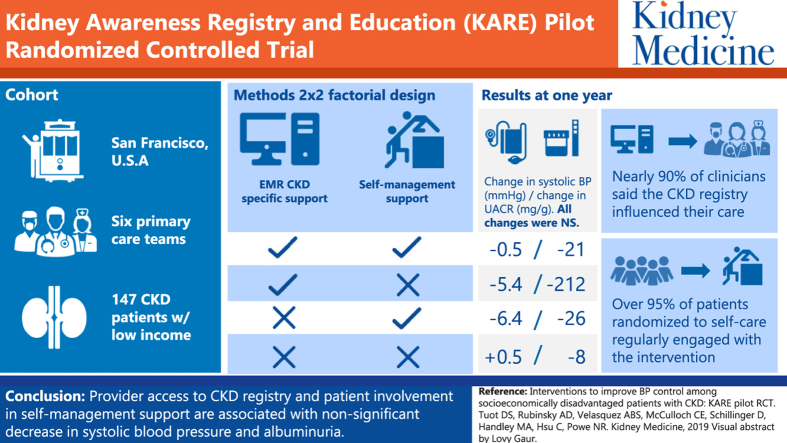
Graphical abstract
Index Words: Chronic kidney disease, randomized controlled trial, registry, self-management support, blood pressure, chronic care model
Abstract
Rationale & Objective
Sustainable interventions that enhance chronic kidney disease (CKD) management are not often studied in safety-net primary care, in which populations bear a disproportionate burden of disease and experience translational gaps between research and practice. We tested the feasibility of implementing and the impact of 2 technology-enhanced interventions designed to enhance CKD care delivery.
Study Design
A 2×2 randomized controlled pilot trial.
Setting & Participants
Primary care provider teams (n = 6) and 137 patients with CKD aged 18 to 75 years from 2 safety-net primary care clinics, 2013 to 2015.
Interventions
Primary care provider teams were randomly assigned to access a CKD registry with point-of-care notifications and quarterly feedback or a usual-care registry for 12 months. Patients within provider teams were randomly assigned to participate in a CKD self-management support program or usual care for 12 months.
Outcomes
We examined recruitment, randomization, and participation in each intervention. We also examined the impact of each intervention and their combination on change in systolic blood pressure (SBP), albuminuria, and patient self-reported behavioral measures after 12 months.
Results
Among potentially eligible patients identified using the electronic health record, 24% were eligible for study participation, of whom 35% (n = 137) were enrolled. Mean age was 55 years, 41% were non–English speaking, and 93% were of racial/ethnic minority. Mean baseline estimated glomerular filtration rate was 70.5 (SD = 30.3) mL/min/1.73 m2; mean baseline SBP was 131 (SD = 21.8) mm Hg. Nearly 90% of clinicians reported that the CKD registry influenced their CKD management. More than 95% of patients randomly assigned to CKD self-management support engaged regularly with the intervention. Estimated changes in SBP over 1 year were nonstatistically different in each of the 3 intervention groups compared with usual care: (usual care: 0.5 [95% CI, −5.2 to 6.3] mm Hg; CKD registry only: −5.4 [95% CI, −12.2 to 1.4] mm Hg; CKD self-management support only: −6.4 [95% CI, −13.7 to 1.0] mm Hg; and CKD registry plus CKD self-management support: −0.5 [−5.5 to 4.5] mm Hg), though differences were larger among those with baseline SBPs > 140/90 mm Hg. Decreases in albuminuria were similarly nonstatistically different in each of the intervention groups compared with usual care. No differences were observed in patient self-reported behaviors.
Limitations
Single health system.
Conclusions
Patient and provider interventions to improve CKD care are feasible to implement in low-income settings with promising results among those with uncontrolled blood pressure.
Funding
National Institute of Diabetes and Digestive and Kidney Diseases.
Trial Registration
ClinicalTrials.gov, number: NCT01530958.
Editorial, p. 229
Chronic kidney disease (CKD) is common, with an estimated prevalence of 11.5% among the US adult population,1 with significant variation by sociodemographic factors,2, 3 and causes excess morbidity and mortality.4, 5 Randomized controlled trials have shown that controlling blood pressure,6 reducing proteinuria using angiotensin-converting enzyme (ACE) inhibitors or angiotensinogen receptor blockers (ARBs),7, 8, 9 and improving glycemic control in people with diabetes10, 11 can delay CKD decline and reduce the morbidity and mortality associated with CKD.12 However, many people with CKD are not receiving the benefit of these findings. There are a number of possible reasons for this, including low levels of CKD awareness among both providers and patients,13, 14, 15 low confidence among primary care clinicians for delivering CKD care,16 and low empowerment of patients to live healthy lifestyles, adhere to medication regimens, and avoid nephrotoxic insults.17, 18
The Chronic Care Model provides a framework for delivering high-quality chronic disease care and can be incorporated into a Patient-Centered Medical Home.19 Implementation of single elements of the Chronic Care Model (eg, health care organization, community resources, patient self-management support [SMS], delivery system redesign, and decision support) can improve care processes for patients with chronic illnesses (ie, decreased hospitalizations among patients with congestive heart failure).20, 21
Many studies of CKD registries (an example of provider decision support) in the United States with computer-assisted prompts/alerts directed toward individual physicians have similarly increased processes of care (serum laboratory testing and increased ACE-inhibitor/ARB use among patients with CKD) and have not been shown to improve clinical outcomes.22, 23, 24, 25 Interventions that have led to improvements in patient outcomes have incorporated several components of the Chronic Care Model.26 The North Carolina Improving Performance in Practice program, a state-wide quality improvement program designed to improve health outcomes among patients with diabetes, for example, showed a positive graded association between improved cholesterol levels and implementation of more Chronic Care Model elements, including chronic disease registries, standardized list of items discussed with every patient with diabetes at each visit, comprehensive care protocols for diabetes management, and support systems for patient self-management.27
Multicomponent sustainable interventions designed to improve the management of CKD in primary care settings are not common, and to our knowledge, none have been studied in US safety-net delivery systems. In these types of systems, individuals of low socioeconomic status, racial/ethnic minority, and/or limited health literacy/English proficiency experience a disproportionate burden of disease28, 29 and large translational gaps between research and practice.30 In the Kidney Awareness Registry and Education (KARE) pilot trial, we examined the feasibility of testing a provider-level intervention (ie, CKD registry) and a patient-level intervention (ie, SMS program) that relied on multiple elements of the Chronic Care Model, as well as their combination, to improve blood pressure among low-income patients with CKD.31, 32
Methods
Study Design, Population, and Setting
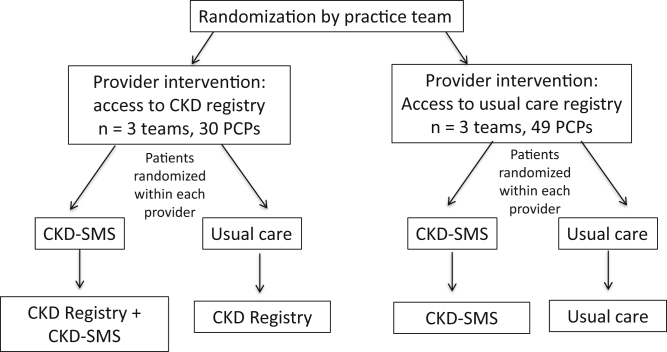
KARE was a 2×2 factorial pilot randomized controlled trial that took place in 2 primary care clinics (1 academic training site and 1 community clinic) in San Francisco’s public health care delivery system between 2013 and 2015. The KARE Study had 2 levels of randomization. First, within each clinic, primary care practice teams consisting of several physicians (including trainees), 1 nurse, nurse practitioners, medical assistants, and behaviorists were randomly assigned 1:1 to 1 of 2 arms with a random number generator: access to a CKD registry versus usual-care registry. Second, within 6 months of the provider-level randomization, eligible patients were recruited to a baseline visit and after receiving written informed consent to participate, were randomly assigned within each provider 1:1 to participate in a year-long comprehensive CKD-SMS program delivered as an adjunct to clinical care or usual clinical care (Fig 1), also with a random number generator administered by the study team. The study period lasted 18 months within each clinic. Study participants (providers and patients) and study personnel (study coordinator and health coaches) were aware of the randomization status of providers and patients, but outcome assessors were blinded to randomization.
Figure 1.
Study design. Academic clinic: May 2013 to November 2014; community clinic: January 2014 to August 2015. Abbreviations: CKD, chronic kidney disease; CKD-SMS, chronic kidney disease self-management support; PCP, primary care provider.
Six primary care provider teams with 79 providers altogether were recruited to participate in the study. Eligible patients included adults (aged ≥18 years) with CKD, defined by 2 values for estimated glomerular filtration rate (eGFR) of 15 to 60 mL/min/1.73 m2 or albuminuria (urine dipstick ≥ 1+ or urinary albumin-creatinine ratio > 30 mg/g) documented in the electronic health record on 2 occasions at least 90 days apart, who had contact with their primary health care team at least once within the past 2 years and spoke English, Spanish, or Cantonese. eGFR using the Modified Diet in Renal Disease Study equation33 was obtained from the electronic health record.
Patients were excluded from the study if they were kidney transplant recipients, pregnant, or unlikely to benefit from the SMS program due to hearing or visual impairment, impaired cognition, severe mental illness, or life expectancy of less than 6 months, as determined by the study team or the primary care provider. Approval to conduct this study was granted by the Institutional Review Board at the University of California, San Francisco (#11-07399), and the trial was registered (ClinicalTrials.gov: NCT01530958).
Interventions
The KARE interventions have been previously described in detail and found to be acceptable among providers and patients.32, 34 Briefly, the provider intervention consisted of an electronic health record–enabled CKD registry tool with “in-reach” and “outreach” elements to support team-based management of CKD. At the point of care during each primary care visit, the CKD registry provided primary care teams with data about patient-specific CKD status (eGFR and CKD on problem list), recent ambulatory clinic blood pressure readings, prescription status of pertinent medications (aspirin, ACE inhibitor/ARB, or statin), and date of last albuminuria quantification. The CKD registry also provided immunization status and data pertinent to age-appropriate cancer screening to align with the electronic health–enabled usual-care registry.
To reach patients who did not regularly visit their primary care provider and would thus not benefit from the in-reach component of the CKD registry, the study team research assistant generated quarterly feedback from the electronic registry to practice teams and individual primary care providers that identified patients for outreach. This included lists of patients with CKD who also had blood pressures ≥ 140/90 mm Hg, were not prescribed an ACE inhibitor/ARB, or had persistent albuminuria. Practice teams randomly assigned to the usual-care registry received point-of-care information about age-appropriate cancer screening and immunization status only; they did not receive quarterly feedback.
The patient intervention was a comprehensive CKD-SMS program based on multiple constructs of Social Cognitive Theory: behavioral capability, self-efficacy, expectations, and reinforcement.35 The CKD-SMS program was delivered over 1 year by 2 full-time bilingual health coaches. The program had 3 distinct elements: (1) language-concordant low-literacy written patient educational materials36 mailed to patients at months 1, 4, and 8; (2) a language-concordant and culturally tailored automated telephone self-management program with 26 modules delivered every other week that reviewed topics pertinent to kidney health: basics of kidney disease, association of kidney disease with high blood pressure, importance of engagement in healthy lifestyle behaviors (diet, physical activity, smoking cessation, and stress reduction), avoidance of nonsteroidal anti-inflammatory medications, participation and preparation for primary care clinic visits, complementary medication use, medication adherence, and glycemic control; and (3) telephone-based health coaching delivered by lay bilingual health coaches trained in motivational interviewing and action planning.
Outcomes
The primary outcome was change in systolic blood pressure (SBP) after 1 year, ascertained by study personnel at the baseline and 12-month study visits using the Omron digital blood pressure monitor model HEM- 907X, using the average of 3 blood pressure measurements in the right arm after the patient sat quietly for 5 minutes.37
Secondary clinical outcomes included changes after 1 year in the proportion of patients with blood pressure control (<140/90 mm Hg) and albuminuria severity (albumin-creatinine ratio), both ascertained at study visits. Secondary behavioral outcomes included changes in patient-reported self-efficacy of chronic disease management, communication with providers, medication adherence, quality of life, and awareness of CKD. Behavioral measures were ascertained at the baseline and 12-month study visits using validated language-concordant instruments, including those validated among similar patients and settings.38, 39, 40, 41
Covariates
At the baseline visit, patient sociodemographic data (age, sex, race/ethnicity, education, income, and insurance status) were self-reported, as were comorbid condition data (diabetes, coronary artery disease, and hyperlipidemia). Food insecurity and health literacy were ascertained using validated screening questionnaires.42, 43
Statistical Analyses
This pilot trial had the goal of assessing the feasibility of recruitment, randomization, and implementation of patient- and provider-level interventions and a multilevel combination of those interventions. No sample size was predetermined for this pilot trial; instead, we executed the pilot trial in 2 different primary care clinics (1 academic training clinic and 1 community-based clinic) and recruited all eligible participants, which ultimately determined the sample size. We examined baseline characteristics of KARE participants and losses to follow-up overall and by study arm.
We also tested for differences across the study arms using χ2 tests for categorical variables and nonparametric Kruskal-Wallis tests for continuous variables. We conducted an intention-to-treat analysis using mixed models to assess the 1-year impact of the CKD registry only, CKD-SMS program only, and CKD registry plus CKD-SMS program compared with usual care for each outcome after an analysis of effect modification for the 2 interventions leveraging the 2×2 factorial design demonstrated nonsignificant interaction for all outcomes. We used the best fitting linear (for continuous outcomes) or logistic (for dichotomous outcomes) mixed model for each outcome based on the lowest Aikake information criterion, considering random intercepts for person, provider, and team, and a random slope for time (with independent, exchangeable, or unstructured covariance structure). A bootstrap analysis was performed to examine change in albuminuria given the non-normally distributed residuals. Cluster robust standard errors were calculated using the sandwich estimator in the final model for each outcome to account for misspecification. A subgroup analysis of the clinical outcomes was performed among individuals with uncontrolled blood pressure at baseline to determine whether a future trial should focus on this subset of patients.
Results
Recruitment
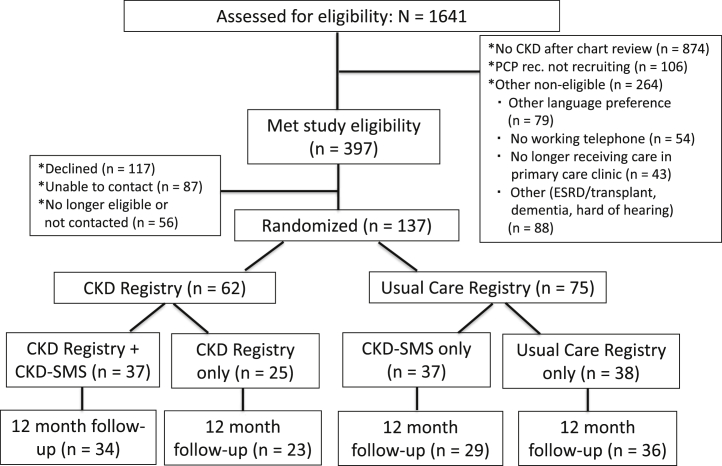
All primary care provider teams (n = 6) were recruited to participate in the study. We assessed 1,641 primary care patients with possible CKD determined by the electronic health record for eligibility. Among those, only 47% (n = 767) had persistent low eGFRs or elevated albuminuria indicating CKD (Fig 2). On electronic health record review to verify eligibility, many of the patients who were inaccurately identified as having probable CKD in the initial data abstraction had experienced multiple episodes of acute kidney injury with improvement thereafter, had experienced an increase in eGFR over time and no longer met study criteria, had false-positive dipstick albuminuria testing results, or had their albuminuria pharmacologically suppressed before recruitment.
Figure 2.
Consolidated Standards of Reporting Trials (CONSORT) diagram. Abbreviations: CKD, chronic kidney disease; CKD-SMS, chronic kidney disease self-management support; ESRD, end-stage renal disease; PCP, primary care provider; rec, recommends.
Approximately 16% of patients (n = 264) were not eligible for other reasons, including speaking an ineligible language despite being identified in the electronic health record as speaking English, Spanish, or Cantonese (n = 79); having previously undergone kidney transplantation (n = 6); not having a working telephone (n = 54); and no longer receiving care from a participating clinic (n = 43). Among the 397 (24.2%) eligible patients, 137 were enrolled and randomly assigned (Fig 2). The inability to contact socially vulnerable patients due to inaccurate telephone numbers in the electronic health record (n = 87) was a common reason for not enrolling eligible patients.
Baseline Patient Characteristics
The study population (n = 137) had a mean age of 55 (SD, 12.2) years, consisted of 51.8% women, and was racially/ethnically diverse (42.3% African American, 36.5% Hispanic, 14.6% Asian/Pacific Islander, and 6.6% white). Nearly 35% of patients spoke a language other than English, 23.4% had limited health literacy, and more than one-half self-reported food insecurity. Approximately 59% had diabetes, 54.0% had hyperlipidemia, 48.9% had advanced CKD stages 3 to 4, and 73.0% of patients had micro- or macroalbuminuria (Table 1). Demographic and comorbid condition data were similar across the 4 groups (P > 0.1), with the exception of baseline SBP because the median SBP was lowest in the CKD registry plus CKD-SMS program group (P = 0.02), and urinary albumin-creatinine ratio because the median urinary albumin-creatinine ratio was lowest in CKD-SMS only group (P = 0.03; Table 1). Demographic characteristics captured from the electronic health record were qualitatively similar among potentially eligible individuals who agreed to participate versus those who were contacted but declined (n = 117), though the decliners were statistically more likely to be female and of Asian race/ethnicity and less likely to be Spanish speaking (Table S1).
Table 1.
Baseline Characteristics of KARE Participants
| Characteristics | All | Usual Care | Intervention Groups |
P | ||
|---|---|---|---|---|---|---|
| CKD Registry Only | SMS Only | SMS + CKD Registry | ||||
| N | n = 137 | n = 38 | n = 25 | n = 37 | n = 37 | |
| Age, y | 58.0 [50.0-64.0] | 56.0 [49.0-64.0] | 60.0 [51.0-66.0] | 57.0 [50.0-61.0] | 56.0 [49.0-63.0] | 0.4 |
| Women | 71 (51.8%) | 18 (47.4%) | 14 (56.0%) | 16 (43.2%) | 23 (62.2%) | 0.4 |
| Race/ethnicity | 0.3 | |||||
| Caucasian/white | 9 (6.6%) | 0 (0%) | 1 (4.0%) | 3 (8.1%) | 5 (13.5%) | |
| Black or African American | 58 (42.3%) | 21 (55.3%) | 8 (32.0%) | 17 (45.9%) | 12 (32.4%) | |
| Asian/Pacific Islander | 20 (14.6%) | 4 (10.5%) | 5 (20.0%) | 4 (10.8%) | 7 (18.9%) | |
| Hispanic | 50 (36.5%) | 13 (34.2%) | 11 (44.0%) | 13 (35.1%) | 13 (35.1%) | |
| Preferred language | 0.1 | |||||
| English | 90 (65.7%) | 27 (71.1%) | 15 (60.0%) | 28 (76.0%) | 20 (54.0%) | |
| Spanish | 40 (29.2%) | 10 (26.3%) | 10 (40.0%) | 8 (21.6%) | 12 (32.4%) | |
| Cantonese | 7 (5.1%) | 1 (2.6%) | 0 (0%) | 1 (2.7%) | 5 (13.5%) | |
| Educational attainment | 0.8 | |||||
| <High school | 46 (33.6%) | 14 (36.8%) | 9 (36%) | 10 (27%) | 13 (35.1%) | |
| High school | 41 (29.9%) | 8 (21.1%) | 8 (32.0%) | 11 (29.7%) | 14 (37.8%) | |
| >High school | 50 (36.5%) | 16 (42.1%) | 8 (32.0%) | 16 (43.2%) | 10 (27%) | |
| Limited health literacy | 32 (23.4%) | 8 (21.1%) | 8 (32.0%) | 5 (13.5%) | 11 (29.7%) | 0.3 |
| Food insecurity | 72 (52.6%) | 17 (44.7%) | 14 (56.0%) | 18 (48.6%) | 23 (62.2%) | 0.5 |
| Insurance | 0.2 | |||||
| None | 39 (28.4%) | 12 (31.6%) | 4 (16.0%) | 11 (29.7%) | 12 (32.4%) | |
| Medicaid only | 61 (44.5%) | 13 (34.2%) | 15 (60.0%) | 18 (48.6%) | 15 (40.5%) | |
| Medicare only | 10 (7.3%) | 3 (7.9%) | 2 (8.0%) | 0 (0.0%) | 5 (13.5%) | |
| Medicare + Medicaid | 27 (19.7%) | 10 (26.3%) | 4 (16.0%) | 8 (21.6%) | 5 (13.5%) | |
| Self-reported diabetesa | 80 (58.4%) | 20 (52.6%) | 17 (68.0%) | 20 (54.1%) | 23 (62.2%) | 0.6 |
| Self-reported coronary diseasea | 21 (15.3%) | 8 (21.1%) | 5 (20.0%) | 4 (10.8%) | 4 (10.8%) | 0.7 |
| Self-reported hyperlipidemiaa | 74 (54.0%) | 16 (42.1%) | 13 (52.0%) | 23 (62.2%) | 22 (59.5%) | 0.4 |
| Baseline systolic blood pressure, mm Hg | 129.0 [115.0-142.0] | 123.0 [115.0-135.0] | 131.0 [113.0-153.0] | 137.0 [129.0-147.0] | 122.0 [112.0-139.0] | 0.02 |
| Estimated glomerular filtration rate, mL/min/1.73 m2 | 60.2 [48.5-94.7] | 51.8 [45.4-91.3] | 60.7 [47.0-97.1] | 63.9 [52.1-96.3] | 62.5 [51.6-90.9] | 0.4 |
| CKD stage | 0.2 | |||||
| 1-2 | 70 (51.1%) | 14 (36.8%) | 14 (56.0%) | 20 (54.0%) | 22 (59.5%) | |
| 3-4 | 67 (48.9%) | 24 (63.2%) | 11 (44.0%) | 17 (46.0%) | 15 (40.5%) | |
| Albumin-creatinine ratio, mg/g | 86.9 [21.0-561.0] | 50.5 [9.0-373] | 438.0 [69.0-1481.0] | 119.0 [36.0-219.0] | 77.5 [14.5-367.5] | 0.03 |
| Albumin-creatinine ratio, mg/ga | 0.03 | |||||
| A1 <30 | 36 (26.3%) | 14 (36.8%) | 3 (12%) | 8 (21.6%) | 11 (29.7%) | |
| A2 30-300 (microalbuminuria) | 57 (41.6%) | 12 (31.6%) | 8 (32%) | 21 (56.8%) | 16 (43.2%) | |
| A3 >300 (macroalbuminuria) | 43 (31.4%) | 12 (31.6%) | 14 (56%) | 8 (21.6%) | 9 (24.3%) | |
Note: Values expressed as median [interquartile range] or number (percent).
Abbreviations: CKD, chronic kidney disease; KARE, Kidney Awareness Registry and Education; SMS, self-management support.
Column and row percentages may not sum to 100% due to missing data.
Retention among study participants was high, with 90% of randomly assigned participants completing the 12-month study visit and no statistically significant difference by study group (P = 0.2). The timing of the 12-month follow-up visit ranged from 9.1 to 27.6 months, with a median of 12.7 (interquartile range, 12.3-13.5) months.
Uptake of Interventions
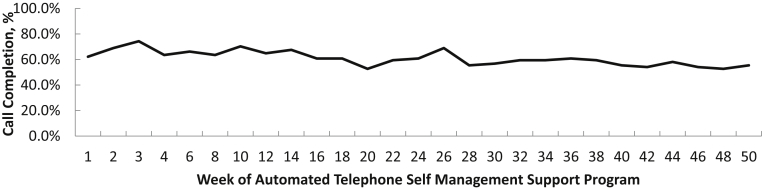
Patient uptake of the intervention was favorable. Average call completion of automated telephone modules was 61% across the 50-week program (Fig 3). Among the 74 participants randomly assigned to receive the intervention, nearly 40% were considered high utilizers, with an average call completion rate ≥ 80%. More than 95% of individuals participated in regular telephone calls with their health coach; 77% completed at least 1 action plan. Among the 38% of clinicians randomly assigned to have access to the CKD registry who completed the satisfaction survey, most (88%) reported that point-of-care notifications influenced the way they managed CKD and 74% reported that quarterly feedback enhanced their ability to manage CKD.34
Figure 3.
Call completion of automated telephone self-management modules.
Clinical Outcomes
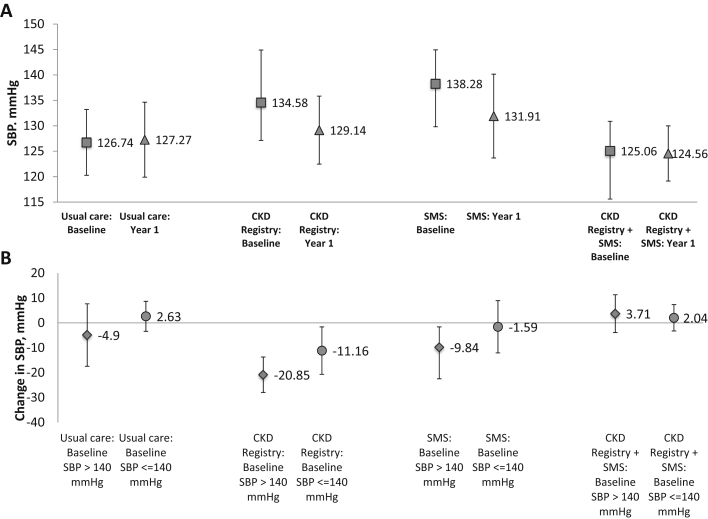
Mean baseline SBP among study participants was 131 (SD, 22.0) mm Hg and mean change at the 12-month follow-up visit was −2.4 (SD, 19.8) mm Hg. Mean estimated change in SBP ranged from −6.37 (95% confidence interval [CI], −13.69 to 0.95) mm Hg (CKD-SMS only) to +0.53 (95% CI, −5.20 to 6.27) mm Hg (usual care) across groups; mean estimated SBP values at baseline and 12 months for each group are shown in Figure 4A.
Figure 4.
(A) Estimated mean systolic blood pressure (SBP) and 95% confidence interval (CI) at baseline and 1 year for each Kidney Awareness Registry and Education (KARE) group. (B) Estimated mean 1-year change and 95% CI in SBP by baseline SBP control for each KARE group. Abbreviations: CKD, chronic kidney disease; SMS, self-management support.
Compared with patients randomly assigned to usual care, who had little change in SBP, those randomly assigned to intervention groups had nonstatistically larger changes in SBP: −6.0 (95% CI, −14.9 to 2.9) mm Hg for CKD registry; −6.9 (95% CI, −16.2 to 2.4) mm Hg for CKD-SMS program; and −1.0 (95% CI, −8.6 to 6.6) mm Hg for CKD registry plus CKD-SMS program. There was no evidence of effect modification by baseline SBP (Pinteraction = 0.16), but among patients with baseline SBP < 140 mm Hg (n = 91), little difference in changes in SBP was observed across trial groups (range, −1.59 to 3.71 mm Hg) compared with usual care (2.63 mm Hg), whereas among patients with baseline SBPs ≥ 140 mm Hg (n = 46), larger decreases in SBP were observed among those randomly assigned to the intervention groups (range, −20.85 to −9.84 mm Hg) compared with usual care (−4.90 mm Hg; Fig 4B).
Similarly, change in the proportion of patients with blood pressure control was nonstatistically larger among patients randomly assigned to 3 intervention groups compared with those randomly assigned to usual care (odds ratio [OR], 2.5 [95% CI, 0.3-18.7] for the CKD registry only; OR, 1.6 [95% CI, 0.2-9.9] for CKD-SMS program only; and OR, 1.2 [95% CI, 0.2-7.0] for CKD registry plus CKD-SMS program). The percentage of patients with blood pressure control did not change substantially among the different groups (76.3% to 75.0% for usual care; 60.0% to 73.9% for CKD registry only; 51.4% to 58.6% for CKD-SMS program only; and 75.7% to 76.5% for CKD registry plus CKD-SMS program).
Randomization to any of the intervention groups was also associated with nonstatistically significant larger decreases in albuminuria compared with usual care: urinary albumin-creatinine ratio, −218.8 (95% CI, −622.1 to 184.5) mg/g larger for CKD registry only; −32.8 (95% CI, −440.8 to 375.1) mg/g larger for CKD-SMS program only; and −28.6 (95% CI, −303.9 to 246.6) mg/g larger for CKD registry plus CKD-SMS program (Fig S1A). There was no evidence of effect modification by baseline SBP (Pinteraction = 0.84), though decreases in albuminuria were greater in the subgroup with baseline SBP ≥ 140 mm Hg (Fig S1B).
Self-reported Patient Outcomes
Compared with usual care, randomization to any of the 3 intervention arms was not associated with changes in medication adherence, reported self-efficacy, communication with providers, quality of life, or CKD awareness (Table 2).
Table 2.
Mean Predicted Values and Probabilities of Behavioral Outcomes at Baseline and at 1-Year Follow-up for Each Group and Mean Predicted Difference and Estimated Change in Intervention Groups Compared to Usual Care
| Behavioral Outcomea | Mean (95% CI) Baseline (n=137) | Mean (95% CI) y 1 (n=122) | Mean Predicted Difference (95% CI) | βb | CI |
|---|---|---|---|---|---|
| Morisky Medication Adherence (scale: 0 [great adherence] to 4 [low adherence]) | |||||
| Usual care | 1.34 (0.98 to 1.71) | 1.11 (0.75 to 1.47) | −0.2 (−0.5 to 0.1) | Ref | Ref |
| CKD registry only | 1.46 (0.93 to 2.00) | 1.2 (0.68 to 1.72) | −0.3 (−0.8 to 0.3) | −0.03 | (−0.63 to 0.57) |
| SMS only | 1.38 (0.92 to 1.83) | 1.17 (0.72 to 1.62) | −0.2 (−0.6 to 0.2) | 0.03 | (−0.48 to 0.53) |
| CKD registry + CKD-SMS | 1.22 (0.91 to 1.53) | 1.07 (0.74 to 1.41) | −0.1 (−0.5 to 0.2) | 0.09 | (−0.36 to 0.53) |
| Stanford Chronic Disease Self-efficacy (scale of 1 [not confident] to 10 [very confident]) | |||||
| Usual care | 7.94 (7.39 to 8.48) | 8.19 (7.63 to 8.74) | 0.3 (−0.3 to 0.8) | Ref | Ref |
| CKD registry only | 6.89 (6.14 to 7.64) | 6.87 (6.07 to 7.68) | 0.0 (−0.7 to 0.7) | −0.27 | (−1.15 to 0.61) |
| SMS only | 7.91 (7.23 to 8.59) | 8.31 (7.78 to 8.85) | 0.4 (−0.2 to 0.9) | 0.15 | (−0.60 to 0.91) |
| CKD registry + CKD-SMS | 7.5 (6.95 to 8.05) | 8.3 (7.81 to 8.80) | 0.8 (0.3 to 2.4) | 0.56 | (−0.25 to 1.36) |
| Lorig Communication (Scale: 0 [poor communication] to 5 [excellent communication]) | |||||
| Usual care | 2.86 (2.46 to 3.26) | 2.79 (2.41 to 3.18) | −0.1 (−0.4 to 0.3) | Ref | Ref |
| CKD registry only | 2.75 (2.30 to 3.21) | 2.33 (1.93 to 2.73) | −0.4 (−0.8 to 0.0) | −0.35 | (−0.90 to 0.19) |
| SMS only | 2.95 (2.56 to 3.35) | 2.7 (2.28 to 3.12) | −0.2 (−0.7 to 0.2) | −0.18 | (−0.74 to 0.38) |
| CKD registry + CKD-SMS | 3.09 (2.78 to 3.40) | 2.91 (2.62 to 3.21) | −0.2 (−0.5 to 0.2) | −0.11 | (−0.61 to 0.39) |
| SF-12 (scale: 0 [lowest] to 100 [highest]) | |||||
| Usual care | 57.4 (50.47 to 64.33) | 61.61 (54.30 to 68.92) | 4.2 (−0.7 to 9.1) | Ref | Ref |
| CKD registry only | 39.07 (30.05 to 48.09) | 41.68 (32.91 to 50.45) | 2.6 (−6.6 to 11.8) | −1.6 | (−12.02 to 8.82) |
| SMS only | 51.52 (41.69 to 61.34) | 53.81 (43.99 to 63.63) | 2.3 (−1.8 to 6.4) | −1.92 | (−8.31 to 4.47) |
| CKD registry + CKD-SMS | 46.58 (39.12 to 54.03) | 47.96 (40.38 to 55.53) | 1.4 (−4.0 to 6.8) | −2.83 | (−10.16 to 4.49) |
| SF-12: Physical Health Component (scale: 0 [lowest] to 100 [highest]) | |||||
| Usual care | 54.33 (46.08 to 62.58) | 58.16 (49.79 to 66.52) | 3.8 (−2.6 to 10.3) | Ref | Ref |
| CKD registry only | 33.03 (22.10 to 43.96) | 36.79 (26.44 to 47.14) | 3.8 (−7.5 to 15.0) | −0.07 | (−13.06 to 12.92) |
| SMS only | 46.99 (36.37 to 57.62) | 49.88 (38.65 to 61.12) | 2.9 (−2.3 to 8.1) | −0.94 | (−9.23 to 7.36) |
| CKD registry + CKD-SMS | 41.61 (32.54 to 50.68) | 43.22 (34.13 to 52.30) | 1.6 (−5.3 to 8.5) | −2.22 | (−11.65 to 7.21) |
| SF-12: Mental Health Component (scale: 0 [lowest] to 100 [highest]) | |||||
| Usual care | 63.57 (56.78 to 70.36) | 68.57 (61.88 to 75.27) | 5.0 (−1.0 to 11.0) | Ref | Ref |
| CKD registry only | 51.12 (42.67 to 59.57) | 51.32 (43.14 to 59.50) | 3.8 (−7.5 to 15.0) | −4.81 | (−15.07 to 5.46) |
| SMS only | 60.59 (51.02 to 70.16) | 61.72 (53.52 to 69.92) | 2.9 (−2.3 to 8.1) | −3.87 | (−11.13 to 3.39) |
| CKD registry + CKD-SMS | 56.5 (50.87 to 62.12) | 57.29 (51.42 to 63.16) | 1.6 (−5.3 to 8.5) | −4.21 | (−11.71 to 3.28) |
| Behavioral Outcomea | Probability at Baseline | Probability at y 1 | Mean Predicted Difference (95% CI) | ORc | CI |
|---|---|---|---|---|---|
| CKD Awareness: “Weak or Failing Kidneys”: (scale: 0 [no one aware] to 1.00 [everyone aware]) | |||||
| Usual care | 0.4 (0.23 to 0.56) | 0.43 (0.26 to 0.60) | 0.03 (−0.11 to 0.17) | Ref | Ref |
| CKD registry only | 0.3 (0.15 to 0.44) | 0.29 (0.15 to 0.43) | −0.01 (−0.16 to 0.17) | 0.68 | (0.1 to 5.3) |
| SMS only | 0.36 (0.22 to 0.50) | 0.36 (0.21 to 0.51) | 0.00 (−0.16 to 0.17) | 0.77 | (0.1 to 6.0) |
| CKD registry + CKD-SMS | 0.35 (0.20 to 0.50) | 0.44 (0.27 to 0.61) | 0.09 (−0.06 to 0.24) | 1.75 | (0.3 to 11.4) |
| CKD Awareness: “Kidney Problem” (scale: 0 [no one aware] to 1.00 [everyone aware]) | |||||
| Usual care | 0.47 (0.28 to 0.66) | 0.58 (0.40 to 0.76) | 0.11 (−0.05 to 0.27) | Ref | Ref |
| CKD registry only | 0.51 (0.29 to 0.72) | 0.5 (0.29 to 0.71) | −0.01 (−0.08 to 0.07) | 0.28 | (0.0 to 2.3) |
| SMS only | 0.36 (0.18 to 0.53) | 0.46 (0.27 to 0.64) | 0.10 (−0.06 to 0.26) | 0.94 | (0.1 to 11.5) |
| CKD registry + CKD-SMS | 0.5 (0.35 to 0.64) | 0.57 (0.41 to 0.72) | 0.07 (−0.07 to 0.21) | 0.65 | (0.1 to 7.6) |
Abbreviations: CI, confidence interval; CKD, chronic kidney disease; OR, odds ratio; Ref, reference; SF-12, 12-Item Short Form Health Survey; SMS, self-management support.
Estimates from mixed model.
Mean difference in 1-year change in outcome for each intervention arm compared to usual care (with 95% CI and P value).
OR comparing time effect on outcome for each intervention compared with usual care (with 95% CI and P value)
Discussion
In this randomized controlled pilot trial of a range of single- and multilevel theory-informed interventions, we demonstrated: (1) the feasibility of implementing and testing a provider- and patient-level intervention, as well as their combination, in a public health care delivery system; (2) the challenges associated with trial recruitment among vulnerable low-income patients; and (3) nonstatistically significant improvement in SBP and albuminuria in the intervention groups compared with usual care among patients with blood pressures ≥ 140/90 mm Hg. However, we did not detect differences in changes in CKD awareness across trial arms or any signal of greater impact with the multilevel intervention compared with the single-level interventions.
Interventions integrated into health care delivery are complex and require buy-in from numerous stakeholders, including providers, nonphysician team members, clinic administrators, and executive leadership. Working with health care delivery partners, we were able to simultaneously embed a practice team–oriented CKD registry directly into primary care workflows and a telephone-based CKD-SMS system that was an adjunct to primary care. This was in part due to some of KARE’s more pragmatic trial elements, including the integration of interventions into real-world settings, flexibility inherent to the health coaching intervention, and nonenforcement of adherence to the intervention. KARE demonstrates that public health care delivery systems can be sites in which novel interventions that reorganize health care delivery and leverage information technology can be studied.
One of the main challenges of KARE implementation was patient identification and recruitment. Among those identified as potentially having CKD from the electronic health record based on at least 2 low eGFRs or abnormal albuminuria values separated in time, only ∼50% had prevalent CKD at trial enrollment, defined as having sustained low eGFRs or elevated albuminuria. This is consistent with data from a recent methodological study that demonstrated that 68% of patients deemed to have CKD based on historic eGFRs had confirmed CKD determined by physician chart review.44 Many potentially eligible patients in our study had experienced repeated episodes of acute kidney injury or had false-positive dipstick albuminuria results.
Identifying eligible patients for trial participation from the electronic health record is a core element of contemporary clinical trials, which will be important to ameliorate in future research. As an example, to improve efficiency and attain higher specificity of CKD diagnosis for our planned efficacy trial, we will eliminate dipstick albuminuria as a criterion for identifying patients with probable CKD, although studies have shown that persistent dipstick albuminuria identifies patients at higher risk for early mortality, CKD progression, and myocardial infarction.45 However, it is important to note that high specificity of diagnosis may be less of an analytical concern in pragmatic trials embedded in health systems with cluster-randomized designs because inaccuracies are likely to be equally distributed among clusters of providers, health care teams, or clinics.
Because this was a pilot trial, we did not have the power to detect significant changes in clinical or behavioral outcomes. However, we were able to glean a few important insights. First, SBP improved among patients with higher baseline blood pressures randomly assigned to any intervention arm compared to usual care, suggesting that a larger trial among individuals with uncontrolled blood pressure at baseline may show statistically significant improvements.
Second, because there was no perceived benefit to the multilevel intervention compared to single intervention, the larger efficacy trial will focus on a refined more robust patient intervention. Data from this pilot study suggest that we will need to enroll 324 patients with uncontrolled blood pressure to detect a clinically meaningful 6–mm Hg difference in SBPs between groups, assuming 2-sided alpha of 0.05 and power of 0.8.
Third, there was no evidence for any behavior change among trial participants. The lack of change in medication adherence was particularly disappointing because this is one of the mainstays of hypertension self-management. Although the CKD-SMS program encouraged individual action planning and highlighted the importance of medication adherence for improved blood pressure control and kidney health, many participants opted to focus on other aspects of their health (ie, healthier diet and greater physical activity) rather than work on their medication adherence. While purely speculative, it is possible that engaging patients in shared decision making around medication adherence during the clinic visit may be more valuable than during out-of-visit discussions.
Fourth, although education about kidney disease is key to self-management, CKD awareness did not change among patients randomly assigned to participate in the CKD-SMS program. To remain generalizable and relevant to provider concerns and priorities, the CKD-SMS program purposely discussed kidney disease within the context of cardiovascular health. This may have diluted the message related to kidney disease. The refined CKD-SMS intervention will include more information about kidney disease and its direct linkage to cardiovascular health and will focus more on diet and physical activity and potential mediators for improved blood pressure control.
Although we successfully conducted a pilot randomized controlled trial, this study is subject to limitations. The study took place in 2 primary care clinics within a single public health care delivery system with low-income participants who were able and willing to be contacted repeatedly by telephone. Results may not be generalizable to other health care systems or patient populations. Despite all efforts, ∼10% of the study population dropped out. Also, provider teams’ adherence to the registry and patient participation in the SMS intervention was variable, mimicking a real-world intervention but limiting the potency of the intervention. We did not collect robust data about providers within each practice team randomly assigned to the usual-care registry versus CKD registry and thus could not incorporate these variables in our analyses.
In summary, we successfully developed and tested 2 technology-enhanced interventions for safety-net primary care patients with mild to moderate CKD in a pilot randomized controlled trial. Because there was no perceived benefit to the multilevel intervention compared to single interventions on patient outcomes or CKD awareness, a larger efficacy trial with sufficient power to examine the effect of a refined self-management support intervention among patients with CKD and uncontrolled blood pressure is warranted.
Article Information
Authors’ Full Names and Academic Degrees
Delphine S. Tuot, MDCM, MAS, Anna D. Rubinsky, PhD, Alexandra Velasquez, BS, Charles E. McCulloch, PhD, Dean Schillinger, MD, Margaret A. Handley, MPH, PhD, Chi-yuan Hsu, MD, MS, and Neil R. Powe, MD, MPH, MBA.
Authors’ Contributions
Research idea and study design: DST, NRP; data analysis/interpretation: DST, ADR, AV, DS, MAH, C-yH, NRP; statistical analysis: ADR. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Support
This work was supported by K23DK094850, R01DK104130, and R34DK093992 Planning Grants for Translating CKD Research into Improved Clinical Outcomes, all from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Dr Hsu is additionally supported by K24DK92291. Drs Schillinger and Handley are additionally supported by P60MD006902 from the National Institute of Minority Health and Health Disparities and P30DK092924 from the NIDDK. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Financial Disclosure
The authors declare that they have no relevant financial interests.
Acknowledgements
We thank the providers and patients of the San Francisco Health Network for participation in this study.
Peer Review
Received January 26, 2019. Evaluated by 2 external peer reviewers, with direct editorial input from the Statistical Editor, an Associate Editor, and the Editor-in-Chief. Accepted in revised form July 7, 2019.
Data Sharing Statement
Deidentifiable patient-level data may be made available on request to the corresponding author.
Footnotes
Complete author and article information provided before references.
Figure S1: (A) Estimated mean albuminuria value and 95% CI at baseline and 1 year for each KARE group. (B) Estimated mean 1-year change and 95% CI in albuminuria value, by baseline systolic blood pressure control, for each KARE group.
Table S1: Demographic characteristics of KARE participants and those who declined to participate.
Supplementary Material
Figure S1; Table S1.
References
- 1.Levey A.S., Stevens L.A., Schmid C.H. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Merkin S.S., Roux A.V., Coresh J., Fried L.F., Jackson S.A., Powe N.R. Individual and neighborhood socioeconomic status and progressive chronic kidney disease in an elderly population: the Cardiovascular Health Study. Soc Sci Med. 2007;65(4):809–821. doi: 10.1016/j.socscimed.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 3.McClellan W.M., Newsome B.B., McClure L.A. Poverty and racial disparities in kidney disease: the REGARDS study. Am J Nephrol. 2010;32(1):38–46. doi: 10.1159/000313883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Go A.S., Chertow G.M., Fan D., McCulloch C.E., Hsu C.Y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 5.McCullough P.A., Li S., Jurkovitz C.T. Chronic kidney disease, prevalence of premature cardiovascular disease, and relationship to short-term mortality. Am Heart J. 2008;156(2):277–283. doi: 10.1016/j.ahj.2008.02.024. [DOI] [PubMed] [Google Scholar]
- 6.Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ. 1998;317(7160):703–713. [PMC free article] [PubMed] [Google Scholar]
- 7.Brenner B.M., Cooper M.E., de Zeeuw D. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345(12):861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 8.Lewis E.J., Hunsicker L.G., Clarke W.R. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345(12):851–860. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- 9.Randomised placebo-controlled trial of effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, non-diabetic nephropathy. The GISEN Group (Gruppo Italiano di Studi Epidemiologici in Nefrologia) Lancet. 1997;349(9069):1857–1863. [PubMed] [Google Scholar]
- 10.Diabetes Control and Complications Trial Research Group. Nathan D.M., Genuth S., Lachin J. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 11.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):837–853. [PubMed] [Google Scholar]
- 12.KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Summary of recommendation statements. Kidney Int Suppl. 2013;3(1):5–14. doi: 10.1038/kisup.2012.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Plantinga L.C., Tuot D.S., Powe N.R. Awareness of chronic kidney disease among patients and providers. Adv Chronic Kidney Dis. 2010;17(3):225–236. doi: 10.1053/j.ackd.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tuot D., Plantinga L., Judd S. Health behaviors, risk factor control and awareness of chronic kidney disease. Am J Nephrol. 2013;37(2):135–143. doi: 10.1159/000346712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tuot D.S., Plantinga L.C., Hsu C.Y. Chronic kidney disease awareness among individuals with clinical markers of kidney dysfunction. Clin J Am Soc Nephrol. 2011;6(8):1838–1844. doi: 10.2215/CJN.00730111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tahir M.A., Dmitrieva O., de Lusignan S. Confidence and quality in managing CKD compared with other cardiovascular diseases and diabetes mellitus: a linked study of questionnaire and routine primary care data. BMC Fam Pract. 2011;12:83. doi: 10.1186/1471-2296-12-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Costantini L., Beanlands H., McCay E., Cattran D., Hladunewich M., Francis D. The self-management experience of people with mild to moderate chronic kidney disease. Nephrol Nurs J. 2008;35(2):147–155. quiz 156. [PubMed] [Google Scholar]
- 18.Bodenheimer T., Lorig K., Holman H., Grumbach K. Patient self-management of chronic disease in primary care. JAMA. 2002;288(19):2469–2475. doi: 10.1001/jama.288.19.2469. [DOI] [PubMed] [Google Scholar]
- 19.American College of Physicians . ACP; Philadelphia, PA: 2010. The Patient-Centered Medical Home Neighbor: the Interface of the Patient Centered Medical Home With Specialty/Subspecialty Practices. [Google Scholar]
- 20.Rich M.W., Beckham V., Wittenberg C., Leven C.L., Freedland K.E., Carney R.M. A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure. N Engl J Med. 1995;333(18):1190–1195. doi: 10.1056/NEJM199511023331806. [DOI] [PubMed] [Google Scholar]
- 21.DeWalt D.A., Schillinger D., Ruo B. Multisite randomized trial of a single-session versus multisession literacy-sensitive self-care intervention for patients with heart failure. Circulation. 2012;125(23):2854–2862. doi: 10.1161/CIRCULATIONAHA.111.081745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tuot D.S., McCulloch C.E., Velasquez A., Schillinger D., Handley M., Powe N.R. Impact of a primary care CKD registry in a US public safety-net healthcare delivery system: a pragmatic randomized trial. Am J Kidney Dis. 2018;72(2):168–177. doi: 10.1053/j.ajkd.2018.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drawz P.E., Miller R.T., Singh S., Watts B., Kern E. Impact of a chronic kidney disease registry and provider education on guideline adherence--a cluster randomized controlled trial. BMC Med Inform Decis Mak. 2012;12:62. doi: 10.1186/1472-6947-12-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abdel-Kader K., Fischer G.S., Li J., Moore C.G., Hess R., Unruh M.L. Automated clinical reminders for primary care providers in the care of CKD: a small cluster-randomized controlled trial. Am J Kidney Dis. 2011;58(6):894–902. doi: 10.1053/j.ajkd.2011.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galbraith L., Jacobs C., Hemmelgarn B.R., Donald M., Manns B., Jun M. Chronic disease management interventions for people with chronic kidney disease in primary care: a systematic review and meta-analysis. Nephrol Dial Transplant. 2018;33(1):112–121. doi: 10.1093/ndt/gfw359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bodenheimer T., Wagner E.H., Grumbach K. Improving primary care for patients with chronic illness: the chronic care model, part 2. JAMA. 2002;288(15):1909–1914. doi: 10.1001/jama.288.15.1909. [DOI] [PubMed] [Google Scholar]
- 27.Halladay J.R., DeWalt D.A., Wise A. More extensive implementation of the chronic care model is associated with better lipid control in diabetes. J Am Board Fam Med. 2014;27(1):34–41. doi: 10.3122/jabfm.2014.01.130070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Volkova N., McClellan W., Klein M. Neighborhood poverty and racial differences in ESRD incidence. J Am Soc Nephrol. 2008;19(2):356–364. doi: 10.1681/ASN.2006080934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tarver-Carr M.E., Powe N.R., Eberhardt M.S. Excess risk of chronic kidney disease among African-American versus white subjects in the United States: a population-based study of potential explanatory factors. J Am Soc Nephrol. 2002;13(9):2363–2370. doi: 10.1097/01.asn.0000026493.18542.6a. [DOI] [PubMed] [Google Scholar]
- 30.Schillinger D. Literacy and health communication: reversing the 'inverse care law'. Am J Bioeth. 2007;7(11):15–18. doi: 10.1080/15265160701638553. discussion W11-W12. [DOI] [PubMed] [Google Scholar]
- 31.Tuttle K.R., Tuot D.S., Corbett C.L., Setter S.M., Powe N.R. Type 2 translational research for CKD. Clin J Am Soc Nephrol. 2013;8(10):1829–1838. doi: 10.2215/CJN.00130113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tuot D.S., Velasquez A., McCulloch C.E. The Kidney Awareness Registry and Education (KARE) study: protocol of a randomized controlled trial to enhance provider and patient engagement with chronic kidney disease. BMC Nephrol. 2015;16:166. doi: 10.1186/s12882-015-0168-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levey A.S., Bosch J.P., Lewis J.B., Greene T., Rogers N., Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 34.Strait A., Velasquez A., Handley M. Acceptability of a multi-level intervention to improve blood pressure control among patients with chronic kidney disease in a public healthcare delivery system. Clin Kidney J. 2018;11(4):540–548. doi: 10.1093/ckj/sfx141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Painter J.E., Borba C.P., Hynes M., Mays D., Glanz K. The use of theory in health behavior research from 2000 to 2005: a systematic review. Ann Behav Med. 2008;35(3):358–362. doi: 10.1007/s12160-008-9042-y. [DOI] [PubMed] [Google Scholar]
- 36.Tuot D.S., Davis E., Velasquez A., Banerjee T., Powe N.R. Assessment of printed patient-educational materials for chronic kidney disease. Am J Nephrol. 2013;38(3):184–194. doi: 10.1159/000354314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pickering T.G., Hall J.E., Appel L.J. Recommendations for blood pressure measurement in humans: an AHA scientific statement from the Council on High Blood Pressure Research Professional and Public Education Subcommittee. J Clin Hypertens (Greenwich) 2005;7(2):102–109. doi: 10.1111/j.1524-6175.2005.04377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morisky D.E., Green L.W., Levine D.M. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24(1):67–74. doi: 10.1097/00005650-198601000-00007. [DOI] [PubMed] [Google Scholar]
- 39.Ware J., Jr., Kosinski M., Keller S.D. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 40.Lorig K., Stewart A.F., Ritter P., Golzalez V., Laurent D., Lynch J. Sage Publications; Thousand Oaks, CA: 1996. Outcome Measures for Health Education and other Health Care Intervensions. [Google Scholar]
- 41.Tuot D.S., Zhu Y., Velasquez A. Variation in patients' awareness of CKD according to how they are asked. Clin J Am Soc Nephrol. 2016;11(9):1566–1573. doi: 10.2215/CJN.00490116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hager E.R., Quigg A.M., Black M.M. Development and validity of a 2-item screen to identify families at risk for food insecurity. Pediatrics. 2010;126(1):e26–e32. doi: 10.1542/peds.2009-3146. [DOI] [PubMed] [Google Scholar]
- 43.Chew L.D., Griffin J.M., Partin M.R. Validation of screening questions for limited health literacy in a large VA outpatient population. J Gen Intern Med. 2008;23(5):561–566. doi: 10.1007/s11606-008-0520-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frigaard M., Rubinsky A., Lowell L. Validating laboratory defined chronic kidney disease in the electronic health record for patients in primary care. BMC Nephrol. 2019;20(1):3. doi: 10.1186/s12882-018-1156-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hemmelgarn B.R., Manns B.J., Lloyd A. Relation between kidney function, proteinuria, and adverse outcomes. JAMA. 2010;303(5):423–429. doi: 10.1001/jama.2010.39. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1; Table S1.