the proposed mechanism for how periodontal pathogens might lead to kidney damage and is consistent with prior studies. Periodontal pathogens can access systemic circulation through normal oral health procedures such as tooth brushing and even chewing.9,10 As a result, circulating bacterial coating (lipopolysaccharide) can bind to Toll-like receptors (TLR4), which are found throughout the kidney.11, 12, 13 Once bound to the lipopolysaccharide ligand, the TLR4 is activated to launch an inflammatory cascade that may lead to endothelial dysfunction14,15 and sustained local tissue inflammation and fibrosis, with deterioration of kidney function.11,12 Also, reactive oxygen species, which are increased in periodontal disease,16 are another potential pathway for endothelial dysfunction through TLR4 activation17 and inhibition of nitric oxide–mediated vascular activity.18 Amar et al15 found that patients with advanced periodontal disease exhibited endothelial dysfunction and systemic inflammation, possibly placing them at increased risk for cardiovascular disease. Further, Tonetti et al19 found that intensive periodontal treatment resulted in improved endothelial function at 6 months compared with the control group.
Our inability to establish preliminary estimates for the magnitude of change in measured biomarker levels in response to intensive periodontal treatment is likely multifactorial. The presence of more severe periodontal disease in our immediate treatment group and the lack of a true control group certainly weakened our ability to determine the full effect of periodontal treatment. In addition to a third of rescue-group participants receiving treatment for progressive periodontal disease, the rescue group also received oral hygiene instruction, above the gumline cleaning, and extraction of hopeless teeth—all of which could have reduced the burden of periodontal disease, thus reducing our ability to achieve significant separation in periodontal status between groups and detect differences in biomarkers. Furthermore, that more than one-third (40%) of immediate-group participants did not receive treatment every 4 months as intended, and more than half were not brushing and flossing at least twice daily as recommended after repeat instruction and had fewer than half the recommended hopeless tooth extractions also diluted separation between groups by periodontal status. However, while bleeding on probing (a marker of active inflammation) appeared to improve more in the immediate group compared with the rescue group, our prior study suggested that the presence of more severe disease as measured by pocket probing depth and attachment loss, rather than active inflammation, were most strongly associated with CKD progression, suggesting that longer follow-up may be needed to observe improvements in more severe periodontal disease and therefore biomarker levels.1
Although KAPD did not meet all of its goals, important lessons can be applied to future studies to maximize the likelihood of determining the effect of intensive periodontal treatment on CKD progression. For example, more frequent visits might result in greater improvement in periodontal status, and provision of dental implants or dentures to replace extracted hopeless teeth might improve adherence to extraction recommendations. Finally, a stepped-wedge study design in which the intervention is rolled out in stages could provide a true and ethically acceptable control group. Such a design would collect blood and urine samples at baseline for all sites, but periodontal examinations and treatments would be done by site every quarter. For a 4-site study, this would mean 0, 3, 6, and 9 months of true control for participants at sites A, B, C, and D, respectively. A primary limitation here would be the lack of a baseline periodontal examination for all participants. With successful replication incorporating these changes, future studies may lead to the treatment of periodontal disease as an important and currently underused intervention for reducing disparities among individuals with CKD.
Article Information
Authors’ Full Names and Academic Degrees
Vanessa Grubbs, MD, Faviola Garcia, BA, Eric Vittinghoff, PhD, Bonnie L. Jue, DDS, Mark Ryder, DMD, David H. Lovett, MD, Steven Offenbacher, DDS, George Taylor, DMD, Peter Ganz, MD, Kirsten Bibbins-Domingo, MD, and Neil R. Powe, MD.
Authors’ Contributions
Research idea and study design: VG, EV, MR, DHL, GT, PG, KB-D, NRP; data acquisition: VG, FG, BLJ, MR; data analysis/interpretation: VG, EV, BLJ, MR, GT; statistical analysis: EV; supervision or mentorship: VG, MR, KB-D, NRP. SO died before the manuscript was submitted. VG affirms that he contributed to the research idea and study design and vouches for his coauthorship status; all other authors approved the final author list. Except as noted, each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Support
KAPD and Dr Grubbs were supported by the National Institute of Diabetes and Digestive and Kidney Disease (Bethesda, MD; grant number 1K23DK093710-01A1) and by the Harold Amos Medical Faculty Development Program of the Robert Wood Johnson Foundation, Princeton, NJ.
Financial Disclosure
The study drug (minocycline HCl, Arestin microspheres) was provided through an investigator-initiated research grant from Valeant Pharmaceuticals, Bridgewater, NJ. Dr Lovett is a member of the Abbvie-UCSF Steering Committee. Dr Offenbacher died before this article was submitted. The corresponding author affirms that to her knowledge Dr Offenbacher had no relevant financial interests. The remaining authors declare that they have no relevant financial interests.
Acknowledgements
We thank the members of the DSMB: K.L. Johansen (chair), G. Armitage, and C.E. McCullouch.
Peer Review
Received June 20, 2019. Evaluated by 2 external peer reviewers, with direct editorial input from the Statistical Editor and the Editor-in-Chief. Accepted in revised form September 12, 2019.
Data Sharing
We will share deidentified individual participant data underlying the results presented in the manuscript and study protocol with researchers on request for meta-analysis or study development. Request by contacting the corresponding author by e-mail.
Footnotes
Complete author and article information provided before references.
References
- 1.Grubbs V., Vittinghoff E., Beck J.D. Association between periodontal disease and kidney function decline in African Americans: the Jackson Heart Study. J Periodontol. 2015;86(10):1126–1132. doi: 10.1902/jop.2015.150195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grubbs V., Vittinghoff E., Taylor G. The association of periodontal disease with kidney function decline: a longitudinal retrospective analysis of the MrOS dental study. Nephrol Dial Transplant. 2016;31(3):466–472. doi: 10.1093/ndt/gfv312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grubbs V., Garcia F., Jue B.L. The Kidney and Periodontal Disease (KAPD) study: a pilot randomized controlled trial testing the effect of non-surgical periodontal therapy on chronic kidney disease. Contemp Clin Trials. 2016;53:143–150. doi: 10.1016/j.cct.2016.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eke P.I., Page R.C., Wei L., Thornton-Evans G., Genco R.J. Update of the case definitions for population-based surveillance of periodontitis. J Periodontol. 2012;83(12):1449–1454. doi: 10.1902/jop.2012.110664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levey A.S., Stevens L.A., Schmid C.H. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harris P.A., Taylor R., Minor B.L. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams R.C., Paquette D.W., Offenbacher S. Treatment of periodontitis by local administration of minocycline microspheres: a controlled trial. J Periodontol. 2001;72(11):1535–1544. doi: 10.1902/jop.2001.72.11.1535. [DOI] [PubMed] [Google Scholar]
- 9.Christensen P.J., Kutty K., Adlam R.T., Taft T.A., Kampschroer B.H. Septic pulmonary embolism due to periodontal disease. Chest. 1993;104(6):1927–1929. doi: 10.1378/chest.104.6.1927. [DOI] [PubMed] [Google Scholar]
- 10.Fisher M.A., Taylor G.W., Papapanou P.N., Rahman M., Debanne S.M. Clinical and serologic markers of periodontal infection and chronic kidney disease. J Periodontol. 2008;79(9):1670–1678. doi: 10.1902/jop.2008.070569. [DOI] [PubMed] [Google Scholar]
- 11.Anders H.J., Banas B., Schlondorff D. Signaling danger: Toll-like receptors and their potential roles in kidney disease. J Am Soc Nephrol. 2004;15(4):854–867. doi: 10.1097/01.asn.0000121781.89599.16. [DOI] [PubMed] [Google Scholar]
- 12.Anders H.J., Schlondorff D. Toll-like receptors: emerging concepts in kidney disease. Curr Opin Nephrol Hypertens. 2007;16(3):177–183. doi: 10.1097/MNH.0b013e32803fb767. [DOI] [PubMed] [Google Scholar]
- 13.Summers S.A., Hoi A., Steinmetz O.M. TLR9 and TLR4 are required for the development of autoimmunity and lupus nephritis in pristane nephropathy. J Autoimmun. 2010;35(4):291–298. doi: 10.1016/j.jaut.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 14.Chen J., John R., Richardson J.A. Toll-like receptor 4 regulates early endothelial activation during ischemic acute kidney injury. Kidney Int. 2011;79(3):288–299. doi: 10.1038/ki.2010.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amar S., Gokce N., Morgan S., Loukideli M., Van Dyke T.E., Vita J.A. Periodontal disease is associated with brachial artery endothelial dysfunction and systemic inflammation. Arterioscler Thromb Vasc Biol. 2003;23(7):1245–1249. doi: 10.1161/01.ATV.0000078603.90302.4A. [DOI] [PubMed] [Google Scholar]
- 16.D'Aiuto F., Nibali L., Parkar M., Patel K., Suvan J., Donos N. Oxidative stress, systemic inflammation, and severe periodontitis. J Dent Res. 2010;89(11):1241–1246. doi: 10.1177/0022034510375830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kong X., Thimmulappa R., Kombairaju P., Biswal S. NADPH oxidase-dependent reactive oxygen species mediate amplified TLR4 signaling and sepsis-induced mortality in Nrf2-deficient mice. J Immunol. 2010;185(1):569–577. doi: 10.4049/jimmunol.0902315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cooke J.P. Asymmetrical dimethylarginine: the Uber marker? Circulation. 2004;109(15):1813–1818. doi: 10.1161/01.CIR.0000126823.07732.D5. [DOI] [PubMed] [Google Scholar]
- 19.Tonetti M.S., D'Aiuto F., Nibali L. Treatment of periodontitis and endothelial function. N Engl J Med. 2007;356(9):911–920. doi: 10.1056/NEJMoa063186. [DOI] [PubMed] [Google Scholar]