Abstract
Rationale & Objective
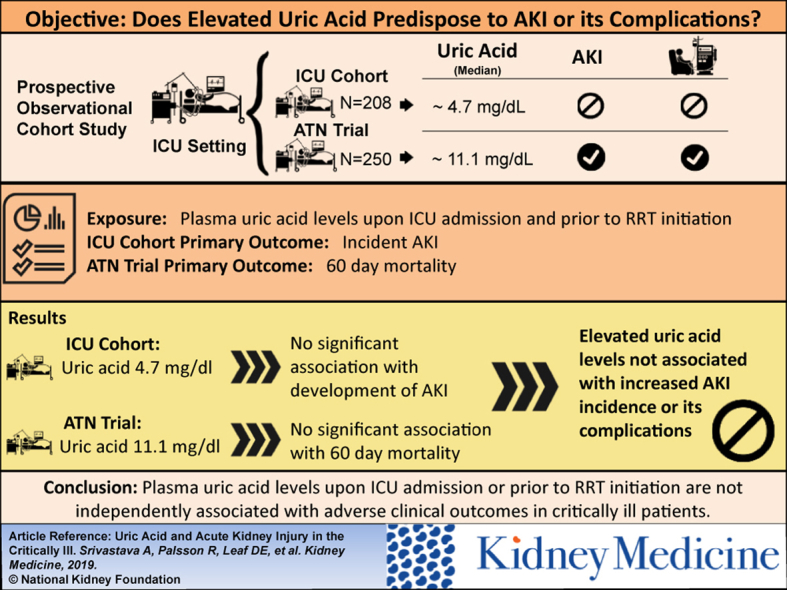
Uric acid is excreted by the kidney and accumulates in acute kidney injury (AKI). Whether higher plasma uric acid level predisposes to AKI or its complications is not known.
Study Design
Prospective observational cohort study.
Setting & Participants
2 independent cohorts of critically ill patients: (1) 208 patients without AKI admitted to the intensive care unit (ICU) at Brigham & Women’s Hospital between October 2008 and December 2016; and (2) 250 participants with AKI requiring renal replacement therapy (RRT) who had not yet initiated RRT enrolled in the Acute Renal Failure Trial Network (ATN) Study.
Exposure
Plasma uric acid level upon ICU admission and before RRT initiation in the ICU and ATN Study cohorts, respectively.
Outcomes
Incident AKI and 60-day mortality in the ICU and ATN Study cohorts, respectively.
Analytical Approach
Logistic regression models were used to test the association of plasma uric acid level with incident AKI and 60-day mortality.
Results
In the ICU cohort, median plasma uric acid level was 4.7 (interquartile range [IQR], 3.6-6.4) mg/dL, and 40 patients (19.2%) developed AKI. Higher plasma uric acid levels associated with incident AKI, but this association was confounded by serum creatinine level and was not significant after multivariable adjustment (adjusted OR per doubling of uric acid, 1.50; 95% CI, 0.80-2.81). In the ATN Study cohort, median plasma uric acid level was 11.1 (IQR, 8.6–14.2) mg/dL, and 125 participants (50.0%) died within 60 days. There was no statistically significant association between plasma uric acid levels and 60-day mortality in either unadjusted models or after multivariable adjustment for demographic, severity-of-illness, and kidney-specific covariates (adjusted OR per doubling of uric acid, 1.15; 95% CI, 0.71-1.86).
Limitations
Heterogeneity of ICU patients.
Conclusions
Plasma uric acid levels upon ICU admission or before RRT initiation are not independently associated with adverse clinical outcomes in critically ill patients.
Index Words: AKI, ICU, uric acid, RRT, dialysis
Graphical abstract
Acute kidney injury (AKI) is estimated to occur in up to 60% of critically ill patients.1, 2, 3 Patients who experience AKI in the intensive care unit (ICU) have a mortality risk of 20% to 50% and patients with severe AKI requiring renal replacement therapy (AKI-RRT) have a mortality risk of 40% to 80%.2, 4, 5, 6 Renal recovery (no longer requiring RRT) can occur in up to 50% of patients, but the probability of recovery is difficult to assess.7, 8 The pathogenesis of AKI-RRT and mechanisms for recovery are incompletely understood.
Elevations in plasma uric acid levels (hyperuricemia) are common in patients with decreased kidney function from chronic kidney disease (CKD) and AKI. Multiple studies have found associations between higher uric acid levels and increased risk for the development of chronic systemic illnesses such as hypertension,9 cardiovascular disease,10, 11 and CKD.12, 13 Prior studies have demonstrated higher uric acid levels as a risk factor for the development of contrast-induced AKI14, 15 and AKI after cardiac surgery.16, 17, 18, 19 Few studies have investigated the potential role of uric acid in inciting or perpetuating AKI in critically ill patients. In the setting of AKI from tumor lysis syndrome, hyperuricemia is thought to cause kidney injury by intratubular obstruction and intrarenal inflammation by the precipitated crystals.20, 21, 22 Mechanisms beyond tubular obstruction are also potential contributors. In animal models, increased uric acid levels may lead to endothelial dysfunction,23, 24 renin-angiotensin system activation,25 and oxidative stress.26
Little is known about the role of uric acid in AKI outside of tumor lysis syndrome. We wanted to test the hypothesis that higher circulating uric acid levels are a risk factor for the development of AKI and its prognosis. To do so, we measured plasma uric acid in stored samples from 2 patient cohorts: (1) critically ill patients admitted to the ICU, and (2) patients with AKI-RRT enrolled into a randomized controlled trial of RRT intensity.
Methods
Study Design and Population
We conducted a prospective study of the association between plasma uric acid levels and adverse clinical outcomes in 2 independent cohorts of critically ill patients. The first was a prospective observational cohort study and biorepository of patients admitted to the ICU at Brigham & Women’s Hospital (BWH) between October 2008 and December 2016. This cohort is comprised of patients admitted to the medical and surgical ICUs at BWH and patients enrolled in the Registry of Critical Illness (ROCI) cohort study27 from the medical ICUs at BWH (collectively named ICU cohort). The second was the Veterans Affairs/National Institutes of Health Acute Renal Failure Trial Network (ATN) Study, a multicenter randomized clinical trial of intensive versus less intensive RRT that enrolled critically ill patients between November 2003 and July 2007.28 All participants provided written informed consent for biomarker measurements. The study protocol was approved by the Partners Human Research Committee (the BWH Institutional Review Board: 2007P000894 and 2016P001542) and is in accordance with the principles of the Declaration of Helsinki.
Enrollment Criteria, Study Procedures, Selection of Time Points, and Data Collection
Inclusion criteria for the ICU cohort were 18 years or older and admission to a medical or surgical ICU with blood samples available within 72 hours of arrival to the ICU. Exclusion criteria were: (1) current receipt of RRT, (2) anticipated ICU stay less than 48 hours, (3) admission to the ICU for a low-risk condition such as airway monitoring or serial neurologic checks, (4) pregnancy, or (5) enrollment in a conflicting research study. ICU patients were screened and enrolled intermittently through the study period. A total of 128 patients were eligible from the medical and surgical ICUs at BWH and 120 patients were eligible from the ROCI cohort. Because worsening kidney function can increase plasma uric acid levels, we adjudicated the medical record of each potentially eligible patient and excluded anyone with clinical evidence of AKI upon study enrollment based on serum creatinine (Scr) values. Eligible patients with an increasing Scr level before or upon study enrollment who met criteria for AKI were excluded. Patients with an elevated Scr value without prior values were excluded if they experienced improvement in Scr levels to a nadir that would have qualified them to meet criteria for AKI. We excluded 40 patients from the medical/surgical ICUs at the BWH and ROCI cohort due to clinical evidence of AKI upon study enrollment, which resulted in 208 patients in the ICU cohort. We obtained baseline demographic information, comorbid conditions, and severity of illness upon enrollment assessed using the Acute Physiology and Chronic Health Evaluation II (APACHE II) score.29 All patients were followed up until hospital discharge, death, or day 28 after enrollment, whichever occurred first.
The ATN Study was an interventional trial of higher versus lower intensity RRT that included critically ill patients diagnosed with AKI-RRT and with failure of at least 1 nonrenal organ system or sepsis. The study excluded patients with: (1) CKD (defined as premorbid Scr > 2 mg/dL in men and > 1.5 mg/dL in women), (2) AKI clinically believed to be due to a cause other than acute tubular necrosis, (3) prior kidney transplantation, and (4) comfort measures only status. Full inclusion and exclusion criteria for the ATN Study are described elsewhere.28 Among the 1,124 ATN Study participants, blood samples were collected in 817 participants. Because receipt of RRT would lower plasma uric acid levels, we excluded 561 participants who received some form of RRT before randomization. Of the remaining samples, 6 were deemed unsatisfactory for measurement, resulting in 250 individuals for inclusion in this study.
We obtained baseline characteristics including demographic information along with other pertinent clinical and laboratory data upon enrollment in the ATN Study. Severity of illness was obtained upon enrollment using APACHE II score and Sequential Organ Failure Assessment (SOFA) cardiovascular score. In the ATN Study, all participants were followed up daily until hospital discharge, death, or day 28 after randomization, whichever occurred first. Outcomes were ascertained daily during hospitalization and at day 60 and 1 year using telephone and/or mail follow-up.
Blood Sample Collection and Laboratory Assays
In the ICU cohort, blood samples were collected in EDTA-containing vacutainers and were processed, aliquoted, and stored at −80°C within 4 hours of collection. In the ATN Study, blood samples were collected and immediately placed on ice and centrifuged at 4°C. They were separated into aliquots and stored frozen (−20°C) at the collection site until shipment. Batched samples were shipped on dry ice by commercial courier to the Massachusetts Veterans Epidemiology Research and Information Center (MAVERIC) Core Laboratory at the Veteran’s Affairs Boston Healthcare System. Samples were then stored at −80°C. Samples were shipped from MAVERIC on dry ice, thawed, and subaliquoted into 0.5-mL vials. Plasma uric acid measurements for the ICU cohort were performed at the laboratories of University of Washington (n = 115) and BWH (ROCI, n = 93). ATN Study samples were processed at the University of California San Diego. All uric acid assays were performed using the colorimetric peroxidase-coupled indirect equilibrium uricase method. The interassay coefficient of variation at each laboratory was <10%.
Clinical Outcomes
Authors A.S., R.P., D.E.L., and A.H. adjudicated outcomes by reviewing the medical record while still blinded to study measurements in the ICU cohort. The prespecified primary outcome was incident AKI (occurring after enrollment/arrival to the ICU). The secondary outcome was 90-day mortality. AKI was defined according to the Scr-based criteria established by the Kidney Disease: Improving Global Outcomes Work Group.30 Incident AKI was defined as a ≥ 0.3-mg/dL increase in enrollment Scr level over any 48-hour period during the first 7 days in the ICU or an increase in Scr level ≥ 1.5 times the enrollment Scr level within 7 days. AKI stage was defined as follows: stage 1, increase in Scr level of 1.5 to 1.9 times the enrollment Scr level or absolute increase ≥ 0.3 mg/dL; stage 2, increase in Scr level of 2.0 to 2.9 times the enrollment Scr level; and stage 3, increase in Scr level of ≥ 3.0 times enrollment, an absolute increase in Scr level ≥ 0.5 mg/dL to a level ≥ 4.0 mg/dL, or initiation of RRT. Urine output data were not available for all patients.
In the ATN Study cohort, the primary outcome was 60-day mortality. Secondary outcomes were 28-day mortality, duration of RRT (RRT-free days), and recovery of kidney function among survivors through day 28. To avoid the competing risk for death, RRT-free days were calculated as 28 minus the number of days requiring RRT assuming survival to 28 days. Any participant who died before 28 days was assigned a score of zero.31, 32, 33
Statistical Analysis
Descriptive statistics were summarized and presented as frequencies, mean ± standard deviation, or median with interquartile range (IQR). We assessed all variables for normality and log-transformed as appropriate. Plasma uric acid level was examined as a continuous variable (log base 2, so that interpretation would be per doubling of the exposure) and categorized as tertiles. We assessed the association between plasma uric acid levels and 2-group comparisons using Wilcoxon rank sum test and Kruskal-Wallis test for multiple group comparisons. We used Spearman correlation to assess the association between plasma uric acid levels and continuous variables. We used χ2 tests to compare plasma uric acid tertiles with categorical variables, and analysis of variance or Kruskal-Wallis tests for normally or non-normally distributed continuous variables, respectively. To assess for differences between the original ATN Study cohort and the subset of patients without RRT before randomization, we used t test for normal continuous data, Wilcoxon rank sum test for non-normal continuous data, and χ2 or Fisher exact test for categorical data. We evaluated predictors of plasma uric acid level in the ATN Study cohort using linear regression.
We fit multivariable logistic regression models to examine associations between plasma uric acid levels and the dichotomous outcomes of incident AKI, mortality, and renal recovery. Model results are reported as odds ratio (OR) with 95% confidence interval (CI). We used a multivariable negative binomial regression model for the outcome of RRT-free days. Model results are reported as relative number of RRT-free days with 95% CI. We assessed for confounding by iteratively generating increasingly adjusted models. All comparisons were 2 tailed, with P < 0.05 considered significant. All statistical analyses were performed using SAS software (version 9.4; SAS Institute Inc.).
Results
Characteristics of Study Participants
In the ICU cohort, mean age was 61.8 ± 15.3 years, 94 (45.2%) were women, and 177 (85.1%) were white. In the ATN Study, mean age was 62.0 ± 14.6 years, 77 (30.8%) were women, and 199 (79.6%) were white. The primary cause of AKI was multifactorial in 18 of 40 (45.0%) patients with AKI in the ICU cohort and in 135 (54.0%) participants in the ATN Study cohort. Additional baseline characteristics for the ICU and ATN Study cohorts are in Tables 1 and 2, respectively. Tables S1 and S2 show baseline characteristics for the ICU and ATN Study cohorts by their primary outcomes, respectively. Compared with characteristics of participants in the parent ATN Study not included in this study (n = 874), participants in the subcohort with plasma uric acid measurements were older, were more commonly white, required less mechanical ventilation, and had higher urine volume, blood urea nitrogen, and Scr values before RRT initiation (Table S3).
Table 1.
Baseline Characteristics of the ICU Cohort by Uric Acid Tertiles
| Characteristic | All Patients (n = 208) | Tertile 1 (n = 67) | Tertile 2 (n = 72) | Tertile 3 (n = 69) |
|---|---|---|---|---|
| Plasma uric acid,a mg/dL | 4.7 [3.6-6.4] (1.5-20.3) | 1.5-3.9 | 4.0-5.8 | 5.9-20.3 |
| Demographics | ||||
| Female sex | 94 (45.2%) | 26 (38.8%) | 35 (48.6%) | 33 (47.8%) |
| Age, y | 61.8 ± 15.3 | 57.6 ± 15.4 | 63.4 ± 15.3 | 64.2 ± 14.4 |
| White | 177 (85.1%) | 61 (91.0%) | 61 (84.7%) | 55 (79.7%) |
| Enrollment characteristics | ||||
| Scr, mg/dL | 0.8 [0.6-1.0] | 0.7 [0.5-0.8] | 0.8 [0.6-1.0] | 0.9 [0.8-1.1] |
| eGFR, mL/min/1.73 m2 | 91.1 ± 26.6 | 102.2 ± 23.6 | 90.4 ± 26.1 | 81.1 ± 26.0 |
| APACHE II score | 17 [12-24] | 16 [11-22] | 16 [12-23] | 18 [13-27] |
| Sepsis | 113 (54.3%) | 42 (62.7%) | 30 (41.7%) | 41 (59.4%) |
| AKI causeb | 40 (19.2%) | 9 (13.4%) | 12 (16.7%) | 19 (27.5%) |
| Multifactorial | 18 (45.0%) | 2 (22.2%) | 6 (50.0%) | 10 (52.6%) |
| Ischemic | 9 (22.5%) | 2 (22.2%) | 3 (25.0%) | 4 (21.0%) |
| Prerenal | 6 (15.0%) | 3 (33.3%) | 2 (16.7%) | 1 (5.3%) |
| Sepsis | 5 (12.5%) | 2 (22.2%) | 1 (8.3%) | 2 (10.5%) |
| Other | 2 (5.0%) | 0 (0%) | 0 (0%) | 2 (10.5%) |
| Comorbid conditions | ||||
| Active malignancy | 84 (40.4%) | 28 (41.8%) | 32 (44.4%) | 24 (34.8%) |
| Chronic lung disease | 49 (23.6%) | 14 (20.9%) | 16 (22.2%) | 19 (27.5%) |
| Diabetes mellitus | 44 (21.2%) | 8 (11.9%) | 10 (13.9%) | 26 (37.7%) |
| Congestive heart failure | 12 (5.8%) | 2 (3.0%) | 2 (2.8%) | 8 (11.6%) |
| Chronic liver disease | 10 (4.8%) | 4 (6.0%) | 3 (4.2%) | 3 (4.4%) |
| ICU type | ||||
| Medical | 112 (53.9%) | 26 (38.8%) | 37 (51.4%) | 49 (71.0%) |
| Surgical | 96 (46.1%) | 41 (61.2%) | 35 (48.6%) | 20 (29.0%) |
Note: Data presented as mean ± standard deviation or median [IQR] for continuous variables and frequencies for binary or categorical variables. eGFR was determined using the Chronic Kidney Disease Epidemiology Collaboration equation. Conversion factors for units: Scr in mg/dL to μmol/L, ×88.4; plasma uric acid in mg/dL to μmol/L, ×59.48.
Abbreviations: AKI, acute kidney injury; APACHE, Acute Physiology and Chronic Health Evaluation; eGFR, estimated glomerular filtration rate; ICU, intensive care unit; IQR, interquartile range; KDIGO, Kidney Disease: Improving Global Outcomes; Scr, serum creatinine.
Plasma uric acid presented as median [IQR] with range for all patients and range for each tertile.
Percentages are per total KDIGO stage 1 or greater AKI in all patients and per tertile.
Table 2.
Baseline Characteristics of ATN Study Participants by Uric Acid Tertiles
| Characteristic | All Participants (n = 250) | Tertile 1 (n = 83) | Tertile 2 (n = 83) | Tertile 3 (n = 84) |
|---|---|---|---|---|
| Plasma uric acid,a mg/dL | 11.1 [8.6-14.2] (3.3-29.4) | 3.3-9.4 | 9.5-12.9 | 13.0-29.4 |
| Demographics | ||||
| Female sex | 77 (30.8%) | 31 (37.4%) | 25 (30.1%) | 21 (25.0%) |
| Age, y | 62.0 ± 14.6 | 65.5 ± 13.1 | 60.5 ± 15.7 | 59.9 ± 14.5 |
| White | 199 (79.6%) | 67 (80.7%) | 67 (80.7%) | 65 (77.4%) |
| Premorbid characteristics | ||||
| Scr, mg/dL | 1.1 [0.8-1.4] | 1.0 [0.8-1.3] | 1.1 [0.8-1.4] | 1.1 [0.9-1.4] |
| eGFR, mL/min/1.73 m2 | 69.1 ± 32.8 | 72.1 ± 32.7 | 68.5 ± 33.7 | 66.8 ± 32.3 |
| Weight, kg | 76.4 ± 15.6 | 73.1 ± 17.3 | 76.5 ± 13.4 | 79.5 ± 15.4 |
| Comorbid conditionsb | ||||
| Cardiovascular disease | 82 (32.8%) | 30 (36.1%) | 21 (25.3%) | 31 (36.9%) |
| Diabetes mellitus | 72 (28.8%) | 20 (24.1%) | 23 (27.7%) | 29 (34.5%) |
| Malignancy | 50 (20.0%) | 15 (18.1%) | 17 (20.5%) | 18 (21.4%) |
| Immunocompromised | 35 (14.0%) | 11 (13.3%) | 11 (13.3%) | 13 (15.5%) |
| Cerebrovascular disease | 29 (11.6%) | 8 (9.6%) | 9 (10.8%) | 12 (14.3%) |
| Chronic hypoxemia | 25 (10.0%) | 11 (13.3%) | 4 (4.8%) | 10 (11.9%) |
| Enrollment characteristics | ||||
| BUN before RRT, mg/dL | 69.5 ± 30.1 | 62.8 ± 31.3 | 67.1 ± 26.5 | 78.5 ± 30.3 |
| Scr before RRT, mg/dL | 4.1 [3.0-5.6] | 3.6 [2.7-4.7] | 4.2 [3.1-5.4] | 4.7 [3.7-6.3] |
| MAP, mm Hg | 76.0 ± 15.4 | 74.8 ± 15.3 | 77.7 ± 15.4 | 75.6 ± 15.5 |
| Urine volume, mL/24 h | 195 [72-545] | 155 [70-455] | 255 [107-532] | 210 [62-638] |
| Urine volume, mL/h | 10.9 [4.2-26.0] | 8.1 [4.9-19.6] | 12.2 [5.3-26.3] | 10.6 [2.8-29.2] |
| Oliguria | 189 (75.6%) | 66 (79.5%) | 63 (75.9%) | 60 (71.4%) |
| Mechanical ventilation | 189 (75.6%) | 65 (78.3%) | 62 (74.7%) | 62 (73.8%) |
| APACHE II score | 26 [21-31] | 25 [21-31] | 27 [22-32] | 26 [22-31] |
| SOFA cardiovascular score | ||||
| 0-2 | 102 (40.8%) | 30 (36.1%) | 34 (41.0%) | 38 (45.2%) |
| 3-4 | 148 (59.2%) | 53 (63.9%) | 49 (59.0%) | 46 (54.8%) |
| Treatment | ||||
| Intensive | 122 (48.8%) | 45 (54.2%) | 35 (42.2%) | 42 (50.0%) |
| Less intensive | 128 (51.2%) | 38 (45.8%) | 48 (57.8%) | 42 (50.0%) |
| ICU type | ||||
| Medical | 122 (48.8%) | 36 (43.4%) | 43 (51.8%) | 43 (51.2%) |
| Surgical | 108 (43.2%) | 38 (45.8%) | 35 (42.2%) | 35 (41.7%) |
| Other | 20 (8.0%) | 9 (10.8%) | 5 (6.0%) | 6 (7.1%) |
| Postsurgical | 126 (50.4%) | 47 (56.6%) | 43 (51.8%) | 36 (42.9%) |
| Cause of AKIc | ||||
| Ischemic | 198 (79.2%) | 66 (79.5%) | 66 (79.5%) | 66 (78.6%) |
| Nephrotoxic | 48 (19.2%) | 16 (19.2%) | 15 (18.1%) | 17 (20.2%) |
| Sepsis | 132 (52.8%) | 41 (49.4%) | 49 (59.0%) | 42 (50.0%) |
| Multifactorial | 135 (54.0%) | 39 (47.0%) | 45 (54.2%) | 51 (60.7%) |
Note: Data presented as mean ± standard deviation or median [IQR] for continuous variables and frequencies for binary or categorical variables. eGFR was determined using the Modification of Diet in Renal Disease Study equation. Conversion factors for units: Scr in mg/dL to μmol/L, ×88.4; plasma uric acid in mg/dL to μmol/L, ×59.48; BUN in mg/dL to mmol/L, ×0.357.
Abbreviations: APACHE, Acute Physiology and Chronic Health Evaluation; ATN, Acute Renal Failure Trial Network; AKI, acute kidney injury; BUN, blood urea nitrogen; eGFR, estimated glomerular filtration rate; ICU, intensive care unit; IQR, interquartile range; MAP, mean arterial pressure; RRT, renal replacement therapy; Scr, serum creatinine; SOFA, Sequential Organ Failure Assessment.
Plasma uric acid presented as median [IQR] with range for all participants and range for each tertile.
Cardiovascular disease includes participants with a history of myocardial infarction, angina, or congestive heart failure. Diabetes mellitus includes participants with a history of end-organ disease from diabetes, use of diabetic diet, or use of diabetic medications. Malignancy includes participants with a history of leukemia and tumors with or without metastasis. Immunocompromised includes participants with a history of human immunodeficiency virus infection, AIDS, or on immunosuppressive therapy. Cerebrovascular disease includes participants with a history of transient ischemic attack or stroke.
Participants could have had more than 1 reason for AKI.
Baseline Plasma Uric Acid Levels
Median enrollment plasma uric acid level was 4.7 mg/dL with a range of 1.5 to 20.3 mg/dL in the ICU cohort. In the ATN Study, median plasma uric acid level before RRT was 11.1 mg/dL with a range of 3.3 to 29.4 mg/dL.
Factors Associated With Plasma Uric Acid Levels
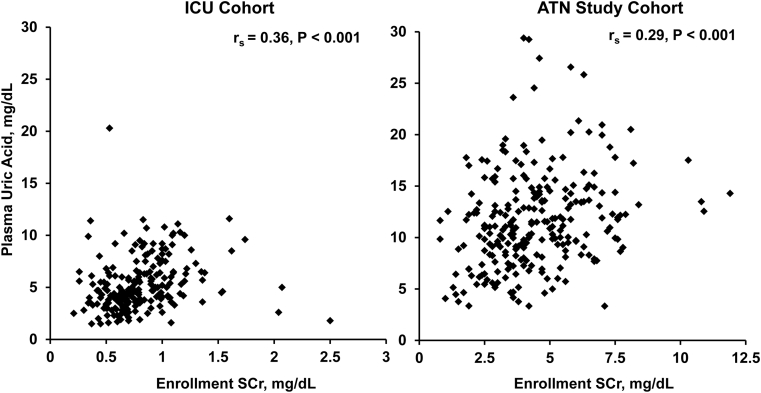
There were no differences between plasma uric acid levels by sex, race, or ICU setting in the ICU or ATN Study cohorts. Plasma uric acid levels were higher in patients with a history of diabetes mellitus (6.4 [IQR, 4.2-9.2] vs 4.5 [IQR, 3.5-6.0] mg/dL; P < 0.001) and congestive heart failure (8.0 [IQR, 5.4-10.0] vs 4.6 [IQR, 3.6-6.3] mg/dL; P = 0.007) in the ICU cohort. Plasma uric acid level had a positive correlation with APACHE II scores in the ICU cohort (rs = 0.19; P = 0.007). In the ATN Study cohort, there were no significant differences in plasma uric acid levels by treatment arm, SOFA cardiovascular score, or presence of oliguria. Age was associated with plasma uric acid levels in the ICU cohort (rs = 0.19; P = 0.006) and ATN Study participants (rs = −0.13; P = 0.03). In the ICU cohort, plasma uric acid levels positively correlated with enrollment Scr levels (rs = 0.36; P < 0.001). In the ATN Study cohort, plasma uric acid levels had a positive correlation with enrollment blood urea nitrogen levels (rs = 0.22; P < 0.001), enrollment Scr levels (rs = 0.29; P < 0.001), and change in Scr level from admission to enrollment (rs = 0.25; P < 0.001). Figure 1 demonstrates the correlation between enrollment Scr and plasma uric acid levels in both cohorts. Table S4 demonstrates unadjusted determinants of plasma uric acid levels in the ATN Study cohort.
Figure 1.
Association between plasma uric acid and serum creatinine (SCr) levels. Scatterplots of enrollment plasma uric acid concentrations and their association with enrollment SCr levels in the intensive care unit (ICU) cohort (rs = 0.36; P < 0.001), and Acute Renal Failure Trial Network (ATN) Study cohort (rs = 0.29; P < 0.001).
Association of Plasma Uric Acid With Study Outcomes
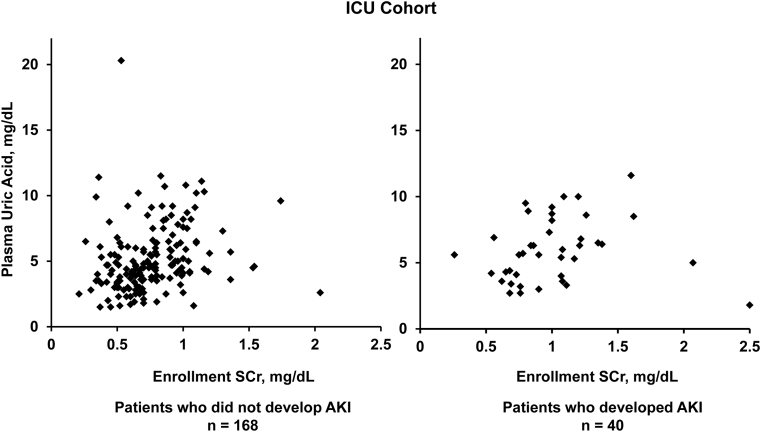
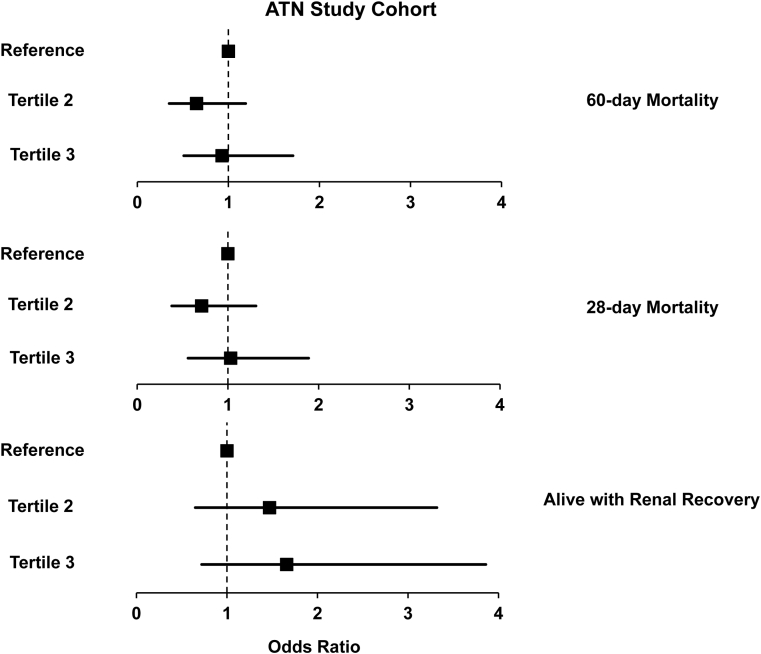
In the ICU cohort, 40 (19.2%) patients developed AKI (52.5% had stage 2 or 3), and 52 (25.0%) patients reached the secondary outcome of 90-day mortality. Plasma uric acid levels were higher upon enrollment in patients who developed AKI compared with patients who did not (5.7 [IQR, 4.1-7.8] vs 4.6 [IQR, 3.5-6.2] mg/dL; P = 0.03; Fig 2). In the ATN Study cohort, 125 (50.0%) participants died at 60 days, 108 (43.2%) died at 28 days, and 65 (26.0%) who survived to day 28 experienced renal recovery. Mean number of RRT-free days was 6.7 ± 8.9. There were no differences in mortality or renal recovery by tertiles of plasma uric acid levels (Fig 3). Tables 3 and 4 demonstrate unadjusted and adjusted associations of plasma uric acid levels with the renal and mortality outcomes, respectively.
Figure 2.
Scatterplots of plasma uric acid levels in patients with and without acute kidney injury (AKI) in the intensive care unit (ICU) cohort. Plasma uric acid levels were higher in patients who developed AKI (5.7 [IQR, 4.1-7.8] mg/dL) compared with patients who did not (4.6 [IQR, 3.5-6.2] mg/dL; P = 0.03).
Figure 3.
Association of plasma uric acid levels with mortality and renal recovery in the Acute Renal Failure Trial Network (ATN) Study cohort. Unadjusted association between plasma uric acid levels and adverse clinical outcomes. There were no differences in 60-day mortality, 28-day mortality, or alive with renal recovery through day 28 between the groups. Reference is tertile 1 and refers to plasma uric acid level range: 3.3 to 9.4 mg/dL; tertile 2: 9.4 to 12.9 mg/dL; tertile 3: 12.9 to 29.4 mg/dL. For 60-day mortality OR with (95% confidence interval [CI]), tertile 3: 0.93 (0.51-1.71), tertile 2: 0.65 (0.35-1.19); For 28-day mortality OR with (95% CI), tertile 3: 1.03 (0.56-1.89), tertile 2: 0.71 (0.38-1.31); For alive with renal recovery OR with (95% CI), tertile 3: 1.66 (0.72-3.86), tertile 2: 1.47 (0.65-3.32).
Table 3.
Association of Uric Acid With Renal Outcomes in the ICU and ATN Study Cohorts
| Outcome | No. of Events | Unadjusted | P | Adjusted | P |
|---|---|---|---|---|---|
| ICU | |||||
| Incident AKIa | 40 | 1.78 (1.03-3.05) | 0.04 | 1.50 (0.80-2.81) | 0.2 |
| ATN Study | |||||
| RRT-free daysb | — | 1.12 (0.70-1.79) | 0.7 | 1.07 (0.67-1.72) | 0.8 |
| Renal recoveryb | 65 | 1.40 (0.79-2.47) | 0.2 | 1.05 (0.54-2.04) | 0.9 |
Note: Plasma uric acid levels are log2 transformed. Measures of association presented as odds ratio with (95% CI) for dichotomous outcomes and relative number of RRT-free days with (95% CI).
Abbreviations: AKI, acute kidney injury; APACHE, Acute Physiology and Chronic Health Evaluation; ATN, Acute Renal Failure Trial Network; CI, confidence interval; ICU, intensive care unit; RRT, renal replacement therapy.
Adjusted for age, diabetes mellitus, APACHE II score, and enrollment serum creatinine level.
Adjusted for age, randomization arm (high vs less intensity), natural log urine output, blood urea nitrogen level at enrollment, APACHE II score, and Sequential Organ Failure Assessment cardiovascular score.
Table 4.
Association of Uric Acid With Mortality in the ICU and ATN Study Cohorts
| Outcome | Events | Unadjusted | P | Adjusted | P |
|---|---|---|---|---|---|
| ICU | |||||
| 90-d mortalitya | 52 | 1.16 (0.72-1.87) | 0.5 | 0.98 (0.57-1.67) | 0.9 |
| ATN Study | |||||
| 60-d mortalityb | 125 | 1.04 (0.69-1.57) | 0.9 | 1.15 (0.71-1.86) | 0.6 |
| 28-d mortalityb | 108 | 1.09 (0.72-1.65) | 0.7 | 1.16 (0.72-1.88) | 0.5 |
Note: Plasma uric acid levels are log2 transformed. Measures of association presented as odds ratio with (95% CI) for dichotomous outcomes. Abbreviations: APACHE, Acute Physiology and Chronic Health Evaluation; ATN, Acute Renal Failure Trial Network; CI, confidence interval; ICU, intensive care unit; RRT, renal replacement therapy.
Adjusted for age, diabetes mellitus, APACHE II score, and enrollment serum creatinine level.
Adjusted for age, randomization arm (high vs less intensity), natural log urine output, blood urea nitrogen level at enrollment, APACHE II score, and Sequential Organ Failure Assessment cardiovascular score.
In the ICU cohort, higher plasma uric acid levels were associated with incident AKI (OR, 1.78; 95% CI, 1.03-3.05) but not 90-day mortality in unadjusted analyses (OR, 1.16; 95% CI, 0.72-1.87). After multivariable adjustment for age, diabetes mellitus, APACHE II score, and enrollment Scr level, plasma uric acid level was no longer associated with incident AKI (adjusted OR, 1.50; 95% CI, 0.80-2.81). Adjustment for enrollment Scr level alone was sufficient to render the association nonsignificant (adjusted OR, 1.49; 95% CI, 0.83-2.66).
In the ATN Study cohort, plasma uric acid levels were not associated with the primary outcome of 60-day mortality in unadjusted models. Adjustment for age, randomization arm (intensive vs less intensive RRT), urine output, enrollment blood urea nitrogen level, SOFA cardiovascular score, and APACHE II score did not change the association with 60-day mortality (adjusted OR, 1.15; 95% CI, 0.71-1.86). Similarly, there was no association between plasma uric acid levels and 28-day mortality (adjusted OR, 1.16; 95% CI, 0.72-1.88) or renal recovery (adjusted OR, 1.05; 95% CI, 0.54-2.04) among survivors through day 28 or change in RRT-free days (incident rate ratio, 1.07; 95% CI, 0.67-1.72) after multivariable adjustment. Substitution of estimated glomerular filtration rate (GFR) for Scr level in the multivariable adjustment did not fundamentally change results for either cohort.
Discussion
In this study involving 2 cohorts of critically ill patients, we were unable to identify an association between uric acid level and subsequent risk for AKI or its complications. In the ICU cohort, higher plasma uric acid levels were associated with incident AKI in unadjusted models, but this effect was attenuated and no longer significant after multivariable adjustment. We found no statistically significant association between plasma uric acid levels upon admission to the ICU and 90-day mortality. In the ATN Study cohort, there was no statistically significant association between plasma uric acid levels and mortality, renal recovery, or duration of RRT in individuals with AKI-RRT.
Our results do not support the hypothesis that uric acid level predisposes to AKI in ICU patients or mortality in patients with established severe AKI. Preclinical evidence in favor of this hypothesis derives from both in vitro and in vivo studies, which established several potential mechanisms of kidney injury from uric acid, including afferent arteriole vasoconstriction,34, 35 decreased renal blood flow due to endothelial dysfunction, oxidative stress,36, 37, 38 and augmentation of the inflammatory response.34 Our results also conflict with a number of published studies on the association between uric acid level and the development of AKI or risk for death.39 Higher uric acid levels have been reported to predict the risk for AKI in hospitalized patients40, 41, 42 and after cardiac surgery,16, 17, 18 with some reports of a J-shaped association showing higher risk at both very low and high uric acid levels.43 In the Atherosclerosis Risk in Communities Study, higher uric acid levels predicted risk for a hospital stay with AKI during a mean follow-up of 12 years, although results from Mendelian randomization analyses did not support a causal association.44 Prior retrospective studies in cardiac surgery have found increased risk for postoperative AKI in patients with higher preoperative uric acid levels.16, 17, 43 However, these prior studies in cardiac surgery adjust for kidney function as a dichotomous variable (estimated GFR < 60 mL/min/1.73 m2), whereas adjustment as a continuous variable may address confounding by kidney function more appropriately. A recent prospective study in 247 patients undergoing cardiac surgery demonstrated that patients with preoperative uric acid levels ≥ 373 μmol/L (≥ 6.3 mg/dL) were at greater than 5-fold increased risk for the development of postoperative AKI even after adjustment for preoperative Scr level. However, this cohort had a relatively low event rate (12.1%) and also had limited power for the multivariable analysis.18 A prior prospective study of 144 critically ill patients suggested that higher plasma uric acid levels were associated with a secondary outcome of incident AKI by univariate analysis, but it is not clear whether individuals already had AKI upon ICU admission.42
Our prospective study investigated whether higher plasma uric acid levels increased risk for the development of AKI and mortality in high-risk critically ill patients admitted to the ICU. We measured plasma uric acid within 72 hours of ICU admission in the ICU cohort and adjudicated the medical records to exclude patients with clinical evidence of AKI upon enrollment to reduce confounding by already worsening GFR in the setting of AKI. Despite reasonably well-preserved kidney function upon enrollment, the association of plasma uric acid level with incident AKI was still confounded by Scr level.
To our knowledge, this is the first study to evaluate the association of plasma uric acid level with mortality, renal recovery, and duration of RRT in patients with severe AKI requiring RRT. Few studies have evaluated the association of uric acid level with mortality in critically ill patients. In a study of 1,140 consecutive patients undergoing coronary artery bypass grafting, Hillis et al45 found 1.5-fold increased risk for all-cause mortality per 1.68-mg/dL increase in uric acid level over a median follow-up of 4.5 years. In the ATN Study cohort, plasma uric acid levels were significantly higher than the normal range. Despite the atypically high plasma uric acid levels and high event rates, we did not detect an association between plasma uric acid levels and the study outcomes even in unadjusted models. Severe AKI likely accounts for the high plasma uric acid levels, but a high inflammatory or hypercatabolic state in critically ill patients could also have contributed.
A recent scientific workshop reviewed the existing evidence for the association between uric acid levels and adverse outcomes in CKD and AKI. They suggested that there was insufficient evidence to recommend routine treatment of asymptomatic hyperuricemia in patients with hypertension, kidney disease, or diabetes mellitus to reduce the risk for AKI. The authors also called for more evidence from well-designed adequately powered clinical trials.46 Two small studies tested whether lowering plasma uric acid levels may be beneficial in reducing the risk for AKI after cardiac surgery and found no benefit when treated with preoperative allopurinol plus vitamin E or rasburicase (recombinant uricase), respectively.47, 48
We acknowledge that this study has several limitations. The single-center recruitment in the ICU cohort, heterogeneity of ICU patients, and lack of racial diversity in both cohorts limit the generalizability of the study results to all critically ill patients. We were unable to account for volume overload in our studies, which could dilute plasma uric acid levels. This may have been most pertinent in the ATN Study cohort because patients with AKI-RRT may develop volume overload with declining urine output. In addition, the modest sample size may have led to wide confidence intervals around the point estimates for the study outcomes, which did not reach significance. Therefore, we cannot exclude the possibility that we were unable to detect a potentially meaningful association between higher plasma uric acid levels and adverse clinical outcomes.
In summary, higher plasma uric acid levels measured in critically ill patients upon ICU admission and before RRT initiation were not observed to be statistically associated with the development of AKI or adverse clinical outcomes after AKI-RRT, respectively.
Article Information
Authors’ Full Names and Academic Degrees
Anand Srivastava, MD, MPH, Ragnar Palsson, MD, David E. Leaf, MD, MMSc, Angelica Higuera, MD, Margaret E. Chen, BS, Polly Palacios, MSPH, Rebecca M. Baron, MD, Venkata Sabbisetti, PhD, Andrew N. Hoofnagle, MD, PhD, Sucheta M. Vaingankar, PhD, Paul M. Palevsky, MD, and Sushrut S. Waikar, MD, MPH.
Authors’ Contributions
Study design: AS, SSW; data acquisition: all authors; data analysis: AS, SSW; data interpretation: all authors. Each author also contributed important intellectual content during manuscript drafting or revision and ensured that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Support
This work was supported by National Institutes of Health (NIH) grant F32DK111066 (Dr Srivastava). Dr Palsson is supported by an American Society of Nephrology Research Fellowship Grant. Dr Leaf is supported by NIH grant K23DK106448, and Dr Waikar is supported by NIH grants U01DK085660, U01DK104308, R01DK103784, and UG3DK114915.
Financial Disclosure
Dr Srivastava has been a consultant for Horizon Pharma. Dr Palevsky has been a consultant for GE Healthcare, Baxter, Novartis, Healthspan Dx, and Durect. Dr Waikar received consulting fees for serving on the data safety monitoring board for Takeda, for trials involving uric acid–lowering agents; and consulting fees from the National Institutes of Health for serving as a medical monitor for a trial involving inosine to raise uric acid levels. The remaining authors declare that they have no relevant financial interests.
Acknowledgements
We thank the ATN Study Investigators and National Institute of Diabetes and Digestive Diseases (NIDDK) data repository for the data used in this study; the following members of the ROCI who helped assemble this cohort: Drs Paul Dieffenbach, Samuel Ash, Laura Fredenburgh, and Joshua Englert; and the members of the Waikar Laboratory group at BWH for invaluable work.
Disclaimer
The ATN Study was performed by the ATN Study Investigators and supported by the Cooperative Studies Program of the Department of Veterans Affairs (VA) Office of Research and Development and the NIDDK. This report does not necessarily reflect the opinions or views of the ATN Study investigators, VA, or NIDDK. The funders of this study had no role in the study design; collection, analysis, or interpretation of data; drafting of the manuscript; or decision to submit the manuscript for publication.
Prior Presentation
Part of this work was presented as a poster at the American Society of Nephrology Scientific Session in November 17, 2016, Chicago, IL.
Peer Review
Received November 1, 2018. Evaluated by 2 external peer reviewers, with direct editorial and statistical input from an Associate Editor and the Editor-in-Chief. Accepted in revised form January 2, 2019.
Footnotes
Complete author and article information provided before references.
Table S1: Baseline Characteristics of the ICU Cohort by AKI Status.
Table S2: Baseline Characteristics of ATN Study Participants by 60-Day Mortality.
Table S3: Baseline Characteristics in the ATN Study: Participants Not in Subcohort vs Subcohort Included in This Study.
Table S4: Unadjusted Predictors of Uric Acid in the ATN Study Cohort.
Supplementary Material
Table S1. Baseline Characteristics of the ICU Cohort by AKI Status.
Table S2. Baseline Characteristics of ATN Study Participants by 60-Day Mortality.
Table S3. Baseline Characteristics in the ATN Study: Participants Not in Subcohort vs Subcohort Included in This Study.
Table S4. Unadjusted Predictors of Uric Acid in the ATN Study Cohort.
References
- 1.Chertow G.M., Christiansen C.L., Cleary P.D., Munro C., Lazarus J.M. Prognostic stratification in critically ill patients with acute renal failure requiring dialysis. Arch Intern Med. 1995;155(14):1505–1511. [PubMed] [Google Scholar]
- 2.Bagshaw S.M., Laupland K.B., Doig C.J. Prognosis for long-term survival and renal recovery in critically ill patients with severe acute renal failure: a population-based study. Crit Care. 2005;9(6):R700–R709. doi: 10.1186/cc3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uchino S., Kellum J.A., Bellomo R. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294(7):813–818. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- 4.Waikar S.S., Liu K.D., Chertow G.M. Diagnosis, epidemiology and outcomes of acute kidney injury. Clin J Am Soc Nephrol. 2008;3(3):844–861. doi: 10.2215/CJN.05191107. [DOI] [PubMed] [Google Scholar]
- 5.Waikar S.S., Curhan G.C., Wald R., McCarthy E.P., Chertow G.M. Declining mortality in patients with acute renal failure, 1988 to 2002. J Am Soc Nephrol. 2006;17(4):1143–1150. doi: 10.1681/ASN.2005091017. [DOI] [PubMed] [Google Scholar]
- 6.Wonnacott A., Meran S., Amphlett B., Talabani B., Phillips A. Epidemiology and outcomes in community-acquired versus hospital-acquired AKI. Clin J Am Soc Nephrol. 2014;9(6):1007–1014. doi: 10.2215/CJN.07920713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wald R. Chronic dialysis and death among survivors of acute kidney injury requiring dialysis. JAMA. 2009;302(1):1179–1185. doi: 10.1001/jama.2009.1322. [DOI] [PubMed] [Google Scholar]
- 8.Schiffl H., Lang S.M., Fischer R. Long-term outcomes of survivors of ICU acute kidney injury requiring renal replacement therapy: a 10-year prospective cohort study. Clin Kidney J. 2012;5(4):297–302. doi: 10.1093/ckj/sfs070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Masuo K., Kawaguchi H., Mikami H., Ogihara T., Tuck M.L. Serum uric acid and plasma norepinephrine concentrations predict subsequent weight gain and blood pressure elevation. Hypertension. 2003;42(4):474–480. doi: 10.1161/01.HYP.0000091371.53502.D3. [DOI] [PubMed] [Google Scholar]
- 10.Bos M.J., Koudstaal P.J., Hofman A., Witteman J.C., Breteler M.M. Uric acid is a risk factor for myocardial infarction and stroke: the Rotterdam study. Stroke. 2006;37(6):1503–1507. doi: 10.1161/01.STR.0000221716.55088.d4. [DOI] [PubMed] [Google Scholar]
- 11.Feig D.I., Kang D.H., Johnson R.J. Uric acid and cardiovascular risk. N Engl J Med. 2008;359(17):1811–1821. doi: 10.1056/NEJMra0800885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cirillo P., Sato W., Reungjui S. Uric acid, the metabolic syndrome, and renal disease. J Am Soc Nephrol. 2006;17(12 suppl 3):S165–S168. doi: 10.1681/ASN.2006080909. [DOI] [PubMed] [Google Scholar]
- 13.Chonchol M., Shlipak M.G., Katz R. Relationship of uric acid with progression of kidney disease. Am J Kidney Dis. 2007;50(2):239–247. doi: 10.1053/j.ajkd.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 14.Mendi M.A., Afsar B., Oksuz F. Uric acid is a useful tool to predict contrast-induced nephropathy. Angiology. 2017;68(7):627–632. doi: 10.1177/0003319716639187. [DOI] [PubMed] [Google Scholar]
- 15.Kanbay M., Solak Y., Afsar B. Serum uric acid and risk for acute kidney injury following contrast. Angiology. 2017;68(2):132–144. doi: 10.1177/0003319716644395. [DOI] [PubMed] [Google Scholar]
- 16.Joung K.W., Jo J.Y., Kim W.J. Association of preoperative uric acid and acute kidney injury following cardiovascular surgery. J Cardiothorac Vasc Anesth. 2014;28(6):1440–1447. doi: 10.1053/j.jvca.2014.04.020. [DOI] [PubMed] [Google Scholar]
- 17.Lee E.H., Choi J.H., Joung K.W. Relationship between serum uric acid concentration and acute kidney injury after coronary artery bypass surgery. J Korean Med Sci. 2015;30(10):1509–1516. doi: 10.3346/jkms.2015.30.10.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaufeld T., Foerster K.A., Schilling T. Preoperative serum uric acid predicts incident acute kidney injury following cardiac surgery. BMC Nephrol. 2018;19(1):161. doi: 10.1186/s12882-018-0970-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hahn K., Kanbay M., Lanaspa M.A., Johnson R.J., Ejaz A.A. Serum uric acid and acute kidney injury: a mini review. J Adv Res. 2017;8(5):529–536. doi: 10.1016/j.jare.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rastegar A., Thier S. The physiologic approach to hyperuricemia. N Engl J Med. 1972;286(9):470–476. doi: 10.1056/NEJM197203022860907. [DOI] [PubMed] [Google Scholar]
- 21.Howard S., Jones D., Pui C.-H. The tumor lysis syndrome. N Engl J Med. 2011;364(19):1844–1854. doi: 10.1056/NEJMra0904569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou Y., Fang L., Jiang L. Uric acid induces renal inflammation via activating tubular NF-kappaB signaling pathway. PLoS One. 2012;7(6):e39738. doi: 10.1371/journal.pone.0039738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kang D.H., Park S.K., Lee I.K., Johnson R.J. Uric acid-induced C-reactive protein expression: implication on cell proliferation and nitric oxide production of human vascular cells. J Am Soc Nephrol. 2005;16(12):3553–3562. doi: 10.1681/ASN.2005050572. [DOI] [PubMed] [Google Scholar]
- 24.Rabadi M.M., Kuo M.C., Ghaly T. Interaction between uric acid and HMGB1 translocation and release from endothelial cells. Am J Physiol Renal Physiol. 2012;302(6):F730–F741. doi: 10.1152/ajprenal.00520.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu M.A., Sanchez-Lozada L.G., Johnson R.J., Kang D.H. Oxidative stress with an activation of the renin-angiotensin system in human vascular endothelial cells as a novel mechanism of uric acid-induced endothelial dysfunction. J Hypertens. 2010;28(6):1234–1242. [PubMed] [Google Scholar]
- 26.Corry D.B., Eslami P., Yamamoto K., Nyby M.D., Makino H., Tuck M.L. Uric acid stimulates vascular smooth muscle cell proliferation and oxidative stress via the vascular renin-angiotensin system. J Hypertens. 2008;26(2):269–275. doi: 10.1097/HJH.0b013e3282f240bf. [DOI] [PubMed] [Google Scholar]
- 27.Dolinay T., Kim Y.S., Howrylak J. Inflammasome-regulated cytokines are critical mediators of acute lung injury. Am J Respir Crit Care Med. 2012;185(11):1225–1234. doi: 10.1164/rccm.201201-0003OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palevsky P.M., Zhang J.H., O'Connor T.Z. Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med. 2008;359(1):7–20. doi: 10.1056/NEJMoa0802639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knaus W., Draper E., Wagner D., Zimmerman J. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–829. [PubMed] [Google Scholar]
- 30.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group KDIGO clinical practice guidelines for acute kidney injury. Kidney Int Suppl. 2012;2:1–138. [Google Scholar]
- 31.Rubenfeld G., Angus D., Pinsky M., Curtis R., Connors A., Bernard G. Outcomes research in critical care: results of the American Thoracic Society Critical Care Assembly Workshop on Outcomes Research. Am J Respir Crit Care Med. 1999;160:358–367. doi: 10.1164/ajrccm.160.1.9807118. [DOI] [PubMed] [Google Scholar]
- 32.Schoenfeld D., Bernard G.R. Statistical evaluation of ventilator-free days as an efficacy measure in clinical trials of treatments for acute respiratory distress syndrome. Crit Care Med. 2002;30(8):1772–1777. doi: 10.1097/00003246-200208000-00016. [DOI] [PubMed] [Google Scholar]
- 33.Schoenfeld D. Survival methods, including those using competing risk analysis, are not appropriate for intensive care unit outcome studies. Crit Care. 2006;10(1):103. doi: 10.1186/cc3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mazzali M., Hughes J., Kim Y.-G. Elevated uric acid increases blood pressure in the rat by a novel crystal-independent mechanism. Hypertension. 2001;38(5):1101–1106. doi: 10.1161/hy1101.092839. [DOI] [PubMed] [Google Scholar]
- 35.Sanchez-Lozada L.G., Tapia E., Santamaria J. Mild hyperuricemia induces vasoconstriction and maintains glomerular hypertension in normal and remnant kidney rats. Kidney Int. 2005;67(1):237–247. doi: 10.1111/j.1523-1755.2005.00074.x. [DOI] [PubMed] [Google Scholar]
- 36.Khosla U.M., Zharikov S., Finch J.L. Hyperuricemia induces endothelial dysfunction. Kidney Int. 2005;67(5):1739–1742. doi: 10.1111/j.1523-1755.2005.00273.x. [DOI] [PubMed] [Google Scholar]
- 37.Zoccali C., Maio R., Mallamaci F., Sesti G., Perticone F. Uric acid and endothelial dysfunction in essential hypertension. J Am Soc Nephrol. 2006;17(5):1466–1471. doi: 10.1681/ASN.2005090949. [DOI] [PubMed] [Google Scholar]
- 38.Sanchez-Lozada L.G., Soto V., Tapia E. Role of oxidative stress in the renal abnormalities induced by experimental hyperuricemia. Am J Physiol Renal Physiol. 2008;295(4):F1134–F1141. doi: 10.1152/ajprenal.00104.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu X., Hu J., Song N., Chen R., Zhang T., Ding X. Hyperuricemia increases the risk of acute kidney injury: a systematic review and meta-analysis. BMC Nephrol. 2017;18(1):27. doi: 10.1186/s12882-016-0433-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheungpasitporn W., Thongprayoon C., Harrison A.M., Erickson S.B. Admission hyperuricemia increases the risk of acute kidney injury in hospitalized patients(.) Clin Kidney J. 2016;9(1):51–56. doi: 10.1093/ckj/sfv086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Otomo K., Horino T., Miki T. Serum uric acid level as a risk factor for acute kidney injury in hospitalized patients: a retrospective database analysis using the integrated medical information system at Kochi Medical School hospital. Clin Exp Nephrol. 2016;20(2):235–243. doi: 10.1007/s10157-015-1156-5. [DOI] [PubMed] [Google Scholar]
- 42.Akbar S.R., Long D.M., Hussain K. Hyperuricemia: an early marker for severity of illness in sepsis. Int J Nephrol. 2015;2015:301021. doi: 10.1155/2015/301021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lapsia V., Johnson R.J., Dass B. Elevated uric acid increases the risk for acute kidney injury. Am J Med. 2012;125(3):e9–e17. doi: 10.1016/j.amjmed.2011.06.021. [DOI] [PubMed] [Google Scholar]
- 44.Greenberg K.I., McAdams-DeMarco M.A., Kottgen A., Appel L.J., Coresh J., Grams M.E. Plasma urate and risk of a hospital stay with AKI: the Atherosclerosis Risk in Communities Study. Clin J Am Soc Nephrol. 2015;10(5):776–783. doi: 10.2215/CJN.05870614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hillis G.S., Cuthbertson B.H., Gibson P.H. Uric acid levels and outcome from coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2009;138(1):200–205. doi: 10.1016/j.jtcvs.2008.12.052. [DOI] [PubMed] [Google Scholar]
- 46.Johnson R.J., Bakris G.L., Borghi C. Hyperuricemia, acute and chronic kidney disease, hypertension, and cardiovascular disease: report of a scientific workshop organized by the National Kidney Foundation. Am J Kidney Dis. 2018;71(6):851–865. doi: 10.1053/j.ajkd.2017.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nouri-Majalan N., Fotouhi-Ardakani E., Forouzannia K., Moshtaghian H. Effects of allopurinol and vitamin E on renal function in patients with cardiac coronary artery bypass grafts. Vasc Health Risk Manag. 2009;5(2):489–494. doi: 10.2147/vhrm.s5761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ejaz A.A., Dass B., Lingegowda V. Effect of uric acid lowering therapy on the prevention of acute kidney injury in cardiovascular surgery. Int Urol Nephrol. 2013;45(2):449–458. doi: 10.1007/s11255-012-0192-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Baseline Characteristics of the ICU Cohort by AKI Status.
Table S2. Baseline Characteristics of ATN Study Participants by 60-Day Mortality.
Table S3. Baseline Characteristics in the ATN Study: Participants Not in Subcohort vs Subcohort Included in This Study.
Table S4. Unadjusted Predictors of Uric Acid in the ATN Study Cohort.