Abstract
Rationale & Objective
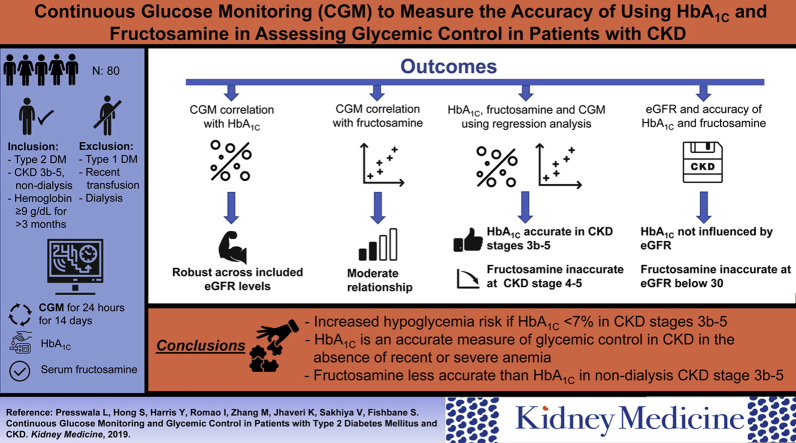
The accuracy of glycated hemoglobin (HbA1c) level for assessment of glycemic control in patients with chronic kidney disease (CKD) is uncertain. This study assessed the accuracy of HbA1c level using continuous glucose monitoring.
Study Design
Diagnostic test study of HbA1c and serum fructosamine. The continuous glucose monitor was worn for 14 days. Glucose was measured every 15 minutes (up to 1,344 measurements). Average glucose concentration was calculated for each patient from the patient’s continuous glucose monitor measurements. Linear regression was applied to estimate the relationship between average glucose concentration and HbA1c and serum fructosamine levels. The influence of patient characteristics on the relationship between HbA1c and average glucose concentrations was examined in a multivariate regression model.
Setting & Participants
Patients with type 2 diabetes and CKD (estimated glomerular filtration rate, 7-45 mL/min, not receiving dialysis) seen in an academic nephrology clinic.
Tests Analyzed
The accuracy of HbA1c level for assessment of chronic glycemia. A secondary objective was to study serum fructosamine levels.
Outcomes
The degree of correlation between continuous glucose monitoring–derived average glucose concentration and HbA1c level; serum fructosamine level was studied as a secondary outcome.
Results
80 patients wore the continuous glucose monitor for a mean of 12.7 ± 2.9 days. Average glucose concentration of all patients was 151.5 ± 55.7 mg/dL. Mean HbA1c level was 7.2% ± 1.5%. HbA1c level was highly correlated with average glucose concentration, described by the equation: average glucose concentration = 30.48 × HbA1c − 68.48; r = 0.82; P < 0.001. Serum fructosamine level was also significantly correlated with average glucose concentration; r = 0.55; P < 0.001. The strong correlation between average glucose concentration and HbA1c level was not affected by the severity of CKD, whereas the performance of serum fructosamine level, in contrast, degraded among patients with more severe CKD.
Limitations
Relatively small sample size.
Conclusions
HbA1c is an accurate measure of glycemic status among patients with CKD and type 2 diabetes. This relationship appears to hold true among patients with more severe CKD.
Index Words: Type 2 diabetes, chronic kidney disease, continuous glucose monitoring, HbA1c, glycated hemoglobin, diagnostic test study
Graphical abstract
Diabetes mellitus (DM) affects more than 30 million people in the United States and is the leading cause of chronic kidney disease (CKD) and end-stage kidney disease.1 Among patients with DM who have CKD, both monitoring and treatment of DM are more complicated.2 An important aspect of management is ongoing assessment of the effectiveness of the treatment plan on long-term glycemic control. A frequently used test for glycemic control is glycated hemoglobin (HbA1c) fraction. This test is generally accepted as a reliable method for evaluating chronic glycemia in populations without kidney disease.3, 4, 5 In contrast, among patients with CKD, limited data are available on the utility of HbA1c level.
In CKD, erythrocyte lifespan is decreased,2 which reduces the time available for glycosylation of hemoglobin. In addition, there is increased carbamylation of hemoglobin,6 a process that tends to increase HbA1c results independent of glucose exposure.7 As a result of uncertainty regarding HbA1c level in CKD, other tests, including serum fructosamine, are occasionally used. Serum fructosamine reflects glycation of several circulating proteins.8 This test provides an estimate of glucose control during the previous 2 weeks but does not have well-established accuracy in the CKD population. Taken together, the perceived inaccuracy of these tests in CKD is significant in that it potentially results in a lack of confidence in test results, management uncertainty, and therapeutic inertia. In addition, there may be risk for harm due to hypoglycemia if treatment is improperly intensified on the basis of falsely high test results.
Continuous glucose monitoring offers an effective method for understanding the totality of glucose exposure. While HbA1c level reflects glucose exposure for up to 90 days, continuous glucose monitor use for 14 days has been shown to be an excellent predictor of 90-day glucose exposure.9 The purpose of the current study was to use continuous glucose monitoring in patients with CKD to provide a full profile of glucose measurements over a 14-day period. These data were then used to: (1) determine the accuracy of HbA1c and serum fructosamine testing as measures of glucose control and (2) better understand the tests’ characteristics in CKD (correlation, linear equation, slope, Y intercept, and average glucose level at different HbA1c levels).
Methods
Patients
We enrolled patients from the academic nephrology practice of the Zucker School of Medicine at Hofstra/Northwell. The practice is comprised of 19 nephrologists and located in western Nassau County, NY. Patients were identified as potentially eligible based on a weekly prescreen of the following week’s patients who matched key entry criteria. All informed consent processes were carried out by the Principal Investigator with a witness present in person in the office. The study was approved by the Northwell Institutional Review Board (IRB # 17-0531).
Patients were eligible for study if they were older than 18 years and had type 2 DM and CKD with estimated glomerular filtration rates (eGFRs) of 0 to 45 mL/min (stages 3b-5 CKD). Exclusion criteria included a diagnosis of type 1 DM, end-stage kidney disease (current dialysis treatment), hemoglobinopathies, red blood cell transfusions in the prior 12 months, hemoglobin level < 9 g/dL documented within the previous 3 months, daily acetaminophen use, steroid treatment within 3 months, any new medication for DM in the previous 2 months or any dose change in DM medications > 50%, and current pregnancy. Treatment with erythropoiesis-stimulating agents was permitted, but the dose and frequency of administration had to be stable for 2 months.
Study Design
The initial data set was collected on day 1 of the study after provision of consent. The continuous glucose monitor used was the Abbott Freestyle Libre Pro (Abbott Laboratories). The device was configured to blind the patient to all glucose results. Glucose was measured every 15 minutes throughout the day and results were stored in the device’s memory, providing 1,344 measurements over 14 days. The device was placed on the patient’s upper arm according to the manufacturer’s instructions. No specific education for diet or medications (beyond what the patient had already received) was provided. While wearing the continuous glucose monitor, no diabetic medication could be changed unless absolutely necessary, in which case the patient would be withdrawn from the study.
After 14 days, patients returned to the office to have the device removed and the data downloaded. At this point, blood sampling was performed, including fasting blood glucose, HbA1c, and serum fructosamine, and additional clinical information was collected. If the device fell off or became inoperable earlier, the same closeout visit procedures were followed.
Statistical Analysis
Outcomes
The primary outcome was the degree of correlation between the continuous glucose monitor–derived average glucose concentration and HbA1c level. Secondary outcomes included degree of correlation between serum fructosamine level and average glucose concentration measured using continuous glucose monitoring, the relationship between HbA1c or fructosamine level and average glucose concentration established using simple regression equations including an estimated slope and intercept for all patients and for patients by severity of CKD, and the relationship between average glucose concentration and HbA1c level stratified by eGFR. Prediction error was defined as the difference between the actual data points and the regression line of best fit.
Hypoglycemia was defined as glucose level < 70 mg/dL; hyperglycemia, by glucose level > 180 mg/dL. The percentage of total time hypo- or hyperglycemic was calculated as the number of individual readings out of range, multiplied by 15 minutes (measurements were made every 15 minutes) divided by the total time wearing the continuous glucose monitor device, multiplied by 100. Glucose variability was studied using coefficient of variation.
Analysis Plan
The primary goal of the study was to evaluate the linear correlation measured using Pearson correlation coefficient between HbA1c level and average glucose concentration measured using continuous glucose monitoring. The sample size required to achieve 80% power to detect a correlation coefficient significantly different from 0 ranges from 7 to 19 at a 5% significance level, depending on the assumptions about the strength of linear association between the 2 variables. Specifically, if a medium strength of linear association was assumed, for example, a correlation coefficient of 0.6, sample size could have been 19. Because of uncertainty and lack of previous data to guide assumptions, we decided to recruit 80 patients.
Patients with fewer than 7 days of continuous glucose monitor measurements would be excluded from analysis. The average glucose concentration was calculated for each patient from all of that patient’s continuous glucose monitor glucose measurements. A simple linear regression model was applied to estimate the relationship between average glucose concentration and HbA1c and serum fructosamine levels. When the relationship was established through the model, prediction of average glucose concentration was calculated at different HbA1c levels. We were interested in determining whether test performance would be different for patients with severe CKD versus more moderate CKD. Accordingly, separate analyses were then performed for patients with stages 4/5 CKD and stage 3b disease.
No heteroscedasticity was found for the simple linear regression using the Breusch-Pagan test10 (P = 0.13). The influence of factors including age (divided by tertiles: 41-68, 69-75, and 76-95 years), sex, race (white, black/African American, or other), body mass index (<30 or ≥30 kg/m2), and eGFR (<20 and ≥20 mL/min) was examined on the relationship between average glucose concentration in a multivariate regression model. For individual subgroups, slopes and intercepts of the regression equations were compared using χ2 test.
Results
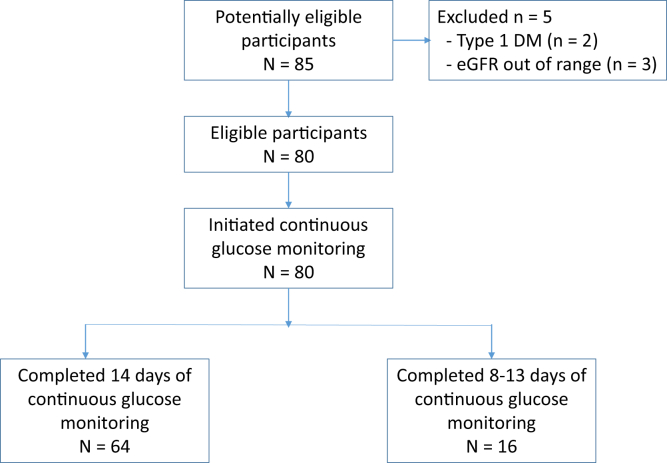
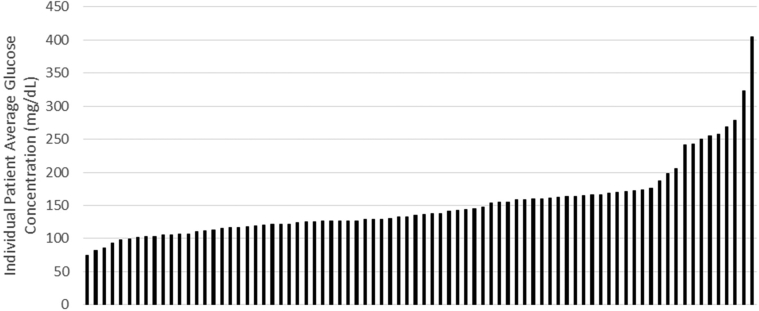
Eighty patients were enrolled and wore the continuous glucose monitor for a mean of 12.7 ± 2.9 days, with 80% completing the full 14 days (Fig 1). Patient characteristics are displayed in Table 1. Although all patients had type 2 DM, the cause of kidney disease was believed to be diabetic nephropathy in only 63 of 80 patients. Medication classes are displayed in Table 2. During the course of continuous glucose monitoring, the average glucose concentration of all patients was 151.5 ± 55.7 mg/dL. The range of average glucose concentrations was from 75 to 405 mg/dL. Individual patient average glucose concentration data are displayed in Figure 2. Mean HbA1c level was 7.2% ± 1.5% with a range of 4.8% to 13.2%. Mean serum fructosamine level was 304.1 ± 57.2 μmol/L, with a range of 177 to 523 μmol/L.
Figure 1.
Patient flow through the study. Abbreviations: DM, diabetes mellitus; eGFR, estimated glomerular filtration rate.
Table 1.
Baseline Characteristics
| Total Enrolled | 80 |
| Age, y | 71.3 ± 10.9 |
| Sex | |
| Male | 61 (76.25%) |
| Female | 19 (23.75%) |
| Race | |
| White | 53 (66.25%) |
| Asian | 11 (13.75%) |
| Black or African American | 10 (12.50%) |
| Unknown/not reported | 4 (5.00%) |
| American Indian/Alaska Native | 1 (1.25%) |
| >1 race/multiracial | 1 (1.25%) |
| Ethnicity | |
| Non-Hispanic | 77 (96.25%) |
| Hispanic | 2 (2.50%) |
| Unknown/not reported | 1 (1.25%) |
| Cause of CKD | |
| DM type 2 | 63 (78.75%) |
| Other | 17 (21.25%) |
| Medical history | |
| CHF | 20 (25.00%) |
| CAD | 26 (32.50%) |
| Hypertension | 77 (96.25%) |
| Stroke | 17 (21.25%) |
| History of malignancy | 6 (7.50%) |
| Estimated GFR, mL/min | |
| 0-≤15 | 15 (18.75%) |
| >15-≤30 | 34 (42.50%) |
| >30-≤45 | 31 (38.75%) |
| BMI, kg/m2 | |
| ≤25 | 14 (17.50%) |
| >25-≤30 | 33 (41.25%) |
| >30-≤35 | 21 (26.25%) |
| >35 | 12 (15.00%) |
Note: Values expressed as number (percent) or mean ± standard deviation.
Abbreviations: BMI, body mass index; CAD, coronary artery disease; CHF, congestive heart failure; CKD, chronic kidney disease; DM, diabetes mellitus; GFR, glomerular filtration rate.
Table 2.
Diabetes Medication Classes
| Any insulin | 42 (52.5%) |
| Short acting only | 10 (12.5%) |
| Long acting only | 14 (17.5%) |
| Short and long acting | 18 (22.5%) |
| Oral agents only | 23 (28.8%) |
| SU | 22 (27.5%) |
| Insulin and SU | 8 (10%) |
| GLP-1 receptor agonists | 10 (12.5%) |
| Oral hypoglycemic agents other than SU and GLP | 27 (33.7%) |
Abbreviations: GLP-1, glucagon-like peptide-1; SU, sulfonylurea.
Figure 2.
Up to 14-day average glucose concentrations for all 80 enrolled patients.
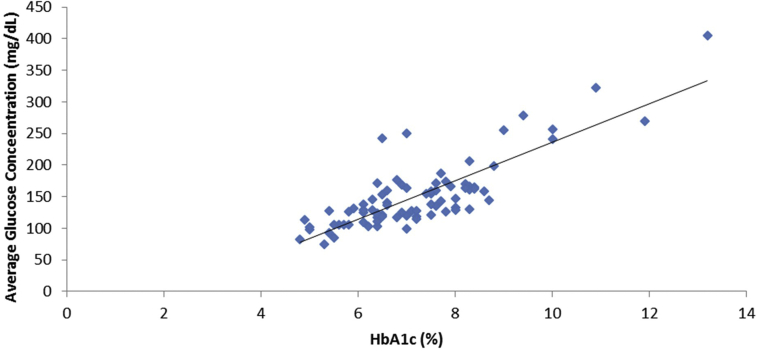
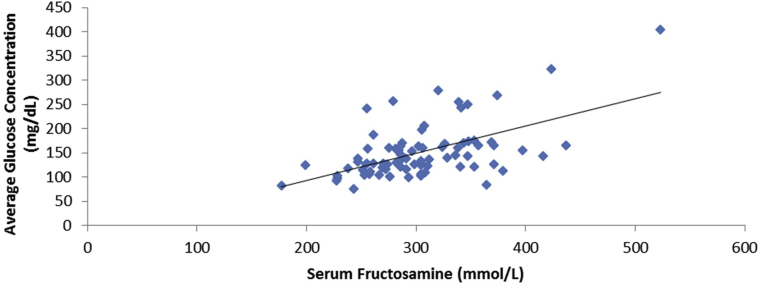
The relationship between average glucose concentration and HbA1c level is displayed in Figure 3. The 2 variables were significantly related as described by the formula average glucose concentration = 30.48 × HbA1c − 68.48; r = 0.82; P < 0.001. The standard deviation (SD) of prediction error based on this established equation was 18.6 mg/dL. The 95% confidence interval for slope was 25.73 to 35.25, and for Y-intercept, −103.6 to −33.42. A Bland-Altman type of analysis examining the difference between estimated and observed glucose level did not vary in any systematic way over the range of measurement. The limits of agreement were mean ± 1.96 × SD = (−62.13 to 62.13). Serum fructosamine level was also significantly correlated with average glucose concentration (Fig 4). The linear equation for this relationship was average glucose concentration = 0.54 × serum fructosamine − 14.76; r = 0.55; P < 0.001.
Figure 3.
A plot of glycated hemoglobin (HbA1c) level against average glucose concentration. The relationship is defined by the equation: average glucose concentration = 30.48 × HbA1c − 68.48; r = 0.82; P < 0.001.
Figure 4.
A plot of serum fructosamine level against average glucose concentration. The relationship is defined by the equation: average glucose concentration = 0.54 × fructosamine − 14.76; r = 0.55; P < 0.001.
Table 3 displays the expected average glucose concentration at different HbA1c levels based on the derived linear equation average glucose concentration = 30.48 × HbA1c − 68.48. To better understand the close relationship between average glucose concentration and HbA1c level, we explored the impact of other patient clinical characteristics, including age, sex, race, body mass index, and eGFR. In a multivariate regression model, no factors were found to be statistically significantly associated with average glucose concentration except for HbA1c level.
Table 3.
Average Expected Glucose Concentration for Each HbA1c Level
| HbA1c, % | Expected Average Glucose Concentration, mg/dL |
|---|---|
| 4 | 53.4 |
| 5 | 83.9 |
| 6 | 114.4 |
| 7 | 144.9 |
| 8 | 175.4 |
| 9 | 205.8 |
| 10 | 236.3 |
| 11 | 266.8 |
| 12 | 297.3 |
Note: The average expected glucose concentration for each HbA1c level as defined by the linear equation determined in the study: average glucose concentration = 30.48 × HbA1c − 68.48.
Abbreviation: HbA1c, glycated hemoglobin.
Table 4 shows the comparison of the regression equations within the specified subgroups. There were no statistically significant differences in the slope or intercept for the regression equations for any of the subgroup comparisons.
Table 4.
Comparison of Regression Equations Between HbA1c and Average Glucose for Subgroups
| Comparison | Difference in Slope | Difference in Intercept | Pa |
|---|---|---|---|
| Age | |||
| 2nd vs 1st tertile | −10.81 ± 5.23 | 78.17 ± 38.47 | 0.13 |
| 3rd vs 2nd tertile | −3.54 ± 8.10 | 31.39 ± 57.07 | 0.68 |
| 3rd vs 1st tertile | −13.57 ± 6.65 | 108.81 ± 48.1 | 0.06 |
| Sex | |||
| Male vs female | −2.82 ± 6.43 | 22.37 ± 45.97 | 0.87 |
| Race | |||
| White vs black | −1.27 ± 9.87 | 0.63 ± 71.08 | 0.85 |
| White vs other | −8.51 ± 11.95 | 67.54 ± 90.02 | 0.74 |
| Other vs black | −6.25 ± 10.67 | 27.45 ± 85.95 | 0.48 |
| BMI | |||
| <30 vs ≥30 kg/m2 | −12.50 ± 4.85 | 94.58 ± 35.59 | 0.53 |
| eGFR | |||
| <20 vs ≥20 mL/min | 1.47 ± 4.81 | −0.89 ± 35.32 | 0.43 |
Note: Values expressed as means ± standard error.
Abbreviations: BMI, body mass index; eGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin.
Chi-squared test with 2 df comparing the intercept and slope simultaneously.
The strong correlation between average glucose concentration and HbA1c level did not appear to be affected by the severity of patients’ CKD. Among 49 patients with eGFRs of 0 to 30 mL/min, HbA1c level remained highly correlated with average glucose concentration. The associated equation was average glucose concentration = 30.6 × HbA1c − 68.6; r = 0.81; P < 0.001. For the 31 patients with eGFRs of 30.1 to 5 mL/min, the 2 variables were similarly significantly related by the equation average glucose concentration = 30.3 × HbA1c − 68.2; r = 0.85; P < 0.001.
The relationship between average glucose concentration and serum fructosamine level was different depending on the severity of CKD. For patients with eGFRs of 0 to 30 mL/min, serum fructosamine level was not significantly correlated with average glucose concentration: r = 0.51; P = 0.96. Correlation was better, but not statistically significant, for patients with eGFRs of 30 to 45 mL/min; r = 0.69; P = 0.09.
Related to HbA1c test accuracy are the frequency of hypo- and hyperglycemia and glucose variability. With hypoglycemia defined as glucose level < 70 mg/dL, patients had a mean of 7.4 ± 9.0 events over the 14 days with a mean percentage of 7.5% ± 10.1% of total time being hypoglycemic. Among 38 patients with HbA1c levels < 7%, mean percentage of time hypoglycemic was 11.2% ± 14.4%. The percentage of time hypoglycemic was significantly less among the 42 patients with HbA1c levels ≥7% at 5.5% ± 8.0%; P = 0.03. Regarding hyperglycemia, the percentage of time that patients experienced glucose levels > 180 mg/dL was 26.1% ± 25.9%. Glucose variability was assessed for the entire study population. The mean coefficient of variation was 33.0 ± 9.3. The range of coefficients of variation was 17.0 to 58.9.
Discussion
We found that HbA1c level, contrary to controversy on the subject, is a highly accurate measure of long-term glycemic control among patients with type 2 DM and CKD with stable hemoglobin concentrations. This was true both in earlier and later stages of CKD, in which the linear relationship between long-term glucose exposure and HbA1c level were almost identical. Serum fructosamine level was also found to be reasonable for the assessment of chronic glycemia in this population; however, its utility may degrade among patients with more severe CKD.
Testing for glycated hemoglobin, or HbA1c, is the most widely used clinical tool for assessing long-term glycemic control in DM.4 The accuracy of this test among patients with CKD has been largely unknown. Previous studies on the subject have not yielded conclusive results due to small sample sizes or lack of an acceptable gold standard for assessment of chronic glycemia, such as continuous glucose monitoring. As a result, practice tends to be guided by a general sense that HbA1c level is probably less accurate in this population and, as a result, either treatment goals are relaxed or other tests, primarily serum fructosamine, are used.
The belief that HbA1c level is not an accurate measure in CKD is based on sound physiologic concerns. The most important is that erythrocyte circulating half-life is diminished in CKD.11 This would be expected to result in less time available for cells to become glycated. However, although erythrocyte circulating half-life is established to be reduced in patients receiving hemodialysis, the same evidence does not exist for patients with CKD not receiving dialysis. Other factors, including carbamylation of hemoglobin, might also degrade HbA1c performance in CKD.7 However, this has not been clearly established and may be less of a concern with modern testing methods.
Our finding that HbA1c level is a highly accurate measure of chronic glycemia in CKD is based on the following findings. First, test results were strongly correlated with average glucose concentration as measured using continuous glucose monitoring. Second, this result was highly statistically significant. Third, results were consistent because the correlation was just as strong in patients with severe CKD (stages 4/5) as it was among patients with CKD stage 3b. Fourth, the relationship between HbA1c level and average glucose concentration was described by a linear equation with slope and Y-intercept very close to the equation most commonly used in the general diabetic population (our derived equation for CKD, average glucose concentration = 30.48 × HbA1c − 68.48, the general diabetes equation from Nathan et al,12 average glucose concentration = 28.7 × HbA1c − 46.7).
We found the comparative accuracy of HbA1c and serum fructosamine levels to be similar. The correlation coefficient between the tests and average glucose concentration was higher for HbA1c than for serum fructosamine, but both linear relationships were statistically significant. Interestingly, although HbA1c level was almost exactly as accurate among patients with less and more severe CKD, the accuracy of serum fructosamine level declined significantly with more severe kidney disease.
Despite the accuracy of HbA1c and serum fructosamine levels for assessing long-term glycemic control, other issues also are important to guide therapy, including hypo- and hyperglycemia risk in the population treated and glucose variability. We found that hypoglycemia was fairly common in our population. The frequency of hypoglycemia was greater than would be expected for patients with type 2 DM, although this is somewhat dependent on the type of treatment received. In CKD there is reduced excretion of endogenous insulin and deranged medication metabolism, both of which may contribute to increased hypoglycemic risk. We found that risk for hypoglycemia was significantly increased among patients with HbA1c levels < 7.0%. Further studies would be required to determine whether this finding could help guide therapy. Hyperglycemia was also common within the study population, together with hypoglycemia reflecting the more difficult nature of managing glycemia in CKD. Given the relatively high rates for hypo- and hyperglycemia, it was not surprising that overall glucose variability was high.
Possible limitations of our study include the relatively small sample size, although results were robust. Second, for unclear reasons, the population skewed toward men, making the results somewhat less applicable to women. Third, this was a single-center study. Fourth, although HbA1c may measure glucose exposure over 60 to 90 days, we performed continuous glucose monitoring for only 14 days. We believe that the duration was sufficient given the highly robust results, which would not be possible otherwise. This is supported by the work of Riddlesworth et al, who found that, “Fourteen days of continuous glucose monitoring data provide a good estimation of glucose metrics for a 3-month period.”9(p 316)
We have found that HbA1c level is highly accurate as a measurement of chronic glycemia among patients with type 2 diabetes and CKD with stable hemoglobin concentrations. Accuracy was similar both for patients with more and less severe CKD. Serum fructosamine level was also accurate, but probably less so than HbA1c level. Further studies in larger and more diverse populations may be helpful and should include patients with end-stage kidney disease.
Article Information
Authors’ Full Names and Academic Degrees
Lubaina Presswala, DO, Susana Hong, MD, Yael Harris, MD, Isabela Romao, MD, Meng Zhang, PhD, Kenar D. Jhaveri, MD, Vipul Sakhiya, MHA, and Steven Fishbane, MD.
Authors’ Contributions
Research idea and study design: SF, SH, YH, LP, IR; data acquisition: SF, SH, VS; data analysis/interpretation: SF, KDJ, VS; statistical analysis: MZ. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Support
None.
Financial Disclosure
The authors declare that they have no relevant financial interests.
Peer Review
Received March 28, 2019. Evaluated by 2 external peer reviewers, with direct editorial input from the Statistical Editor, an Associate Editor, and the Editor-in-Chief. Accepted in revised form July 30, 2019.
Footnotes
Complete author and article information provided before references.
References
- 1.Centers for Disease Control and Prevention . US Department of Health and Human Services, Centers for Disease Control and Prevention; Atlanta, GA: 2017. National Chronic Kidney Disease Fact Sheet, 2017. [Google Scholar]
- 2.Carretero Gómez J., Arévalo Lorido J.C. Clinical assessment and treatment of diabetes in patients with chronic kidney disease. Rev Clin Esp. 2018;218(6):305–315. doi: 10.1016/j.rce.2018.03.016. [DOI] [PubMed] [Google Scholar]
- 3.Saudek C.D., Derr R.L., Kalyani R.R. Assessing glycemia in diabetes using self-monitoring blood glucose and hemoglobin A1c. JAMA. 2006;295(14):1688–1697. doi: 10.1001/jama.295.14.1688. [DOI] [PubMed] [Google Scholar]
- 4.American Diabetes Association. Standards of medical care in diabetes—2007. Diabetes Care. 2007;30(suppl 1):S4–S41. doi: 10.2337/dc07-S004. [DOI] [PubMed] [Google Scholar]
- 5.Goldstein D.E., Little R.R., Lorenz R.A. Tests of glycemia in diabetes. Diabetes Care. 2004;27(7):1761–1773. doi: 10.2337/diacare.27.7.1761. [DOI] [PubMed] [Google Scholar]
- 6.Kwan J.T., Carr E.C., Barron J.L., Bending M.R. Carbamylated haemoglobin in normal, diabetic and uraemic patients. Ann Clin Biochem. 1992;29(pt 2):206–209. doi: 10.1177/000456329202900215. [DOI] [PubMed] [Google Scholar]
- 7.Weykamp C.W., Miedema K., de Haan T., Doelman C.J. Carbamylated hemoglobin interference in glycohemoglobin assays. Clin Chem. 1999;45(3):438–440. [PubMed] [Google Scholar]
- 8.Kruseman A.C., Mercelina L., Degenaar C.P. Value of fasting blood glucose and serum fructosamine as a measure of diabetic control in non-insulin-dependent diabetes mellitus. Horm Metab Res Suppl. 1992;26:59–62. [PubMed] [Google Scholar]
- 9.Riddlesworth T.D., Beck R.W., Gal R.L. Optimal sampling duration for continuous glucose monitoring to determine long-term glycemic control. Diabetes Technol Ther. 2018;20(4):314–316. doi: 10.1089/dia.2017.0455. [DOI] [PubMed] [Google Scholar]
- 10.Breusch T.S., Pagan A.R. A simple test for heteroskedasticity and random coefficient variation. Econometrica. 1979;47(5):1287–1294. [Google Scholar]
- 11.Ma J., Dou Y., Zhang H. Correlation between inflammatory biomarkers and red blood cell life span in chronic hemodialysis patients. Blood Purif. 2017;43(1-3):200–205. doi: 10.1159/000452728. [DOI] [PubMed] [Google Scholar]
- 12.Nathan D.M., Kuenen J., Borg R., Zheng H., Schoenfeld D., Heine R.J., A1c-Derived Average Glucose Study Group Translating the A1C assay into estimated average glucose values. Diabetes Care. 2008;31(8):1473–1478. doi: 10.2337/dc08-0545. [DOI] [PMC free article] [PubMed] [Google Scholar]