Abstract
Rationale & Objective
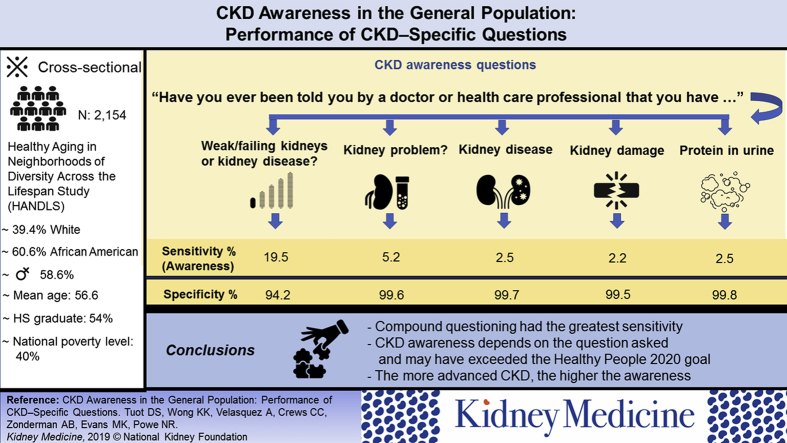
Data from patients in one delivery system have suggested that the prevalence of chronic kidney disease (CKD) awareness differs by how the question is asked. We examined the sensitivity and specificity of different CKD awareness questions among diverse community-dwelling adults who were not necessarily engaged in primary care to determine the generalizability of prior results.
Study Design
Cross-sectional study.
Setting & Participants
Participants in the Healthy Aging in Neighborhoods of Diversity Across the Life Span (HANDLS) Study.
Predictor
CKD awareness, ascertained using 5 different questions.
Outcome
Sensitivity and specificity of each awareness question, using laboratory results as the gold standard.
Analytic Approach
Logistic regression was used to compare sensitivities of different awareness questions.
Results
Among 2,046 participants, mean (SD) age was 56.5 (9.1) years, 41.5% were men, and 61.3% were African American. More than 40% were poor, 35% reported not having health insurance, and 16.9% had low health literacy. More than 20% (n = 424) had CKD. Sensitivities of single CKD awareness questions ranged from 2.2% for “kidney damage” to 5.2% for “kidney problem.” Sensitivity of the compound question asking about “weak kidneys, failing kidneys, or kidney disease” was 19.5%. Sensitivity of this compound CKD awareness question was higher among study participants with more advanced CKD and low health literacy, and those who lived below the poverty level.
Limitations
Single measures of estimated glomerular filtration rate and albuminuria; study participants may have been more engaged in their health care than the average US adult, potentially limiting the generalizability of results.
Conclusions
CKD awareness is low among community-dwelling adults with kidney disease, though data using a sensitive compound question ascertaining awareness suggest that we have met the Healthy People 2020 goal related to CKD awareness of 13.4%. Understanding the phrases about kidney disease that are most understandable to patients with and at risk for CKD is important to further increase CKD awareness.
Index Words: CKD, CKD awareness, sensitivity, literacy, knowledge, HANDLS
Graphical abstract
Chronic kidney disease (CKD) affects more than 30 million people in the United States1 and is associated with cardiovascular morbidity and increased mortality.2 Risk modification (ie, glycemic control, blood pressure control, and avoidance of nephrotoxic substances) can decrease CKD-associated morbidity, including progression to end-stage kidney disease (ESKD), and mortality.3, 4, 5 Such risk modification is presumed to rely heavily on patient understanding of disease, engagement in health care, and empowerment to participate in healthy lifestyles. National efforts have tried to increase individual awareness of CKD with the assumption that increased awareness would lead to improved health outcomes.6, 7 Paradoxically, studies have demonstrated that CKD awareness is not associated with participation in healthy behaviors,8 achievement of risk-reduction targets, such as blood pressure control or use of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers,9 or slower CKD progression.10 Reasons for these observations are likely multifactorial, including the way in which CKD awareness has been ascertained in these studies.
Prior work has suggested that the prevalence of CKD awareness differs by how the question is asked.11 In one study of English- and Spanish-speaking adults receiving primary care at an urban safety-net hospital in the western United States, CKD awareness was ascertained using the question in the National Health and Nutrition Examination Survey (NHANES): “Have you ever been told by a doctor or health care provider that you have weak or failing kidneys (excluding stones, bladder infections, or incontinence)?” and additional questions asking whether patients had been told about “kidney disease,” “protein in the urine,” “kidney problem,” or that “your kidneys are damaged.” The sensitivity of each CKD awareness question ranged from 26.4% for “kidney damage” to 33.2% for “weak or failing kidneys” to 40.1% for “kidney problem.”11 These data suggested that the NHANES question used for national estimates was relatively insensitive to identify awareness of CKD among patients engaged with a health care system and that actual awareness of CKD using different verbiage was potentially higher than anticipated.
To determine whether these prior results are generalizable to other groups and thus should inform changes in the way in which CKD awareness is ascertained nationally, we examined the sensitivity and specificity of different ways to ascertain CKD awareness among diverse community-dwelling adults who were not necessarily engaged in primary care.
Methods
Study Design and Participants
This was a cross-sectional study of individual awareness of CKD among participants who completed wave 4 of the Healthy Aging in Neighborhoods of Diversity Across the Life Span (HANDLS) Study. The purpose of HANDLS was to identify the influences and interaction of race and socioeconomic status on the development of cardiovascular and cerebrovascular health disparities. HANDLS participants are community-dwelling African Americans and whites aged 30 to 64 years at enrollment drawn from 12 neighborhoods, each of which is composed of contiguous US census tracts in Baltimore City that reflect socioeconomic and racial diversity. Methods for recruitment in HANDLS have been detailed elsewhere.12
Overall, 3,720 participants between the ages of 30 and 64 years were recruited into HANDLS between August 2004 and March 2009. They all participated in a wave 1 (baseline) study visit and were invited to participate in follow-up visits every 3 years. Wave 2 visits occurred between April 2006 and October 2011 and provided interim contact with participants, as well as updated health-related information. Wave 3 visits occurred between June 2009 and July 2013 and consisted of the first in-person follow-up visits per protocol. Wave 4 visits occurred between September 2013 and September 2017 (Fig S1). Wave 3 and wave 4 visits consisted of health examinations, a telephone dietary recall, kidney function assessments, evaluation of the subjective experience of diabetes mellitus, and participation in other optional studies. Approximately 58% of the original cohort (n = 2171) had a wave 4 visit, during which data were collected for this ancillary study. Our study population excluded individuals missing serum creatinine and urine albuminuria values (n = 108), as well as those with an estimated glomerular filtration rate (eGFR) < 15 mL/min/1.73 m2 (n = 17) at wave 4, for a total study population of 2,046.
The National Institute of Environmental Health Sciences, National Institutes of Health, approved the study protocol, as did the University of California, San Francisco Institutional Review Board (#10-02885). All participants provided written informed consent to participate.
Data Collection and Definitions
The primary predictor was individual awareness of CKD ascertained by a trained interviewer. In wave 1, CKD awareness was asked with the following question: “Have you ever been told by a doctor or health care provider that you have weak kidneys, failing kidneys, or kidney disease?” In wave 4, participants were asked: “Have you ever been told by a doctor or health care professional that” … “you have kidney disease,” “protein in the urine,” “a kidney problem,” and “kidney damage?” These 4 different questions (with the same mentioned question stem) were chosen because they were used in a prior single-site study.11 As part of this ancillary study, these questions were intermixed with other questions about self-rated health (asked per original HANDLS protocol) and were asked in the same order for each participant.
Self-reported demographic information (age, sex, race, educational attainment, health insurance, annual household income, and comorbid conditions) was obtained by interview during wave 1. Health literacy was determined using the short Test of Functional Health Literacy in Adults (TOFHLA) during wave 3, using a cutoff score of 60 to differentiate between individuals with adequate versus inadequate literacy levels.13 Reading level was ascertained using the Wide Range Achievement Test–3 (WRAT3) during wave 3; individuals with scores > 40 were considered to have at least an 8th grade reading level.14
Physical examination (blood pressure, height, and weight) and laboratory measures (serum creatinine, urine microalbumin, serum glycated hemoglobin, and fasting glucose) were obtained during wave 4. Each participant underwent sitting and standing blood pressure measurements on each arm using the brachial artery auscultation method with an inflatable cuff of appropriate size. Hypertension was defined as an average seated systolic blood pressure > 140 mm Hg, an average seated diastolic blood pressure > 90 mm Hg, a history of blood pressure medication use, and/or a self-report of hypertension. Diabetes was defined as a fasting glucose level > 126 mg/dL, self-report of diabetes, or use of diabetic medication. CKD was defined by single values of eGFR < 60 mL/min/1.73 m2 calculated using the CKD-EPI (CKD Epidemiology Collaboration) equation,15 or a urine microalbumin-creatinine ratio > 30 mg/g.16
Statistical Analysis
Characteristics of participants were compared by CKD status using χ2, analysis of variance, and Mann-Whitney-Wilcoxon tests as appropriate. Using laboratory measurements as the gold standard, sensitivity (true positive rate or the proportion of participants with CKD who answered yes correctly, otherwise known as CKD awareness) and specificity (true negative rate or the proportion of participants without CKD who answered no correctly) of each awareness question were calculated for the entire study population. Then logistic regression analysis was used to determine the independent associations of sociodemographic characteristics (age, sex, race, educational attainment, poverty level, insurance status, regular source of health care, literacy status, and reading level) and comorbid conditions (diabetes, hypertension, and CKD stage) with awareness of CKD in the entire cohort, defined as a correct “yes” answer to any of the CKD awareness questions.
Model 1 included age, sex, race, educational attainment, poverty status, health insurance status, and regular source of health care. Model 2 included age, sex, race, other variables from model 1 that were moderately associated with awareness at P < 0.20, and reading level and health literacy status. Model 3 included age, race, sex, other variables from model 2 that were moderately associated with awareness at P < 0.20, and comorbid conditions. Calculations of sensitivity for each awareness question were then performed separately among subgroups of the study population, stratified by the variables moderately associated with CKD awareness (P < 0.2) in the final logistic regression model (model 3). STATA, version 14.2 (StataCorp LLC), was the statistical software used for this analysis.
Results
Study Participants
Among the study population of 2,046 people, mean (SD) age was 56.5 (9.1) years, 41.5% were men, 38.7% were white, and 61.3% were African American. Nearly one-third did not complete their high school education, 16.9% had low health literacy, and 39.5% had lower than an 8th grade reading level. More than 40% lived below the 125% national poverty level, 64.8% reported having health insurance, and 63.2% cited a regular source of health care. Compared with individuals without kidney disease (n = 1,622), individuals with CKD (n = 424) were slightly older (60.1 vs 55.5 years; P = 0.001), more likely to be African American and poor, and have low health literacy and a reading level below 8th grade (Table 1). Nearly one-quarter of the overall cohort carried a diagnosis of diabetes and 64% had hypertension. The proportion of individuals with both these comorbid conditions was higher among individuals with CKD (diabetes, 46.5% vs 18.5%; hypertension, 86.2% vs 59.2%; P = 0.001 for both comparisons) compared with those without CKD (Table 1).
Table 1.
Characteristics of the Healthy Aging in Neighborhoods of Diversity Across the Life Span Study Population
| Characteristics | All (N = 2,046) | No CKD (N = 1,622) | CKD (N = 424) |
|---|---|---|---|
| Age, y | 56.5 (9.1) | 55.6 (9.0) | 60.1 (8.6) |
| Male sex | 850 (41.5%) | 672 (41.4%) | 178 (42.0%) |
| Race/ethnicity | |||
| White | 792 (38.7%) | 662 (40.8%) | 130 (30.7%) |
| African American | 1,254 (61.3%) | 960 (59.2%) | 294 (69.3%) |
| Educational attainment | |||
| <High school | 657 (32.8%) | 509 (32.2%) | 148 (35.3%) |
| High school graduate | 1,089 (54.4%) | 858 (54.3%) | 231 (55.1%) |
| College graduate | 254 (12.7%) | 214 (13.5%) | 40 (9.6%) |
| Poverty (<125% poverty level) | 825 (40.3%) | 627 (38.7%) | 198 (46.7%) |
| Low health literacy | 260 (16.9%) | 195 (15.8%) | 65 (21.5%) |
| <8th grade reading level | 787 (39.5%) | 603 (38.1%) | 184 (45.1%) |
| Health insurance | 1,326 (64.8%) | 1,036 (63.9%) | 290 (68.4%) |
| Regular source of health care | 1,266 (63.2%) | 978 (61.8%) | 288 (68.7%) |
| Diagnosed diabetes | 496 (24.3%) | 299 (18.4%) | 197 (46.5%) |
| Diagnosed hypertension | 1,319 (64.7%) | 956 (59.2%) | 363 (86.2%) |
| Hemoglobin A1c, % | 6.2 (1.3) | 6.0 (1.1) | 6.8 (1.9) |
| SBP, mm Hg | 117.3 (20.3) | 116.2 (19.1) | 121.4 (23.8) |
| DBP, mm Hg | 65.5 (11.3) | 65.4 (10.6) | 66.0 (13.2) |
| eGFR, mL/min/1.73 m2 | 87.9 (19.9) | 92.0 (15.4) | 72.0 (26.3) |
| eGFR < 60 mL/min/1.73 m2 | 193 (9.3%) | 0 (0%) | 193 (45.5%) |
| Albuminuria, mg/g | 5.4 [3.5-12.8] | 4.7 [3.2-7.7] | 55.3 [16.2-146.5] |
| Albuminuria > 30 mg/g | 295 (14.4%) | 0 (0%) | 295 (70.2%) |
Note: Values expressed as mean (standard deviation), number (percent), or median [interquartile range]. n = 2,154 for all rows except educational attainment (total N = 2,000; no CKD n = 1,581; CKD n = 419); health literacy (total N = 1,534; no CKD n = 1,231; CKD n = 303); reading level (total N = 1,991; no CKD n = 1,583; CKD n = 408); regular source of health care (total N = 2,002; no CKD n = 1,583; CKD n = 419); diagnosed hypertension (total N = 2,037; no CKD n = 1,616; CKD n = 421); diagnosed diabetes (total N = 2,045; no CKD n = 1,621; CKD n = 424); hemoglobin A1c (total N = 2,034; no CKD n = 1,617; CKD n = 417); SBP and DBP (total N = 2,028; no CKD n = 1,610; CKD n = 418); and albuminuria (total N = 2,042; no CKD n = 1,622; CKD n = 420).
Abbreviations: CKD, chronic kidney disease; DBP, diastolic blood pressure; SBP, systolic blood pressure.
Sensitivity/Specificity of CKD Awareness Questions
Sensitivities of single CKD awareness questions ranged from 2.2% for “kidney damage” to 5.2% for “kidney problem.” Sensitivity of the compound question asking about “weak kidneys, failing kidneys, or kidney disease” was 19.5%. Specificities ranged from 94.2% for the compound question to 99.8% for “protein in the urine” (Table 2).
Table 2.
Sensitivity and Specificity of Different Questions Ascertaining CKD Awareness
| Awareness Question | Overall Cohort |
|
|---|---|---|
| True Positive: Sensitivity | True Negative: Specificity | |
| Diagnosed with weak kidneys, failing kidneys, or kidney disease | 19.5% (82/421) | 94.2% (1,553/1,649) |
| Kidney problem | 5.2% (21/405) | 99.6% (1,556/1,562) |
| Kidney disease | 2.5% (10/408) | 99.7% (1,596/1,601) |
| Kidney damage | 2.2% (9/408) | 99.5% (1,594/1,601) |
| Protein in the urinea | 2.5% (7/284) | 99.8% (1,653/1,656) |
Abbreviation: CKD, chronic kidney disease.
Among those with proteinuria only.
In multivariate logistic regression, awareness of CKD using any of the questions was moderately associated with higher (more severe) CKD stage, inadequate health literacy status, greater poverty status, and having insurance (Table 3). Sensitivity of each awareness question was then calculated among subgroups of the study population, stratified by the mentioned variables that were moderately associated with CKD awareness (Table 4).
Table 3.
Independent Associations of Sociodemographic Variables and Comorbid Conditions With Awareness of CKD, Defined by a Correct Response to Any of the CKD Awareness Questions
| Variable | Model 1 |
Model 2 |
Model 3 |
|---|---|---|---|
| Odds Ratio (95% CI) | Odds Ratio (95% CI) | Odds Ratio (95% CI) | |
| Age, per y | 1.05 (1.02-1.08)a | 1.06 (1.03-1.10)a | 1.00 (0.96-1.04) |
| Female sex, vs male | 0.99 (0.64-1.55) | 0.98 (0.58-1.68) | 0.67 (0.35-1.27) |
| African American, vs white | 1.09 (0.69-1.71) | 1.08 (0.62-1.86) | 0.96 (0.52-1.79) |
| High school graduate, vs <high school graduate | 0.96 (0.60-1.52) | __ | __ |
| Below 125% poverty level, vs above | 1.82 (1.16-2.87)a | 1.92 (1.13-3.29)a | 1.55 (0.85-2.81)a |
| Having health insurance, vs none | 1.53 (0.84-2.79) | 2.36 (1.19-4.67)a | 2.05 (0.97-4.35)a |
| Having a regular source of health care, vs not | 1.13 (0.64-2.00) | __ | __ |
| Above 8th grade reading level, vs below | __ | 1.05 (0.58-1.84) | __ |
| Adequate health literacy level, vs inadequate | __ | 0.56 (0.31-1.03)a | 0.62 (0.32-1.20)a |
| Presence of diabetes | __ | __ | 1.17 (0.84-1.65) |
| Presence of hypertension | __ | __ | 1.00 (0.40-2.50) |
| CKD stages 3/4, vs stages 1/2 | __ | __ | 2.48 (1.29-4.75)a |
Note: All models are multivariate logistic regression models and include age, sex, race and variables from the prior model that were associated with awareness at P < 0.2.
Abbreviations: CI, confidence interval; CKD, chronic kidney disease.
Variables associated with awareness with P < 0.2.
Table 4.
Unadjusted Sensitivities of Each Awareness Question Stratified by Severity of CKD, Poverty Level, Health Literacy Status, and Having Health Insurance
|
Severity of CKD | |||
|---|---|---|---|
| Awareness Question | CKD Stages 1-2 |
CKD Stages 3-4 |
Unadjusted P |
| Sensitivity | Sensitivity | ||
| Diagnosed with weak kidneys, failing kidneys, or kidney disease | 26/230 (11.3%) | 56/191 (29.3%) | 0.001 |
| Kidney problem | 5/222 (2.3%) | 16/183 (8.7%) | 0.003 |
| Kidney disease | 3/224 (1.3%) | 7/184 (3.8%) | 0.1 |
| Kidney damage | 2/224 (0.89%) | 7/184 (3.8%) | 0.05 |
| Protein in the urinea | 3/222 (1.4%) | 4/62 (6.45%) | 0.5 |
|
Poverty Level | |||
|---|---|---|---|
| Awareness Question | Above Poverty Level |
Below Poverty Level |
Unadjusted P |
| Sensitivity | Sensitivity | ||
| Diagnosed with weak kidneys, failing kidneys, or kidney disease | 36/224 (16.1%) | 46/197 (23.8%) | 0.003 |
| Kidney problem | 9/218 (4.1%) | 12/187 (6.4%) | 0.1 |
| Kidney disease | 3/221 (1.4%) | 6/187 (3.2%) | 0.9 |
| Kidney damage | 6/221 (2.7%) | 4/187 (2.1%) | 0.1 |
| Protein in the urinea | 3/151 (2.0%) | 4/133 (3.0%) | 0.4 |
|
Health Literacy | |||
|---|---|---|---|
| Awareness Question | Adequate Health Literacy |
Inadequate Health Literacy |
Unadjusted P |
| Sensitivity | Sensitivity | ||
| Diagnosed with weak kidneys, failing kidneys, or kidney disease | 39/236 (16.5%) | 17/65 (26.2%) | 0.006 |
| Kidney problem | 6/231 (2.6%) | 0/62 (0.0%) | 0.3 |
| Kidney disease | 3/232 (1.3%) | 1/62 (1.6%) | 0.7 |
| Kidney damage | 3/232 (1.3%) | 0/62 (0.0%) | 0.4 |
| Protein in the urinea | 4/158 (2.5%) | 0/45 (0.0%) | 0.4 |
|
Health Insurance | |||
|---|---|---|---|
| Awareness Question | Has Health Insurance |
Does Not Have Health Insurance |
Unadjusted P |
| Sensitivity | Sensitivity | ||
| Diagnosed with weak kidneys, failing kidneys, or kidney disease | 61/288 (21.2%) | 21/133 (15.8%) | 0.07 |
| Kidney problem | 13/278 (4.7%) | 8/127 (6.3%) | 0.8 |
| Kidney disease | 6/279 (2.1%) | 3/129 (2.3%) | 0.7 |
| Kidney damage | 6/279 (2.1%) | 4/129 (3.1%) | 0.9 |
| Protein in the urinea | 5/190 (2.6%) | 2/94 (2.1%) | 0.7 |
Note: P values identify significant differences in sensitivity of awareness questions, in unadjusted models.
Abbreviation: CKD, chronic kidney disease.
Among those with proteinuria only.
Higher sensitivities of CKD awareness questions were noted among study participants with more advanced CKD. Among study participants with CKD stages 1 and 2, sensitivities ranged from 1% (“kidney damage”) to 11.3% (“weak kidneys, failing kidneys, or kidney disease”). Among those with CKD stages 3 and 4, sensitivities ranged from 3.8% (“kidney disease”) to 29.3% (“weak kidneys, failing kidneys, or kidney disease”). Sensitivities of the compound question “weak kidneys, failing kidneys, or kidney disease” and the questions asking about a “kidney problem” or “kidney damage” were higher among individuals with more severe CKD (P values ranging from 0.001 to 0.05). Only sensitivity of the compound question was higher among individuals who lived below, compared with those who lived above, the poverty level (23.8% vs 16.1%; P = 0.003), without statistically significant differences in sensitivities of other awareness questions by poverty level. Similarly, sensitivity of the compound question was higher among individuals with inadequate health literacy compared with those with adequate health literacy (26.2% vs 16.5%; P = 0.006) and among individuals with insurance compared with those without health insurance (21.1% vs 15.8%; P = 0.07; Table 4).
Discussion
This population-based study has 3 key findings. First, it corroborates prior work that awareness of CKD is low among community-dwelling adults who may or may not have continuing ongoing contact with a health care delivery system.11, 17, 18 Second, it demonstrates that the detection of awareness among those truly having CKD varies with how the question is asked. The question currently used in national health surveys (“Have you ever been told by a doctor or health care provider that you have weak or failing kidneys [excluding stones, bladder infections, or incontinence]?”) may be poorly estimating true CKD awareness in the general population and may be partially responsible for the divergent results between low awareness of CKD and high awareness of other common chronic diseases such diabetes, hypertension, and hypercholesterolemia. Third, it provides additional evidence that using a compound question to ascertain awareness has greater sensitivity at the expense of a modest decrease in specificity.
Low individual awareness of CKD has implications for clinical care, as well as for research. From a clinical perspective, individuals who do not know about their kidney disease may be less motivated to participate in self-management activities that are directly related to kidney health. Such activities could include medication adherence, healthy dietary patterns, tobacco cessation, and increased physical activity, among other healthy habits. Additionally, individuals who do not know that they have CKD may not know to avoid potentially nephrotoxic medications or supplements, both of which are commonly used among individuals with kidney disease.19, 20 It is thus not surprising that patients with advanced CKD and ESKD wish that they had known more about their kidney disease at earlier stages.21, 22 Additionally, data from some pre-ESKD programs with robust educational components have been associated with decreased incidence in dialysis, better dialysis preparation, and lower mortality, including programs that focused on individuals with less severe CKD.23, 24 Ensuring that patients with CKD at highest risk for progression to ESKD are aware of their CKD and have resources to translate that awareness into action is thus one important component of CKD care delivery.
From a research perspective, low individual awareness of CKD could inhibit participation in clinical trials that seek to recruit individuals with kidney disease. This may be of particular importance for therapies aimed at changing the trajectory of kidney disease decline, which necessitate inclusion of individuals with mild-moderate kidney disease (as opposed to individuals with severe CKD), which are the populations in whom CKD awareness is lowest.25 Low awareness of kidney disease at the population level may also lead to low perceived importance of the disease and is likely one contributing factor to the lower level of federal funding set aside for kidney disease research compared with other chronic conditions26 and the relative paucity of clinical trials in nephrology compared with other medical disciplines.27
Not only is achieving high levels of CKD awareness essential for clinical care and clinical research, but accurately measuring CKD awareness is important from the public health perspective as well. Public health care officials or insurance plan executives may launch health campaigns with the goal of promoting healthy behaviors among individuals with a specific disease.28 Change in CKD awareness at the population level, as well as self-reported participation in healthy behaviors, could be used to evaluate the success of such campaigns targeted at improving kidney health. Also, in the absence of a perfect measure of CKD awareness, the potential tradeoff between sensitivity and specificity of a measure of CKD awareness should be acknowledged. Maximizing the sensitivity of a CKD awareness metric may be important to ensure a campaign’s overall reach or penetration within an at-risk population; maximizing specificity of the measure can ensure accuracy of a campaign’s message. Using a metric of CKD awareness with highest sensitivity and little tradeoff with respective to specificity would be important to evaluate which local campaigns to spread at a regional or national level.
With that in mind, the most common question related to kidney disease awareness (that used in NHANES) may benefit from updating, particularly in light of the evolving terminology of kidney disease in the nephrology field. Results from our prior work suggested that individuals with CKD who responded “yes” to having “protein in the urine” were different from those who responded “yes” to having “kidney disease” or a “kidney problem.”11 Using a compound question including the term kidney disease and also including a descriptor of proteinuria, in addition to the current terms used in the NHANES survey (“weak or failing kidneys”), could thus be useful in future attempts to accurately ascertain CKD awareness. One possibility could be the awareness question: “Have you ever been told by a doctor or other health provider that you have weak or failing kidneys, kidney disease, or protein in the urine?” While this question was not explicitly tested in our study, sensitivity of the one compound question in this study asking about “weak kidneys, failing kidneys, or kidney disease” was high, even among individuals who were poor and those with limited health literacy.
Educational literature suggests that compound questions may score less well with readability instruments because of their length and may be more difficult for individuals with limited health literacy to answer.29 Limited health literacy is common among patients with CKD.30, 31, 32 However, similar to the compound question that was tested in this study, our proposed compound question incorporates the essential elements that define CKD, has few syllables and short words, and includes terminology about kidney disease that is used in public health campaigns and educational materials from national societies and nonprofit organizations.33, 34
The results of this study should be considered within the context of its limitations. Sensitivity/specificity estimates of CKD awareness rely on single measurements of eGFR and albuminuria (similar to NHANES and other population-based studies) rather than 2 repeated measurements in clinical care. This could overestimate the number of individuals with kidney disease and underestimate CKD awareness and could potentially explain the large difference in prevalence of CKD awareness in this study compared to our prior work among individuals engaged with primary care in one health care delivery system.
Also, there was a relatively small number of study participants deemed to have CKD in the overall HANDLS cohort. HANDLS study participants completing the fourth wave of follow-up may have been more engaged in their health care than the average adult in the United States due to their participation in a longitudinal study. For example, >64% of participants in this study cited a regular source of health care. The prevalence and distribution of sociodemographic characteristics of wave 4 study participants were not substantially different from those of HANDLS baseline study participants (including the proportion of individuals with a regular source of health care), except for a smaller proportion of college graduates and a larger percentage of individuals with less than high school education in the baseline cohort (data not shown).
The study population was representative of whites and African Americans of varying socioeconomic status residing in the Baltimore metropolitan area and thus results may not be generalizable to all US adults, particularly those residing in rural areas. Nevertheless, this study shows that by using a more sensitive combination question to ascertain CKD awareness, asking about “weak kidneys, failing kidneys, or kidney disease,” estimates of CKD awareness may be closer to 20%. Given the results seen in this study population, CKD awareness may have already exceeded the Healthy People 2020 target of 13.4%.35
In summary, we corroborate that CKD awareness is low not only in populations engaged with primary care but also among community-dwelling adults with kidney disease. We also demonstrate that we have likely met the Healthy People 2020 goal related to CKD awareness when using a more sensitive measure of awareness. Understanding the phrases about kidney disease that are most understandable to patients with and at risk for CKD and targeting the population with such language in awareness campaigns are important next steps to further improve upon awareness metrics in the United States. Devising new tools to help primary care providers discuss kidney disease is another important step. These efforts will allow us to better target future awareness messages and more accurately evaluate campaign successes.
Article Information
Authors’ Full Names and Academic Degrees
Delphine S. Tuot, MDCM, MAS, Karen K. Wong, BA, Alexandra Velasquez, BS, Deidra C. Crews, MD, ScM, Alan B. Zonderman, PhD, Michele K. Evans, MD, and Neil R. Powe, MD, MPH, MBA.
Authors’ Contributions
Research idea and study design: DST, NRP; data acquisition: AZ, ME; data analysis/interpretation: DST, AV, KW, DCC, NRP; statistical analysis: DST; supervision or mentorship: NRP. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Support
This work is supported by the Intramural Research Program of the National Institute on Aging, National Institutes of Health, grant Z01-AG000513. Dr Tuot is supported by grant R01DK104130 from the National Institute of Diabetes and Digestive and Kidney Diseases. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Financial Disclosure
The authors declare that they have no relevant financial interests.
Acknowledgements
We thank the participants of the HANDLS cohort study.
Peer Review
Received November 23, 2018. Evaluated by 2 external peer reviewers, with direct editorial input from the Statistical Editor, an Associate Editor, and the Editor-in-Chief. Accepted in revised form January 21, 2019.
Footnotes
Complete author and article information provided before references.
Figure S1: Timeline of study visits for the Healthy Aging in Neighborhoods of Diversity Across the Life Span (HANDLS) Study
Supplementary Material
Timeline of study visits for the Healthy Aging in Neighborhoods of Diversity Across the Life Span (HANDLS) Study.
References
- 1.US Renal Data System . National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health; Bethesda, MD: 2018. USRDS 2018 Annual Data Report: epidemiology of kidney disease in the United States. [Google Scholar]
- 2.Go A.S., Chertow G.M., Fan D., McCulloch C.E., Hsu C.Y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 3.Barrett B.J. Applying multiple interventions in chronic kidney disease. Semin Dial. 2003;16(2):157–164. doi: 10.1046/j.1525-139x.2003.16032.x. [DOI] [PubMed] [Google Scholar]
- 4.Sarnak M.J., Greene T., Wang X. The effect of a lower target blood pressure on the progression of kidney disease: long-term follow-up of the Modification of Diet in Renal Disease Study. Ann Intern Med. 2005;142(5):342–351. doi: 10.7326/0003-4819-142-5-200503010-00009. [DOI] [PubMed] [Google Scholar]
- 5.Nissenson A.R., Collins A.J., Hurley J., Petersen H., Pereira B.J., Steinberg E.P. Opportunities for improving the care of patients with chronic renal insufficiency: current practice patterns. J Am Soc Nephrol. 2001;12(8):1713–1720. doi: 10.1681/ASN.V1281713. [DOI] [PubMed] [Google Scholar]
- 6.McCullough P.A., Brown W.W., Gannon M.R. Sustainable community-based CKD screening methods employed by the National Kidney Foundation's Kidney Early Evaluation Program (KEEP) Am J Kidney Dis. 2011;57(3suppl 2):S4–S8. doi: 10.1053/j.ajkd.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 7.Narva A.S., Briggs M. The National Kidney Disease Education Program: improving understanding, detection, and management of CKD. Am J Kidney Dis. 2009;53(3suppl 3):S115–S120. doi: 10.1053/j.ajkd.2008.05.038. [DOI] [PubMed] [Google Scholar]
- 8.Tuot D.S., Plantinga L.C., Judd S.E. Healthy behaviors, risk factor control and awareness of chronic kidney disease. Am J Nephrol. 2013;37(2):135–143. doi: 10.1159/000346712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tuot D.S., Plantinga L.C., Hsu C.Y., Powe N.R. Is awareness of chronic kidney disease associated with evidence-based guideline-concordant outcomes? Am J Nephrol. 2012;35(2):191–197. doi: 10.1159/000335935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whaley-Connell A., Shlipak M.G., Inker L.A. Awareness of kidney disease and relationship to end-stage renal disease and mortality. Am J Med. 2012;125(7):661–669. doi: 10.1016/j.amjmed.2011.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tuot D.S., Zhu Y., Velasquez A. Variation in patients' awareness of CKD according to how they are asked. Clin J Am Soc Nephrol. 2016;11(9):1566–1573. doi: 10.2215/CJN.00490116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evans M.K., Lepkowski J.M., Powe N.R., LaVeist T., Kuczmarski M.F., Zonderman A.B. Healthy Aging in Neighborhoods of Diversity Across the Life Span (HANDLS): overcoming barriers to implementing a longitudinal, epidemiologic, urban study of health, race, and socioeconomic status. Ethn Dis. 2010;20(3):267–275. [PMC free article] [PubMed] [Google Scholar]
- 13.Parker R.M., Baker D.W., Williams M.V., Nurss J.R. The Test of Functional Health Literacy in Adults: a new instrument for measuring patients' literacy skills. J Gen Intern Med. 1995;10(10):537–541. doi: 10.1007/BF02640361. [DOI] [PubMed] [Google Scholar]
- 14.Wilkinson G. 3rd ed. Wide Range; Wilmington, DE: 1993. The Wide Range Achievement Test: Manual. [Google Scholar]
- 15.Lamb E.J., Stevens P.E. Estimating and measuring glomerular filtration rate: methods of measurement and markers for estimation. Curr Opin Nephrol Hypertens. 2014;23(3):258–266. doi: 10.1097/01.mnh.0000444813.72626.88. [DOI] [PubMed] [Google Scholar]
- 16.Inker L.A., Astor B.C., Fox C.H. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis. 2014;63(5):713–735. doi: 10.1053/j.ajkd.2014.01.416. [DOI] [PubMed] [Google Scholar]
- 17.Plantinga L.C., Tuot D.S., Powe N.R. Awareness of chronic kidney disease among patients and providers. Adv Chronic Kidney Dis. 2010;17(3):225–236. doi: 10.1053/j.ackd.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lopez-Vargas P.A., Tong A., Phoon R.K., Chadban S.J., Shen Y., Craig J.C. Knowledge deficit of patients with stage 1-4 CKD: a focus group study. Nephrology (Carlton) 2014;19(4):234–243. doi: 10.1111/nep.12206. [DOI] [PubMed] [Google Scholar]
- 19.Plantinga L., Grubbs V., Sarkar U. Nonsteroidal anti-inflammatory drug use among persons with chronic kidney disease in the United States. Ann Fam Med. 2011;9(5):423–430. doi: 10.1370/afm.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grubbs V., Plantinga L.C., Tuot D.S. Americans' use of dietary supplements that are potentially harmful in CKD. Am J Kidney Dis. 2013;61(5):739–747. doi: 10.1053/j.ajkd.2012.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lewis A.L., Stabler K.A., Welch J.L. Perceived informational needs, problems, or concerns among patients with stage 4 chronic kidney disease. Nephrol Nurs J. 2010;37(2):143–148. quiz 149. [PubMed] [Google Scholar]
- 22.Teasdale E.J., Leydon G., Fraser S., Roderick P., Taal M.W., Tonkin-Crine S. Patients' experiences after CKD diagnosis: a meta-ethnographic study and systematic review. Am J Kidney Dis. 2017;70(5):656–665. doi: 10.1053/j.ajkd.2017.05.019. [DOI] [PubMed] [Google Scholar]
- 23.Wu I.W., Wang S.Y., Hsu K.H. Multidisciplinary predialysis education decreases the incidence of dialysis and reduces mortality--a controlled cohort study based on the NKF/DOQI guidelines. Nephrol Dial Transplant. 2009;24(11):3426–3433. doi: 10.1093/ndt/gfp259. [DOI] [PubMed] [Google Scholar]
- 24.Kurella Tamura M., Li S., Chen S.C. Educational programs improve the preparation for dialysis and survival of patients with chronic kidney disease. Kidney Int. 2014;85(3):686–692. doi: 10.1038/ki.2013.369. [DOI] [PubMed] [Google Scholar]
- 25.Tuot D.S., Plantinga L.C., Hsu C.Y. Chronic kidney disease awareness among individuals with clinical markers of kidney dysfunction. Clin J Am Soc Nephrol. 2011;6(8):1838–1844. doi: 10.2215/CJN.00730111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mendu M.L., Erickson K.F., Hostetter T.H. Federal funding for kidney disease research: a missed opportunity. Am J Public Health. 2016;106(3):406–407. doi: 10.2105/AJPH.2015.303009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chatzimanouil M.K.T., Wilkens L., Anders H.J. Quantity and reporting quality of kidney research. J Am Soc Nephrol. 2019;30(1):13–22. doi: 10.1681/ASN.2018050515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abroms L.C., Maibach E.W. The effectiveness of mass communication to change public behavior. Annu Rev Public Health. 2008;29:219–234. doi: 10.1146/annurev.publhealth.29.020907.090824. [DOI] [PubMed] [Google Scholar]
- 29.Tuot D.S., Cavanaugh K.L. Evaluating the merits of CKD patient educational materials: readability is necessary but not sufficient. Am J Kidney Dis. 2015;65(6):814–816. doi: 10.1053/j.ajkd.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 30.Green J.A., Mor M.K., Shields A.M. Prevalence and demographic and clinical associations of health literacy in patients on maintenance hemodialysis. Clin J Am Soc Nephrol. 2011;6(6):1354–1360. doi: 10.2215/CJN.09761110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cavanaugh K.L., Wingard R.L., Hakim R.M. Low health literacy associates with increased mortality in ESRD. J Am Soc Nephrol. 2010;21(11):1979–1985. doi: 10.1681/ASN.2009111163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Devraj R., Gordon E.J. Health literacy and kidney disease: toward a new line of research. Am J Kidney Dis. 2009;53(5):884–889. doi: 10.1053/j.ajkd.2008.12.028. [DOI] [PubMed] [Google Scholar]
- 33.Tuot D.S., Davis E., Velasquez A., Banerjee T., Powe N.R. Assessment of printed patient-educational materials for chronic kidney disease. Am J Nephrol. 2013;38(3):184–194. doi: 10.1159/000354314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saab G., Whaley-Connell A.T., McCullough P.A., Bakris G.L. CKD awareness in the United States: the Kidney Early Evaluation Program (KEEP) Am J Kidney Dis. 2008;52(2):382–383. doi: 10.1053/j.ajkd.2008.05.026. [DOI] [PubMed] [Google Scholar]
- 35.US Department of Health and Human Services Healthy People 2020 topics and objectives. https://www.healthypeople.gov/2020/topics-objectives/topic/chronic-kidney-disease/objectives
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Timeline of study visits for the Healthy Aging in Neighborhoods of Diversity Across the Life Span (HANDLS) Study.