Abstract
Patients with end-stage renal disease treated with dialysis are often prescribed complex medication regimens, placing them at risk for drug-drug interactions and other medication-related problems. Particularly in the context of a broader interest in more patient-centered value-based care, improving medication management is an increasingly important focus area. However, current medication management metrics, designed for the broader patient population, may not be well suited to the specific needs of patients with kidney disease, especially given the complexity of medication regimens used by dialysis patients. We propose a kidney pharmacy-focused quality pyramid that is intended to provide a framework to guide dialysis organizations, health care providers, and/or clinicians with respect to an optimal medication management approach for dialysis patients. Incorporation of core programs in medication management, including medication reconciliation, safety programs, and medication therapy management for patients at high risk for medication-related problems, may result in improved outcomes. Although a growing body of evidence supports the concept that active medication management can improve medication adherence and reduce medication-related problems, these strategies are viewed as costly and are not widely deployed. However, if done effectively, pharmacy-led medication management has the potential to be one of the more cost-effective disease management strategies and may greatly improve outcomes for these complex patients.
Index Words: end-stage renal disease, medication management, pharmacy
A Changing Landscape and the Medication Management Opportunity
In the context of a broader interest in more holistic value-based care, the potential for improvements in medication management is an important focus area. Within the drug and medical supply industries, pharmacy/prescription drugs (retail) represents the seventh largest medical sector spend in the United States.1 Despite this tremendous expenditure, there is significant room for cost savings and improvement in outcomes. Poor medication management is thought to be responsible for 125,000 deaths annually and contributes to an estimated 21% of hospitalizations,2 resulting in $100 to $289 billion per year of costs to the US health care system.3, 4, 5, 6, 7, 8, 9, 10 Currently, the Centers for Medicare & Medicaid Services (CMS) requires Medicare Part D plans to have medication therapy management programs, but it remains unclear whether such programs have an effect on clinical outcomes.11, 12
As the dialysis industry begins to move from a fee-for-service platform toward integrated value-based care models such as End-Stage Renal Disease (ESRD) Seamless Care Organizations (ESCOs),13 nephrology professionals have an opportunity to simultaneously drive cost efficiencies and clinical outcomes. Medication management programs are one important mechanism by which to achieve these goals. Key components of a medication therapy management program are described in Table 1. A recent study found that among patients enrolled in ESCOs operated by a large dialysis organization and who were recently discharged from the hospital, participation in a multidisciplinary medication therapy management program was associated with a 50% lower hospital readmission rate compared to nonparticipation.14
Table 1.
Medication Therapy Management Program Components and Definitions
| Component | Description | Timing |
|---|---|---|
| Medication therapy management | Pharmacists or advanced practitioners address potential medication-related problems with patients and physicians in 3 steps: medication reconciliation, medication review, and issue resolution | |
| Medication reconciliation | Generate accurate and complete list of what medications a patient is taking, including prescription medications, over-the-counter medications, herbals, and supplements | Monthly |
| Medication review | Review of medication list by advanced practitioners to identify medication-related problems such as gaps or duplications in therapy, kidney dosing/frequency issues, and contraindications | After medication reconciliation; also target recently discharged patients, patients with adherence issues, and patients with multiple comorbid conditions |
| Issue resolution | Issues identified during the medication review are escalated to prescribers to resolve medication-related problems | As needed, based on identified potential medication-related problems |
| Deprescribing | The process of tapering, stopping, discontinuing, or withdrawing drugs, with the goal of managing polypharmacy and improving outcomes | Ongoing based on identified issues |
| Kidney-specific drug utilization review | Automated kidney clinical protocol performed runs on the patient’s medication list that flags potential medication-related problems that require resolution | Each time an updated medication list is produced |
Medication Metrics
Nephrology was one of the first medical specialties to use scientifically based clinical practice guidelines to inform population-based patient care.15 These are complimented by a set of performance measures that are intended to monitor the effect of guidelines on population-level outcomes. However, within the realm of medication management, quality oversight bodies such as CMS, the National Committee for Quality Assurance, the Pharmacy Quality Alliance, and the National Quality Forum have generally focused on non-nephrology disciplines to develop quality measures.
Quality measures developed by these organizations are currently used to create a “star rating” for Part D plans and/or Medicare Advantage plans. These quality measures emphasize elements such as customer service, patient experience, drug pricing accuracy, and safety (Table 2). Importantly, Medicare Advantage Plans, which are responsible for both medical and pharmacy benefits, have consistently higher scores than stand-alone Part D plans, which are responsible for the pharmacy benefit only.16 There is considerably more incentive for Medicare Advantage plans to deliver high-quality medication-related services because they can reap the benefit of reduced downstream health care costs.
Table 2.
Quality Measures Contributing to 2016 Star Rating for Medicare Part D Plans
| Weight | 2016 PDP Average Score | 2016 MA/PDP Plan Average Score | |
|---|---|---|---|
| Drug Plan Customer Service | |||
| Call center; foreign language interpreter and TTY available | 1.5 | 4.0 | 4.2 |
| Appeals autoforward | 1.5 | 4.1 | 4.5 |
| Appeals upheld | 1.5 | 3.1 | 3.3 |
| Member Complaints, Problems Getting Services, and Choosing to Leave the Plan | |||
| Complaints about the drug plan | 1.5 | 3.5 | 3.9 |
| Members choosing to leave the plan | 1.5 | 3.6 | 4.2 |
| Beneficiary access and performance problems | 1.0 | 3.9 | 4.2 |
| Drug plan quality improvement | 5.0 | 3.8 | 3.8 |
| Member Experience With Drug Plan | |||
| Rating of drug plan | 1.5 | 3.2 | 3.3 |
| Getting needed prescription drugs | 1.5 | 3.6 | 3.4 |
| Drug Pricing and Patient Safety | |||
| MPF price accuracy | 1.0 | 3.7 | 4.5 |
| High-risk medication | 3.0 | 3.1 | 4.1 |
| Medication adherence for diabetes medications | 3.0 | 2.7 | 3.9 |
| Medication adherence for hypertension (RAS antagonists) | 3.0 | 3.6 | 4.1 |
| Medication adherence for cholesterol (statins) | 3.0 | 3.5 | 4.0 |
| MTM program completion rate for CMRa | 1.0 | 2.3 | 2.3 |
Abbreviations: CMR, comprehensive medication review; MA, Medicare Advantage; MPF, Medicare Plan Finder; MTM, medication therapy management; PDP, Part D Plan; RAS, renin-angiotensin system; TTY, teletype writer.
Data source:70.
Measure added in 2016 with a default weight of 1.0; weight may increase for 2017 star ratings.
At present, there are a handful of metrics that pertain specifically to medication management for dialysis patients. First, the CMS ESRD Conditions for Coverage require documentation of a quarterly medication reconciliation.17, 18 Second, CMS requires that each dialysis patient’s comprehensive plan of care include a medication history, developed within 30 days of admittance to a dialysis facility, and that the medication history be updated at least annually for stable patients or monthly for unstable patients.19 Although medication reconciliation is considered important and associated with improved outcomes,20, 21 such reviews are often completed by dialysis facility nursing staff under the operational and financial constraints of dialysis providers. Furthermore, quarterly medication reconciliation may not be frequent enough. Some have suggested shorter intervals, although the optimal interval and post–transition of care episode for medication reconciliation have not been determined.22
Beginning in 2022, CMS will require dialysis facilities to report on a new Quality Incentive Program measure with respect to medication reconciliation. This measure will describe the percentage of patient-months in a dialysis facility for which a medication reconciliation was performed and documented by an eligible professional.23 Within each facility, patients eligible for the reporting measure will be those who received a minimum of 7 hemodialysis treatments at the facility in the reporting month. Personnel who may perform medication reconciliation include physicians, registered nurses, nurse practitioners, physician assistants, pharmacists, or pharmacy technicians. Implementation of this new measure is consistent with the relatively high risk for medication-related problems among dialysis patients due to factors including polypharmacy, multiple comorbid conditions, and lower health literacy.
Although dialysis-specific measures are being developed, some of the Medicare Part D quality measures developed for the general patient population may be inappropriate for dialysis patients. For example, some pharmacy benefit management companies impose direct and indirect remuneration fees based on nonadherence to renin-angiotensin-aldosterone system inhibitors, statin medications, and diabetes medications, and although dialysis patients may qualify for these measures based on their comorbid conditions, data are lacking regarding the efficacy of these agents in the dialysis population, resulting in challenges in designing metrics that are clinically appropriate for dialysis patients. For example, although a direct and indirect remuneration fee may be tied to nonadherence to statin therapy, current evidence suggests that despite lowering low-density lipoprotein cholesterol levels, statins have little or no effect on cardiovascular outcomes among dialysis patients. Clinical guidelines in dialysis were recently updated to reflect these findings.24, 25, 26, 27, 28, 29, 30 It is clear that a more kidney-specific approach to medication management, accompanied by more thoughtfully designed metrics, is required.
Medication-Related Issues in Dialysis
The clinical complexity of dialysis patients places them at risk for polypharmacy and medication-related problems. Dialysis patients typically have 10 to 12 prescription medications, resulting in an average burden of 19 pills per day. These prescriptions arise from an average of 4 to 5 different prescribers.31 Many of the oral medications are large pills that may be difficult to swallow.32, 33, 34
Polypharmacy has been variously defined as the use of multiple medications, potentially inappropriate multiple medications, and the use of multiple pharmacies.35 Each of the items forming these definitions has been associated with poor outcomes, including higher costs, higher rates of adverse drug reactions, reduced medication adherence, lower quality of life, and increased hospitalizations and mortality.36, 37, 38, 39 Most dialysis patients interact with multiple health care providers, increasing the risk for using multiple pharmacies and the risk for therapeutic duplication of medications.40 Polypharmacy, combined with a high pill burden, may have a considerable impact on medication adherence.41, 42, 43 Programs geared toward the reduction of polypharmacy can be effective independently of other medication management programs (reviewed in31). Deprescribing is the process of tapering, stopping, or withdrawing drugs with the goal of managing polypharmacy and improving outcomes.44 A recent quality improvement study demonstrated that a targeted deprescribing program reduced inappropriate use of quinine, diuretics, α1-blockers, and proton pump inhibitors among hemodialysis patients.45
Medication-related problems are often defined as “undesirable events experienced by the patient that involve, or are suspected to involve, drug therapy and that interferes with achieving the desired goals of therapy.”46(p 143) Medication-related problems can be further classified into groups, such as issues with dosing, adverse drug reactions, high-risk medication identification, and drug-drug interactions.47 Medication-related problems are both common and costly: a review of more than 677,000 elderly patients receiving prescriptions through Medicare Part D in 2008 revealed that nearly one-third were receiving medications deemed potentially inappropriate for this age group.48 A recent systematic review of both prospective and retrospective studies showed that the median rate of hospital readmissions due to medications was 21% (range, 3%-64%), with a median of 69% of readmissions (range, 5%-87%) considered preventable.2 Recent estimates suggest that 2.4% to 4.1% of all hospitalizations are related to possible adverse drug events and poor adherence, and a high proportion of adverse drug events (up to 69%) are thought to be preventable.21
Disease-specific programs that allow for pharmacist review of medications may be able to reduce medication-related problems and improve outcomes.47 For example, one study of more than 120,000 incident hemodialysis patients found that digoxin use was associated with a 28% increased risk for death, and that an elevated serum digoxin concentration was significantly associated with mortality, most markedly in patients with lower predialysis serum potassium levels.49 A recent review found guideline nonadherence in dosing for kidney function ranges from 19% to as high as 67%.50
In the dialysis setting, studies have supported the use of medication therapy management as an effective tool for the identification and resolution of medication-related problems.22,51 In a 2-year randomized controlled trial, 104 hemodialysis patients were either given in-depth bimonthly medication therapy management conducted by a clinical pharmacist (pharmaceutical care) or brief medication therapy reviews conducted by a nurse (usual care). The pharmaceutical care group was associated with fewer hospitalizations per year.52 Similarly, a meta-analysis of dialysis patients who had been discharged from the hospital within the prior 30 days found that those who underwent a pharmacist-led medication therapy management encounter tended to have lower rates of rehospitalization than those who did not.53
A Kidney Pharmacy Quality Pyramid
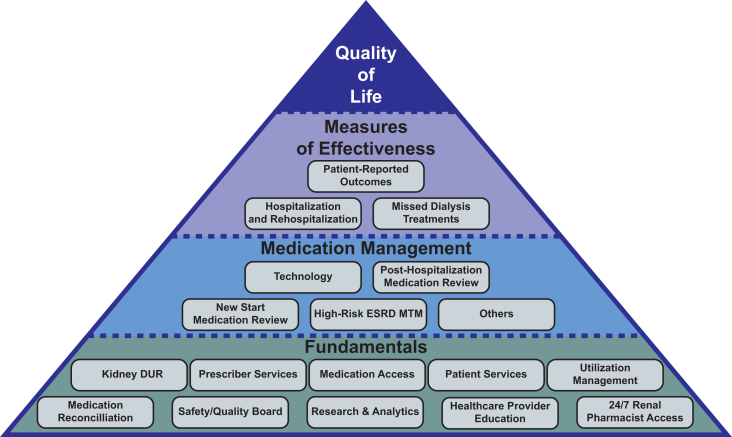
In the rest of this article, we describe a dialysis pharmacy-focused quality pyramid (Fig 1), similar in concept and design to a previously developed dialysis-focused quality pyramid.54 The programs and initiatives included in the pyramid are based on the latest clinical evidence and are mapped to dialysis-specific outcomes that are most likely to be affected by optimal pharmacy care. The pyramid is intended to provide a framework to guide a dialysis organization, health care providers, and/or clinicians with respect to an optimal medication management approach for dialysis patients. Ultimately, the goal of the pyramid is to improve the patient’s health and quality of life by increasing adherence while reducing polypharmacy and identifying and resolving medication-related problems. These aims are achieved by leveraging specially designed medication management programs within the pyramid structure.
Figure 1.
Kidney pharmacy quality pyramid consists of 4 levels. Fundamentals support Medication Management programs. The impact of the Fundamentals and Medication Management programs is assessed using Measures of Effectiveness, with the ultimate goal of improving patients’ Quality of Life. Abbreviations: DUR, drug utilization review; ESRD, end-stage renal disease; MTM, medication therapy management.
The “Fundamental Programs” at the base of the pyramid support patient safety, program quality, and the overall patient and prescriber experience, representing the baseline level of care that must be delivered. Without these programs, the chances of successfully improving dialysis patient outcomes through medication management are slim.
Medication reconciliation, or the process of creating an accurate list of the medications a patient is taking, is the key process that paves the way for optimizing medication management. Medication reconciliation programs should be offered in accordance with CMS and other governing body requirements, but with an eye toward creating encounters that maximize clinical benefit. Medication reconciliation may be most effective when carried out by pharmacy personnel who are familiar with trade name and generic medications and understand how best to develop a reconciled list. Patients considered to be high risk due to being new to dialysis, having recently been discharged from the hospital or a rehabilitation or nursing facility, or having a particularly high number of medications should be prioritized. Such patients may be particularly likely to have received inappropriate medications or conflicting/unclear medication instructions arising from contact with more than 1 prescriber; such issues should be a particular focus for the medication reconciliation. Engagement with nondialysis providers may be of particular importance in this circumstance. Personnel conducting medication reconciliation should be trained in behavioral and motivational interview techniques. Toolkits are available to assist dialysis units in developing a robust medication reconciliation process.55 Patients need to be educated to bring their medications to dialysis at least monthly or after a hospitalization so that medication reconciliation can be efficiently accomplished. The patient should be given a copy of their reconciled list to share with other prescribers.
A safety and quality board with representation from across the care team should be convened on a routine basis. The board should review matters such as adverse events, new medication offerings, drug warnings, and opportunities to educate patients and prescribers, and prescription dispensing occurrences. A robust medication-related research program and an analytics platform should be maintained to develop new programs, study the impact of current programs, stratify patients by risk, and describe their experience. Monthly reporting of adherence, occurrence of medication errors, and performance with respect to quality metrics is important to guide intervention and outreach. The ability to stratify patients based on comorbid conditions, polypharmacy, and nonadherence risk is critical. Reviewing the success and failures of current programs is important to continue to refine and optimize care.
Pharmacists with specific training in dialysis can play a significant role in tackling the challenges of medication management in this population. In Canadian dialysis and chronic kidney disease care models, pharmacy providers often interact with patients more frequently than their primary nephrologists,56 making them a key point of intervention. Drug and disease counseling can facilitate patients’ understanding of the need for specific medications and prepare them for possible adverse events. Efforts should be made to reduce polypharmacy, improve coordination of care, reduce the use of inappropriate drugs (utilization management), and reduce risk factors for medication-related problems.35,57
Programs that facilitate medication access can reduce barriers to medication adherence. Access may be limited by factors such as financial or transportation issues, language barriers, or low health care literacy. Facilitating access to co-pay assistance and manufacturer patient assistant programs, offering patient education programs, and providing clinical supports can enable patients to access their medications in circumstances that will facilitate medication adherence.
Kidney-specific drug utilization reviews are another important mechanism to ensure patient safety. While drug utilization review platforms such as Medi-Span are commonly applied, a kidney-specific drug utilization review platform is paramount to identify and study the specific medication-related problems that are associated with poor outcomes. These platforms should also reflect the pharmacokinetic and pharmacodynamic challenges that are inherent with using medications in patients with kidney disease.
Medication Review and Medication Therapy Management
After medication reconciliation takes place, medication therapy management can be conducted. Medication therapy management is the process of producing an accurate medication list, followed by evaluating the list for problems, keying in on medication indication, effectiveness, safety, and adherence issues. Medication therapy management implies longitudinal activities intended to optimize medication use and health outcomes, targeting the more challenging aspects of care for specifically selected groups of patients. Medication review and medication therapy management should be conducted by highly trained clinicians (pharmacists, nephrologists, and advanced practitioners).
While evaluating clinical risk factors is critical, estimating patient engagement and receptivity to intervention may enable tailoring of programs to maximize efficacy and cost-efficiency. Receptivity and engagement are important in understanding the root cause of nonadherence, and higher engagement promotes adherence.58 A recent study demonstrated that higher patient activation was associated with higher medication adherence among young dialysis patients.59
It is important to build on the analytics platform emphasized in the pyramid base, that not only uses conventional data (comorbid conditions and discharge history) to understand clinical risk, but also focuses on nontraditional data elements, including social determinants of health,60 to understand patient motivation and behavior.61 For example, understanding economic status may offer insights into a patient’s ability to pay; knowing where a patient lives may provide insights into pharmacy access or availability of public transportation for physician visits. In the era of value-based care, being able to predict which interventions or programs are most likely to be impactful for specific patients is critical to guide resource utilization.60 Medication therapy management should focus on opportunities that generate the greatest impact from a clinical and cost perspective.53,62 Because medication therapy management programs are considered expensive in the capitated fee-for-service landscape, care must be taken to apply them where most beneficial. Target populations may include patients initiating dialysis, those recently discharged from the hospital or other care facilities, those with multiple comorbid conditions, and those with multiple medications, known medication adherence issues, poor health literacy, and/or significant financial constraints.63
Medication therapy management services should aim to increase adherence to medications that, in the context of a patient’s particular circumstances, seem most likely to improve either clinical outcomes or quality of life. For example, renin-angiotensin system antagonists may diminish harmful pathophysiologic processes in some dialysis patients.64, 65, 66 In addition, proper use of high-risk medications such as anticoagulants could help avoid complications such as excessive bleeding. Programs that drive selective adherence to critical medications may bring more value than generalized adherence strategies. Adherence technologies such as smart pill bottles and mobile phone apps may also be beneficial, although direct evidence supporting the use of these technologies in the dialysis population is not yet available.
Measures of Effectiveness
Measures of effectiveness allow for assessment of the impact of the lower tiers of the pyramid on key health outcomes.67 Given that there is significant mortality risk associated with a single missed dialysis treatment68 and hospitalization has long been a surrogate marker for heightened mortality risk, these metrics are important readouts for the efficacy of medication management programs. Alleviation of symptoms is important to dialysis patients because this can improve their quality of life. Hemodialysis patients believe that the symptoms of fatigue, insomnia, and muscle cramping should be prioritized for therapeutic intervention, either with improved dialysis methods or with medications.69 There is a critical need in the dialysis field for the development and implementation of patient-reported outcomes measures to evaluate medication therapies.
Discussion
Patients being treated with dialysis take numerous daily medications and are more likely than other patient groups to experience drug-drug interactions and adverse effects due to changes in medication pharmacokinetics and pharmacodynamics in patients with severe decreased kidney function who receive dialytic therapies. Although a growing body of evidence supports the concept that active medication management can increase adherence, reduce medication-related problems, and improve outcomes, these strategies are viewed as costly and are not widely deployed. However, if done effectively, pharmacy-led medication management has the potential to be one of the more cost-effective disease management strategies with the potential to greatly improve outcomes for these complex patients.
Going forward, it will be critical to target resource-intensive medication management programs to patients who are at highest risk for adverse events and who stand to benefit the most from such interventions. Among high-risk patients, the cost of applying medication management programs may be offset by reductions in hospital admissions or readmissions or through reduced medication cost. Recognition of the benefits of a more holistic approach to medication management is reflected in the current trend toward vertical integration of insurers and pharmacy benefit managers. Although there are clear upfront costs with respect to optimization of medication utilization, these may be covered by downstream savings that far outweigh the initial investment. The ability to pay for these programs may be realized further with the merging of pharmacy and medical benefits and a focus on the total cost of care as seen by the recent consolidation of pharmacy benefit managers and payors within the health care landscape.
In contrast to the requirement that Medicare Part D plans offer medication therapy management services, out-of-clinic medication management is currently not included in the dialysis reimbursement bundle. Medication reconciliation services are currently not reimbursed. Rather, they are completed by the dialysis facility nursing staff under the operational and financial constraints of dialysis providers. Medication therapy management and adherence technologies face similar reimbursement challenges. Provision of health technologies and services without discrete reimbursement has been stymied by questions as to whether they may be subject to concern under current Medicare patient inducement limits. For example, if a dialysis provider were to provide a smart pill bottle to improve adherence, it may be viewed as an item of value being bestowed to the patient. The same question applies to medication therapy management programs, which are costly in terms of staff time. Given the potential benefits of these resource-intensive programs, regulatory clarification of the implications of providing such programs to patients is needed, especially as CMS considers adopting quality metrics associated with medication reconciliation and management.
The issues imposed by a fee-for-service payment environment may become less prominent as the ESRD payment system moves toward an integrated care model. ESCO programs, Chronic Condition Special Needs Plans, and future integrated care models in dialysis may overcome these limitations and allow for medication management programs to become more prevalent. The use of medication management programs, particularly in complex patient populations such as those on dialysis, will be a needed tool that can be wielded to influence health care outcomes.
Article Information
Authors’ Full Names and Academic Degrees
John Wigneswaran, MD, Wendy L. St. Peter, PharmD, Allen R. Nissenson, MD, Mahesh Krishnan, MD, Richard Faris, PhD, Bryan Becker, MD, and Jonathan Lorch, MD.
Support
None.
Financial Disclosure
Dr Wigneswaran is an employee of Express Scripts/Cigna and holds stock and options in that company. Drs Nissenson, Krishnan, and Becker are employees of DaVita, Inc, and hold stock and options in that company. Dr Becker is also a member of the board of the Forward Health Group. Dr St. Peter serves on the board of directors of the Kidney Health Initiative. Dr Faris was an employee of DaVita Rx, is currently an employee of PANTHERx Specialty Pharmacy, and has held shares in DaVita, Inc. Dr Lorch is an employee of Rogosin Institute and has served as a consultant to DaVita, Inc.
Acknowledgements
The authors acknowledge the assistance of Dena E. Cohen, PhD, a medical writer employed by DaVita Clinical Research, with the preparation of this manuscript.
Peer Review
Received March 25, 2019. Evaluated by 3 external peer reviewers, with direct editorial input from an Associate Editor and the Editor-in-Chief. Accepted in revised form June 23, 2019.
Footnotes
Complete author and article information provided before references.
References
- 1.Alexa, How Much Is This Going to Hurt? Calibrating Amazon's Entry Into Healthcare. 2017. https://www.zerohedge.com/news/2017-11-20/alexa-going-hurt-these-companies-will-be-destroyed-amazon-next [Google Scholar]
- 2.El Morabet N., Uitvlugt E.B., van den Bemt B.J.F., van den Bemt P., Janssen M.J.A., Karapinar-Carkit F. Prevalence and preventability of drug-related hospital readmissions: a systematic review. J Am Geriatr Soc. 2018;66(3):602–608. doi: 10.1111/jgs.15244. [DOI] [PubMed] [Google Scholar]
- 3.National Pharmaceutical Council Noncompliance with medications: an economic tragedy with important implications for health care reform. 1994. http://www.npcnow.org/publication/noncompliance-medications-economic-tragedy-important-implications-health-care-reform
- 4.Network for Excellence in Health Initiative. Thinking Outside the Pillbox: A System-wide Approach to Improving Patient Medication Adherence for Chronic Disease. New England Healthcare Initiative; 2009. https://www.nehi.net/publications/17-thinking-outside-the-pillbox-asystem-wide-approach-to-improving-patient-medication-adherence-for-chronic-disease/view [Google Scholar]
- 5.DiMatteo M.R., Giordani P.J., Lepper H.S., Croghan T.W. Patient adherence and medical treatment outcomes: a meta-analysis. Med Care. 2002;40(9):794–811. doi: 10.1097/00005650-200209000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Mahoney J., Ansell B., Fleming W., Butterworth S. The unhidden cost of noncompliance. J Manag Care Pharm. 2008;14(6b):S3–S29. [Google Scholar]
- 7.Osterberg L., Blaschke T. Adherence to medication. N Engl J Med. 2005;353(5):487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 8.Peterson A.M., Takiya L., Finley R. Meta-analysis of trials of interventions to improve medication adherence. Am J Health Syst Pharm. 2003;60(7):657–665. doi: 10.1093/ajhp/60.7.657. [DOI] [PubMed] [Google Scholar]
- 9.Schiff G.D., Fung S., Speroff T., McNutt R.A. Decompensated heart failure: symptoms, patterns of onset, and contributing factors. Am J Med. 2003;114(8):625–630. doi: 10.1016/s0002-9343(03)00132-3. [DOI] [PubMed] [Google Scholar]
- 10.Viswanathan M., Golin C.E., Jones C.D. Interventions to improve adherence to self-administered medications for chronic diseases in the United States: a systematic review. Ann Intern Med. 2012;157(11):785–795. doi: 10.7326/0003-4819-157-11-201212040-00538. [DOI] [PubMed] [Google Scholar]
- 11.Barlas S. CMS to test enhanced medication therapy management model: aims for greater use of pharmacists, cost savings, and better outcomes. P T. 2016;41(7):423–441. [PMC free article] [PubMed] [Google Scholar]
- 12.Perlroth D., Marrufo G., Montesinos A. Acumen, LLC; Burlingame, CA: 2013. Medication Therapy Management in Chronically Ill Populations: Final Report. [Google Scholar]
- 13.Centers for Medicare & Medicaid Services Comprehensive ESRD care model. https://innovation.cms.gov/initiatives/comprehensive-esrd-care/
- 14.Manley H.J., Aweh G.N., Lacson E.K. Poster presented at: National Kidney Foundation Spring Clinical Meeting.; Austin, TX: 2018. Multidisciplinary medication therapy management (MTM) program impact on 30-day hospital readmissions. April 10-14, 2018. [Google Scholar]
- 15.Kliger A.S. Quality measures for dialysis: time for a balanced scorecard. Clin J Am Soc Nephrol. 2016;11(2):363–368. doi: 10.2215/CJN.06010615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoadley J., Cubanski J., Neuman T. Henry J. Kaiser Family Foundation Report; Menlo Park, CA: 2016. Medicare Part D in 2016 and Trends Over Time. [Google Scholar]
- 17.Centers for Medicare & Medicaid Services (CMS) HHS. Medicare and Medicaid programs; conditions for coverage for end-stage renal disease facilities. Final rule. Fed Regist. 2008;73(73):20369–20484. [PubMed] [Google Scholar]
- 18.Centers for Medicare & Medicaid Services National Quality Forum. Medication reconciliation for patients receiving care at dialysis facilities. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/ESRDQIP/Downloads/NQF-2988-Patients-Receiving-Care-at-Dialysis-Facilities.pdf
- 19.RTI International Accountable Care Organization 2017: quality measure narrative specifications. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/sharedsavingsprogram/Downloads/2017-Reporting-Year-Narrative-Specifications.pdf
- 20.Mekonnen A.B., McLachlan A.J., Brien J.A. Effectiveness of pharmacist-led medication reconciliation programmes on clinical outcomes at hospital transitions: a systematic review and meta-analysis. BMJ Open. 2016;6(2) doi: 10.1136/bmjopen-2015-010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Najafzadeh M., Schnipper J.L., Shrank W.H., Kymes S., Brennan T.A., Choudhry N.K. Economic value of pharmacist-led medication reconciliation for reducing medication errors after hospital discharge. Am J Manag Care. 2016;22(10):654–661. [PubMed] [Google Scholar]
- 22.Pai A.B., Cardone K.E., Manley H.J. Medication reconciliation and therapy management in dialysis-dependent patients: need for a systematic approach. Clin J Am Soc Nephrol. 2013;8(11):1988–1999. doi: 10.2215/CJN.01420213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Centers for Medicare & Medicaid Services HHS Medicare Program; End-Stage Renal Disease Prospective Payment System, Payment for Renal Dialysis Services Furnished to Individuals With Acute Kidney Injury, End-Stage Renal Disease Quality Incentive Program, Durable Medical Equipment, Prosthetics, Orthotics and Supplies (DMEPOS) Competitive Bidding Program (CBP) and Fee Schedule Amounts, and Technical Amendments To Correct Existing Regulations Related to the CBP for Certain DMEPOS. Final rule. Fed Regist. 2018;83(220):56922–57073. [PubMed] [Google Scholar]
- 24.Baigent C., Landray M.J., Reith C. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377(9784):2181–2192. doi: 10.1016/S0140-6736(11)60739-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fellstrom B.C., Jardine A.G., Schmieder R.E. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med. 2009;360(14):1395–1407. doi: 10.1056/NEJMoa0810177. [DOI] [PubMed] [Google Scholar]
- 26.Markossian T., Burge N., Ling B. Controversies regarding lipid management and statin use for cardiovascular risk reduction in patients with CKD. Am J Kidney Dis. 2016;67(6):965–977. doi: 10.1053/j.ajkd.2015.12.030. [DOI] [PubMed] [Google Scholar]
- 27.Marz W., Genser B., Drechsler C. Atorvastatin and low-density lipoprotein cholesterol in type 2 diabetes mellitus patients on hemodialysis. Clin J Am Soc Nephrol. 2011;6(6):1316–1325. doi: 10.2215/CJN.09121010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nemerovski C.W., Lekura J., Cefaretti M., Mehta P.T., Moore C.L. Safety and efficacy of statins in patients with end-stage renal disease. Ann Pharmacother. 2013;47(10):1321–1329. doi: 10.1177/1060028013501997. [DOI] [PubMed] [Google Scholar]
- 29.Wanner C., Krane V., Marz W. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med. 2005;353(3):238–248. doi: 10.1056/NEJMoa043545. [DOI] [PubMed] [Google Scholar]
- 30.Wanner C., Tonelli M. KDIGO clinical practice guideline for lipid management in CKD: summary of recommendation statements and clinical approach to the patient. Kidney Int. 2014;85(6):1303–1309. doi: 10.1038/ki.2014.31. [DOI] [PubMed] [Google Scholar]
- 31.St Peter W.L. Management of polypharmacy in dialysis patients. Semin Dial. 2015;28(4):427–432. doi: 10.1111/sdi.12377. [DOI] [PubMed] [Google Scholar]
- 32.Collins A.J., Foley R.N., Chavers B. US Renal Data System 2013 annual data report. Am J Kidney Dis. 2014;63(1 suppl 1):e1–e420. doi: 10.1053/j.ajkd.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 33.Chiu Y.W., Teitelbaum I., Misra M., de Leon E.M., Adzize T., Mehrotra R. Pill burden, adherence, hyperphosphatemia, and quality of life in maintenance dialysis patients. Clin J Am Soc Nephrol. 2009;4(6):1089–1096. doi: 10.2215/CJN.00290109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manley H.J., Garvin C.G., Drayer D.K. Medication prescribing patterns in ambulatory haemodialysis patients: comparisons of USRDS to a large not-for-profit dialysis provider. Nephrol Dial Transplant. 2004;19(7):1842–1848. doi: 10.1093/ndt/gfh280. [DOI] [PubMed] [Google Scholar]
- 35.Rodrigues M.C., Oliveira C. Drug-drug interactions and adverse drug reactions in polypharmacy among older adults: an integrative review. Rev Lat Am Enfermagem. 2016;24:e2800. doi: 10.1590/1518-8345.1316.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gnjidic D., Le Couteur D.G., Kouladjian L., Hilmer S.N. Deprescribing trials: methods to reduce polypharmacy and the impact on prescribing and clinical outcomes. Clin Geriatr Med. 2012;28(2):237–253. doi: 10.1016/j.cger.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 37.Riker G.I., Setter S.M. Polypharmacy in older adults at home: what it is and what to do about it--implications for home healthcare and hospice. Home Healthc Nurse. 2012;30(8):474–485. doi: 10.1097/NHH.0b013e31826502dd. quiz 86-87. [DOI] [PubMed] [Google Scholar]
- 38.Rollason V., Vogt N. Reduction of polypharmacy in the elderly: a systematic review of the role of the pharmacist. Drugs Aging. 2003;20(11):817–832. doi: 10.2165/00002512-200320110-00003. [DOI] [PubMed] [Google Scholar]
- 39.Wehling M., Burkhardt H. Polypharmacy. In: Wehling M., editor. Drug Therapy for the Elderly. Springer; Vienna, Austria: 2013. pp. 319–329. [Google Scholar]
- 40.Sherman J.J., Davis L., Daniels K. Addressing the polypharmacy conundrum. US Pharm. 2017;42(6):14–20. [Google Scholar]
- 41.Browne T., Merighi J.R. Barriers to adult hemodialysis patients' self-management of oral medications. Am J Kidney Dis. 2010;56(3):547–557. doi: 10.1053/j.ajkd.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 42.Holley J.L., DeVore C.C. Why all prescribed medications are not taken: results from a survey of chronic dialysis patients. Adv Perit Dial. 2006;22:162–166. [PubMed] [Google Scholar]
- 43.Matteson M.L., Russell C. Interventions to improve hemodialysis adherence: a systematic review of randomized-controlled trials. Hemodial Int. 2010;14(4):370–382. doi: 10.1111/j.1542-4758.2010.00462.x. [DOI] [PubMed] [Google Scholar]
- 44.Thompson W., Farrell B. Deprescribing: what is it and what does the evidence tell us? Can J Hosp Pharm. 2013;66(3):201–202. doi: 10.4212/cjhp.v66i3.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McIntyre C., McQuillan R., Bell C., Battistella M. Targeted deprescribing in an outpatient hemodialysis unit: a quality improvement study to decrease polypharmacy. Am J Kidney Dis. 2017;70(5):611–618. doi: 10.1053/j.ajkd.2017.02.374. [DOI] [PubMed] [Google Scholar]
- 46.Cipolle R.J., Strand L., Morley P. 3rd ed. McGraw-Hill; New York, NY: 2012. Pharmaceutical Care Practice: The Patient-Centered Approach to Medication Management Services. [Google Scholar]
- 47.Manley H.J., Carroll C.A. The clinical and economic impact of pharmaceutical care in end-stage renal disease patients. Semin Dial. 2002;15(1):45–49. doi: 10.1046/j.1525-139x.2002.00014.x. [DOI] [PubMed] [Google Scholar]
- 48.Holmes H.M., Luo R., Kuo Y.F., Baillargeon J., Goodwin J.S. Association of potentially inappropriate medication use with patient and prescriber characteristics in Medicare Part D. Pharmacoepidemiol Drug Saf. 2013;22(7):728–734. doi: 10.1002/pds.3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chan K.E., Lazarus J.M., Hakim R.M. Digoxin associates with mortality in ESRD. J Am Soc Nephrol. 2010;21(9):1550–1559. doi: 10.1681/ASN.2009101047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Long C.L., Raebel M.A., Price D.W., Magid D.J. Compliance with dosing guidelines in patients with chronic kidney disease. Ann Pharmacother. 2004;38(5):853–858. doi: 10.1345/aph.1D399. [DOI] [PubMed] [Google Scholar]
- 51.Possidente C.J., Bailie G.R., Hood V.L. Disruptions in drug therapy in long-term dialysis patients who require hospitalization. Am J Health Syst Pharm. 1999;56(19):1961–1964. doi: 10.1093/ajhp/56.19.1961. [DOI] [PubMed] [Google Scholar]
- 52.Pai A.B., Boyd A., Depczynski J., Chavez I.M., Khan N., Manley H. Reduced drug use and hospitalization rates in patients undergoing hemodialysis who received pharmaceutical care: a 2-year, randomized, controlled study. Pharmacotherapy. 2009;29(12):1433–1440. doi: 10.1592/phco.29.12.1433. [DOI] [PubMed] [Google Scholar]
- 53.Faris R., Wigneswaran J., Brunelli S.M., Maheshwari V., Meng A. Poster presented at: National Kidney Foundation. Spring Clinical Meetings; Orlando, FL: 2017. Impact of a Medication Review Program on Hospitalization Rate Among High-Risk Dialysis Patients. April 18-22, 2017. [Google Scholar]
- 54.Nissenson A.R. Improving outcomes for ESRD patients: shifting the quality paradigm. Clin J Am Soc Nephrol. 2014;9(2):430–434. doi: 10.2215/CJN.05980613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gleason K.M., Brake H., Agramonte V., Perfetti C. Agency for Healthcare Research and Quality; Rockville, MD: 2012. Medications at Transitions and Clinical Handoffs (MATCH) Toolkit for Medication Reconciliation. Contract No.: 0059. [Google Scholar]
- 56.Raymond C.B., Wazny L.D., Sood A.R. Standards of clinical practice for renal pharmacists. Can J Hosp Pharm. 2013;66(6):369–374. doi: 10.4212/cjhp.v66i6.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vonbach P., Dubied A., Krahenbuhl S., Beer J.H. Prevalence of drug-drug interactions at hospital entry and during hospital stay of patients in internal medicine. Eur J Intern Med. 2008;19(6):413–420. doi: 10.1016/j.ejim.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 58.Bakken S., Holzemer W.L., Brown M.A. Relationships between perception of engagement with health care provider and demographic characteristics, health status, and adherence to therapeutic regimen in persons with HIV/AIDS. AIDS Patient Care STDS. 2000;14(4):189–197. doi: 10.1089/108729100317795. [DOI] [PubMed] [Google Scholar]
- 59.Hamilton A.J., Caskey F.J., Casula A., Inward C.D., Ben-Shlomo Y. Associations with wellbeing and medication adherence in young adults receiving kidney replacement therapy. Clin J Am Soc Nephrol. 2018;13(11):1669–1679. doi: 10.2215/CJN.02450218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bell C.C., Deleon P.H., Diaz A. Perspectives on Health Equity & Social Determinants of Health. National Academy of Medicine; Washington, D.C.: 2017. https://nam.edu/wp-content/uploads/2017/12/Perspectives-on-Health-Equity-and-Social-Determinants-of-Health.pdf [Google Scholar]
- 61.Arora N.K., McHorney C.A. Patient preferences for medical decision making: who really wants to participate? Med Care. 2000;38(3):335–341. doi: 10.1097/00005650-200003000-00010. [DOI] [PubMed] [Google Scholar]
- 62.Howland J, Hoang M, Lara-Nevarez M, Chillingworth K, Furgiuele T. Targeted Medication Therapy Management (MTM) Improves Outcomes for Dialysis Patients and the Healthcare System, Poster presented at: American Society of Nephrology Kidney Week, November 5-10, 2013. Atlanta, GA.
- 63.Palosky C., Singh R. New Kaiser/New York Times survey finds one in five working-age Americans with health insurance report problems paying medical bills. 2016. https://www.kff.org/health-costs/press-release/new-kaisernew-york-times-survey-finds-one-in-five-working-age-americans-with-health-insurance-report-problems-paying-medical-bills Accessed August 17, 2018.
- 64.Efrati S., Zaidenstein R., Dishy V. ACE inhibitors and survival of hemodialysis patients. Am J Kidney Dis. 2002;40(5):1023–1029. doi: 10.1053/ajkd.2002.36340. [DOI] [PubMed] [Google Scholar]
- 65.Suzuki H., Kanno Y., Sugahara S. Effect of angiotensin receptor blockers on cardiovascular events in patients undergoing hemodialysis: an open-label randomized controlled trial. Am J Kidney Dis. 2008;52(3):501–506. doi: 10.1053/j.ajkd.2008.04.031. [DOI] [PubMed] [Google Scholar]
- 66.Takahashi A., Takase H., Toriyama T. Candesartan, an angiotensin II type-1 receptor blocker, reduces cardiovascular events in patients on chronic haemodialysis--a randomized study. Nephrol Dial Transplant. 2006;21(9):2507–2512. doi: 10.1093/ndt/gfl293. [DOI] [PubMed] [Google Scholar]
- 67.Nissenson A.R. Delivering better quality of care: relentless focus and starting with the end in mind at DaVita. Semin Dial. 2016;29(2):111–118. doi: 10.1111/sdi.12462. [DOI] [PubMed] [Google Scholar]
- 68.Gray K.S., Cohen D.E., Brunelli S.M. In-center hemodialysis absenteeism: prevalence and association with outcomes. Clinicoecon Outcomes Res. 2017;9:307–315. doi: 10.2147/CEOR.S136577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Flythe J.E., Hilliard T., Castillo G. Symptom prioritization among adults receiving in-center hemodialysis: a mixed methods study. Clin J Am Soc Nephrol. 2018;13(5):735–745. doi: 10.2215/CJN.10850917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Trends in Part C & D Star Rating Measure Cut Points. Centers for Medicare & Medicaid Services; Bethesda, MD: 2016. [Google Scholar]