Related Article, p 248
Angiotensin-converting enzyme (ACE) inhibitors1 and angiotensin receptor blockers (ARBs)2 were major therapeutic advances, with strong evidence from large randomized controlled trials supporting the use of renin–angiotensin system (RAS) blockade to lower blood pressure (BP) and prevent-target organ damage in hypertension, reduce mortality in heart failure, and lower proteinuria and slow the progressive loss of kidney function in patients with kidney disease.3 Given that ACE inhibitors and ARBs are well tolerated, these agents are recommended as first-line antihypertensive therapies by international hypertension, heart failure, diabetes, and kidney disease guidelines.4, 5, 6
Both ACE inhibitors and ARBs induce what could be considered “functional” side effects linked to RAS inhibition, specifically hyperkalemia due to inhibition of aldosterone secretion and an acute reversible decline in glomerular filtration rate (GFR) at initiation of therapy, generally around 10% to 20% depending on the baseline GFR.7,8 This GFR reduction is due to the ability of ACE inhibitors and ARBs both to lower systemic BP and vasodilate the renal efferent arterioles, thereby lowering intraglomerular pressure.9,10 Although these events occur at any level of GFR, the risk for meaningful reductions in GFRs is clearly highest in individuals with advanced chronic kidney disease (CKD). Thus, the incidence of hyperkalemia (serum potassium ≥ 5.5 mmol/L) associated with RAS blockade is ∼30% in patients with CKD stages 4 to 5.10 Concerns regarding GFR decline are clearly greater in patients with advanced CKD. However, GFR decline is a frequent cause of RAS blocker treatment withdrawal in patients with earlier CKD stages.7,8 In the general population, an increase in serum creatinine level of 10% to 30% occurs in 14% of patients and an increase >30% affects 1.7% of patients following RAS blockade.8
The risks for hyperkalemia and GFR decline have generated several concerns and questions regarding maintaining RAS blocker therapy in patients with advanced CKD. Should RAS blockers be stopped below certain levels of GFR? Are RAS blockers still useful to prevent cardiovascular mortality and kidney disease progression in advanced CKD? Is mortality increased if RAS blockade is stopped? Although the answers to these questions are uncertain, reflecting the absence of clinical trials specifically addressing these issues, several studies have provided insights on how to answer these questions and help guide clinical practice. In this issue of Kidney Medicine, Arora et al11 add important insight to these questions by assessing the impact of patterns of use of RAS blockers on all-cause mortality and progression to end-stage kidney disease in a subset of patients with advanced CKD participating in the Chronic Renal Insufficiency Cohort (CRIC) Study.
Arora et al11 performed a retrospective investigation of 678 patients of the CRIC cohort with an estimated GFR (eGFR) < 30 mL/min/1.73 m2 at baseline. In contrast to previous studies, these investigators defined 4 treatment strategy patterns of RAS blocker use during the first year of inclusion in CRIC; specifically “always users,” “never users,” “dynamic users” (on and off users of RAS inhibitors), and “new users.” Propensity scores were used to match participants by treatment strategy, with always users serving as the reference group. Cox regression models were used to investigate associations between treatment strategies and kidney and mortality outcomes.
The most frequent treatment strategy was always users (57%), followed by never users (23%), dynamic users (13%), and new users (7%). Participants were matched for several parameters, including age, sex, race, diabetes, hypertension, serum potassium level, and systolic BP. Overall, no difference in the risk for kidney failure or mortality was observed between the patterns even after further adjustment for proteinuria, heart failure, and atherosclerotic cardiovascular diseases and although always users had the lowest BPs and proteinuria. However, there were some nonsignificant trends in these analyses, such as an increased rate of progression to kidney failure in dynamic users and lower mortality in new users compared with always users.
The results of this study suggest that RAS inhibition in patients with advanced CKD is rather safe. However, because RAS blockade appears neither beneficial nor detrimental in terms of hard end points, one could also question the pertinence of maintaining RAS blocker therapy in advanced CKD. The answer is not so simple. The retrospective design used by Arora et al11 cannot fully inform this question. To answer this question, always users of RAS blockers would need to be randomly assigned to continue or stop RAS blocker therapy and be followed up for sufficient time to allow for progression to kidney failure.
Today, it is well accepted that RAS blockers are most effective in slowing CKD progression when prescribed in the early stages of kidney disease, but whether RAS blockers are as effective in advanced CKD is now increasingly challenged.12 In post hoc analyses of the REIN (Ramipril Efficacy in Nephropathy) and RENAAL (Reduction in End Points in Non-Insulin-Dependent Diabetes With the Angiotensin II Antagonist Losartan) trials, Ruggenenti et al13 and Remuzzi et al14 reported that the reduction of kidney failure events with ACE inhibitors and ARBs was not a function of the baseline GFR. In these analyses, the reduction in the absolute number of patients reaching kidney failure was higher in patients among the lowest tertile of GFR as compared with those with higher GFRs at baseline.13,14 A similar finding was seen in patients with CKD stage 4 treated with benazepril,15 in which carefully selected patients derived a benefit from ACE inhibitor therapy, even at very low eGFRs. In another post hoc analysis of RENAAL,16 the magnitude of the acute decrease in eGFRs observed when losartan therapy was started was predictive of the long-term GFR decline. Viewed in sum, these trials suggest that RAS blockade is effective in reducing the risk for kidney failure in both earlier and advanced CKD.
This position was reinforced in a 2012 KDIGO (Kidney Disease: Improving Global Outcomes) clinical practice guideline for the management of BP in CKD, in which, in the absence of severe hyperkalemia (potassium > 6 mmol/L), acute kidney injury, or a decrease in eGFR > 30%,17 continuing RAS blockade was suggested.
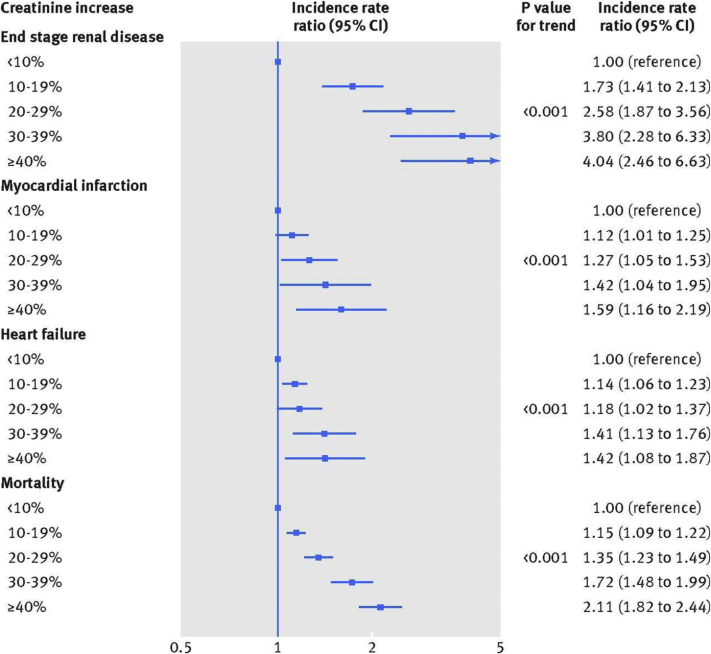
Since 2012, new data have become available that may modulate these recommendations. New data from general population studies suggest that the acute decline in GFR observed on starting treatment with an RAS inhibitor is associated with kidney and cardiac risks in a “dose-response” relation, with no distinct cutoff at a 30% increase in serum creatinine level8 (Fig 1). The risk for adverse cardiac and kidney outcomes associated with a change in kidney function was highest during the first year after initiation but persisted up to 10 years for end-stage kidney disease and all-cause mortality. Patients with the greatest GFR decline were those with multiple comorbid conditions, such as heart failure and diabetes. This meta-analysis suggests that one should consider the risk of RAS blockade among all individuals with an acute decline in kidney function rather than only in those with a 30% change.
Figure 1.
Kidney, cardiac, and mortality risks associated with creatinine level increase after blockade of the renin-angiotensin system in UK primary care (n = 122,363 patients; from reference 8, CC BY 4.0 license). Abbreviation: CI, confidence interval.
A post hoc analysis of the ADVANCE (Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation) trial provides interesting additional insights on the risk associated with an acute decline in kidney function following RAS blockade initiation.18 As observed in the general population,8 any increase in serum creatinine level > 10% was associated with significant greater risks for subsequent major clinical outcomes. Nevertheless, when viewing the entire group of participants randomly assigned to receive an ACE inhibitor, ACE inhibitor use was associated with lower long-term risk for major clinical outcomes when compared with placebo, irrespective of the severity of the acute increase in serum creatinine level.18 This suggests that even if drug-induced increases in serum creatinine levels are associated with higher cardiorenal risk, patients should be maintained on the RAS blocker therapy because their long-term outcome is better.
Similar observations were made in a post hoc analysis of the ONTARGET (Ongoing Telmisartan Alone and in Combination With Ramipril Global Endpoint Trial) and TRANSCEND (Telmisartan Randomised Assessment Study in ACE Intolerant Subjects With Cardiovascular Disease) trials performed in patients with high cardiovascular risk.19 The data from Arora et al11 also suggest that being on and off RAS blockade treatment, for example, because of fluctuating GFRs or hyperkalemia, is associated with higher risk for progressing to kidney failure. This observation may be explained by the higher percentage of patients with CKD with severe heart failure in the group of dynamic users. In heart failure, a decline in GFR may reflect worsening of cardiac function rather than an intrinsic kidney process.
In the Arora et al11 study, 23% of patients with advanced CKD never used an RAS blocker, although in this group, 92% of patients had hypertension and ∼50% had diabetes. Unfortunately, the analysis does not provide insight on the reasons why these patients did not receive RAS blockers despite guideline recommendations.
Interestingly, this percentage is lower than recent figures reported by Murphy et al.20 Among 7,085 adults with CKD participating in the National Health and Nutrition Examination Survey in 2011 to 2014, a total of 40.1% of patients were receiving an ACE inhibitor or an ARB. In the subset of participants with CKD stage 4, a total of 45% of patients discontinued their RAS blocker therapy within 1 year and the rate of discontinuation at 5 years was 83%; similar findings were seen in a study from the United Kingdom.21,22 The primary reasons for discontinuation were hyperkalemia, CKD progression, hospitalization for acute kidney injury, and the presence of multiple comorbid conditions. Very few individuals who discontinued subsequently resumed RAS blockade.
These relatively disappointing utilization data in advanced CKD confirm the global underuse of RAS blockers, which may reflect the lack of confidence of physicians, including nephrologists, on the real benefits of inhibiting the RAS in advanced CKD. Reflecting that no trials have specifically assessed the clinical benefits of RAS blockade in CKD stage 4, physicians may feel uncomfortable when clinical issues arise, such as a sudden worsening of kidney function leading to hospitalization or significant hyperkalemia. In these situations, physicians may prefer withdrawing any drug that may cause problems for the patients, minimizing perceived acute risk but potentially at the cost of increasing their long-term kidney failure and mortality risk. In that respect, the data presented in the study by Arora et al11 should reassure clinicians.
Some studies have demonstrated that stopping RAS blockade treatment in advanced CKD may be beneficial in some patients, increasing the time to dialysis initiation. Ahmed et al23 showed in a cohort of 52 patients with CKD grade 4 that RAS blockade withdrawal was associated with a 10-mL/min/1.73 m2 increase in eGFR and an increase in BP. Given the tremendous burden of dialysis, this finding requires additional investigation.
Given the existing uncertainty and the absence of clear guideline recommendations, the most prudent approach should be evaluating the benefit-risk ratio of maintaining RAS blockade on an individual basis. Consideration of RAS blockade discontinuation should occur only when serious clinical problems arise, such as a substantial GFR decline, severe hypokalemia that cannot be readily modified, symptomatic hypotension, severe acidosis in the setting of very low GFR, and, potentially, in the very elderly. We desperately need more data from prospective randomized controlled trials assessing the risks of withdrawing RAS blocker treatment in advanced CKD. The results of the ongoing STOP-ACEi (Multi-centre Randomised Controlled Trial of Angiotensin Converting Enzyme Inhibitor [ACEi]/Angiotensin Receptor Blocker [ARB] Withdrawal in Advanced Renal Disease) trial,24 a multicenter, open-label, randomized, controlled, clinical trial, may answer some of our questions and are eagerly expected.
In the meantime, new opportunities exist for the management of patients with CKD. Hyperkalemia can be effectively controlled with the newer orally active potassium binders, potentially enabling ongoing RAS blockade.25,26 New agents, including sodium-glucose co-transporter 2 inhibitors, appear to slow CKD progression and heart failure when used in conjunction with RAS blockade.27,28 These therapeutic developments, along with other anticipated breakthroughs, offer broader therapeutic options to treat CKD, emphasizing the critical need for kidney providers to optimize the safe and effective use of RAS blockade as one aspect rather than our only current option for slowing progression in advanced CKD.
Article Information
Author’s Full Name and Academic Degrees
Michel Burnier, MD.
Support
None.
Financial Disclosure
The author declares that he has no relevant financial interests.
Peer Review
Received January 16, 2020, in response to an invitation from the journal. Direct editorial input by the Editor-in-Chief. Accepted in revised form March 30, 2020.
References
- 1.Brunner H.R., Gavras H., Waeber B. Oral angiotensin-converting enzyme inhibitor in long-term treatment of hypertensive patients. Ann Intern Med. 1979;90(1):19–23. doi: 10.7326/0003-4819-90-1-19. [DOI] [PubMed] [Google Scholar]
- 2.Burnier M. Angiotensin II type 1 receptor blockers. Circulation. 2001;103(6):904–912. doi: 10.1161/01.cir.103.6.904. [DOI] [PubMed] [Google Scholar]
- 3.Werner C., Baumhakel M., Teo K.K. RAS blockade with ARB and ACE inhibitors: current perspective on rationale and patient selection. Clin Res Cardiol. 2008;97(7):418–431. doi: 10.1007/s00392-008-0668-3. [DOI] [PubMed] [Google Scholar]
- 4.Williams B., Mancia G., Spiering W. 2018 ESC/ESH guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Cardiology and the European Society of Hypertension: the Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens. 2018;36(10):1953–2041. doi: 10.1097/HJH.0000000000001940. [DOI] [PubMed] [Google Scholar]
- 5.Taler S.J., Agarwal R., Bakris G.L. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for management of blood pressure in CKD. Am J Kidney Dis. 2013;62(2):201–213. doi: 10.1053/j.ajkd.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ponikowski P., Voors A.A., Anker S.D. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 7.Jackevicius C.A., Wong J., Aroustamian I., Gee M., Mody F.V. Rates and predictors of ACE inhibitor discontinuation subsequent to elevated serum creatinine: a retrospective cohort study. BMJ Open. 2014;4(8) doi: 10.1136/bmjopen-2014-005181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmidt M., Mansfield K.E., Bhaskaran K. Serum creatinine elevation after renin-angiotensin system blockade and long term cardiorenal risks: cohort study. BMJ. 2017;356:j791. doi: 10.1136/bmj.j791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maddirala S., Khan A., Vincent A., Lau K. Effect of angiotensin converting enzyme inhibitors and angiotensin receptor blockers on serum potassium levels and renal function in ambulatory outpatients: risk factors analysis. Am J Med Sci. 2008;336(4):330–335. doi: 10.1097/MAJ.0b013e3181836ac7. [DOI] [PubMed] [Google Scholar]
- 10.Bandak G., Sang Y., Gasparini A. Hyperkalemia after initiating renin-angiotensin system blockade: the Stockholm Creatinine Measurements (SCREAM) Project. J Am Heart Assoc. 2017;6(7):e005428. doi: 10.1161/JAHA.116.005428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arora N., Katz R., Bansal N. ACE inhibitor/angiotensin receptor blocker use patterns in advanced CKD and risk of kidney failure and death. Kidney Med. 2020;2(3):248–257. doi: 10.1016/j.xkme.2019.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahmed A., Jorna T., Bhandari S. Should we STOP angiotensin converting enzyme inhibitors/angiotensin receptor blockers in advanced kidney disease? Nephron. 2016;133(3):147–158. doi: 10.1159/000447068. [DOI] [PubMed] [Google Scholar]
- 13.Ruggenenti P., Perna A., Remuzzi G., Gruppo Italiano di Studi Epidemiologici in Nefrologia ACE inhibitors to prevent end-stage renal disease: when to start and why possibly never to stop: a post hoc analysis of the REIN trial results. Ramipril Efficacy in Nephropathy. J Am Soc Nephrol. 2001;12(12):2832–2837. doi: 10.1681/ASN.V12122832. [DOI] [PubMed] [Google Scholar]
- 14.Remuzzi G., Ruggenenti P., Perna A. Continuum of renoprotection with losartan at all stages of type 2 diabetic nephropathy: a post hoc analysis of the RENAAL trial results. J Am Soc Nephrol. 2004;15(12):3117–3125. doi: 10.1097/01.ASN.0000146423.71226.0C. [DOI] [PubMed] [Google Scholar]
- 15.Hou F.F., Zhang X., Zhang G.H. Efficacy and safety of benazepril for advanced chronic renal insufficiency. N Engl J Med. 2006;354(2):131–140. doi: 10.1056/NEJMoa053107. [DOI] [PubMed] [Google Scholar]
- 16.Holtkamp F.A., de Zeeuw D., Thomas M.C. An acute fall in estimated glomerular filtration rate during treatment with losartan predicts a slower decrease in long-term renal function. Kidney Int. 2011;80(3):282–287. doi: 10.1038/ki.2011.79. [DOI] [PubMed] [Google Scholar]
- 17.KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int Suppl. 2012;2(5):372–376. [Google Scholar]
- 18.Ohkuma T., Jun M., Rodgers A. Acute increases in serum creatinine after starting angiotensin-converting enzyme inhibitor-based therapy and effects of its continuation on major clinical outcomes in type 2 diabetes mellitus. Hypertension. 2019;73(1):84–91. doi: 10.1161/HYPERTENSIONAHA.118.12060. [DOI] [PubMed] [Google Scholar]
- 19.Clase C.M., Barzilay J., Gao P. Acute change in glomerular filtration rate with inhibition of the renin-angiotensin system does not predict subsequent renal and cardiovascular outcomes. Kidney Int. 2017;91(3):683–690. doi: 10.1016/j.kint.2016.09.038. [DOI] [PubMed] [Google Scholar]
- 20.Murphy D.P., Drawz P.E., Foley R.N. Trends in angiotensin-converting enzyme inhibitor and angiotensin II receptor blocker use among those with impaired kidney function in the United States. J Am Soc Nephrol. 2019;30(7):1314–1321. doi: 10.1681/ASN.2018100971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mahmoudpour S.H., Asselbergs F.W., Souverein P.C., de Boer A., Maitland-van der Zee A.H. Prescription patterns of angiotensin-converting enzyme inhibitors for various indications: a UK population-based study. Br J Clin Pharmacol. 2018;84(10):2365–2372. doi: 10.1111/bcp.13692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qiao Y., Shin J.I., Sang Y. Discontinuation of angiotensin converting enzyme inhibitors and angiotensin receptor blockers in chronic kidney disease. Mayo Clin Proc. 2019;94(11):2220–2229. doi: 10.1016/j.mayocp.2019.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahmed A.K., Kamath N.S., El Kossi M., El Nahas A.M. The impact of stopping inhibitors of the renin-angiotensin system in patients with advanced chronic kidney disease. Nephrol Dial Transplant. 2010;25(12):3977–3982. doi: 10.1093/ndt/gfp511. [DOI] [PubMed] [Google Scholar]
- 24.Bhandari S., Ives N., Brettell E.A. Multicentre randomized controlled trial of angiotensin-converting enzyme inhibitor/angiotensin receptor blocker withdrawal in advanced renal disease: the STOP-ACEi trial. Nephrol Dial Transplant. 2016;31(2):255–261. doi: 10.1093/ndt/gfv346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kosiborod M., Rasmussen H.S., Lavin P. Effect of sodium zirconium cyclosilicate on potassium lowering for 28 days among outpatients with hyperkalemia: the HARMONIZE randomized clinical trial. JAMA. 2014;312(21):2223–2233. doi: 10.1001/jama.2014.15688. [DOI] [PubMed] [Google Scholar]
- 26.Weir M.R., Bakris G.L., Bushinsky D.A. Patiromer in patients with kidney disease and hyperkalemia receiving RAAS inhibitors. N Engl J Med. 2015;372(3):211–221. doi: 10.1056/NEJMoa1410853. [DOI] [PubMed] [Google Scholar]
- 27.Neuen B.L., Young T., Heerspink H.J.L. SGLT2 inhibitors for the prevention of kidney failure in patients with type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2019;11:845–854. doi: 10.1016/S2213-8587(19)30256-6. [DOI] [PubMed] [Google Scholar]
- 28.Zelniker T.A., Braunwald E. Cardiac and renal effects of sodium-glucose co-transporter 2 inhibitors in diabetes: JACC state-of-the-art review. J Am Coll Cardiol. 2018;72(15):1845–1855. doi: 10.1016/j.jacc.2018.06.040. [DOI] [PubMed] [Google Scholar]