Abstract
Rationale & Objective
We aimed to elucidate whether a balanced salt solution decreases the occurrence of contrast-induced acute kidney injury (CI-AKI) after contrast-enhanced computed tomography (CE-CT) as compared to 0.9% saline solution.
Study Design
A randomized clinical trial.
Setting & Participants
The study was performed in 14 tertiary hospitals in South Korea. Patients with estimated glomerular filtration rates (eGFRs) < 45 or <60 mL/min/1.73 m2 and additional risk factors (age ≥ 60 years or diabetes) who were undergoing scheduled CE-CT were included from December 2016 to December 2018.
Intervention
An open-label intervention was performed. The study group received a balanced salt solution and the control group received 0.9% saline solution as prophylactic fluids for CE-CT.
Outcomes
The primary outcome was CI-AKI, defined by creatinine level elevation ≥ 0.5 mg/dL or 25% from baseline within 48 to 72 hours after CE-CT. Secondary outcomes included AKI defined based on the KDIGO (Kidney Disease: Improving Global Outcomes) guideline, eGFR changes, death, or requiring dialysis within 6 months after CE-CT.
Results
493 patients received the study fluids. The control and study groups included 251 and 242 patients, respectively. The occurrence of CI-AKI in the study (10 [4.2%]) and control (17 [6.8%]) groups was not significantly different (P = 0.27). No significant difference was present for the secondary outcomes; AKI by the KDIGO definition (study: 19 [7.9%], control: 27 [10.8%]; P = 0.33), death/dialysis (study: 11 [4.7%], control: 9 [3.7%]; P = 0.74), and eGFR changes (study: 0.1 ± 0.2 mg/dL, control: 0.3 ± 2.8 mg/dL; P = 0.69).
Limitations
This study failed to meet target enrollment.
Conclusions
The risk for CI-AKI was similar after administration of a balanced salt solution and after use of 0.9% saline solution during CE-CT in higher-risk patients.
Funding
This study was funded by CJ Healthcare (CS2015_0046).
Trial Registration
Registered at ClinicalTrials.gov with study number NCT02799368.
Index Words: Contrast-induced acute kidney injury, computed tomography, acute kidney injury, saline, balanced salt solution, acute renal failure, fluid
Graphical abstract
Contrast-induced acute kidney injury (CI-AKI) is one of the most common iatrogenic kidney injuries.1 Moreover, CI-AKI has been associated with increased risk for mortality, longer hospital stays, and end-stage kidney disease in patients, particularly those with chronic kidney disease.1, 2, 3, 4 Therefore, early diagnosis or prevention of CI-AKI remains an important medical issue.
The prophylactic measures for CI-AKI have long been debated. Intravenous administration of 0.9% saline solution has been reported as an essential part of CI-AKI prevention.5,6 However, effort has been made to find an alternative because 0.9% saline solution contains supraphysiologic levels of chloride and has a relatively acidic pH, which may lead to kidney vasoconstriction.7 No current evidence supports the use of different fluids to prevent CI-AKI, although there have been reports showing that a balanced salt solution,8, 9, 10, 11, 12 which contains a physiologic amount of chloride and has neutral pH, was beneficial in other clinical settings. However, a recent trial also demonstrated that implementing 0.9% saline solution in CI-AKI prevention did not provide any benefits to patients when compared with those who received no preventive prophylactics before contrast-enhanced computed tomography (CE-CT).13 Therefore, the necessity for a better prophylactic measure for CI-AKI is evident, particularly for high-risk patient groups in which prevention may still have clinical benefits.
In this multicenter randomized controlled trial, we aimed to compare the effectiveness of a balanced salt solution and 0.9% saline solution in preventing CI-AKI. We hypothesized that the balanced salt solution may be a similar or better prophylactic method for CI-AKI after CE-CT than 0.9% saline solution.
Methods
Ethical Considerations
All patients were aware of the nature of the study at the time of enrollment and provided written informed consent. The study was performed in accordance with the Declarations of Helsinki. The Ministry of Food and Drug Safety of the Republic of Korea and the institutional review boards of the study hospitals approved this study (Seoul National University Hospital: H-1605-156-766, Kyungpook National University Hospital: KNUH 2016-09-007, National Medical Center: H-1608-069-004, National Health Insurance Service Ilsan Hospital: NHIMC 2016-10-010-002, Seoul National University Bundang Hospital: B-1609/363-401, Bundang Cha Hospital: 2016-09-011-002, Seoul St. Mary’s Hospital: KC16MIMT0741, Seoul National University Boramae Hospital: 16-2016-126, Severance Hospital: 4-2016-0691, Ewha Womans University Mokdong Hospital: 2016-10-014, Incheon St. Mary’s Hospital: IS16MIMT0033, Gachon University Gil Medical Center: GAIRB2016-322, Samsung Medical Center: 2018-01-024, and Ajou University Hospital: AJIRB-MED-CT3-18-008).
Study Design and Study Population
This was a randomized, open-label, active-control, 2–parallel-group, multicenter, phase 3 study. Details of the study design and protocol have been published previously.14 In brief, the study included participants from 14 tertiary hospitals in the Republic of Korea. We recruited patients who were scheduled for CE-CT in outpatient settings but had risk factors for CI-AKI. Inclusion criteria were: (1) adult patients (aged ≥18 years); (2) baseline estimated glomerular filtration rate (eGFR), measured within 3 months, <45 mL/min/1.73 m2 or both having a baseline eGFR < 60 mL/min/1.73 m2 and either one of the following risk factors: diabetes mellitus or age 60 years or older15; and (3) ability to provide informed consent and adequate information for the end point assessment. We excluded those who had end-stage kidney disease, significant heart failure, and other conditions that made receiving contrast materials or the study fluids inappropriate.
Exclusion criteria were: (1) baseline eGFR < 15 mL/min/1.73 m2 or receiving dialysis; (2) heart failure with left ventricular ejection fraction < 45% or severe symptoms (New York Heart Association functional classification III or IV); (3) co-existing acute pulmonary edema or decompensated heart failure requiring the following medications: dobutamine, dopamine, milrinone, amrinone, or nesiritide; (4) serum potassium level > 5.5 mEq/L or serum sodium level > 145 mEq/L at the screening period or within 3 months before the CT; (5) history of intravenous or intra-arterial contrast agent administration within 1 week; (6) previous history of hypersensitivity reaction to the iodinated contrast agent; (7) history of multiple myeloma; (8) women who are currently pregnant/breastfeeding or planning to become pregnant; (9) patients with an expected survival duration less than 6 months; and (10) enrollment in another clinical trial.
Intervention and Randomization
The main intervention was intravenous administration of specific fluids for CI-AKI prevention. The study group received a balanced salt solution (Plasma solution A; CJ Healthcare) with 98 mEq/L of chloride at pH 7.4, and the control group received 0.9% saline solution with 154 mEq/L of chloride at pH 6.0. Both fluids were manufactured by the same company. Both fluids were infused at a rate of 3 mL/kg per hour for 1 hour before and 1.5 mL/kg per hour for 4 hours after the CE-CT. Although the volume or infusion protocol of contrast materials used for the CE-CT was not controlled, all study hospitals used iso- or low-osmolar iodinated contrast materials. N-Acetylcysteine was not used as a supplemental medication due to limited benefits for CI-AKI prevention. Restrictions were not applied for other coadministered medications.
Study randomization was performed in a 1:1 manner with simple randomization. The randomization list was generated using a professional on-line randomization service (Sealed Envelope, London, UK).
Sample Size and Patient Enrollment
CI-AKI incidence was predicted to be 11.5% in the control group according to the previous prospective study.7 Because there was no study regarding the incidence of CI-AKI with prevention by using a balanced solution at the time of study planning, the expected CI-AKI incidence of the study group was adapted from a previous study in another clinical setting and was predicted to be 8.4%.11 The sample size goal was 1,328 study participants, 664 in each group, which was calculated from a noninferiority limit of 1.5% with power of at least 80% and 1-side type 1 error rate of 2.5%. Allowing a 20% withdrawal rate in each group, a total of 1,660 participants were needed. Patient enrollment was scheduled for 2 years from the first patient registration.
Study Outcomes
The primary end point was the occurrence of CI-AKI, which was defined as serum creatinine level elevation ≥ 0.5 mg/dL or ≥25% from baseline at the follow-up visit 48 to 72 hours after CE-CT. Secondary end points included: (1) eGFR reduction at the follow-up visit; (2) AKI according to the KDIGO (Kidney Disease: Improving Global Outcomes) criteria, which was serum creatinine level elevation ≥ 0.3 mg/dL or 50% from baseline at the follow-up visit5; and (3) death or requiring dialysis within 6 months of the CE-CT. Safety assessment was done primarily by collecting data for adverse events or determined by clinical assessments, including abnormalities in laboratory test results or vital signs.
Statistical Analyses
Among randomly assigned patients, all who received the allocated intervention were included in the full analysis set. The per-protocol set consisted of patients who ended the trial period without a violation of the study protocol. Results from the full analysis set are presented, and results from the per-protocol set are not described unless significantly different.
We presented categorical variables as number with percent and continuous variables as mean ± standard deviation. Categorical variables were analyzed using χ2 test. Continuous variables were analyzed using t test. The noninferiority of the study group for the primary outcome was investigated with the noninferiority test with a 1-sided P value. Additional adjustments for baseline characteristics were performed for a multivariable logistic regression model, and the model was adjusted for age, sex, histories of hypertension and diabetes, baseline eGFR, and urine protein-creatinine ratio. We performed complete-case analysis, and patients with missing values were not included in the analysis, accordingly. Multivariable adjustment for continuous variables was performed using analysis of covariance adjusted for the same covariates described. All analyses were performed using R software (version 3.6.2; the R Foundation). Except for the noninferiority test, 2-sided P < 0.05 was considered statistically significant.
Results
Patient Enrollment
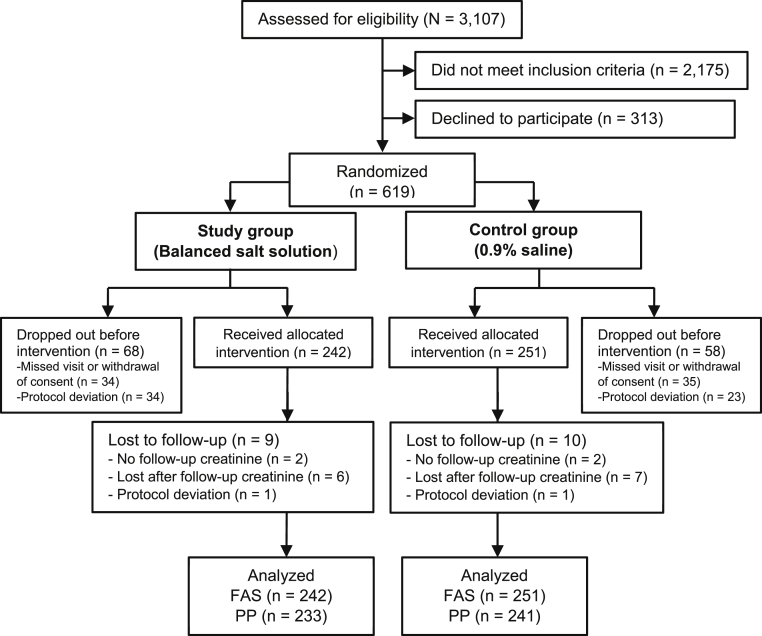
The first patient enrolled on December 14, 2016. At the end of the enrollment period, 619 patients were randomly assigned and 493 patients received the study fluids (Fig 1). Enrollment did not increase despite significant efforts to facilitate patient recruitment and expansion of the inclusion criteria, and the study was closed without further patient registration. The study results were decided to be reported because the data were considered to have certain clinical value and may be referred to by future studies.
Figure 1.
The Consolidated Standards of Reporting Trials (CONSORT) study flow diagram. Abbreviations: FAS, full analysis set; PP, per protocol.
Baseline Characteristics
Among patients who received fluids, 251 patients in the control group received 0.9% saline solution and 242 patients in the study group received a balanced salt solution. Baseline characteristics, demographics, baseline coadministration of other medications, and laboratory result profiles were well balanced between the 2 groups (Table 1), except that the control group had a higher proportion of patients with hypertension or diabetes mellitus.
Table 1.
Baseline Characteristics of the Study Population
| 0.9% Saline Solution (N = 251) | Balanced Salt Solution (N = 242) | |
|---|---|---|
| Demographic Findings | ||
| Age, y | 70.6 ± 10.2 | 71.3 ± 10.4 |
| Male sex | 178 (70.9%) | 167 (69.0%) |
| Height, cm | 162.2 ± 7.9 | 162.3 ± 8.1 |
| Weight, kg | 63.7 ± 11.0 | 63.9 ± 10.2 |
| Comorbid Conditions and Medication History | ||
| Hypertension | 200 (79.7%) | 171 (70.7%) |
| Diabetes mellitus | 135 (53.8%) | 106 (43.8%) |
| RAAS inhibitor | 107 (42.6%) | 93 (38.4%) |
| β-Blocker | 51 (20.9%) | 46 (19.3%) |
| Calcium channel blocker | 99 (40.6%) | 90 (37.8%) |
| Metformin | 37 (15.0%) | 29 (12.2%) |
| Insulin | 25 (10.1%) | 18 (7.6%) |
| Other diabetes medications | 84 (34.1%) | 60 (25.3%) |
| Statin | 97 (39.6%) | 98 (41.2%) |
| Diuretics | 54 (22.0%) | 46 (19.3%) |
| NSAID | 30 (12.2%) | 25 (10.5%) |
| CE-CT contrast amount, mL | 119.0 ± 29.0 | 116.6 ± 27.8 |
| Baseline Laboratory Values | ||
| Systolic BP, mm Hg | 130.8 ± 16.6 | 130.7 ± 16.3 |
| Diastolic BP, mm Hg | 73.7 ± 10.9 | 74.4 ± 12.2 |
| Hemoglobin, g/dL | 11.8 ± 2.0 | 11.8 ± 2.0 |
| Sodium, mEq/L | 139.7 ± 3.0 | 139.5 ± 3.0 |
| Potassium, mEq/L | 4.6 ± 0.5 | 4.6 ± 0.6 |
| Chloride, mEq/L | 105.2 ± 3.7 | 105.4 ± 4.5 |
| Total CO2, mEq/L | 23.8 ± 3.5 | 23.8 ± 3.6 |
| SUN, mg/dL | 26.0 ± 11.7 | 26.0 ± 10.9 |
| Creatinine, mg/dL | 1.6 ± 0.6 | 1.6 ± 0.6 |
| eGFR, mL/min/1.73 m2 | 45.2 ± 13.1 | 46.1 ± 13.1 |
| Protein, g/dL | 7.0 ± 0.7 | 7.0 ± 0.7 |
| Albumin, g/dL | 4.0 ± 0.6 | 4.0 ± 0.5 |
| Urinary PCR, mg/g | 178.6 ± 837.6 | 231.0 ± 1159.2 |
Note: Values for categorical variables are given as number (percent); values for continuous variables are given as mean ± standard deviation. Conversion factors for units: creatinine in mg/dL to μmol/L, ×88.4; SUN in mg/dL to mmol/L, × 0.357.
Abbreviations: BP, blood pressure; CE-CT, contrast-enhanced computed tomography; CO2, carbon dioxide; eGFR, estimated glomerular filtration rate; NSAID, nonsteroidal anti-inflammatory drug; PCR, protein-creatinine ratio; RAAS, renin-angiotensin-aldosterone system; SUN, serum urea nitrogen.
Patient Outcomes
Four patients missed their follow-up creatinine measurements. CI-AKI occurred in 10 (4.2%) patients in the study group and 17 (6.8%) in the control group (Table 2). The balanced salt solution showed noninferior risks for CI-AKI when compared with the control group (P for noninferiority < 0.001). However, no statistical significance was identified with the incidence of CI-AKI (P = 0.27), AKI using the KDIGO definition (P = 0.33), or death/ dialysis within 6 months from CE-CT (P = 0.74). Absolute changes in eGFRs at the follow-up visit were also similar (P = 0.69).
Table 2.
Univariable Analysis Results for the Study Outcomes
| 0.9% Saline Solution (N = 251)a | Balanced Salt Solution (N = 242)a | P | |
|---|---|---|---|
| CI-AKI | 17 (6.8%) | 10 (4.2%) | 0.27 |
| Absolute change in eGFR, mg/dL | 0.3 ± 2.8 | 0.1 ± 0.2 | 0.69 |
| AKI by KDIGO definition | 27 (10.8%) | 19 (7.9%) | 0.33 |
| Death/dialysis within 6 mo | 9 (3.7%) | 11 (4.7%) | 0.74 |
Note: Values for categorical variables are given as number (percent); values for continuous variables are given as mean ± standard deviation.
Abbreviations: AKI, acute kidney injury; CI-AKI, contrast-induced acute kidney injury; eGFR, estimated glomerular filtration rate; KDIGO, Kidney Disease: Improving Global Outcomes.
Two patients in the study group and 2 patients in the control group missed the follow-up visits for creatinine measurement. Six patients in the study group and 7 patients in the control group did not receive assessment for death/dialysis within 6 months.
When we adjusted for age, sex, history of diabetes and hypertension, baseline eGFR, and urinary protein-creatinine ratio values (Table 3), the difference in outcomes between the 2 groups did not reach statistical significance; CI-AKI (adjusted odds ratio [OR], 78 [95% CI, 0.31-1.89]; P = 0.58), AKI by KDIGO definition (adjusted OR, 0.93 ([95% CI, 0.45-1.87]; P = 0.83), death/dialysis (adjusted OR, 1.29 [95% CI, 0.49-3.50]; P = 0.61], and eGFR changes (P = 0.61).
Table 3.
Multivariable Logistic Regression Analysis Results
| Adjusted OR (95% CI) | P | |
|---|---|---|
| CI-AKI | 0.78 (0.31-1.89) | 0.58 |
| AKI by KDIGO definition | 0.93 (0.45-1.87) | 0.83 |
| Death/dialysis within 6 mo | 1.29 (0.49-3.50) | 0.61 |
Note: ORs were adjusted for baseline age, sex, history of diabetes, hypertension, baseline estimated glomerular filtration rate, and urinary protein-creatinine ratio. The reference group was the control group that received 0.9% saline solution, and the study group received a balanced salt solution.
Abbreviations: AKI, acute kidney injury; CI, confidence interval; CI-AKI, contrast-induced acute kidney injury; KDIGO, Kidney Disease: Improving Global Outcomes; OR, odds ratio.
Clinical characteristics including age (interaction P = 0.44), sex (interaction P = 0.19), history of diabetes (interaction P = 0.38) or hypertension (interaction P = 0.99), eGFR (interaction P = 0.17), or urinary protein-creatinine ratio (interaction P = 0.69) values did not show significant interaction with the association between preventive fluid use and risk for CI-AKI. Therefore, no subgroup analysis was performed.
Safety
Four adverse events occurred from the time of fluid administration and CE-CT until the initial follow-up visits, and no deaths occurred. There were 2 cases of gastrointestinal symptoms or pneumonia that were not considered to be related to the fluid administration or CE-CT. There was 1 case of suspected pulmonary edema in the study group that resolved after a week of hospitalization. There was 1 patient requiring acute dialysis due to possible CI-AKI in the study group that resolved after a few dialysis sessions without proceeding to maintenance dialysis.
Discussion
In this randomized clinical trial, risk for CI-AKI was similar after the use of a balanced salt solution and after use of 0.9% saline solution during CE-CT within the limited number of high-risk patients. However, the primary and secondary outcomes did not improve with the balanced salt solution, and a similar safety profile was observed. Although our trial registration did not meet the initially planned enrollment number, our study suggests that use of a balanced salt solution may be noninferior to the use of 0.9% saline solution regarding the risk for CI-AKI. However, no additional benefits of the administration of a balanced salt solution was proven for CI-AKI prevention after CE-CT.
CI-AKI remains an important clinical issue because use of iodine contrast is common in many interventions and diagnostic procedures, and it has been reported to be associated with an increased risk for adverse patient outcomes.1,2,6,16,17 CI-AKI after CE-CT has different characteristics than AKI after intra-arterial contrast material administration because most patients in the former are in a relatively stable medical condition and the amount of the contrast material is controlled, leading to a low incidence.1 However, CI-AKI after CE-CT has been reported to be associated with worse patient prognosis.3,18 Contrarily, the recent multicenter AMACING clinical trial showed that use of 0.9% saline solution was similar to taking no preventive measures with respect to the incidence of CI-AKI after CE-CT.13 Therefore, the efficacy of 0.9% saline solution has been questioned, and additional investigation for a better prophylactic method is warranted, particularly in a patient group with higher risk for CI-AKI.
In this clinical trial, we tested the efficacy and safety of a balanced salt solution, which has shown better outcomes than normal saline solution in other clinical settings,11 for CI-AKI prevention in patients undergoing CE-CT. Our patient group had multiple risk factors or severely decreased kidney function; thus, we aimed to assess the use of a balanced salt solution in a patient group with a prominent risk for CI-AKI. Although the study failed to meet the target number of participants, in the non-negligible number of the finally studied patients, the balanced salt solution group showed similar risk for CI-AKI and other secondary outcomes when compared with the 0.9% saline solution group. The study suggested that a balanced salt solution may be noninferior to 0.9% saline solution regarding the risk for CI-AKI, but whether the specific benefits of the fluid for CI-AKI after CE-CT exist has not been answered here.
The balanced salt solution may provide benefits over 0.9% saline solution because it contains physiologic levels of chloride, decreasing the possibility of developing metabolic acidosis.8,9 Similar expectations were present regarding the use of sodium bicarbonate fluids, but recent trials have shown that the benefits of sodium bicarbonate are uncertain.7,19,20 Recent studies in critical care settings or with health volunteers have suggested the benefits of the balanced salt solution.8,9,11 A clinical trial was performed using perioperative fluids, but the trial did not demonstrate any benefits of using a balanced salt solution for patients who underwent surgery.12 Our patients were more clinically stable than those in other settings, and we considered that in such a clinical environment, the pure effect of a prophylactic fluid administration could be revealed. However, use of a balanced salt solution did not show additional benefits for CI-AKI prevention in this trial with a considerable number of patients, and thus the advantage of a balanced solution over 0.9% saline solution is still in question.
Our study has several limitations. First, the study did not meet the calculated number of patients needed to be appropriately powered. The robust statistical confirmation of our trial cannot be guaranteed due to its low power; thus, our study results can only address the possibilities. With the reported incidence of CI-AKI in our study, 968 patients with follow-up completion would be necessary for an adequately powered noninferiority trial. However, our study results may be referred to when designing a further trial or be implemented in a meta-analysis searching for an appropriate measure in the field of CI-AKI prophylaxis.
Second, our study did not address potential benefits of prevention of CI-AKI during CE-CT over no prevention. After the recent report from the AMACING trial, the benefits of CI-AKI prophylactic measure during CE-CT itself has been questioned. Thus, a future clinical trial may consider including a control group without intervention and testing whether a prophylactic method, even the use of 0.9% saline solution, is practically necessary before performing CE-CT in clinically stable patients. In addition, because CI-AKI risk after CE-CT is relatively low, such a future trial could target a patient group with higher risks of CI-AKI or those who receive cardiovascular procedures using iodine contrasts.
Last, the study was performed in South Korea with little ethnic diversity; thus, our study cannot be generalized to patients of non-Asian ethnicity.
In conclusion, in this trial with limited study enrollment, use of a balanced salt solution during CE-CT may be noninferior to the use of 0.9% saline solution regarding the risk for CI-AKI. Whether there are clinical benefits of a balanced salt solution over 0.9% saline solution remains uncharacterized. Further studies demonstrating an efficacy-proven CI-AKI prevention method are warranted.
Article Information
Authors’ Full Names and Academic Degrees
Sehoon Park, MD, Dong Ki Kim, MD, PhD, Hee-Yeon Jung, MD, PhD, Chan-Duck Kim, MD, PhD, Jang-Hee Cho, MD, PhD, Ran-hui Cha, MD, PhD, Jong Cheol Jeong, MD, PhD, Sejoong Kim, MD, PhD, Hyung-Jong Kim, MD, PhD, Tae Hyun Ban, MD, Byung Ha Chung, MD, PhD, Jung Pyo Lee, MD, PhD, Jung Tak Park, MD, PhD, Seung Hyeok Han, MD, PhD, Tae-Hyun Yoo, MD, PhD, Dong-Ryeol Ryu, MD, PhD, Sung Jin Moon, MD, PhD, Jung Eun Lee, MD, PhD, Wooseong Huh, MD, PhD, Ea Wha Kang, MD, PhD, Tae Ik Chang, MD, PhD, and Kwon Wook Joo, MD, PhD.
Authors’ Contributions
Research idea and study design: DKK; study protocol: KWJ, DKK, SP; patient enrollment and data acquisition: SP, TIC, EWK, H-YJ, C-DK, J-HC, R-hC, JCJ, SK, H-JK, THB, BHC, JPL, JTP, SHH, T-HY, D-RR, SJM, JEL, WH, DKK, and KWJ; data interpretation and statistical analysis: SP, TIC, DKK; principal investigator and supervision: KWJ. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Support
This study is funded by CJ Healthcare Corporation (CS2015_0046), Seoul, Korea. The funder did not have any role in study design; collection, analysis, or interpretation of data; writing the report; or the decision to submit the report for publication.
Financial Disclosure
The authors declare that they have no relevant financial interests.
Data Sharing
The study protocol has been published before. The anonymized data that support the findings of this study are available from the corresponding author on reasonable request.
Peer Review
Received September 1, 2019. Evaluated by 2 external peer reviewers, with direct editorial input from the Statistical Editor, an Associate Editor, and the Editor-in-Chief. Accepted in revised form December 12, 2019.
Footnotes
Complete author and article information provided before references.
Contributor Information
Tae Ik Chang, Email: kidneyjang@gmail.com.
Kwon Wook Joo, Email: junephro@gmail.com.
References
- 1.Weisbord S.D., Mor M.K., Resnick A.L., Hartwig K.C., Palevsky P.M., Fine M.J. Incidence and outcomes of contrast-induced AKI following computed tomography. Clin J Am Soc Nephrol. 2008;3(5):1274–1281. doi: 10.2215/CJN.01260308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.James M.T., Ghali W.A., Tonelli M. Acute kidney injury following coronary angiography is associated with a long-term decline in kidney function. Kidney Int. 2010;78(8):803–809. doi: 10.1038/ki.2010.258. [DOI] [PubMed] [Google Scholar]
- 3.Kim S.M., Cha R.H., Lee J.P. Incidence and outcomes of contrast-induced nephropathy after computed tomography in patients with CKD: a quality improvement report. Am J Kidney Dis. 2010;55(6):1018–1025. doi: 10.1053/j.ajkd.2009.10.057. [DOI] [PubMed] [Google Scholar]
- 4.Park S., Kim M.H., Kang E. Contrast-induced nephropathy after computed tomography in stable CKD patients with proper prophylaxis: 8-year experience of outpatient prophylaxis program. Medicine (Baltimore) 2016;95(18):e3560. doi: 10.1097/MD.0000000000003560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.KDIGO Clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2(1):116–121. [Google Scholar]
- 6.ACR Committee on Drugs and Contrast Media ACR Manual on Contrast Media. 2016. https://www.acr.org/Clinical-Resources/Contrast-Manual
- 7.Brar S.S., Shen A.Y., Jorgensen M.B. Sodium bicarbonate vs sodium chloride for the prevention of contrast medium-induced nephropathy in patients undergoing coronary angiography: a randomized trial. JAMA. 2008;300(9):1038–1046. doi: 10.1001/jama.300.9.1038. [DOI] [PubMed] [Google Scholar]
- 8.Chowdhury A.H., Cox E.F., Francis S.T., Lobo D.N. A randomized, controlled, double-blind crossover study on the effects of 2-L infusions of 0.9% saline and plasma-lyte(R) 148 on renal blood flow velocity and renal cortical tissue perfusion in healthy volunteers. Ann Surg. 2012;256(1):18–24. doi: 10.1097/SLA.0b013e318256be72. [DOI] [PubMed] [Google Scholar]
- 9.Chowdhury A.H., Cox E.F., Francis S.T., Lobo D.N. A randomized, controlled, double-blind crossover study on the effects of 1-L infusions of 6% hydroxyethyl starch suspended in 0.9% saline (Voluven) and a balanced solution (Plasma Volume Redibag) on blood volume, renal blood flow velocity, and renal cortical tissue perfusion in healthy volunteers. Ann Surg. 2014;259(5):881–887. doi: 10.1097/SLA.0000000000000324. [DOI] [PubMed] [Google Scholar]
- 10.Shaw A.D., Schermer C.R., Lobo D.N. Impact of intravenous fluid composition on outcomes in patients with systemic inflammatory response syndrome. Crit Care. 2015;19:334. doi: 10.1186/s13054-015-1045-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yunos N.M., Bellomo R., Hegarty C., Story D., Ho L., Bailey M. Association between a chloride-liberal vs chloride-restrictive intravenous fluid administration strategy and kidney injury in critically ill adults. JAMA. 2012;308(15):1566–1572. doi: 10.1001/jama.2012.13356. [DOI] [PubMed] [Google Scholar]
- 12.Semler M.W., Wanderer J.P., Ehrenfeld J.M. Balanced crystalloids versus saline in the intensive care unit. The SALT randomized trial. Am J Respir Crit Care Med. 2017;195(10):1362–1372. doi: 10.1164/rccm.201607-1345OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nijssen E.C., Rennenberg R.J., Nelemans P.J. Prophylactic hydration to protect renal function from intravascular iodinated contrast material in patients at high risk of contrast-induced nephropathy (AMACING): a prospective, randomised, phase 3, controlled, open-label, non-inferiority trial. Lancet. 2017;389(10076):1312–1322. doi: 10.1016/S0140-6736(17)30057-0. [DOI] [PubMed] [Google Scholar]
- 14.Jo H.A., Park S., Kim C.D. Efficacy and safety of a balanced salt solution versus a 0.9% saline infusion for the prevention of contrast-induced acute kidney injury (BASIC trial): a study protocol for a randomized controlled trial. Trials. 2017;18(1):461. doi: 10.1186/s13063-017-2202-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jeon J., Kim S., Yoo H. Risk prediction for contrast-induced nephropathy in cancer patients underoing computed tomography under preventive measures. J Oncol. 2019;2019:8736163. doi: 10.1155/2019/8736163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barrett B.J., Parfrey P.S. Preventing nephropathy induced by contrast medium. N Engl J Med. 2006;354(4):379–386. doi: 10.1056/NEJMcp050801. [DOI] [PubMed] [Google Scholar]
- 17.Rihal C.S., Textor S.C., Grill D.E. Incidence and prognostic importance of acute renal failure after percutaneous coronary intervention. Circulation. 2002;105(19):2259–2264. doi: 10.1161/01.cir.0000016043.87291.33. [DOI] [PubMed] [Google Scholar]
- 18.Mitchell A.M., Kline J.A., Jones A.E., Tumlin J.A. Major adverse events one year after acute kidney injury after contrast-enhanced computed tomography. Ann Emerg Med. 2015;66(3):267–274. doi: 10.1016/j.annemergmed.2015.04.028. [DOI] [PubMed] [Google Scholar]
- 19.Zoungas S., Ninomiya T., Huxley R. Systematic review: sodium bicarbonate treatment regimens for the prevention of contrast-induced nephropathy. Ann Intern Med. 2009;151(9):631–638. doi: 10.7326/0003-4819-151-9-200911030-00008. [DOI] [PubMed] [Google Scholar]
- 20.Choi H., Jo H.A., Park S. Efficacy and safety of a balanced salt solution versus a 0.9% saline infusion for the prevention of contrast-induced acute kidney injury (BASIC trial): a study protocol for a randomized controlled trial. Trials. 2017;18:461. doi: 10.1186/s13063-017-2202-2. [DOI] [PMC free article] [PubMed] [Google Scholar]