Abstract
Rationale & Objective
The use of renin-angiotensin system (RAS) inhibitors is standard of care in people with early to moderate chronic kidney disease (CKD). Less is known regarding the efficacy of RAS inhibitors in very advanced CKD. In this study, we describe patterns of use of RAS inhibitors and associations of these patterns of use with risk for CKD progression and mortality in patients with advanced CKD.
Study Design
Propensity-matched cohort study.
Settings & Participants
We identified 678 participants who were enrolled in the multicenter Chronic Renal Insufficiency Cohort (CRIC) Study with estimated glomerular filtration rates (eGFRs) < 30 mL/min/1.73 m2 at the baseline visit.
Exposure
Use of RAS inhibitors within the first year after the baseline visit, characterized by 4 patterns of use: never users, always users, dynamic users, and new users.
Outcome(s)
Progression to end-stage renal disease (ESRD) and all-cause mortality.
Analytical Approach
We generated propensity scores and matched participants in the always users group with a 1:1 ratio with a participant from the other 3 groups, matching by age, sex, race, diabetes, hypertension, systolic blood pressure, eGFR, urinary protein-creatinine ratio, and serum potassium level. Cox models were used to test the association of patterns of RAS inhibitor use with risk for kidney failure and death.
Results
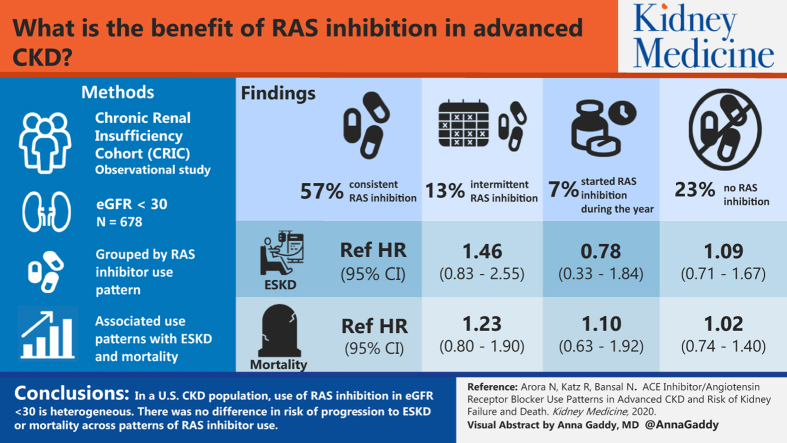
Of the 678 participants with eGFRs < 30 mL/min/1.73 m2, 57% were identified as always users of RAS inhibitors during the 1 year, 23% as never users, 13% as dynamic users, and 7% as new users. We found no differences in risk for ESRD across patterns of RAS inhibitor use (never users [HR, 1.09; 95% CI, 0.71-1.67], dynamic users [HR, 1.46; 95% CI, 0.83-2.55], new users [HR, 0.78; 95% CI, 0.33-1.84] vs the always users reference group). Similarly, there was no association of patterns of RAS inhibitor use with death (never users [HR, 1.02; CI, 0.74-1.40], dynamic users [HR, 1.23; 95% CI, 0.80-1.90], new users [HR, 1.10; 95% CI, 0.63-1.92] vs always users).
Limitations
Observational study.
Conclusions
Use of RAS inhibitors in patients with eGFRs < 30 mL/min/1.73 m2 is heterogeneous..We found no difference in risk for progression to ESRD or mortality across patterns of RAS inhibitor use. Further research is required to identify optimal prescribing strategies of RAS inhibitors during advanced stages of CKD.
Index Words: CKD, RAS inhibition, mortality, progression
Graphical Abstract
Editorial, p 231
Strategies targeted to delay progression of kidney disease and reduce mortality remain paramount for the treatment of patients with chronic kidney disease (CKD). The use of renin-angiotensin system (RAS) inhibitors is effective in slowing CKD progression,1,2 as well as reducing cardiovascular events,3,4 independent of blood pressure effects.1,5, 6, 7
Although RAS inhibition is standard of care in early to moderate CKD, there is a lack of consensus on RAS inhibitor prescription at more advanced stages of CKD. Despite studies that have suggested that RAS blockade may be both safe and beneficial in patients with advanced CKD,7, 8, 9 physicians remain reticent to prescribe such therapy, likely attributable to undesired side effects, particularly hyperkalemia and a further decline in glomerular filtration rate (GFR), potentially hastening progression to end-stage renal disease (ESRD).10 Most studies have excluded patients with advanced CKD (estimated GFR [eGFR] < 30 mL/min/1.73 m2), resulting in a paucity of data regarding the efficacy of these agents in this population.
Prior studies have examined the use of RAS inhibitors in patients with advanced CKD, yielding conflicting findings. Ruggenenti et al8 published a post hoc analysis of the Ramipril Efficacy in Nephropathy trial, demonstrating decreased incidence of ESRD with angiotensin-converting enzyme (ACE) inhibitor and angiotensin receptor blocker (ARB) use in 322 nondiabetic participants with proteinuric stage 4 CKD. Conversely, a study by Ahmed et al11 involving 52 participants with a mean eGFR of 16 mL/min/1.73 m2 showed an improvement in eGFR after discontinuation of ACE inhibitor/ARB therapy. Most recently, Hsu et al9 showed that ACE inhibitors and ARBs are associated with lower risk for progression to ESRD in patients with advanced CKD using a Taiwanese research database. Important limitations of previous studies include possible misclassification of kidney function, lack of adjustment for important confounders such as blood pressure and proteinuria, and study of non-US populations that may not be generalizable. A recent National Kidney Foundation Kidney Disease Outcomes Quality Initiative (NKF-KDOQI) controversies report highlighted the paucity of data among patients with advanced CKD and identified study of RAS inhibitors in advanced CKD as a research priority.12
Therefore, in this study we described the use of ACE inhibitors/ARBs in a multicenter US cohort of participants with advanced CKD and examined the association of different patterns of ACE inhibitor/ARB use during advanced CKD with risks for progression to ESRD and death.
Methods
Study Population
We studied participants enrolled in the Chronic Renal Insufficiency Cohort (CRIC) Study. CRIC is a National Institutes of Health–funded multicenter prospective observational cohort study that enrolled 3,939 participants 21 to 74 years of age with CKD with eGFRs of 20 to 70 mL/min/1.73 m2 using the Modification in Diet in Renal Disease (MDRD) Study equation between 2003 and 2008.13 CRIC participants had annual in-person visits and telephone calls every 6 months to update medications and medical history. At the study visits, updated data for medical history, medications, physical examination, laboratory measures, and cardiovascular testing were collected. Exclusion criteria included New York Heart Association class III or IV heart failure and severe liver disease. A subset of enrolled CRIC participants progressed to ESRD. Informed consent was obtained from each participating site.
For this study, we identified 678 CRIC participants enrolled in the study with stage 4 or 5 CKD at cohort entry, defined as eGFR < 30 mL/min/1.73 m2 at the baseline visit (but not receiving kidney replacement therapy or a kidney transplant).
RAS Inhibitor Use
CRIC participants had annual in-person and every 6-month telephone contacts during which medication use was verified by a review of the participant’s medication containers by study coordinators or through self. For the purpose of our study, RAS inhibitor use was defined as ACE inhibitor or ARB use only.
A single study physician reviewed the use of RAS inhibitors within the first year after the baseline visit (for which eGFR was <30 mL/min/1.73 m2) and characterized 4 patterns of use; never users, always users, dynamic users, and new users. Participants were grouped into never users if RAS inhibitor was not used during this 1-year period, always users if RAS inhibitors were used for the entire period, dynamic users if treated with periods on and off therapy, and new users if participants reported new RAS inhibitor use within the first year after the baseline visit.
Outcomes
The study outcomes included: (1) progression to ESRD, defined as initiation of kidney replacement therapy or receipt of a kidney transplant, and (2) all-cause mortality. ESRD was ascertained by participant report or linkage to the US Renal Data System. Mortality was ascertained by regular contact with participants and next of kin and linkage to the National Death Index.
Covariates
At the baseline and subsequent visits, participants provided information regarding medical history, medication use, lifestyle behaviors (eg, tobacco use), and sociodemographic characteristics. Race/ethnicity was categorized as non-Hispanic white, non-Hispanic black, Hispanic, or other. Diabetes mellitus was defined as fasting glucose concentration > 126 mg/dL, nonfasting glucose concentration > 200 mg/dL, or use of antidiabetic agents. Serum creatinine was measured using an enzymatic method on an Ortho Vitros 950 (Ortho Clinical Diagnostics) at the CRIC Study Central Laboratory and standardized to isotope-dilution mass spectrometry–traceable values.14, 15, 16 eGFR was calculated from serum creatinine level using the MDRD Study equation. Body mass index was derived and blood pressure was assessed using standard protocols.17
Statistical Analyses
We first described characteristics of the study participants overall and by categories of RAS inhibitor use (never users, always users, dynamic users, and new users). To address potential confounders, we used propensity score matching. Each participant in the always users group was matched in a 1:1 ratio with a participant from the other 3 groups. Propensity scores were estimated using logistic regression models to predict the assignment to the groups. Participants were matched on the following confounders, selected a priori: age (±5 years), sex, race (white vs nonwhite), diabetes, hypertension, systolic blood pressure (±5 mm Hg), eGFR (±5 mL/min/1.73 m2), urinary protein-creatinine ratio (UPCR; ±0.2 g), and serum potassium level (± 0.2 mg/dL). UPCR was log-transformed due to the skewed distribution. Complete cases (with no missing covariates) were used for propensity score analyses. For each participant, we computed the logit of the estimated propensity score. We used greedy matching to match participants using calipers that were defined to have a maximum width of 0.2 standard deviation of the logit of the estimated propensity score. Adequacy of the propensity score to adjust for effects of covariates was assessed by testing each of the 3 groups for differences in individual covariates between always/never (always/dynamic and always/new user) participants after stratifying by propensity score quintiles. Each covariate was modeled as a function of always/never and propensity score quintiles. We further calculated the standardized mean differences and the ratio of the standard deviations.
Kaplan-Meier curves for both ESRD and mortality were generated comparing never users, dynamic users, and new users individually with the always users group. Hazard ratios (HRs) and 95% confidence intervals (CIs) were estimated using Cox proportional hazards regression in the matched population, for which we adjusted for the matched pair design. We tested for an interaction by baseline history of atherosclerotic cardiovascular disease (CVD) or heart failure because these comorbid conditions may modify the observed associations.
We performed 2 sensitivity analyses. In the first, we evaluated models accounting for the competing risk for death using the method of Fine and Gray18 when examining the association of patterns of RAS inhibitor use with ESRD. In the second, we additionally adjusted for UPCR in our Cox models because imbalances were noted in propensity score matching for this variable.
In secondary analyses, we repeated our Cox models using the entire cohort of interest rather than propensity score matching. In these analyses, we adjusted for proteinuria in addition to atherosclerotic CVD and heart failure.
Analyses were performed using SPSS (IBM Corp; 2017, version 25.0) and R, version 3.4.3 (R Core Team, 2017).
Results
Characteristics of Study Population
Among the 678 total participants in our study, mean age was 59 years, 53% were women, and 38% were white (Table 1). Mean eGFR was 25 mL/min/1.73 m2 and median UPCR and albumin-creatinine ratio were 0.54 g/g and 261 mg/g, respectively. Most (91%) had hypertension, 54% had diabetes, 13% had heart failure, and 39% had coronary artery disease. Mean blood pressure was 131 mm Hg systolic and 70 mm Hg diastolic.
Table 1.
Baseline Characteristics of CRIC Participants With Baseline eGFRs < 30 mL/min/1.73 m2 and RAS Inhibitor Use
| Overall (N = 678) | No ACEi/ARB (N = 159) | Always User (N = 386) | Dynamic User (N = 85) | New User (N = 48) | Missing | |
|---|---|---|---|---|---|---|
| Age, y | 59 ± 11 | 59 ± 12 | 59 ± 11 | 59 ± 11 | 56 ± 14 | 0 |
| Male sex | 315 (47%) | 67 (42%) | 185 (48%) | 39 (46%) | 24 (50%) | 0 |
| Race | 0 | |||||
| Non-Hispanic white | 256 (38%) | 55 (35%) | 160 (42%) | 31 (37%) | 10 (21%) | |
| Non-Hispanic black | 269 (40%) | 62 (39%) | 152 (39%) | 32 (38%) | 23 (48%) | |
| Hispanic | 122 (18%) | 31 (20%) | 60 (16%) | 19 (22%) | 12 (25%) | |
| Other | 31 (5%) | 11 (7%) | 14 (4%) | 3 (4%) | 3 (6%) | |
| Diabetes | 364 (54%) | 68 (43%) | 225 (58%) | 45 (53%) | 26 (54%) | 0 |
| Hypertension | 619 (91%) | 137 (86%) | 359 (93%) | 79 (93%) | 44 (92%) | 0 |
| No. of antihypertensive medications | 3.0 ± 1.5 | 2.1 ± 1.4 | 3.4 ± 1.4 | 3.4 ± 1.5 | 2.4 ± 1.5 | 0 |
| No. of antihypertensive medications | 0 | |||||
| 0 | 32 (5%) | 22 (14%) | 0 (0%) | 2 (2%) | 8 (17%) | |
| 1 | 82 (12%) | 34 (21%) | 36 (9%) | 7 (8%) | 5 (10%) | |
| 2 | 147 (22%) | 40 (25%) | 76 (20%) | 20 (24%) | 11 (23%) | |
| 3 | 154 (23%) | 32 (20%) | 98 (25%) | 12 (14%) | 12 (25%) | |
| ≥4 | 263 (39%) | 31 (20%) | 176 (46%) | 44 (52%) | 12 (25%) | |
| β-Blockers | 370 (55%) | 86 (54%) | 207 (54%) | 48 (57%) | 29 (60%) | 0 |
| Calcium channel blockers | 346 (51%) | 83 (52%) | 191 (50%) | 45 (53%) | 27 (56%) | 0 |
| Diuretics | 476 (70%) | 97 (61%) | 283 (73%) | 62 (73%) | 34 (71%) | 0 |
| Systolic blood pressure, mm Hg | 131 ± 23 | 134 ± 21 | 128 ± 23 | 133 ± 24 | 139 ± 23 | 0 |
| Diastolic blood pressure, mm Hg | 70 ± 13 | 72 ± 12 | 69 ± 12 | 70 ± 16 | 74 ± 13 | 0 |
| Potassium level, mEq/L | 4.53 ± 0.57 | 4.42 ± 0.55 | 4.60 ± 0.57 | 4.56 ± 0.58 | 4.37 ± 0.56 | 0 |
| Ejection fraction categories | 175 (26%) | |||||
| >50% | 390 (78%) | 96 (79%) | 228 (79%) | 37 (65%) | 29 (83%) | |
| 46%-50% | 46 (9%) | 14 (12%) | 24 (8%) | 8 (14%) | 0 (0%) | |
| 36%-45% | 42 (8%) | 9 (7%) | 23 (8%) | 7 (12%) | 3 (9%) | |
| ≤35% | 25 (5%) | 3 (3%) | 14 (5%) | 5 (9%) | 3 (9%) | |
| Left ventricular mass, g | 222 ± 66 | 213 ± 65 | 221 ± 61 | 244 ± 84 | 233 ± 72 | 230 (34%) |
| Heart failure | 86 (13%) | 10 (6%) | 56 (15%) | 14 (17%) | 6 (13%) | 0 |
| Coronary artery disease | 263 (39%) | 44 (28%) | 160 (42%) | 40 (47%) | 19 (40%) | 0 |
| Current smoker | 99 (15%) | 37 (23%) | 41 (11%) | 13 (15%) | 8 (17%) | 0 |
| Body mass index, kg/m2 | 32.28.3 | 30.17.9 | 33.0±8.4 | 32.6±8.6 | 32.5±6.9 | 3 (0.4%) |
| eGFR, mL/min/1.73 m2 | 25 ± 3 | 25 ± 4 | 25 ± 3 | 24 ± 3 | 25 ± 4 | 0 |
| Urinary protein-creatinine ratio, g/g | 0.54 [0.12-1.81] | 0.62 [0.17-2.17] | 0.46 [0.11-1.40] | 0.65 [0.11-2.76] | 1.02 [0.22-3.04] | 0 |
| Urinary albumin-creatinine ratio, mg/g | 261 [43-1,151] | 252 [50-1,406] | 237 [38-915] | 418 [49-1,655] | 575 [63-2,277] | 0 |
Note: Values expressed as number (percent), mean ± standard deviation, or median [interquartile range] unless otherwise noted.
Abbreviations: ACEi/ARB, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker; CRIC, Chronic Renal Insufficiency Cohort; eGFR, estimated glomerular filtration rate; RAS, renin-angiotensin system.
Characteristics of Study Population Across Patterns of RAS Inhibitor Use in CKD
Before propensity score matching, we identified 159 (23%) patients who were not prescribed an ACE inhibitor/ARB for the duration of the study period (never users), 386 (57%) were consistently taking an ACE inhibitor/ARB (always users), 85 (13%) were dynamic users with periods on and off therapy, and 48 (7%) were initiated on ACE inhibitor/ARB therapy (new users). Never users had a lower proportion of hypertension, diabetes, heart failure, and coronary artery disease than the other groups. Most always users had diabetes and this group had the lowest blood pressure and degree of proteinuria compared with the other groups. Dynamic users had slightly higher proportions of heart failure and coronary artery disease, whereas rates of diabetes and hypertension and serum potassium levels were similar in comparison to the other groups. New users had similar rates of diabetes and hypertension, though the highest degree of proteinuria and blood pressure in comparison to the other groups (Table 1).
When characteristics of the propensity score–matched groups were compared, the groups were well matched in regard to systolic blood pressure, eGFR, age, and ethnicity (Table 2). The greatest imbalance was seen with proteinuria, which was true in all propensity-matched groups (Table 2).
Table 2.
Baseline Characteristics of Propensity Score–Matched Participants
| Always User | Never User | Standardized Mean Difference in Propensity Score Means | Ratio of SDs | |
|---|---|---|---|---|
| N | 141 | 141 | 0.0003 | 1.0018 |
| Age, y | 60 ± 11 | 59 ± 11 | ||
| Male sex | 61 (43%) | 60 (43%) | ||
| Black | 59 (42%) | 55 (39%) | ||
| Diabetes | 52 (37%) | 61 (43%) | ||
| Hypertension | 123 (87%) | 125 (89%) | ||
| Systolic blood pressure, mm Hg | 130 ± 24 | 133 ± 21 | ||
| Potassium level, mEq/L | 4.45 ± 0.55 | 4.46 ± 0.55 | ||
| eGFR, mL/min/1.73 m2 | 25 ± 3 | 25 ± 4 | ||
| Urinary protein-creatinine ratio, g/g | 0.40 [0.11, 1.25] | 0.68 [0.15, 2.11] |
| Always User | Dynamic User | |||
|---|---|---|---|---|
| N | 76 | 76 | 0.0008 | 1.0010 |
| Age, y | 60 ± 11 | 60 ± 11 | ||
| Male sex | 40 (53%) | 35 (46%) | ||
| Black | 30 (40%) | 29 (38%) | ||
| Diabetes | 32 (42%) | 40 (53%) | ||
| Hypertension | 70 (92%) | 71 (93%) | ||
| Systolic blood pressure, mm Hg | 128 ± 24 | 132 ± 24 | ||
| Potassium level, mEq/L | 4.61 ± 0.58 | 4.56 ± 0.58 | ||
| eGFR mL/min/1.73 m2 | 25 ± 3 | 25 ± 4 | ||
| Urinary protein-creatinine ratio, g/g | 0.42 [0.12, 1.61] | 0.60 [0.12, 2.68] |
| Always User | New User | |||
|---|---|---|---|---|
| N | 39 | 39 | 0.0014 | 1.0114 |
| Age, y | 57 ± 11 | 59 ± 13 | ||
| Male sex | 23 (59%) | 18 (46%) | ||
| Black | 19 (49%) | 19 (49%) | ||
| Diabetes | 19 (49%) | 23 (59%) | ||
| Hypertension | 37 (95%) | 36 (92%) | ||
| Systolic blood pressure, mm Hg | 131 ± 16 | 137 ± 22 | ||
| Potassium level, mEq/L | 4.41 ± 0.50 | 4.50 ± 0.51 | ||
| eGFR, mL/min/1.73 m2 | 25 ± 3 | 25 ± 4 | ||
| Urinary protein-reatinine ratio, g/g | 0.33 [0.12, 1.16] | 0.85 [0.22, 2.80] |
Note: Values expressed as number (percent), mean ±SD, or mean [minimum, maximum] unless otherwise noted.
Abbreviations: eGFR, estimated glomerular filtration rate; SD, standard deviation.
Association of RAS Inhibitor Use With Progression to ESRD
There were 368 ESRD events with an overall rate of 14.3% per year in our study population, with a mean follow-up time of 3.3 years.
Never Users Compared With Always Users: Primary Analysis
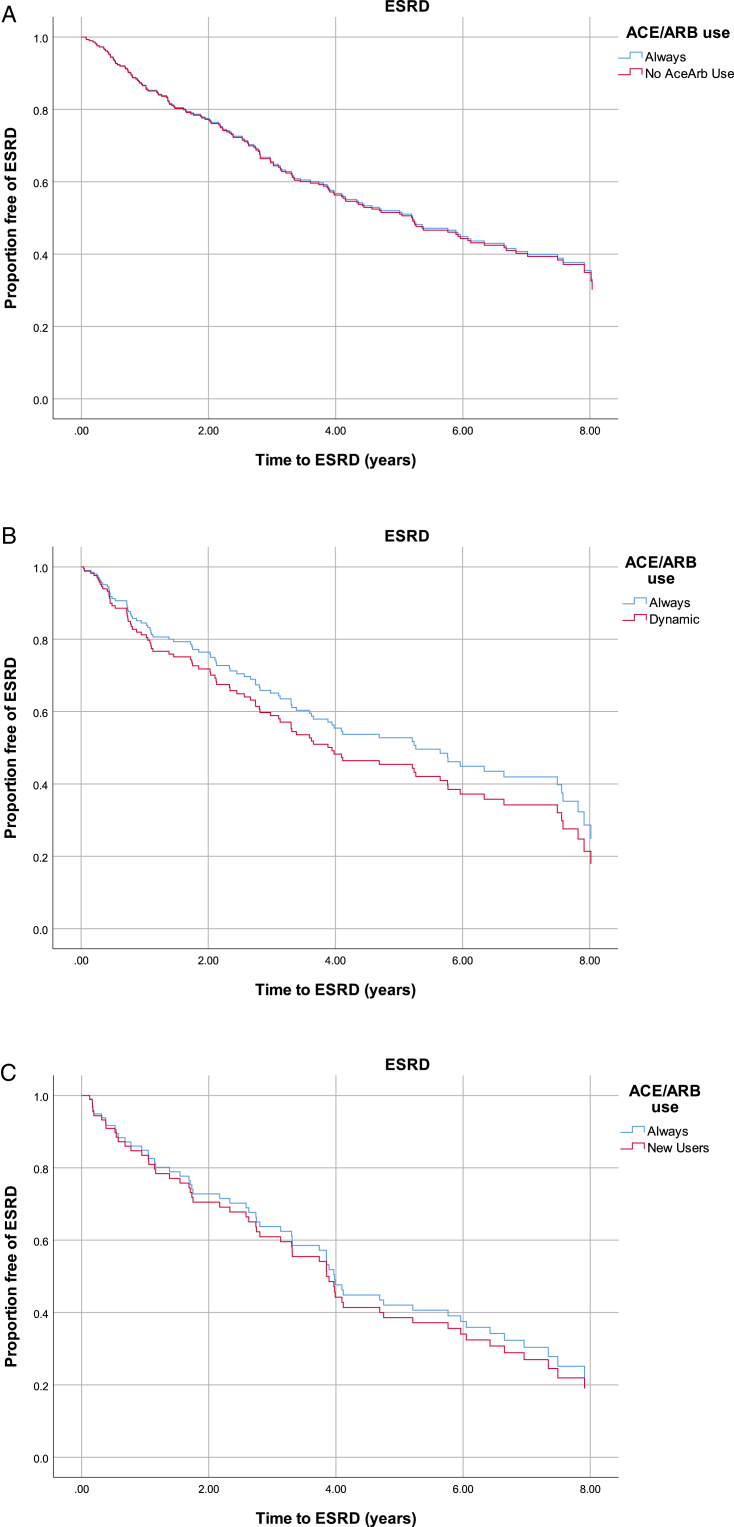
In the propensity score–matched analysis; there were no differences in cumulative risk for ESRD between always users versus never users (Fig 1A). In the propensity score–matched Cox models, we did not observe a statistically significant difference in progression to ESRD between always and never users (HR, 1.02; 95% CI, 0.74-1.40; Table 3). Interaction by history of atherosclerotic CVD or heart failure was not statistically significant (P = 0.12).
Figure 1.
Kaplan-Meier curves display proportions of end-stage renal disease (ESRD)-free survival comparing always users with (A) never users (log-rank P = 0.812), (B) dynamic users (log-rank P = 0.369), and (C) new users (log-rank P = 0.362). Abbreviations: ACEi/ARB, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker; ESRD, end-stage renal disease.
Table 3.
Association of Patterns of ACEi/ARB Use With Risk for ESRD and Mortality: Propensity Score–Matched Participants
| Hazard Ratio (95% CI) | N | |
|---|---|---|
| ESRD | ||
| Always user | 1.00 (reference) | 256 |
| Never user | 1.02 (0.74-1.40) | 141 |
| Dynamic user | 1.23 (0.80-1.90) | 76 |
| New user | 1.10 (0.63-1.92) | 39 |
| Mortality | ||
| Always user | 1.00 (reference) | 256 |
| Never user | 1.09 (0.71-1.67) | 141 |
| Dynamic user | 1.46 (0.83-2.55) | 76 |
| New user | 0.78 (0.33-1.84) | 39 |
Abbreviations: ACEi/ARB, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker; CI, confidence interval; ESRD, end-stage renal disease.
Dynamic Users Compared With Always Users: Primary Analysis
In the propensity score–matched analysis, there were no differences in cumulative risk for ESRD between always and dynamic users (Fig 1B). In the propensity score–matched Cox models, we found no significant difference in progression to ESRD between groups. However, we observed a trend toward increased risk for progression to ESRD in dynamic users compared with always users of ACE inhibitors/ARBs (HR, 1.23; 95% CI, 0.80-1.90; Table 3). Interaction by history of atherosclerotic CVD or heart failure was not statistically significant (P = 0.14).
New Users Compared With Always Users
Primary Analysis
There was no difference in cumulative risk for ESRD between always and new users in the propensity score–matched analysis (Fig 1C). There was no significant difference between groups in propensity score–matched Cox models in risk for ESRD between new users compared with always users (HR, 1.10; 95% CI, 0.63-1.92; Table 3). Interaction by history of atherosclerotic CVD or heart failure was not statistically significant (P = 0.26).
Sensitivity Analyses
In sensitivity analysis, we performed a competing-risk model for death, in addition to adjusting for UPCR, which did not affect our results (Tables S1 and S2).
Secondary Analysis
We repeated our analyses without propensity score matching. Incidence rates of progression to ESRD were 13.6% per year, 13.8% per year, 16.4% per year, and 19.4% per year for always, never, dynamic, and new users, respectively. In Cox models, there was no significant difference between groups in the unadjusted model or after adjustment for all covariates (Table S3).
Association of RAS Inhibitor Use With Risk for Mortality
Among the 678 participants in our study, the overall number of deaths was 204 and the incidence rate of death was 5.3% per year, during a mean follow-up of 5.4 years.
Never Users Compared With Always Users: Primary Analysis
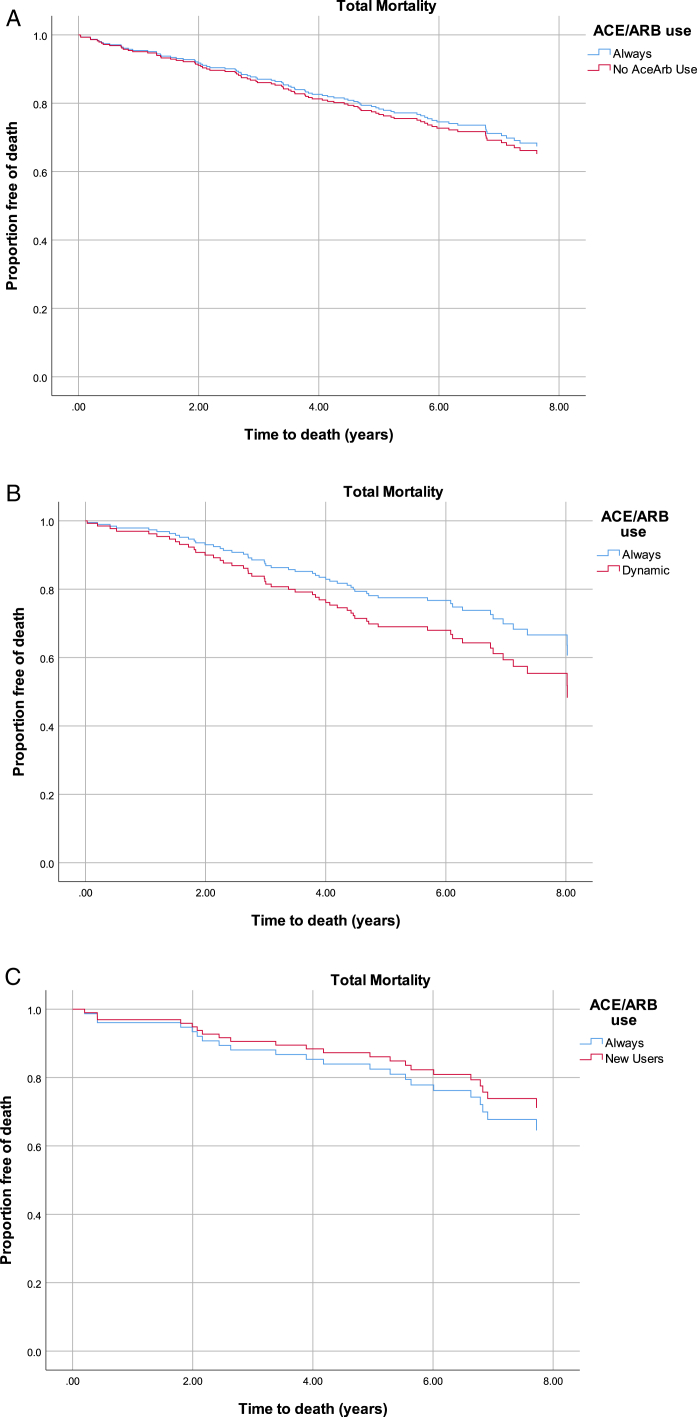
We found no difference in cumulative risk for mortality between always and never users in the propensity score–matched analysis (Fig 2A). Similarly, there was no significant difference between the 2 groups in the propensity score–matched Cox models (HR, 1.09; 95% CI, 0.71-1.67; Table 3). Interaction by history of atherosclerotic CVD or heart failure was not statistically significant (P = 0.89).
Figure 2.
Kaplan-Meier curves display proportions free of death comparing always users with (A) never users (log-rank P = 0.619), (B) dynamic users (log-rank P = 0.138), and (C) new users (log-rank P = 0.376). Abbreviation: ACEi/ARB, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker.
Dynamic Users Compared With Always Users: Primary Analysis
In the propensity score–matched analysis there was no difference in cumulative risk for mortality between always and dynamic users of ACE inhibitors/ARBs (Fig 2B). We found no significant difference between always and dynamic users in the propensity score–matched Cox models (HR, 1.46; 95% CI, 0.83-2.55; Table 3). Interaction by history of atherosclerotic CVD or heart failure was not statistically significant (P = 0.44).
New Users Compared With Always Users
Primary Analysis
We found no difference in cumulative risk for mortality between always and new users in the propensity score–matched analysis (Fig 2C). We noted decreased risk for mortality in new users as compared with always users in the propensity score–matched Cox models that did not reach statistical significance (HR, 0.78; 95% CI, 0.33-1.84; Table 3). Interaction by history of atherosclerotic CVD or heart failure was not statistically significant (P = 0.45).
Sensitivity Analysis
Further adjustment for UPCR did not change our results (Table S4).
Secondary Analysis
Mortality rates for always, never, dynamic, and new users were 5.1% per year, 5.5% per year, 7.4% per year, and 3.6% per year, respectively. When performing Cox models in the non–propensity score cohort, there was no difference between groups in the unadjusted model or after adjustment for all covariates, though there was a decreased rate of mortality in new users compared with always users, which did not reach statistical significance (Table S4).
Discussion
In this observational study of RAS inhibitor use in a large multicenter cohort of participants with advanced CKD, our results demonstrate significant variability in the use of RAS inhibitors, even among high-risk subgroups. In study participants with eGFRs < 30 mL/min/1.73 m2, 23% were not taking an ACE inhibitor/ARB despite at least 43% of these with common nonrenal indications for RAS inhibitors, such as diabetes or heart failure. We found that 57% of the study population were consistently using ACE inhibitors/ARBs and 13% and 7% were either dynamic users or started ACE inhibitor/ARB therapy at eGFRs < 30 mL/min/1.73 m2, respectively. Using propensity score–matched analysis, we did not find differences in risk for progression to ESRD or mortality across patterns of RAS inhibitor use in participants with advanced CKD. It is possible that therapies before advanced CKD have the greatest impact on clinical outcomes. However, more definitive clinical trials are needed to answer this question more conclusively.
Our study is consistent with prior published data indicating underuse of RAS inhibitors in high-risk populations.19, 20, 21, 22 Although data regarding patients with advanced CKD are sparse, it has been suggested that there is an increasing prevalence of prescriptions for RAS inhibitors among this population,19,22 although use appears to have reached a plateau in the past decade.23 We identified irregular prescribing patterns among participants with traditional nonrenal indications for RAS inhibitors, such as heart failure, despite evidence that these agents decrease cardiovascular events.13 Despite this, the prescription rate in our study was significantly higher than previously published data, suggesting that <40% of patients with CKD stages 4 and 5 and concurrent heart failure received RAS inhibition,24 which is consistent with a large study assessing older adults transitioning from CKD to ESRD.25 Overall, our study is indicative of a high but heterogeneous pattern of RAS inhibitor use in patients with advanced CKD, which continues to highlight the lack of robust data to guide RAS inhibitor use in this population.
We found no difference in the rate of progression to ESRD between participants treated continuously with RAS inhibitors or not treated at all for the duration of the study, which may be due to the suggestion that RAS inhibition confers the greatest benefit when used early in the course of CKD.7 Initiation of RAS inhibitors in participants with advanced CKD did not seem to confer benefit or cause detriment. Prior studies have yielded conflicting results. Notably, a study by Hou et al7 demonstrated improved outcomes in participants with mild to moderate CKD treated with ACE inhibitors, which was not sustained in participants with advanced disease. This study is limited by its lack of reporting of eGFR and a population consisting of nondiabetic patients only, which encompassed >50% of our study population. More recently, a study by Hsu et al9 demonstrated that ACE inhibitors/ARBs are associated with lower risk for progression to KRT in participants with advanced CKD. However, the degree of decreased kidney function was extrapolated by the use of erythropoiesis-stimulating agents. Furthermore, glomerulonephritis is the most common cause of CKD in these countries and may not be generalizable to a United States–based population in which diabetes and hypertension are the leading causes of CKD.26 In a post hoc analysis of the RENAAL (Reduction of Endpoints in NIDDM With the Angiotensin II Antagonist Losartan) trial, investigators examined the efficacy of losartan among patients with diabetic nephropathy, which revealed benefit in ESRD risk across all levels of eGFR. However, no mortality benefit was seen.27 Thus, observational and post hoc clinical trial data remain conflicting on the efficacy of RAS inhibitors to slow progression to ESRD among patients with advanced CKD.
Similar to the association with ESRD, we found no differences in mortality rates between participants treated continuously with RAS inhibitors or not for the duration of the study. Although this study is not designed to determine causation, we observed lower risk for mortality among participants initiated on RAS inhibitor therapy during advanced CKD, which was not statistically significant. This may be secondary to cardiovascular benefit versus maintenance of residual kidney function, which has previously been shown to confer mortality benefit.28 Additionally, participants who initiated ACE inhibitor/ARB therapy had a higher degree of proteinuria, which is a known predictor of both cardiovascular and noncardiovascular mortality.29
There are few studies addressing RAS inhibition and effect on mortality in participants with advanced CKD. A post hoc analysis of the SAVE (Survival and Ventricular Enlargement) trial showed a risk ratio reduction of 31% on the composite end point of all-cause mortality, cardiovascular mortality, myocardial infarction, and heart failure among participants with CKD, as defined by a baseline creatinine level < 2.5 mg/dL, treated with captopril.30 A study by Brar et al31 of the Alberta Kidney Disease Network demonstrated a reduction in mortality, though increased hospitalizations for a kidney cause, that is, acute kidney injury or hyperkalemia, if RAS inhibitors were prescribed within 6 months of a hospitalization complicated by AKI. This effect was consistent among prior users of RAS inhibitors.31 However, the mean eGFR of ACE inhibitor or ARB users was 62 mL/min/1.73 m2, and 98% of these participants had CKD stage 3 or better, so it is unclear whether these findings can be extrapolated to those with advanced CKD.
Based on our data, it remains unclear whether patients with advanced CKD should be resumed on RAS inhibitor therapy if they develop an acute indication for discontinuation. A question that may be better addressed when results of the STOP-ACEi (Multicentre Randomised Controlled Trial of Angiotensin-Converting Enzyme inhibitor [ACEi]/Angiotensin Receptor Blocker [ARB] Withdrawal in Advanced Renal Disease) trial, which is assessing discontinuation of ACE inhibitors/ARBs in patients with stage 4 or 5 CKD, are published.32 Finally, it is plausible that in advanced stages of CKD, other therapies may be more effective in improving clinical outcomes. Recent evidence has shown that sodium-glucose cotransporter 2 inhibitors slow progression to ESRD among patients with CKD and diabetes,33, 34, 35, 36 though patients with advanced CKD were excluded. Ongoing studies will evaluate for potential benefit in other populations of patients with CKD.
Strengths of our study include a large multicenter cohort of participants with a long follow-up period and availability of important confounders. Unique to our study is the assessment of patterns of RAS inhibitor use, as opposed to static use, over time.
We acknowledge several limitations in our study. Medication use was ascertained only every 6 months and did not include detailed information regarding dose, use of medications before study entry, indication or contraindication for RAS inhibitor prescription, complications of RAS inhibitor use (eg, hospitalizations for hyperkalemia), reason for medication changes, or measures of medication adherence. We studied a selective population of research volunteers from nephrology clinics from US medical centers, which may not be generalizable to other populations. The study was not designed to determine rationale for medication initiation or discontinuation or to assess adverse events. We had a relatively small sample size and the possibility of residual confounding exists, despite the use of propensity score analysis. We are not able to determine causation or rule out confounding by indication.
In conclusion, we found that use of RAS inhibitors was heterogeneous after patients advanced to an eGFR < 30 mL/min/1.73 m2. However, we did not find differences in risk for progression to ESRD or mortality based on patterns of RAS inhibitor use during advanced stages of CKD. Investigation of therapies to improve clinical outcomes in this vulnerable and high-risk period should be prioritized.
Article Information
Authors’ Full Names and Academic Degrees
Nayan Arora, MD, Ronit Katz, PhD, and Nisha Bansal, MD
Authors’ Contributions
Research idea and study design: NB; data acquisition: NB, NA, RK; data analysis and interpretation: NB, NA, RK; statistical analysis: RK; supervision and mentorship: NB. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Support
The CRIC Study was conducted by the CRIC and supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). The data from CRIC reported here were supplied by the NIDDK Central Repositories. This manuscript was not prepared in collaboration with CRIC Steering Committee and does not necessarily reflect the opinions or views of the CRIC study, the NIDDK Central Repositories, or the NIDDK.
Financial Disclosure
The authors declare that they have no relevant financial interests.
Disclaimer
This manuscript was not prepared in collaboration with Investigators of the CRIC Study and does not necessarily reflect the opinions or views of the CRIC Study, the NIDDK Central Repositories, or the NIDDK.
Peer Review
Received July 3, 2019. Evaluated by 2 external peer reviewers, with direct editorial input from the Statistical Editor, an Associate Editor, and the Editor-in-Chief. Accepted in revised form December 12, 2019.
Footnotes
Complete author and article information provided before references.
Table S1: Association of patterns of ACE inhibitor/ARB use with ESRD in propensity-matched participants, accounting for competing risk of death
Table S2: Association of patterns of ACE inhibitor/ARB use with ESRD and mortality in propensity-matched participants, adjusted for urine protein-creatinine ratio
Table S3: Association of patterns of ACE inhibitor/ARB use with risk of ESRD, non–propensity score–matched analysis
Table S4: Association of patterns of ACE inhibitor/ARB use with risk of mortality, non–propensity score–matched analysis
Supplementary Material
. Tables S1-S4
References
- 1.Lewis E.J., Hunsicker L.G., Bain R.P., Rohde R.D. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. N Engl J Med. 1993;329(20):1456–1462. doi: 10.1056/NEJM199311113292004. [DOI] [PubMed] [Google Scholar]
- 2.Maschio G., Alberti D., Janin G. Effect of the angiotensin-converting–enzyme inhibitor benazepril on the progression of chronic renal insufficiency. N Engl J Med. 1996;334(15):939–945. doi: 10.1056/NEJM199604113341502. [DOI] [PubMed] [Google Scholar]
- 3.Matsushita K., Selvin E., Bash L.D., Franceschini N., Astor B.C., Coresh J. Change in estimated GFR associates with coronary heart disease and mortality. J Am Soc Nephrol. 2009;20(12):2617–2624. doi: 10.1681/ASN.2009010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shlipak M.G., Katz R., Kestenbaum B. Rapid decline of kidney function increases cardiovascular risk in the elderly. J Am Soc Nephrol. 2009;20(12):2625–2630. doi: 10.1681/ASN.2009050546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lewis E.J., Hunsicker L.G., Clarke W.R. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345(12):851–860. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- 6.Dahlöf B., Devereux R.B., Kjeldsen S.E. Cardiovascular morbidity and mortality in the Losartan Intervention for Endpoint Reduction in Hypertension Study (LIFE): a randomised trial against atenolol. Lancet. 2002;359(9311):995–1003. doi: 10.1016/S0140-6736(02)08089-3. [DOI] [PubMed] [Google Scholar]
- 7.Hou F.F., Zhang X., Zhang G.H. Efficacy and safety of benazepril for advanced chronic renal insufficiency. N Engl J Med. 2006;354(2):131–140. doi: 10.1056/NEJMoa053107. [DOI] [PubMed] [Google Scholar]
- 8.Ruggenenti P., Perna A., Remuzzi G. ACE inhibitors to prevent end-stage renal disease: when to start and why possibly never to stop: a post hoc analysis of the REIN trial results. J Am Soc Nephrol. 2001;12(12):2832–2837. doi: 10.1681/ASN.V12122832. [DOI] [PubMed] [Google Scholar]
- 9.Hsu T.-W., Liu J.-S., Hung S.-C. Renoprotective effect of renin-angiotensin-aldosterone system blockade in patients with predialysis advanced chronic kidney disease, hypertension, and anemia. JAMA Intern Med. 2014;174(3):347–354. doi: 10.1001/jamainternmed.2013.12700. [DOI] [PubMed] [Google Scholar]
- 10.Bakris G.L., Weir M.R. Angiotensin-converting enzyme inhibitor–associated elevations in serum creatinine: is this a cause for concern? Arch Intern Med. 2000;160(5):685–693. doi: 10.1001/archinte.160.5.685. [DOI] [PubMed] [Google Scholar]
- 11.Ahmed A.K., Kamath N.S., El Kossi M., El Nahas A.M. The impact of stopping inhibitors of the renin–angiotensin system in patients with advanced chronic kidney disease. Nephrol Dial Transplant. 2010;25(12):3977–3982. doi: 10.1093/ndt/gfp511. [DOI] [PubMed] [Google Scholar]
- 12.Weir M.R., Lakkis J.I., Jaar B. Use of renin-angiotensin system blockade in advanced CKD: an NKF-KDOQI Controversies Report. Am J Kidney Dis. 2018;72(6):873–884. doi: 10.1053/j.ajkd.2018.06.010. [DOI] [PubMed] [Google Scholar]
- 13.Xie X., Liu Y., Perkovic V. Renin-angiotensin system inhibitors and kidney and cardiovascular outcomes in patients with CKD: a Bayesian network meta-analysis of randomized clinical trials. Am J Kidney Dis. 2016;67(5):728–741. doi: 10.1053/j.ajkd.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 14.Joffe M., Hsu C.Y., Feldman H.I., Weir M., Landis J.R., Hamm L.L. Chronic Renal Insufficiency Cohort (CRIC) Study Group. Variability of creatinine measurements in clinical laboratories: results from the CRIC study. Am J Nephrol. 2010;31:426–434. doi: 10.1159/000296250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levey A.S., Coresh J., Greene T. Chronic Kidney Disease Epidemiology Collaboration. Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem. 2007;53:766–772. doi: 10.1373/clinchem.2006.077180. [DOI] [PubMed] [Google Scholar]
- 16.Anderson A.H., Yang W., Hsu C.Y., CRIC Study Investigators Estimating GFR among participants in the Chronic Renal Insufficiency Cohort (CRIC) study. Am J Kidney Dis. 2012;60:250–261. doi: 10.1053/j.ajkd.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Center for Health Statistics (NCHS): National Health and Nutrition Examination Survey Anthropometry Procedures Manual. Centers for Disease Control and Prevention; Atlanta, GA: 2000. [Google Scholar]
- 18.Fine J.P., Gray R.J. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 19.Tylicki L., Jakubowska A., Lizakowski S., Świetlik D., Rutkowski B. Management of renin-angiotensin system blockade in patients with chronic kidney disease under specialist care. retrospective cross-sectional study. J Renin Angiotensin Aldosterone Syst. 2015;16(1):145–152. doi: 10.1177/1470320314550018. [DOI] [PubMed] [Google Scholar]
- 20.Ku E., McCulloch C.E., Vittinghoff E., Lin F., Johansen K.L. Use of antihypertensive agents and association with risk of adverse outcomes in chronic kidney disease: focus on angiotensin-converting enzyme inhibitors and angiotensin receptor blockers. J Am Heart Assoc. 2018;7(19):e009992. doi: 10.1161/JAHA.118.009992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Winkelmayer W.C., Fischer M.A., Schneeweiss S., Wang P.S., Levin R., Avorn J. Underuse of ACE inhibitors and angiotensin II receptor blockers in elderly patients with diabetes. Am J Kidney Dis. 2005;46(6):1080–1087. doi: 10.1053/j.ajkd.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 22.Tonelli M., Gill J., Pandeya S., Bohm C., Levin A., Kiberd B.A. Barrier to blood pressure control and angiotensin enzyme inhibitor use in Canadian patients with chronic renal insufficiency. Nephrol Dial Transplant. 2002;17:1426–1433. doi: 10.1093/ndt/17.8.1426. [DOI] [PubMed] [Google Scholar]
- 23.Murphy D.P., Drawz P.E., Foley R.N. Trends in angiotensin-converting enzyme inhibitor and angiotensin II receptor blocker use among those with impaired kidney function in the United States. J Am Soc Nephrol. 2019;30(7):1314–1321. doi: 10.1681/ASN.2018100971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berger A.K., Duval S., Manske C. Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in patients with congestive heart failure and chronic kidney disease. Am Heart J. 2007;153(6):1064–1073. doi: 10.1016/j.ahj.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 25.Chang T.I., Zheng Y., Montez-Rath M.E., Winkelmayer W.C. Antihypertensive medication use in older patients transitioning from chronic kidney disease to end-stage renal disease on dialysis. Clin J Am Soc Nephrol. 2016;11(8):1401–1412. doi: 10.2215/CJN.10611015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Imai E., Seiichi M. Chronic kidney disease in Asia. Lancet. 2008;371(9631):2147–2148. doi: 10.1016/S0140-6736(08)60928-9. [DOI] [PubMed] [Google Scholar]
- 27.Remuzzi G. Continuum of renoprotection with losartan at all stages of type 2 diabetic nephropathy: a post hoc analysis of the RENAAL trial results. J Am Soc Nephrol. 2004;15(12):3117–3125. doi: 10.1097/01.ASN.0000146423.71226.0C. [DOI] [PubMed] [Google Scholar]
- 28.Shemin D., Bostom A.G., Laliberty P., Dworkin L.D. Residual renal function and mortality risk in hemodialysis patients. Am J Kidney Dis. 2001;38(1):85–90. doi: 10.1053/ajkd.2001.25198. [DOI] [PubMed] [Google Scholar]
- 29.Hillege H.L., Fidler V., Diercks G.F.H. Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in general population. Circulation. 2002;106(14):1777–1782. doi: 10.1161/01.cir.0000031732.78052.81. [DOI] [PubMed] [Google Scholar]
- 30.Tokmakova M.P., Skali H., Kenchaiah S. Chronic kidney disease, cardiovascular risk, and response to angiotensin-converting enzyme inhibition after myocardial infarction: the Survival and Ventricular Enlargement (SAVE) Study. Circulation. 2004;110(24):3667–3673. doi: 10.1161/01.CIR.0000149806.01354.BF. [DOI] [PubMed] [Google Scholar]
- 31.Brar S., Ye F., James M.T., Hemmelgarn B., Klarenbach, Pannu N., for the Interdisciplinary Chronic Disease Collaboration Association of angiotensin-converting enzyme inhibitor or angiotensin receptor blocker use with outcomes after acute kidney injury. JAMA Intern Med. 2018;178(12):1681–1690. doi: 10.1001/jamainternmed.2018.4749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bhandari S., Ives N., Brettell E.A. Multicentre randomized controlled trial of angiotensin-converting enzyme inhibitor/angiotensin receptor blocker withdrawal in advanced renal disease: the STOP-ACEi Trial. Nephrol Dial Transplant. 2016;31(2):255–261. doi: 10.1093/ndt/gfv346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wanner C., Inzucchi S.E., Lachin J.M. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375:323–334. doi: 10.1056/NEJMoa1515920. [DOI] [PubMed] [Google Scholar]
- 34.Perkovic V., de Zeeuw D., Mahaffey K.W. Canagliflozin and renal outcomes in type 2 diabetes: results from the CANVAS Program randomised clinical trials. Lancet Diabetes Endocrinol. 2018;6:691–704. doi: 10.1016/S2213-8587(18)30141-4. [DOI] [PubMed] [Google Scholar]
- 35.Perkovic V., Jardine M.J., Neal B. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295–2306. doi: 10.1056/NEJMoa1811744. [DOI] [PubMed] [Google Scholar]
- 36.Heerspink H.J.L., Desai M., Jardine M., Balis D., Meininger G., Perkovic V. Canagliflozin slows progression of renal function decline independently of glycemic effects. J Am Soc Nephrol. 2017;28(1):368–375. doi: 10.1681/ASN.2016030278. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
. Tables S1-S4