Abstract
Proliferative glomerulonephritis with monoclonal immunoglobulin G (IgG) deposits is a rare monoclonal gammopathy of renal significance with dense deposits on electron microscopy similar to polyclonal immune complex–mediated glomerulonephritis. 70% of patients with proliferative glomerulonephritis with monoclonal IgG are negative for a monoclonal (M) spike, and patients with this condition rarely develop an M spike during follow-up. We report a Chinese man in his 50s who presented with nephrotic syndrome and normal glomerular filtration rate. His first kidney biopsy showed masked IgG3 deposition, such that IgG3 staining was apparent only after digestion by enzyme on paraffin tissue, with a membranoproliferative pattern. During follow-up, his glomerular filtration rate worsened and proteinuria increased. 18 months after the first biopsy, the patient developed an M spike; a second kidney biopsy showed proliferative glomerulonephritis with monoclonal IgG deposits with unmasked IgG3λ deposition. The patient was successfully treated with bortezomib and dexamethasone, followed by lenalidomide and dexamethasone maintenance therapy.
Index Words: MPGN, monoclonal gammopathy, monoclonal gammopathy of renal significance, proliferative glomerulonephritis with monoclonal IgG deposits (PGNMID)
Background
Membranoproliferative glomerulonephritis (MPGN) describes a glomerular pathologic pattern characterized by mesangial hypercellularity, mesangial matrix proliferation, and remodeling and thickening of the capillary wall with double-contour formation. MPGN is further classified based on immunofluorescence staining and pathogenesis.1 Polyclonal immunoglobulin and complement deposition indicate a likely underlying systemic disease such as autoimmune disease or chronic infection. Monoclonal immunoglobulin deposition indicates the presence of plasma cell or B-cell proliferative disorders, for example, monoclonal gammopathy of renal significance.2 Complement 3 (C3)-dominant staining indicates C3 glomerulopathy (C3G), whereas C4-dominant staining indicates C4 glomerulopathy.3
Nonsignificant deposits, in contrast, are more consistent with microangiopathy. Accurate immunostaining of kidney biopsy tissue using both frozen and paraffin specimens is therefore the most critical step for identifying the causes of MPGN and guiding subsequent treatment because some glomerular-deposited monoclonal immunoglobulins are masked such that they cannot be detected on frozen tissue and are revealed only after digestion by enzyme on paraffin tissue. When accompanied by prominent C3 deposition, C3 glomerulonephritis (C3GN) may be falsely diagnosed in these patients.
Monoclonal gammopathy of renal significance is a term used to defined a group of hematologic disorders with related kidney lesions.2 Importantly, the underlying plasma cell or B-cell dyscrasia of patients with monoclonal gammopathy of renal significance does not meet any current hematologic criteria for immediate specific treatment, such as multiple myeloma. Monoclonal gammopathy of renal significance is caused either by direct deposition of the whole monoclonal immunoglobulins or its fragments or by indirect mechanisms acting as autoantibodies, such as autoantibodies to the complement regulatory proteins leading to C3G or thrombotic microangiopathy.4
Proliferative glomerulonephritis with monoclonal immunoglobulin deposits (PGNMID) is a rare type of glomerular monoclonal gammopathy of renal significance in which 70% of cases do not have detectable blood or bone marrow monoclonal immunoglobulins. The management of PGNMID remains unclear and the absence of a detectable clonal disorder in most cases affects the choice of therapy.
We report a patient with an MPGN pattern on kidney biopsy who first had C3G diagnosed due to masked immunoglobulin G (IgG) deposition. He subsequently developed detectable serum and urine monoclonal immunoglobulins during follow-up, and PGNMID (IgG3λ) ultimately was diagnosed following a second kidney biopsy.
Case Presentation
A Chinese man aged in his 50s presented with a 1-month history of edema and hypertension after an upper respiratory tract infection. He had a 10-year history of type 2 diabetes mellitus that was well controlled. There was no family history of kidney disease. On admission, blood pressure was 150/90 mm Hg, temperature was 36.9°C, heart rate was 70 beats/min, and respiratory rate was 14 breaths/min. Mild edema was observed in the lower extremities.
Urinalysis showed markedly elevated proteinuria (protein excretion, 5.7 g/d) and hematuria (10-20 red blood cells/high-powered field). Serum albumin level was 2.9 g/dL, and serum creatinine (Scr) level was 1.12 (reference range, 0.50-1.50) mg/dL, with estimated glomerular filtration rate of 71 mL/min/1.73 m2. White blood cell count was 5.10 (reference range, 3.5-9.5) ×109 cells/L, hemoglobin level was 138 (reference range, 115-150) g/L, and platelet count was 205 (reference range, 125-300) ×109 cells/L. Serum IgG level was reduced at 6.57 (reference range, 7.23-16.85) g/L, IgA level was 2.55 (reference range, 0.69-3.82) g/L, and IgM level was 0.72 (reference range, 0.63-2.77) g/L. Plasma C3 level was 0.62 (reference range, 0.60-1.50) g/L, and C4 level was 0.29 (reference range, 0.12-0.36) g/L. Hepatitis B surface antigen, anti-human immunodeficiency virus, and Treponema pallidum antibody were all negative. Antinuclear antibodies, antineutrophil cytoplasmic antibodies, and anti-phospholipase A2 receptor (PLA2R) antibodies were also negative.
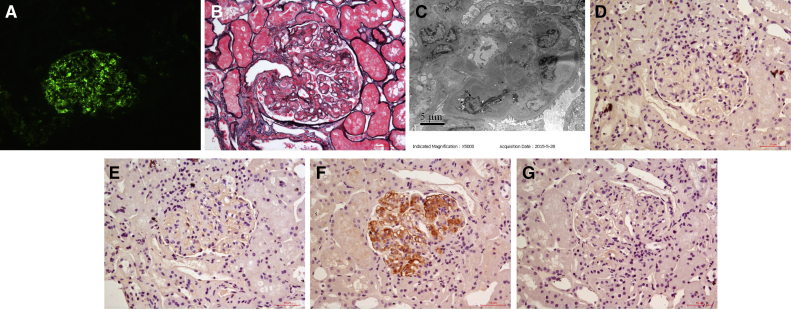
The patient underwent kidney biopsy. Direct immunofluorescence examination of frozen tissue revealed IgM (+), C3 (+++), and C1q (+) depositing in the mesangial area and along the capillary wall (Fig 1A). IgG and IgA were negative. IgG subclasses and light chain staining were not done at that time due to negative immunoglobulin staining. Light microscopy examination had 3 glomeruli that showed an MPGN pattern (Fig 1B). Electron microscopy examination revealed electron-dense deposits in the mesangial, subendothelial, and subepithelial areas (Fig 1C). Based on these findings, we arrived at a diagnosis of C3GN with an MPGN pattern. To identify the cause of C3GN, the following tests were performed: serum and urine immunofixation electrophoresis did not identify monoclonal immunoglobulins, serum complement factor H and complement factor I levels were normal, and anti-complement factor H autoantibodies and C3 nephritic factor were all negative.
Figure 1.
Patient’s first kidney biopsy findings. (A) Immunofluorescence shows granular C3 deposition in the mesangial area and along the capillary wall on frozen tissue (original magnification, ×200). (B) Light microscopy shows membranoproliferative glomerulonephritis change in glomeruli (periodic methenamine silver and Masson trichrome staining; original magnification, ×400). (C) Electron-dense deposits in the mesangial and subendothelial areas on electronic microscopy (original magnification, ×5,000). (D) Immunoglobulin G1 (IgG1) and (E) IgG2 were negative by immunohistochemistry staining on paraffin tissue (D, E: original magnification, ×400). (F) IgG3 deposition in the mesangial area and along the capillary wall by immunohistochemistry staining on paraffin tissue (original magnification, ×400). (G) IgG4 was negative by immunohistochemistry staining on paraffin tissue (original magnification, ×400).
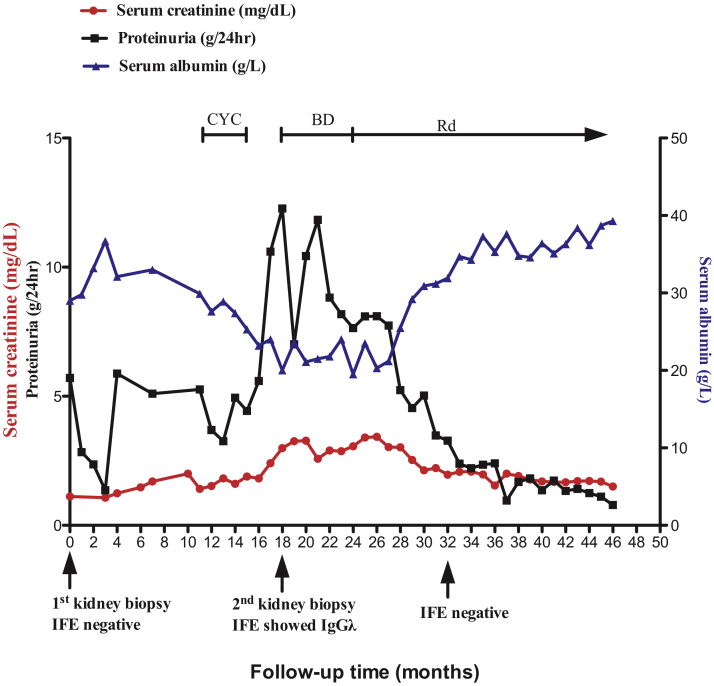
The patient was treated with fosinopril and supportive treatments, and blood pressure was controlled at ∼130/80 mm Hg. Proteinuria decreased to protein excretion of 1.4 to 2.4 g/d, and serum albumin level increased to 3.3 to 3.6 g/dL. However, several months later, his Scr level began to gradually increase (Fig 2) with worsening proteinuria, and serum C3 level decreased to 0.49 g/L with normal C4 level. He was treated with oral cyclophosphamide (total, 6.3 g) and prednisone without remission. Eighteen months after the first kidney biopsy, Scr level increased to 2.99 mg/dL; serum albumin, 2.0 g/dL; protein excretion of 12.3 g/d; and C3, 0.58 g/L; and monoclonal IgGλ was identified in serum and urine. Serum free κ chain level was 51.8 (reference range, 3.30-19.40) mg/L, free λ chain was 35.3 (reference range, 5.71-26.3) mg/L, and κ/λ ratio was 1.4674 (reference range, 0.26-1.65).
Figure 2.
Clinical data for the patient. Abbreviations: BD, bortezomib and dexamethasone; CYC, cyclophosphamide; IFE, immunofixation electrophoresis; Rd, lenalidomide and dexamethasone.
Bone marrow aspiration smear revealed 1% mature plasma cells. Bone marrow biopsy showed a few plasma cells with equal κ and λ expression. CD38-positive cells accounted for 0.1% of bone marrow cells without evidence of monoclonal light chain restricted expression as determined using flow cytometry. A repeat kidney biopsy was done 18 months after the first kidney biopsy.
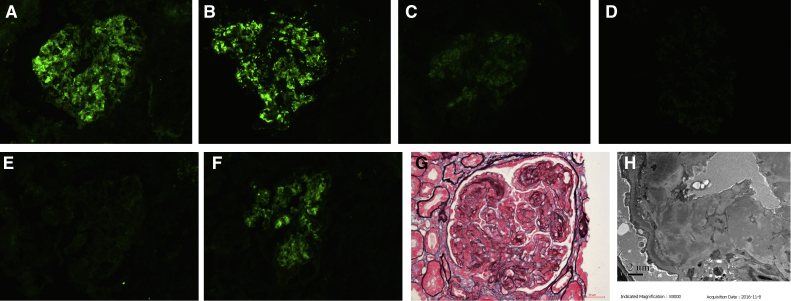
Immunofluorescence of frozen tissue revealed IgG (+++), IgM (negative), C3 (+++), C1q (+), κ (negative), λ (+++), IgG1 (negative), IgG2 (negative), IgG3 (+), and IgG4 (negative) depositing in the mesangial area and along the capillary wall (Fig 3A-F). Light microscopy examination revealed that 2 of 19 glomeruli were globally sclerosed, and 1 glomerulus was segmentally sclerosed. Other glomeruli showed an MPGN pattern (Fig 3G) with 2 cellular crescents. Electron microscopy revealed electron-dense deposits in the mesangial, subendothelial, and subepithelial areas. Subendothelial edema was observed in segmental capillary loops (Fig 3H). PGNMID was diagnosed. Due to the new finding of serum monoclonal IgG, monoclonal IgG deposition was strongly suspected in the first kidney biopsy. Accordingly, we performed immunohistochemistry staining for IgG subclass and light chain of the first kidney biopsy on paraffin tissue. This showed IgG3 (++) but was otherwise negative (Fig 1D-G).
Figure 3.
Patient’s second kidney biopsy findings. Immunofluorescence showed (A) granular immunoglobulin G (IgG), (B) granular C3, (C) granular C1q, and (D) granular IgG3 deposition in the mesangial area and along the capillary wall on frozen tissue; (E) trace κ light chain deposition on frozen tissue; and (F) strong granular λ light chain deposition in the mesangial area and along the capillary wall on frozen tissue (A-F: original magnification, ×200). (G) Light microscopy shows membranoproliferative glomerulonephritis change in glomeruli (periodic methenamine silver and Masson trichrome stain; original magnification, ×400). (H) Electron-dense deposits in the mesangial, subendothelial, and segmental subepithelial areas on electron microscopy (original magnification, ×5,000).
The patient was treated with 9 courses of bortezomib and dexamethasone with decreasing monoclonal immunoglobulins, followed by lenalidomide and dexamethasone maintenance therapy. Fourteen months after the chemotherapy, he was negative for monoclonal immunoglobulins. Scr level and proteinuria gradually improved with a parallel increase in serum albumin level (shown in Fig 2). Three years 10 months after the first biopsy, Scr level was 1.50 mg/dL; serum albumin level, 3.9 g/dL, protein excretion of 0.8 g/d, and C3 was 0.69 g/L.
Discussion
Proliferative glomerulonephritis with monoclonal IgG deposits is a rare monoclonal gammopathy of renal significance with dense deposits on electron microscopy similar to polyclonal immune complex–mediated glomerulonephritis.
We report a patient with 2 consecutive kidney biopsies. The first biopsy showed masked IgG3 deposition, with IgG3 revealed only after enzyme digestion on paraffin tissue. Eighteen months after the first biopsy, the patient developed monoclonal immunoglobulins, and the second biopsy showed PGNMID with now unmasked IgG3λ deposition.
C3G is a recently defined glomerular disease with dominant C3 deposition (C3c ≥ 2+ than others) after exclusion of postinfectious diseases and other well-defined glomerular diseases.5 Based on electron microscopy, C3G is further classified as dense deposit disease and C3GN. C3G is due to genetic or acquired complement alternative pathway dysregulation. Rarely, C3G is due to monoclonal immunoglobulins acting as autoantibodies to complement components.6 Although these patients have no monoclonal immunoglobulin direct deposition in kidney tissue, they may be categorized as having monoclonal gammopathy of renal significance.
Moreover, some glomerular-deposited monoclonal immunoglobulins are masked, such that they cannot be detected on frozen tissue and are revealed only after digestion by enzyme on paraffin tissue.7, 8, 9 These masked monoclonal immunoglobulins may still activate the complement system and may be accompanied by prominent C3 deposition, resulting in a high likelihood of C3G being falsely diagnosed in these patients. Therefore, the diagnosis of C3G should only be made after immunostaining of the paraffin tissue after enzyme digestion, especially in older patients.
The first biopsy of our patient was negative for IgG on frozen tissue stained by polyclonal anti-IgG antibody, and IgG subclass staining on paraffin tissue revealed monoclonal IgG3 deposition, which indicated that the patient’s IgG was masked. However, on the second biopsy, on the frozen tissue, IgG and λ light chain were strongly positive, indicating that the deposited monoclonal IgG3λ was not masked, which means that the pathogenesis of this patient may be far more complicated and further studies are needed. The glomerular-deposited immunoglobulins are in dynamic changes due to multiple factors, such as the intrinsic characteristics of the deposited immunoglobulins, local decoration, and digestion. The presence of predominantly subendothelial deposits and the absence of subepithelial humps in C3G should prompt further study to exclude masked monoclonal immunoglobulin deposition.
About 97% of patients with PGNMID have strong glomerular C3 deposition, and 27% have low serum C3 and/or C4 levels,10 indicating local complement activation. The deposited monoclonal immunoglobulin was intact IgG; about 47% to 66% of patients were IgG3, and about 28% to 37% were IgG1,10, 11, 12 which both have the strongest activity of activating complement. Sixty-four percent of patients with PGNMID have positive glomerular C1q staining, indicating complement activation through the classic pathway. Our patient had strong glomerular C3 deposition, C1q was positive, and serum C3 level was low, indicating complement classic pathway activation. Even the masked IgG of the first biopsy activated the complement classic pathway, which is hard to explain and needs further study. Serum C3 level decreased as the proteinuria and Scr levels increased, and with remission of kidney disease, serum C3 level returned to normal range, indicating that complement activation contributed to the severity of glomerular disease. Thus, anti-complement therapy (eg, anti-C5 monoclonal antibody eculizumab) could have a theoretical benefit in patients with PGNMID, especially those with active changes (endocapillary exudation and cellular/fibrocellular crescents).
About 70% of patients with PGNMID are initially negative for monoclonal immunoglobulins,10, 11, 12 though some subsequently develop monoclonal immunoglobulins as occurred with the patient in this case report. This is unusual, with 1 prior case report describing a patient with PGNMID who developed monoclonal immunoglobulins 3 years after the biopsy.10
There is no guideline for the treatment of PGNMID; the treatment is based on expert consensus depending on the underlying clone, the presence or absence of monoclonal immunoglobulins, and the risk for kidney disease progression.13, 14 The recommended treatment for patients without monoclonal immunoglobulins and with a low risk for kidney disease progression (chronic kidney disease stages 1-2 and proteinuria with protein excretion < 1.0 g/d) is renin-angiotensin system inhibitors and supportive treatments. If there is the presence of monoclonal immunoglobulins or the patient has a high risk of kidney disease progression (proteinuria with protein excretion > 1.0 g/d or increasing Scr level), chemotherapy is suggested. Plasma cell–derived PGNMID (usually IgG) is treated with bortezomib-based chemotherapy, and B-cell–derived PGNMID is treated with a rituximab-based regimen. Also, rituximab has been reported to be effective in patients with PGNMID (monoclonal IgG deposition) without monoclonal immunoglobulins.12 Our patient had monoclonal IgGλ with high risk for kidney disease progression (nephrotic syndrome with increasing Scr level). Therefore, chemotherapy was indicated. His bone marrow study did not reveal monoclonal secreting B cells or plasma cells. It was speculated that the monoclonal IgGλ was derived from plasma cells, and he was treated with 9 courses of bortezomib and dexamethasone followed by lenalidomide and dexamethasone maintenance therapy. The patient showed complete hematologic remission followed by complete kidney remission.
In conclusion, we report a patient with MPGN with 2 consecutive kidney biopsies. The first biopsy showed masked IgG3 deposition. Eighteen months after the first biopsy, the patient developed monoclonal immunoglobulins, and the second biopsy showed PGNMID with unmasked IgG3λ deposition. The patient was successfully treated with bortezomib and dexamethasone followed by lenalidomide and dexamethasone maintenance therapy.
Article Information
Authors’ Full Names and Academic Degrees
Xiao-juan Yu, MD, Mang-ju Wang, MD, Zi-hao Yong, BA, Yi-yi Ma, MM, Su-xia Wang, MD, Fu-de Zhou, MD, and Ming-hui Zhao, MD, PhD.
Support
This study was supported by grants from National Natural Science Foundation of China (No. 81470956 and No. 81500543). The funders had a role in data collection, analysis, and reporting.
Financial Disclosure
The authors declare that they have no relevant financial interests.
Peer Review
Received April 24, 2019. Evaluated by 1 external peer reviewer, with direct editorial input from an Associate Editor and the Editor-in-Chief. Accepted in revised form June 12, 2019.
Footnotes
Complete author and article information provided before references.
References
- 1.Sethi S., Fervenza F.C. Membranoproliferative glomerulonephritis--a new look at an old entity. N Engl J Med. 2012;366(12):1119–1131. doi: 10.1056/NEJMra1108178. [DOI] [PubMed] [Google Scholar]
- 2.Leung N., Bridoux F., Batuman V. The evaluation of monoclonal gammopathy of renal significance: a consensus report of the International Kidney and Monoclonal Gammopathy Research Group. Nat Rev Nephrol. 2019;15(1):45–59. doi: 10.1038/s41581-018-0077-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sethi S., Sullivan A., Smith R.J. C4 dense-deposit disease. N Engl J Med. 2014;370(8):784–786. doi: 10.1056/NEJMc1309449. [DOI] [PubMed] [Google Scholar]
- 4.Bridoux F., Leung N., Hutchison C.A. Diagnosis of monoclonal gammopathy of renal significance. Kidney Int. 2015;87(4):698–711. doi: 10.1038/ki.2014.408. [DOI] [PubMed] [Google Scholar]
- 5.Pickering M.C., D'Agati V.D., Nester C.M. C3 glomerulopathy: consensus report. Kidney Int. 2013;84(6):1079–1089. doi: 10.1038/ki.2013.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jokiranta T.S., Solomon A., Pangburn M.K., Zipfel P.F., Meri S. Nephritogenic lambda light chain dimer: a unique human miniautoantibody against complement factor H. J Immunol. 1999;163(8):4590–4596. [PubMed] [Google Scholar]
- 7.Howlader A., Thajudeen B., Sussman A.N., Bracamonte E., Krahl L., Nasr S.H. Proliferative glomerulonephritis with masked monoclonal deposits responsive to myeloma therapy. Kidney Int Rep. 2017;2(6):1233–1237. doi: 10.1016/j.ekir.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Larsen C.P., Ambuzs J.M., Bonsib S.M. Membranous-like glomerulopathy with masked IgG kappa deposits. Kidney Int. 2014;86(1):154–161. doi: 10.1038/ki.2013.548. [DOI] [PubMed] [Google Scholar]
- 9.Larsen C.P., Boils C.L., Cossey L.N., Sharma S.G., Walker P.D. Clinicopathologic features of membranous-like glomerulopathy with masked IgG kappa deposits. Kidney Int Rep. 2016;1(4):299–305. doi: 10.1016/j.ekir.2016.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nasr S.H., Satoskar A., Markowitz G.S. Proliferative glomerulonephritis with monoclonal IgG deposits. J Am Soc Nephrol. 2009;20(9):2055–2064. doi: 10.1681/ASN.2009010110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nasr S.H., Markowitz G.S., Stokes M.B. Proliferative glomerulonephritis with monoclonal IgG deposits: a distinct entity mimicking immune-complex glomerulonephritis. Kidney Int. 2004;65(1):85–96. doi: 10.1111/j.1523-1755.2004.00365.x. [DOI] [PubMed] [Google Scholar]
- 12.Guiard E., Karras A., Plaisier E. Patterns of noncryoglobulinemic glomerulonephritis with monoclonal Ig deposits: correlation with IgG subclass and response to rituximab. Clin J Am Soc Nephrol. 2011;6(7):1609–1616. doi: 10.2215/CJN.10611110. [DOI] [PubMed] [Google Scholar]
- 13.Fermand J.P., Bridoux F., Kyle R.A. How I treat monoclonal gammopathy of renal significance (MGRS) Blood. 2013;122(22):3583–3590. doi: 10.1182/blood-2013-05-495929. [DOI] [PubMed] [Google Scholar]
- 14.Gumber R., Cohen J.B., Palmer M.B. A clone-directed approach may improve diagnosis and treatment of proliferative glomerulonephritis with monoclonal immunoglobulin deposits. Kidney Int. 2018;94(1):199–205. doi: 10.1016/j.kint.2018.02.020. [DOI] [PubMed] [Google Scholar]