To the Editor:
Inflammatory glomerular diseases, including immunoglobulin A nephropathy (IgAN), Henoch-Schönlein purpura nephritis (IgA vasculitis), membranoproliferative glomerulonephritis, and crescentic glomerulonephritis, are an important cause of end-stage kidney disease. Glomerular inflammation with leukocyte influx and subsequent glomerular basement membrane (GBM) disruption lead to hematuria and proteinuria, eventually resulting in glomerular sclerotic scar lesions. Although some studies have reported on GBM disruption such as gaps shown using transmission electron microscopy (TEM),1,2 the interaction and pathologic changes among glomerular constituent cells resulting from GBM injury remain unclear at the 3-dimensional (3D) ultrastructural level, especially in human biopsy samples.
We analyzed human biopsy samples from patients with IgAN using serial block-face scanning electron microscopy (SBF-SEM) by reconstructing the images on automated serial sections.3 We assessed 3D images of GBM disruption and investigated the relationships among glomerular components in disrupted areas. Serial images of renal glomeruli were examined using SBF-SEM for 7 Japanese patients with biopsy-proven IgAN (Table S1). Fresh kidney tissues from kidney biopsy were cut into small pieces. One piece was fixed in 10% formalin/phosphate buffer solution, and the other pieces were fixed in 2.5% glutaraldehyde/phosphate buffer solution for TEM and SEM. The preparation, imaging, and analysis of SBF-SEM samples have been described previously4, 5, 6 and are detailed in Item S1.
Briefly, tissues were treated with reduced osmium tetroxide, thiocarbohydrazide, and osmium tetroxide. Following en bloc staining with uranyl acetate and lead aspartate, tissues were dehydrated and embedded in conductive resin. They were then imaged in a field emission–SEM (Merlin or Sigma, Carl Zeiss) equipped with 3View (Gatan). The serial images were processed with the Fiji image processing platform (http://fiji.sc/wiki/index.php/Fiji). Segmentation and image analyses were performed with the Microscopy Image Browser7 and Amira (FEI Visualization Science Group). After studying the GBM on each serial image, GBM disruption was identified in 2 of 7 patients.
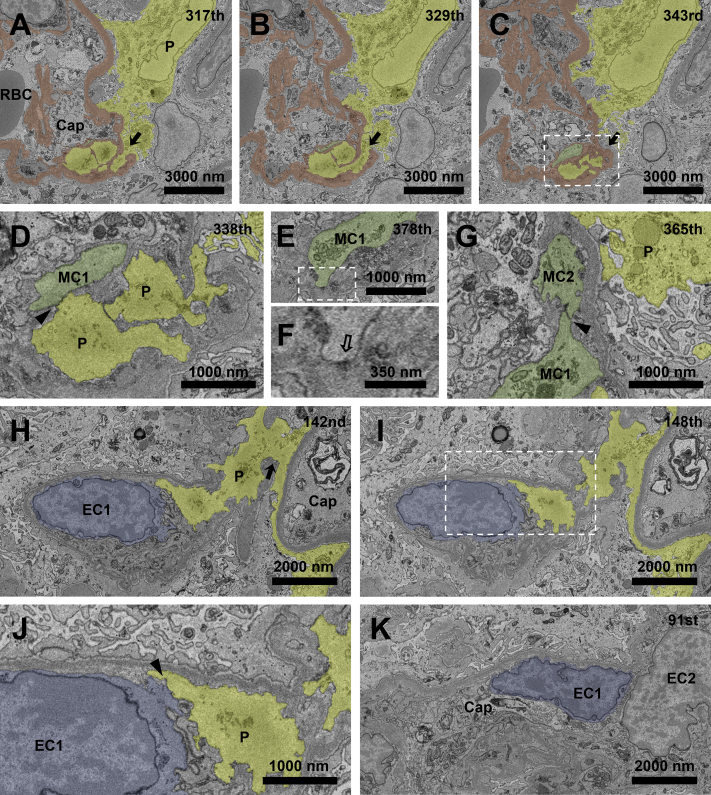
Case 1 was a man in his 20s (Table S1, patient 1) who had microscopic hematuria (>100 red blood cells/high-power field) and proteinuria (protein-creatinine ratio, 4.11 g/g creatinine) diagnosed at an annual health examination 4 years prior. Using SBF-SEM, we detected GBM disruption in 6 different glomerular capillary segments (Fig 1). The cytoplasmic processes of the podocytes penetrated the GBM in all disrupted areas (Fig 1A-C; Movie S1). The penetrating cytoplasm of the podocyte contacted the mesangial cell (Fig 1D), which had the hallmark dense patch on the cytoplasmic membrane (Fig 1E and F), in the area of mesangial interposition. Furthermore, these mesangial cells contacted other mesangial cells, forming a gap junction within the glomerular tuft (Fig 1G). In the other glomerular loop, the penetrating cytoplasm of a podocyte with a flattened foot process passed underneath the GBM through the disruption (Fig 1H) and contacted the endothelial cell cytoplasm (Fig 1I and J), which had no dense patch on the cell membrane and was exposed directly to the glomerular capillary lumen (Fig 1K).
Figure 1.
Penetrating podocytes and mesangial or endothelial cells make intercellular contact through the disrupted glomerular basement membrane (GBM). (A-C) GBM disruption is detected with cytoplasmic penetration of a podocyte at the 317th and 329th sections (A, B, arrows), whereas the GBM regains continuity at the 343rd section (C, arrow). (D) Podocyte cytoplasmic process contacts a mesangial cell (MC1) in the area of mesangial interposition at the 338th section (arrowhead). (E, F) The corresponding mesangial cell has a hallmark dense patch (hollow arrow) on the cytoplasmic membrane. (G) This mesangial cell (MC1) contacts another mesangial cell (MC2), forming a gap junction in the capillary lumen at the 365th section (arrowhead). (H) In the other glomerular loop, a cytoplasmic process of the podocyte penetrates the disrupted GBM (arrow). (I-K) The podocyte cytoplasm contacts the cytoplasm of the endothelial cell (arrowhead) that is exposed directly to the glomerular capillary lumen. The areas marked with a dotted rectangle line in (C, E, and I) are magnified in D, F, and J, respectively. Podocytes, GBM, mesangial cells, and endothelial cells are yellow, brown, green, and blue, respectively. Abbreviations: Cap, capillary lumen; EC, endothelial cell; P, podocytes; RBC, red blood cell.
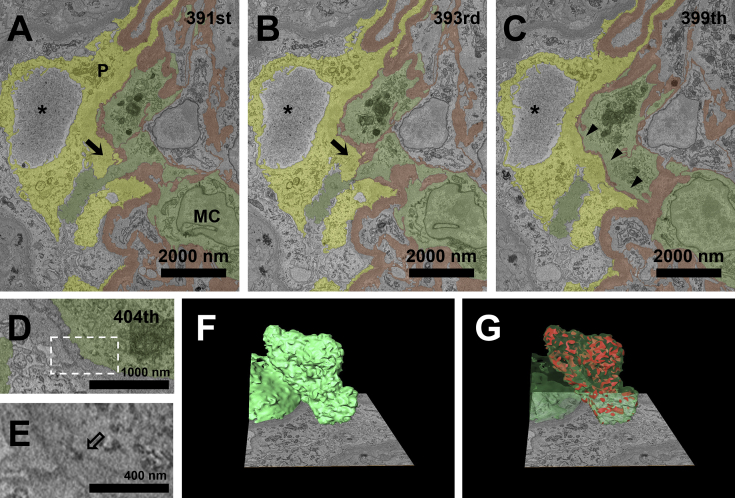
Case 2 was a woman in her 30s (Table S1, patient 2) who had persistent microscopic hematuria (30-50 red blood cells/high-power field) and proteinuria (protein-creatinine ratio, 4.17 g/g creatinine) for 6 years after the delivery of her first daughter. Using SBF-SEM, we detected 2 areas of disrupted GBM with cytoplasmic penetration (Fig 2A-C). Unlike case 1, the nucleus and most of the cytoplasm of the penetrating cell were present in the area of mesangial interposition of the GBM. The dense patch beneath the cytoplasmic membrane showed that the penetrating cytoplasm belonged to a mesangial cell (Fig 2D and E). The mesangial cytoplasmic process protruded into the urinary space through the disrupted GBM and made contact with the podocytes (Fig 2A-C). The 3D reconstruction of the penetrating mesangial cell and podocytes showed multiple contacting points outside the GBM (Fig 2F and G; Movie S2).
Figure 2.
A penetrating mesangial cell (MC) and podocytes (P) make intercellular contact through the disrupted glomerular basement membrane (GBM). (A, B) Cytoplasm of an MC penetrates the urinary space through the disrupted GBM (arrows) at the 391st and 393rd sections. (C) The disrupted GBM regains continuity at the 399th section, showing marked attenuation along the cytoplasm of the penetrating MC (arrowheads). Podocytes surrounding the disrupted area exhibit foot-process effacement and pseudocyst formation (asterisks). (D, E) The corresponding MC has the hallmark dense patch (hollow arrow) beneath the cytoplasmic membrane. (F, G) Reconstructed 3-dimensional images of the penetrating cytoplasm of the MC show multiple contacting points with surrounding podocytes in the urinary space. The area marked with a dotted rectangle line in D is magnified in E. Podocytes, GBM, MCs, and intercellular contact points are yellow, brown, green, and red, respectively.
In our SBF-SEM images, we could clearly detect evidence of cytoplasmic penetration and the direct intercellular contact among glomerular cells through the disrupted GBM, the findings of which are not easy to detect using conventional TEM because 2-dimensional images cannot follow the entire 3D structure of the glomerulus. Direct contacts between the podocytes and mesangial or endothelial cells are unlikely to occur in a normal state because the GBM separates podocytes in the urinary space from the endocapillary compartment. Few studies have investigated the physical interaction of the glomerular constituents between the inner and outer sides of the GBM that occur regardless of the obstacle-like nature of the GBM, although there are several reports of cytokine and chemokine signaling across the GBM in vitro.8, 9, 10 Using SBF-SEM, we found that the cellular glomerular components consisting of podocytes, mesangial cells, and endothelial cells are synergistically involved in the repair process through physical contact with each other following GBM rupture. We hypothesize that the use of SBF-SEM can improve knowledge of the 3D ultrastructural relationships of each glomerular constituent, and we expect further application of this invaluable method in the field of nephrology.
Article Information
Authors’ Contributions
Research idea and study design: OH, NO, KJ; data acquisition: MN, TO; data analysis/interpretation: MN, SS, TT; supervision or mentorship: OH, NO, KJ. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Support
This work was supported by JSPS KAKENHI (Dr Joh), Grant-in-Aid for Scientific Research on Innovative Areas-Platforms for Advanced Technologies (Drs Joh, Ohno, and Nagai), and Research Resources “Advanced Bioimaging Support” and a grant from Japan Agency for Medical Research and Development (Dr Joh). The funders had no role in study design, data collection, analysis, or the decision to submit for publication.
Financial Disclosure
The authors declare that they have no relevant financial interests.
Acknowledgements
We thank Drs Truc Quynh Thai and Huy Bang Nguyen (Division of Neurobiology and Bioinformatics, National Institute for Physiological Sciences, Okazaki, Japan) for providing technical assistance; Ms Atsuko Imai (Division of Cell Structure, National Institute for Physiological Sciences, Okazaki, Japan) for kind support in the 3D reconstruction; and Dr Takashi Oite (Niigata University of Health and Welfare, Niigata, Japan) for valuable advice.
Patient Consent
The authors declare that they have obtained consent from the patients discussed in the article.
Peer Review
Received September 3, 2019. Evaluated by 1 external peer reviewer, with direct editorial input from an Associate Editor and the Editor-in-Chief. Accepted in revised form November 14, 2019.
Footnotes
Item S1: Detailed methods.
Table S1: Clinical and histologic findings from the study participants.
Movie S1: Disrupted GBM and penetration of the podocyte cytoplasmic process.
Movie S2: Reconstructed 3D image of the contact points between the penetrating mesangial cell and podocytes.
Supplementary Material
Item S1;Table S1.
Movie S1.
Movie S2.
References
- 1.Terasaki T., Sano M., Narita M. Ultrastructural study of gaps of the glomerular basement membrane in IgA nephropathy. Am J Nephrol. 1986;6(6):443–449. doi: 10.1159/000167250. [DOI] [PubMed] [Google Scholar]
- 2.Collar J.E., Ladva S., Cairns T.D. Red cell traverse through thin glomerular basement membranes. Kidney Int. 2001;59(6):2069–2072. doi: 10.1046/j.1523-1755.2001.00721.x. [DOI] [PubMed] [Google Scholar]
- 3.Denk W., Horstmann H. Serial block-face scanning electron microscopy to reconstruct three-dimensional tissue nanostructure. PLoS Biol. 2004;2(11):e329. doi: 10.1371/journal.pbio.0020329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thai T.Q., Nguyen H.B., Saitoh S. Rapid specimen preparation to improve the throughput of electron microscopic volume imaging for three-dimensional analyses of subcellular ultrastructures with serial block-face scanning electron microscopy. Med Mol Morphol. 2016;49(3):154–162. doi: 10.1007/s00795-016-0134-7. [DOI] [PubMed] [Google Scholar]
- 5.Nguyen H.B., Thai T.Q., Saitoh S. Conductive resins improve charging and resolution of acquired images in electron microscopic volume imaging. Sci Rep. 2016;6:23721. doi: 10.1038/srep23721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takaki T., Ohno N., Saitoh S. Podocyte penetration of the glomerular basement membrane to contact on the mesangial cell at the lesion of mesangial interposition in lupus nephritis: a three-dimensional analysis by serial block-face scanning electron microscopy. Clin Exp Nephrol. 2019;23(6):773–781. doi: 10.1007/s10157-019-01701-0. [DOI] [PubMed] [Google Scholar]
- 7.Belevich I., Joensuu M., Kumar D. Microscopy image browser: a platform for segmentation and analysis of multidimensional datasets. PLoS Biol. 2016;14(1) doi: 10.1371/journal.pbio.1002340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schrijvers B.F., Flyvbjerg A., De Vriese A.S. The role of vascular endothelial growth factor (VEGF) in renal pathophysiology. Kidney Int. 2004;65(6):2003–2017. doi: 10.1111/j.1523-1755.2004.00621.x. [DOI] [PubMed] [Google Scholar]
- 9.Sison K., Eremina V., Baelde H. Glomerular structure and function require paracrine, not autocrine, VEGF-VEGFR-2 signaling. J Am Soc Nephrol. 2010;21(10):1691–1701. doi: 10.1681/ASN.2010030295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Banas B., Wörnle M., Berger T. Roles of SLC/CCL21 and CCR7 in human kidney for mesangial proliferation, migration, apoptosis, and tissue homeostasis. J Immunol. 2002;168(9):4301–4307. doi: 10.4049/jimmunol.168.9.4301. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Item S1;Table S1.
Movie S1.
Movie S2.