Abstract
Rationale & Objective
Measurement of residual kidney function is recommended for the adjustment of the dialysis prescription, but timed urine collections are difficult and prone to errors. Equations to calculate residual kidney function from serum concentrations of endogenous filtration markers and demographic parameters would simplify monitoring of residual kidney function. However, few equations to estimate residual kidney function using serum concentrations of small solutes and low-molecular-weight proteins have been developed and externally validated.
Study Design
Study of diagnostic test accuracy.
Setting & Participants
823 Chinese peritoneal dialysis (PD) patients (development cohort) and 826 PD and hemodialysis patients from the Netherlands NECOSAD study (validation cohort).
Tests Compared
Equations to estimate residual kidney function (estimated clearance [eCl]) using serum creatinine, urea nitrogen, cystatin C, β2-microglobulin (B2M), β-trace protein (BTP), and combinations, as well as demographic variables (age, sex, height, and weight). Equations were developed using multivariable linear regression analysis in the development cohort and then tested in the validation cohort. Equations were compared with published validated equations.
Outcomes
Residual kidney function measured as urinary clearance (mCl) of urea nitrogen (mClUN) and average of creatinine and urea nitrogen clearance (mClUN-cr).
Results
In external validation, bias (difference between mCl and eCl) was within ± 1.0 unit for all equations. Accuracy (percent of differences within ± 2.0 units) was significantly better for eClBTP, eClB2M, and eClBTP-B2M than eClUN-cr for both mClUN (78%, 80%, and 81% vs 72%; P < 0.05 for all) and mClUN-cr (72%, 78%, and 79% vs 68%; P < 0.05 for all). The area under the curve for predicting mClUN > 2.0 mL/min was highest for eClB2M (0.853) and eClBTP-B2M (0.848). Results were similar for other validated equations.
Limitations
Development cohort only consisted of PD patients, no gold-standard method for residual kidney function measurement.
Conclusions
These results confirm the validity and extend the generalizability of residual kidney function estimating equations from serum concentrations of low-molecular-weight proteins without urine collection.
Index Words: Residual kidney function, creatinine, low-molecular-weight proteins, peritoneal dialysis, hemodialysis
Residual kidney function is associated with morbidity and mortality in patients with chronic kidney failure treated by peritoneal dialysis (PD).1, 2, 3, 4, 5 Guidelines recommend regular assessment of residual kidney function in PD patients to adjust the dialysis prescription.6 Residual kidney function is generally quantified as measured clearance (mCl) using timed urine collections of small solutes, such as mCl of urea nitrogen (mClUN) or mCl of the average of urea nitrogen and creatinine (mClUN-cr).6, 7, 8 However, timed urine collections are difficult and prone to errors. Therefore, estimated clearance (eCl) from serum concentrations of endogenous filtration markers without urine collection, as routinely performed in earlier stages of chronic kidney disease,9 could simplify clinical practice.
In principle, eCl from serum concentrations of small solutes would not be expected to perform well in dialysis patients, due in part to extrarenal elimination of the solutes during dialysis.10 Serum concentrations of low-molecular-weight proteins (LMWPs), such as β-trace protein (BTP [molecular weight, 23-29 kDa], β2-microglobulin (B2M [molecular weight, 11.6 kDa]), and cystatin C (molecular weight, 13.3 kDa), could be useful for eCl because LMWPs are eliminated by glomerular filtration as efficiently as small solutes, but less efficiently by dialysis.7, 11, 12, 13, 14, 15, 16, 17, 18, 19
Prior studies have developed estimating equations for residual kidney function using serum concentrations of small solutes or LMWPs,10, 20, 21, 22, 23 but only the study by Shafi et al10 included an external validation cohort. In that study, estimating equations were developed in a small cohort of dialysis patients in the United States (Residual Kidney Function [RKF] Study) and validated in hemodialysis (HD) and PD patients in the Netherlands Cooperative Study on the Adequacy of Dialysis (NECOSAD).10 Equations using BTP, B2M, and cystatin C levels were more accurate than equations using small solute levels, but to our knowledge, they have not been evaluated in other populations.
Residual kidney function estimating equations could be of particular interest in countries with a high prevalence of PD patients and limited resources, such as China, to simplify medical treatment and reduce costs.24 The aim of our study was to assess the validity and generalizability of residual kidney function estimating equations using LMWPs without urine collections. We developed equations using serum concentrations of small solutes and LMWPs in a large Chinese cohort of prevalent PD patients (Guangzhou PD Study), validated the equations in NECOSAD, and compared them with the residual kidney function estimating equations previously developed by Shafi et al.10
Methods
Study Design
This is a cross-sectional study for the development and internal validation of residual kidney function estimation equations in a Chinese PD cohort and external validation in a European HD and PD cohort. The study was approved by the Institutional Review Board (IRB) of the First Affiliated Hospital of Sun Yat-sen University (IRB approval no. [2013] 051), the Tufts Medical Center IRB (no. 10890), and medical ethics boards involved in NECOSAD. The study adheres to the ethical principles of the Declaration of Helsinki. All patients who took part in these studies gave their written informed consent.
Participants
The Guangzhou PD Study was used for equation development and internal validation. It consists of prevalent patients treated by continuous ambulatory PD (CAPD) from the First Affiliated Hospital at Sun Yat-Sen University in Guangzhou and affiliated outpatient dialysis units between January 2013 and December 2015. Inclusion criteria were 18 years or older and treatment with CAPD for 3 or more months. Exclusion criteria were critical illness or major surgery at the time of study enrollment, active bleeding within the previous 3 days before enrollment, advanced stage of malignancy, peritonitis within 4 weeks before enrollment, untreated clinical disorders of the thyroid gland, and medications that significantly affect tubular secretion of creatinine. We further excluded patients with missing demographic data, missing samples on 24-hour urine and dialysate collections, and anuric patients (ie, urine output of 0 mL/d).
After completion of data collection, we randomly divided the study population into a development data set (two-thirds of patients) and an internal validation data set (one-third of patients). NECOSAD was used for external validation of the equations. It is a large multicenter cohort of incident HD and PD patients older than 18 years recruited from 38 dialysis units in the Netherlands between January 1997 and January 2005.2, 25, 26 The present analysis includes 826 patients at 3 or 12 months after dialysis initiation with stored specimens and available data for residual kidney function.
Test Methods
The reference test is mCl of small solutes (urea nitrogen and creatinine) and the index tests are equations for eCl based on serum concentrations of small solutes, LMWPs (BTP, B2M, and cystatin C), demographic variables (age and sex), and body size (height and weight). Because clinical laboratories might not be able to assay all 3 LMWPs, we developed single-marker and multiple-marker LMWP equations.
Clearance Measurements
In both study populations, mCl of small solutes was ascertained as UV/P, where UV is urine solute excretion rate (urine solute concentration × urine volume) and P is plasma (serum) solute concentration. mClUN was expressed as mL/min and mClUN-cr was expressed as mL/min/1.73 m2 body surface area. In Guangzhou PD Study patients and NECOSAD PD patients, samples from 24-hour collections of dialysate and urine and a serum sample were obtained before a routine visit to the PD clinic. In NECOSAD HD patients, all urine during the interdialytic interval was collected, and blood samples were drawn at the end of the preceding HD session and directly before the next session, with the mean of these 2 values used for clearance calculations.27 Aliquots of all specimens were stored at −80°C until analyses were performed.
Filtration Marker Assessment
Small Solutes
For the Guangzhou PD study, urea nitrogen and creatinine measurements were performed at the University of Minnesota Advanced Research and Diagnostic Laboratory, Minneapolis, MN (Table S1). Serum and urinary urea nitrogen were measured on the Roche Cobas 6000 using a standardized enzymatic method. Serum and urinary creatinine were measured using an isotope-dilution mass spectrometry–traceable enzymatic method on the Roche Cobas 6000. In NECOSAD, urea nitrogen and creatinine (mainly using the alkaline picrate method) had previously been measured at the local laboratories.10 Earlier analyses in NECOSAD had shown that the method of creatinine measurement had a negligible effect on creatinine concentrations and that the interlaboratory variation at low ranges of creatinine measurements is low.
Low-Molecular-Weight Proteins
For both studies, LMWP measurements were performed at the University of Minnesota Advanced Research and Diagnostic Laboratory, Minneapolis, MN (Table S1). In the Guangzhou PD Study, BTP was measured using an immunonephelometric assay. B2M and cystatin C were measured using an immunoturbidimetric method. In the NECOSAD cohort, all measurements were performed using an immunonephelometric assay.10
Analyses in the Guangzhou PD Study
Development Data Set
For both mClUN and mClUN-cr, our goal was to develop an equation containing only small solutes (eClUN-cr) that could readily be used in clinical practice without measurement of additional filtration markers, 3 single-marker LMWP equations (eClBTP, eClB2M, or eCl of cystatin C [eClcys]), and 1 multiple-marker LMWP equation. Small solutes were considered for inclusion in equations containing LMWPs, and demographic characteristics and body size were considered for inclusion in all equations.
We prespecified a process for equation development similar to methods published previously.9, 10, 28 We transformed serum concentrations of filtration markers and mCls to natural logarithmic scale to stabilize variance. We used least squares linear regression and analysis of variance to assess linearity between the filtration markers and clearances. In case nonlinearity was detected, we determined the optimal number and location of breakpoints for spline functions for each filtration marker. For the small-solute equation, we forced urea nitrogen level, creatinine level, age, and sex into the equation. For the single-marker LMWP equations, we forced urea nitrogen level, creatinine level, age, and sex into the equation. For the multiple-marker LMWP equation, we evaluated all three 2-marker combinations and one 3-marker combination. At each step we retained a variable if it was significant in the model and improved (reduced) the root-mean-square error (RMSE; standard deviation of the mean difference between mCl and eCl) of the model by ≥2% compared to the model without the variable (a lower RMSE implies better model fit).
Internal Validation Data Set
All equations selected in the development data set were evaluated in the internal validation data set. Equations were excluded from further analysis if 1 of the filtration markers had a nonsignificant coefficient (P > 0.05) or the polynomial form of the filtration marker did not improve the RMSE of the model by ≥1% compared to the model with the linear form of the marker. In this case, the linear form was retained.
Combined Data Set
The remaining equations were refitted in the combined data set to determine coefficients for the final equations. The final multiple-marker LMWP equation was selected as the model with the lowest RMSE.
Analyses in the NECOSAD (external validation) Data Set
We compared mCl versus eCl graphically by plotting the residuals of the regression model (difference between mCl and eCl) against eCl. We defined bias as the median of the residuals and precision as the interquartile range of the residuals.9, 10, 29 As in the study by Shafi et al,10 we defined accuracy as the percentage of eCls within ± 2.0 mL/min of mClUN and 2.0 mL/min/1.73 m2 of mClUN-cr, respectively. We acknowledge that the range of ± 2.0 units for accuracy is wide but considered that a narrower range was not practical given the uncertainty in the reference test. We also considered a definition of accuracy based on a relative scale, as is generally used at higher glomerular filtration rates (GFRs), but concluded that it was not necessary because the GFR range is narrow. We calculated 95% confidence intervals for bias, precision, and accuracy by bootstrapping with 2,000 replicates.30 We compared accuracy between equations using the McNemar test for paired data. We compared the accuracy of each equation between HD versus PD patients using χ2 test for independent data. We assessed the area under the receiver operating characteristic curve (AUC) for estimating mClUN < 2.0 mL/min and mClUN-cr < 2.5 mL/min/1.73 m2. mClUN > 2 mL/min has been proposed by the Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines as a residual kidney function threshold, below which the treating physician should perform a thrice-weekly HD regimen.31 The threshold for mClUN-cr was chosen based on published literature to compare results.10 The optimal cutoff was defined as the value with the highest combined sensitivity and specificity (Youden index32). Finally, we compared the accuracy of the equations developed in the Guangzhou PD Study with equations previously published by Shafi et al10 using χ2 test. Analyses were performed using R, version 3.4.1 (R Development Core Team). P < 0.05 was considered to be significant. Results have not been adjusted for multiple testing.
Results
Participants
A total of 1,241 participants were included in the Guangzhou PD Study. After excluding participants with missing demographic data (n = 13), missing filtration marker measurements (n = 248), and anuria (n = 157; Table S2), 823 participants were selected (Fig S1). Mean age was 50 years and 63% were men (Table 1). Mean urinary output was 710 mL/d, mClUN was 2.0 mL/min, and mClUN-cr was 3.1 mL/min/1.73 m2. All patients were on CAPD treatment with 4 exchanges per day; total dwell volume used was 7.7 ± 1.2 L/d. Mean ultrafiltration was 505 ± 595 mL/d. NECOSAD included 826 participants (587 HD and 239 PD; Table 1). Mean age was 60 years and 60% were men. Mean urinary output was 897 mL/d, mClUN was 2.9 mL/min, and mClUN-cr was 3.8 mL/min/1.73 m2.
Table 1.
Participants’ Baseline Characteristics in the Guangzhou PD Study and NECOSAD Study
| Guangzhou PD Study (n = 823) | NECOSAD |
|||
|---|---|---|---|---|
| Total (n = 826) | HD (n = 587) | PD (n = 239) | ||
| Demographics | ||||
| Characteristics | ||||
| Age, y | 49.9 ± 14.5 | 60.2 ± 14.4 | 63.4 ± 13.3 | 52.2 ± 14.0 |
| Men | 62.8% | 60.0% | 59.1% | 67.8% |
| White | 0% | 87.7% | 91.8% | 77.4% |
| Body mass index, kg/m2 | 22.2 ± 3.2 | 25.1 ± 4.1 | 24.9 ± 4.2 | 25.5 ± 4.1 |
| Height, cm | 162.9 ± 7.4 | 171.3 ± 9.9 | 170.4 ± 9.8 | 173.6 ± 9.8 |
| Weight. kg | 59.1 ± 10.3 | 73.8 ± 14.2 | 72.5 ± 14.0 | 77.0 ± 14.2 |
| Body surface area, m2 | 1.6 ± 0.2 | 1.9 ± 0.2 | 1.8 ± 0.2 | 1.9 ± 0.2 |
| Total-body water, L | 32.9 ± 05.5 | 38.0 ± 6.7 | 37.1 ± 6.4 | 40.1 ± 7.0 |
| Diabetes mellitus | 16.7% | 21.3% | 23.7% | 15.5% |
| Residual kidney function | ||||
| Urinary output, mL/d | 710 ± 538 | 897 ± 675 | 826 ± 623 | 1,069 ± 762 |
| Measured clearances | ||||
| Urea nitrogen, mL/min | 2.0 ± 1.9 | 2.9 ± 2.1 | 2.8 ± 2.1 | 3.1 ± 2.2 |
| Urea nitrogen, mL/min/1.73 m2 | 2.1 ± 2.1 | 2.7 ± 1.9 | 2.6 ± 1.9 | 2.8 ± 1.9 |
| Cr, mL/min | 3.7 ± 3.9 | 4.6 ± 3.5 | 4.6 ± 3.7 | 4.6 ± 3.2 |
| Cr, mL/min/1.73 m2 | 4.0 ± 4.2 | 4.9 ± 3.7 | 4.8 ± 3.7 | 5.0 ± 3.5 |
| Mean of urea nitrogen and Cr, mL/min | 2.9 ± 2.9 | 3.6 ± 2.6 | 3.6 ± 2.7 | 3.6 ± 2.4 |
| Mean of urea nitrogen and Cr, mL/min/1.73 m2 | 3.1 ± 3.1 | 3.8 ± 2.7 | 3.7 ± 2.7 | 3.9 ± 2.6 |
| Weekly kidney Kt/V | 0.6 ± 0.6 | 0.7 ± 0.5 | 0.7 ± 0.5 | 0.7 ± 0.5 |
| Filtration marker serum concentrations | ||||
| SUN, mg/dL | 49.3 ± 15.5 | 64.9 ± 17.1 | 66.9 ± 16.7 | 60.1 ± 17.2 |
| Cr, mg/dL | 9.9 ± 3.4 | 8.7 ± 2.8 | 8.5 ± 2.7 | 9.2 ± 3.0 |
| β2-microglobulin, mg/L | 29.9 ± 10.5 | 25.5 ± 9.5 | 25.6 ± 9.6 | 25.2 ± 9.3 |
| β-Trace protein, mg/L | 8.6 ± 2.9 | 6.9 ± 2.6 | 6.8 ± 2.4 | 7.3 ± 2.9 |
| Cystatin C, mg/L | 6.6 ± 1.4 | 5.1 ± 1.1 | 5.0 ± 1.1 | 5.2 ± 1.2 |
Note: Values expressed as mean ± standard deviation or percent. Conversion factors for units: SUN in mg/dL to mmol/L, ×357; Cr in mg/dL to μmol/L, ×88.4.
Abbreviations: Cr, creatinine; HD, hemodialysis; NECOSAD, Netherlands Cooperative Study on the Adequacy of Dialysis; PD, peritoneal dialysis; SUN, serum urea nitrogen.
Test Results: Correlations Among Clearances and Serum Concentrations of Filtration Markers
In both studies, B2M level showed the strongest correlation with mClUN (r = −0.56 and −0.69 in the Guangzhou PD Study combined data set and NECOSAD, respectively) and mClUN-cr (r = −0.58 and r = −0.75), whereas urea nitrogen level had the weakest correlation with mClUN (r = −0.19 and r = −0.24) and mClUN-cr (r = −0.23 and r = −0.26; Tables S3 and S4). Correlation among filtration markers was strongest between B2M and cystatin C levels (r = 0.85 and r = 0.75) and weakest for BTP and urea nitrogen levels (r = 0.27 and r = 0.20). Adjusting for clearances moderately attenuated the correlations among filtration markers (Table S4).
Equation Development in the Guangzhou PD Study
Development Data Set, Internal Validation, and Combined Data Set
Equation development in the development data set and internal validation are described in Tables S5 and S6. Table 2 shows the final equations developed in the combined data set. Performance of the final equations in the combined data set is shown in Table S7.
Table 2.
Guangzhou PD Study Equations for Estimation of Residual Kidney Function in Dialysis Patients
| Markers | Covariables | Equation |
|---|---|---|
| Equations to estimate mClUN, mL/min | ||
| UN-creatinine | Age, sex | 60 × cr-2.271 × UN0.369 × 0.989Age (× 1.536 if male) |
| BTP | 98 × BTP-2.128 | |
| B2M | For B2M ≤ 24 mg/L: 2 × (B2M/24)-0.678 For B2M > 24 mg/L: 2 × (B2M/24)-2.880 |
|
| Cystatin C | 571 × cys -3.349 | |
| BTP-B2M | For B2M ≤ 24 mg/L: 16 × BTP-1.02 × (B2M/24)0.159 For B2M > 24 mg/L: 16 × BTP-1.02 × (B2M/24)-2.187 |
|
| Equations to estimate mClUN-cr, mL/min/1.73 m2) | ||
| UN-creatinine | Age, sex | 207 × cr-2.539 × UN0.334 × 0.988Age (× 1.427 if male) |
| BTP | Creatinine | 445 × BTP-1.301 × cr-1.274 |
| B2M | Creatinine | For B2M ≤ 23 mg/L: 39 × (B2M/23)0.144 × cr-1.152 For B2M > 23 mg/L: 39 × (B2M/23)-2.129 × cr-1.152 |
| Cystatin C | Creatinine | 1,53 × cys-2.082 × cr-1.228 |
| BTP-B2M | For B2M ≤ 23 mg/L: 32 × BTP-1.126 × (B2M/23)0.271 For B2M > 23 mg/L: 32 × BTP-1.126 × (B2M/23)-2.133 |
|
Note: Coefficients for creatinine are in mg/dL; for BTP, B2M, and cystatin C, in mg/L.
Abbreviations and definitions: B2M, β2-microglobulin; BTP, β-trace protein; cr, creatinine; cys, cystatin C; mClUN, measured clearance of urea nitrogen in mL/min; mClUN-cr, average measured clearance of urea nitrogen and creatinine in mL/min/1.73 m2; UN, urea nitrogen.
Comparison to Published Equations
Coefficients of equations containing similar LMWP markers in the equations that we developed differ from equations published by Shafi et al,10 in part due to the use of spline B2M and the absence of a coefficient for sex in equations for both mClUN and mClUN-cr and the presence of a coefficient for creatinine in the single-marker LMWP equations for mClUN-cr (Table S8). The performance of equations published by Shafi et al10 in the Guangzhou PD Study combined data set is shown in Table S9.
Equation Validation in NECOSAD
Estimating mClUN and mClUN-cr
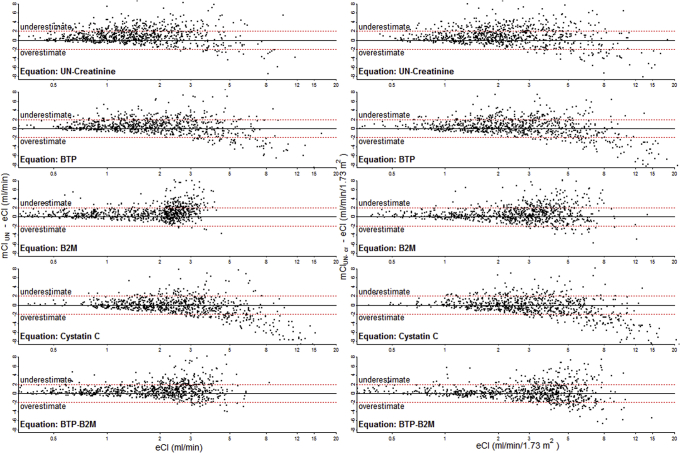
Bias was within ± 1.0 mL/min and within ± 1.0 mL/min/1.73 m2, respectively, for all equations (Table 3). eClB2M and eClBTP-B2M were unbiased across the range of eCl; other equations overestimated mCl at higher levels of eCl (Fig 1). Precision was between 1.5 and 2.1 mL/min for mClUN and 1.8 and 2.3 mL/min/1.73 m2 for mClUN-cr. Accuracy was nominally highest for eClB2M and eClBTP-B2M for mClUN (80% and 81%, respectively) and mClUN-cr (78% and 79%, respectively) and significantly higher than eClUN-cr for mClUN (72%) and mClUN-cr (68%; Table 3). Accuracy was similar for HD and PD patients, except for eClB2M and eClBTP-B2M, which were more accurate in HD patients estimating mClUN (Table 4).
Table 3.
Performance of Estimating Equations in the NECOSAD Cohort
| Variables | Guangzhou PD Study Equations |
Shafi et al10 Equations |
|||
|---|---|---|---|---|---|
| RMSEa | Biasb | Precisionc | Accuracy (95% CI)d | Accuracy (95% CI)d | |
| Equations to estimate mClUN(mL/min) | |||||
| mL/min (95% CI) | mL/min (95% CI) | ||||
| UN-creatinine | 0.694 (0.656 to 0.730) | 0.8 (0.7 to 0.9) | 2.1 (1.9 to 2.3) | 72% (69% to 75%) | 75% (72% to 78%)e |
| BTP | 0.630 (0.594 to 0.665) | 0.4 (0.3 to 0.5) | 1.8 (1.6 to 1.9) | 78% (75% to 80%)f | 81% (78% to 83%)e,g |
| B2M | 0.588 (0.549 to 0.622) | 0.7 (0.6 to 0.8) | 1.7 (1.5 to 1.8) | 80% (77% to 83%)f | 79% (76% to 81%)g |
| Cystatin C | 0.667 (0.626 to 0.702) | −0.3 (−0.4 to −0.2) | 2.0 (1.8 to 2.2) | 75% (72% to 78%) | 79% (76% to 82%)e,g |
| BTP-B2M | 0.514 (0.483 to 0.545) | 0.5 (0.4 to 0.6) | 1.5 (1.4 to 1.8) | 81% (78% to 84%)f | 81% (78% to 84%)g |
| Equations to estimate mClUN-cr(mL/min/1.73 m2) | |||||
| mL/min/1.73 m2 (95% CI) | mL/min/1.73 m2 (95% CI) | ||||
| UN-creatinine | 0.606 (0.569 to 0.642) | 0.7 (0.6 to 0.9) | 2.2 (2.0 to 2.4) | 68% (65% to 71%) | 68% (65% to 72%) |
| BTP | 0.550 (0.519 to 0.582) | 0.2 (0.1 to 0.4) | 2.1 (1.9 to 2.3) | 72% (69% to 75%)h | 71% (68% to 74%) |
| B2M | 0.513 (0.482 to 0.546) | 0.5 (0.4 to 0.6) | 1.8 (1.7 to 2.0) | 78% (75% to 80%)f | 69% (66% to 72%)i |
| Cystatin C | 0.572 (0.536 to 0.607) | −0.2 (−0.4 to −0.1) | 2.3 (2.1 to 2.5) | 71% (68% to 74%) | 72% (69% to 75%)g |
| BTP-B2M | 0.511 (0.478 to 0.543) | 0.2 (0.1 to 0.4) | 1.8 (1.6 to 2.0) | 79% (77% to 82%)f | 75% (72% to 78%)e,g |
Note: n = 826. All associations between filtration marker and outcome are linear except for B2M (2-slope polynomial model, breakpoint for B2M at 24 [mClUN] and 23 [mClUN-cr] mg/L). UN-creatinine equations also contain age and sex as covariables. Of note, equations developed by Shafi et al10 contained in part different covariables.
Abbreviations: B2M, β2-microglobulin; BTP, β-trace-protein; CI, confidence interval; RMSE, root-mean-square error; mClUN, measured clearance of urea nitrogen in mL/min; mClUN-cr, average measured clearance of urea nitrogen and creatinine in mL/min/1.73 m2; NECOSAD, Netherlands Cooperative Study on the Adequacy of Dialysis; PD, peritoneal dialysis; UN, urea nitrogen.
RMSE defined as the standard deviation of mean difference between (ln) measured and (ln) estimated clearance.
Bias defined as the median difference between measured and estimated clearance.
Precision defined as interquartile range of the differences between measured and estimated total clearance.
Accuracy defined as the percentage of estimates within ± 2 units of measured clearance.
Significance level of P < 0.05.
Significance level of P < 0.001 for the difference between the accuracy of the corresponding equation and the UN-creatinine equation.
Significance level of P < 0.05 for difference between the corresponding equation and the UN-creatinine equation published by Shafi et al.10
Significance level of P < 0.05.
Significance level of 0.01 for the difference between the accuracy of the corresponding equation and the similar Guangzhou PD Study equation (ie, same row).
Figure 1.
Associations between estimated clearances (eCls) and difference between measured (mCl) and eCl in the total Netherlands Cooperative Study on the Adequacy of Dialysis (NECOSAD) cohort (n = 826). Differences between mCls and eCls are presented on the y-axis; eCl, on the x-axis. The specific markers used in the eCl equations are indicated within the graphs. Positive differences indicate underestimation of mCl by the eCl; negative differences, overestimation. All associations between filtration marker and outcome are linear except for β2-microglobulin (B2M; 2-slope polynomial model, breakpoint at 24 [mCl of urea nitrogen; mClUN] and 23 [mCl or urea nitrogen-creatinine; mClUN-cr] mg/L). Abbreviations: BTP, β-trace-protein; eCl (mL/min), estimated clearance of urea nitrogen in mL/min; eCl (mL/min/1.73 m2), estimated average clearance of urea nitrogen and creatinine in mL/min/1.73 m2; UN, urea nitrogen.
Table 4.
Performance of mClUN and mClUN-cr Estimating Equations Developed in the Guangzhou PD Study Data Set and in NECOSAD, Comparing HD Versus PD Patients
| Markers | Modality | Biasa | Precisionb | Accuracy (95% CI)c | |
|---|---|---|---|---|---|
| Equations to estimate mClUN(mL/min) | |||||
| mL/min (95% CI) | mL/min (95% CI) | P | |||
| UN-creatinine | HD | 0.8 (0.6 to 0.8) | 2.1 (1.8 to 2.2) | 73% (69% to 76%) | 0.4 |
| PD | 1.2 (0.8 to 1.3) | 2.1 (1.8 to 2.5) | 69% (63% to 75%) | ||
| BTP | HD | 0.2 (0.1 to 0.3) | 1.7 (1.5 to 1.9) | 79% (76% to 82%) | 0.2 |
| PD | 0.8 (0.6 to 1.0) | 1.8 (1.6 to 2.2) | 74% (69% to 80%) | ||
| B2M | HD | 0.6 (0.6 to 0.7) | 1.5 (1.4 to 1.7) | 82% (79% to 85%) | 0.02 |
| PD | 0.9 (0.6 to 1.1) | 1.9 (1.6 to 2.3) | 75% (69% to 80%) | ||
| Cystatin C | HD | −0.5 (−0.6 to −0.3) | 1.9 (1.8 to 2.3) | 75% (71% to 78%) | 0.6 |
| PD | 0.1 (−0.2 to 0.3) | 1.8 (1.6 to 2.2) | 77% (71% to 82%) | ||
| BTP-B2M | HD | 0.4 (0.3 to 0.5) | 1.5 (1.3 to 1.6) | 84% (81% to 87%) | 0.001 |
| PD | 0.8 (0.6 to 1.0) | 1.9 (1.6 to 2.2) | 74% (69% to 80%) | ||
| Equations to estimate mCLUN-cr(mL/min/1.73 m2) | |||||
| mL/min/1.73 m2 (95% CI) | mL/min/1.73 m2 (95% CI) | ||||
| UN-creatinine | HD | 0.7 (0.5 to 0.8) | 2.0 (2.0 to 2.5) | 67% (63% to 71%) | 0.4 |
| PD | 0.9 (0.7 to 1.2) | 2.0 (1.7 to 2.4) | 71% (65% to 77%) | ||
| BTP | HD | 0.1 (−0.1 to 0.2) | 2.0 (1.8 to 2.3) | 73% (69% to 76%) | 0.9 |
| PD | 0.7 (0.5 to 1.0) | 2.0 (1.7 to 2.2) | 72% (66% to 77%) | ||
| B2M | HD | 0.4 (0.4 to 0.5) | 1.8 (1.6 to 2.0) | 79% (76% to 82%) | 0.2 |
| PD | 0.8 (0.6 to 1.0) | 2.0 (1.7 to 2.3) | 74% (69% to 79%) | ||
| Cystatin C | HD | −0.4 (−0.6 to −0.3) | 2.2 (2.0 to 2.5) | 71% (68% to 75%) | 0.99 |
| PD | 0.1 (−0.1 to 0.4) | 2.1 (1.8 to 2.5) | 72% (66% to 78%) | ||
| BTP-B2M | HD | 0.1 (0.0 to 0.3) | 1.8 (1.6 to 2.0) | 79% (76% to 82%) | 1.00 |
| PD | 0.5 (0.3 to 0.7) | 1.8 (1.4 to 2.1) | 79% (74% to 84%) | ||
Note: Total N = 826; HD, n = 587; PD, n = 239. UN-creatinine equations also contain age and sex as covariables. All associations between filtration marker and mCl are linear except for B2M (2-slope polynomial model, breakpoint for B2M at 24 [mClUN] and 23 [mClUN-cr] mg/L). P value for the difference of accuracy of the corresponding equation in HD versus PD subcohort.
Abbreviations: B2M, β2-microglobulin; BTP, β-trace-protein; CI, confidence interval; mClUN, measured clearance of urea nitrogen in mL/min; mClUN-cr, average measured clearance of urea nitrogen and creatinine clearance in mL/min/1.73 m2; HD, hemodialysis; NECOSAD, Netherlands Cooperative Study on the Adequacy of Dialysis; PD, peritoneal dialysis. UN, urea nitrogen.
Bias defined as the median difference between measured and estimated clearance.
Precision defined as interquartile range of the differences between measured and estimated clearance.
Accuracy defined as the percentage of estimates within ± 2 units of measured clearance.
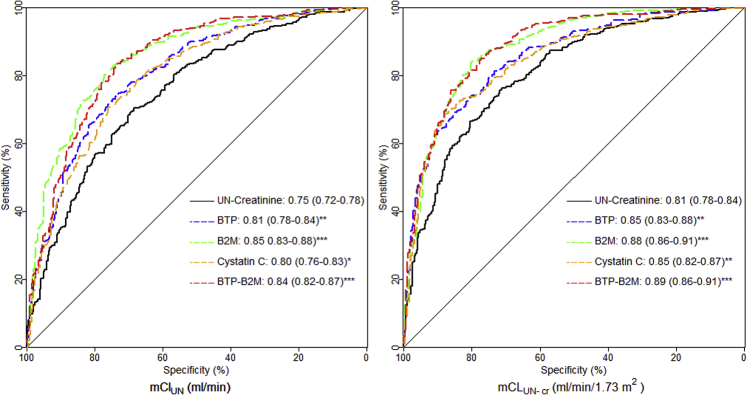
The AUC to detect mClUN > 2.0 mL/min and mClUN-cr > 2.5 mL/min/1.73 m2 was highest for eClB2M (0.85 and 0.88, respectively) and eClBTP-B2M (0.84 and 0.89, respectively (Fig 2; Table S10). Findings were similar in HD and PD patients (Table S10). Among LMWP equations, eClB2M and eClBTP-B2M were more accurate than eClcys to detect mCLUN > 2.0 mL/min (P = 0.02 and P < 0.01, respectively) and mClUN-cr > 2.5 mL/min/1.73 m2 (P < 0.01 and P = 0.04, respectively).
Figure 2.
Receiver operating characteristic curves for the diagnostic accuracy of estimating equations to detect urea clearance in mL/min (ClUN) < 2 mL/min and average clearance of urea and creatinine in mL/min/1.73 m2 (ClUN-cr) < 2.5 mL/min/1.73 m2 in the Netherlands Cooperative Study on the Adequacy of Dialysis (NECOSAD) data set (n = 826), respectively. Sensitivity is presented on the y-axis; specificity, on the x-axis. Equations can be identified by the markers that were used. The area under the curve result for every equation is presented with confidence intervals in brackets. All associations between filtration marker and outcome are linear except for β2-microglobulin (B2M; 2-slope polynomial model, breakpoint at 24 measured ClUN [mClUN] and 23 [mClUN-cr] mg/L). ∗P < 0.05; ∗∗P < 0.01; ∗∗∗P < 0.001 of the difference between area under the curve of corresponding equation and UN-creatinine equation. Abbreviations: BTP, β-trace protein; UN, urea nitrogen.
Comparison to Published Equations
In general, the LMWP equations of Shafi et al10 were more accurate than the small-solute equations of Shafi et al10 for both mClUN and mClUN-cr (Table 3). Our eClB2M and eClBTP-B2M equations had similar accuracy to the equations of Shafi et al10 for mClUN, but were significantly more accurate for mClUN-cr. Our eClBTP and eClcys equations were significantly less accurate than the equations of Shafi et al10 for mClUN, but had similar accuracy for mClUN-cr.
Discussion
We developed equations containing small solutes (urea nitrogen and creatinine) and LMWPs (BTP, B2M, and cystatin C) for the estimation of residual kidney function, assessed as mClUN and mClUN-cr, in a large cohort of prevalent Chinese CAPD patients and externally validated these equations in a large European cohort of incident HD and PD patients. All LMWP equations performed moderately well in terms of bias, precision, and accuracy and outperformed the small-solute equation for the detection of clinically relevant residual kidney function thresholds. Equation performance was generally similar to results of Shafi et al,10 except in our data, equations with B2M appeared to be consistently more accurate than equations with other LMWP markers. Results were generally consistent between PD and HD patients. These results add substantially to the evidence of validity and generalizability of estimation of residual kidney function from serum levels of endogenous LMWP filtration markers without urine collection.
Both small solutes and LMWPs are eliminated by glomerular filtration, and creatinine and cystatin C are recommended for use in GFR estimating equations in patients not treated by dialysis.33 We hypothesized that LMWPs would be more useful than small solutes to estimate residual kidney function because there is less extra-renal elimination of LMWPs by dialysis than small solutes. BTP is produced primarily in the central nervous system.34, 35 Moderate removal by HD has only been reported for high-flux HD.12, 36 Clearance through PD is unknown. B2M and cystatin C are produced by nucleated cells.37, 38 Compared to BTP, B2M and cystatin C have lower molecular weight; low-flux HD eliminates neither marker but both can be removed by high-flux HD and PD.16, 36, 39, 40, 41
Prior studies have shown better performance of LMWPs than small solutes in estimating residual kidney function,10, 20, 21, 23 including one study of 160 CAPD patients in China using cystatin C.22 Comparison of our results with these studies is limited due to differences in assays for the filtration markers and absence of external validation, except in the study by Shafi et al.10 The generally similar performance of equations previously developed by Shafi et al10 in a US population of predominantly HD patients and our study in a Chinese population of CAPD patients is strong evidence for the validity and generalizability of these equations. Of note, in contrast to our study, Shafi et al10 did not detect substantial differences among LMWP equations. The equations that we developed differ slightly from the equations of Shafi et al,10 which likely reflects differences in study populations and dialysis modality in the cohorts used for equation development and equation development methods.
It is noteworthy that the BTP-B2M equations that we developed did not perform substantially better than the B2M equations without BTP. In principle, a multiple-marker LMWP equation would perform better than a single-marker equation due to a smaller contribution to error from variation in the non-GFR determinants of each marker.42 Prior studies in people not treated by dialysis have shown better performance of a GFR estimating equation including both BTP and B2M compared to B2M alone.28, 43, 44 Possibly the inability to detect improvement with a multiple-marker equation reflects measurement error in the reference test (nonsupervised timed urine collections). Similar findings in the study by Shafi et al10 with more accurate measurements (supervised timed urine collections) may reflect the limited number of participants in the development database in that study.
We anticipate 2 clinical settings in which residual kidney function estimating equations might be useful. First, they could be used to reduce the frequency of urine collections for adjustment of the dialysis prescription, thereby reducing patient burden and potentially reducing costs. These considerations may be more relevant for countries with a high prevalence of PD patients, such as China. However, the accuracy of equations is not sufficient to make fine adjustments in PD prescription recommended by guidelines (eg, a 15% reduction in weekly effluent volume for each 1–mL/min/1.73 m2 higher mClUN45). This suggests that residual kidney function estimating equations may be most useful as a screening test to determine whether urine collection for clearance measurement is necessary. Clinical trials will be necessary to evaluate these strategies.
Second, residual kidney function estimating equations could be used in settings when urine collection is not practical, such as in patients with voiding difficulties, but ascertainment of residual kidney function is important for medical decision making, such as whether iodinated contrast media can be administered. Our results suggest that a single-marker LMWP may be as accurate as a multiple-marker equation. The differences in accuracy among LMWPs appears small, so the decision as to which LMWP to measure would be influenced by the availability of laboratory methods and costs. Of note, the assays for BTP and B2M are not standardized, so it will be important to harmonize laboratory measurement procedures for application of the equations. Our study has several strengths. We developed equations in a large data set, which enabled us to assess the form of variables for the filtration markers and the need for covariables. We included patients with minimal urine output, providing a wide range for serum concentrations of endogenous filtration markers and allowing application of the equations to patients with even a low level of residual kidney function. We assessed 2 small solutes and 3 different LMWPs, enabling us to test a wide spectrum of currently available filtration markers. All patients in the development data set were treated with CAPD; in contrast to HD, stable serum concentrations of endogenous filtration markers can be assumed. Because the external validation cohort included both HD and PD patients, we were able to address the question of applicability of our equations to both treatment modalities. The cohorts differed substantially in terms of age, body size, race, and level of residual kidney function, which enhances the generalizability of results. We used the same laboratory as Shafi et al10 for measurement of serum LMWP concentrations, thus eliminating an important source of bias in comparing estimating equations from different studies.
Our study also has limitations. The reference tests, mClUN and mClUN-cr, reflect only small-solute clearances and may differ from measured GFR. However, standardized methods for assessing other measures of residual kidney function have not been defined, and few studies of residual kidney function have included measurement of exogenous filtration markers for assessment of GFR. As mentioned, urine collection was not supervised in both cohorts; therefore, errors in urine collection cannot be excluded. Modalities other than CAPD and low-flux HD were not frequently used in NECOSAD, so our results apply primarily to these modalities. We did not adjust for clinical conditions that could affect non-GFR determinants such as inflammation. This could have had an impact on equation performance. Finally, we did not have longitudinal data to evaluate the performance of the equations in detecting change in mCl.
In conclusion, we present equations developed in a Chinese PD cohort to estimate residual kidney function from serum concentrations of LMWPs without urine collection in both European HD and PD patients. These findings confirm the findings of Shafi et al10 and may have clinical implications for routine care for dialysis patients. Studies in other cohorts are necessary to compare the accuracy and clinical utility of these equations. In addition, future research should evaluate these equations to assess other measures of residual kidney function.
Article Information
Authors’ Full Names and Academic Degrees
Dominik Steubl, MD, Li Fan, MD, PhD, Wieneke M. Michels, MD, PhD, Lesley A. Inker, MD, MS, Hocine Tighiouart, MS, Friedo W. Dekker, PhD, Raymond T. Krediet, MD, PhD, Andrew L. Simon, ScM, Meredith C. Foster, ScD, MPH, Amy B. Karger, MD, PhD, John H. Eckfeldt, MD, PhD, Hongyan Li, MD, Jiamin Tang, RN, Yongcheng He, MD, Minyan Xie, MD, Fei Xiong, MD, Hongbo Li, MD, Hao Zhang, MD, Jing Hu, MD, PhD, Yunhua Liao, MD, PhD, Xudong Ye, MD, Tariq Shafi, MBBS, MHS, Wei Chen, MD, PhD, Xueqing Yu, MD, PhD, and Andrew S. Levey, MD.
Authors’ Contributions
Research idea and study design: LAI, XYu, ASL; data acquisition: LF, WMM, FWD, RTK, HL, JT, YH, MX, FX, HL, HZ, JH, YL, XYe, WC; data analysis/interpretation: DS, LF, LAI, HT, ALS, MCF, TS, XYu, ASL; biomarker measurement: ABK, JHE. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Support
Grant 2002B60118 from Guangdong Provincial Key Laboratory of Nephrology, Guangzhou, China; Key Laboratory of Nephrology, Guangdong Province, Guangzhou, China; Operational grant 2017B030314019 of Guangdong Provincial Key Laboratory; Guangdong Provincial Programme of Science and Technology, grant 2017A050503003; Paul Teschan Research Fund – Dialysis Clinic Inc (“Monitoring PD Adequacy Using Serum Levels of Endogenous Filtration Marker“); Siemens Healthcare (“Monitoring PD Adequacy Using Serum Levels of Endogenous Filtration Marker). None of the funders of this study had any role in study design; collection, analysis, and interpretation of data; writing the report; and the decision to submit the report for publication.
Financial Disclosure
Dr Eckfeldt was a consultant for Gentian AS until December 2017; Dr Inker receives grant support from Retrophin, Reata, and Omeros Corp; Dr Michels received payment for a PD advisory counsil 06/2018. The remaining authors declare that they have no relevant financial interests.
Acknowledgements
We express our appreciation to the nurses in the PD center of the First Affiliated Hospital, Sun Yat-sen University, who collected demographic and clinical characteristics and follow-up data for patients, as well as all patients enrolled in the Guangzhou PD Study.
Peer Review
Received December 20, 2018. Evaluated by 2 external peer reviewers and a statistician, with editorial input from an Acting Editor-in-Chief (Editorial Board Member Roberto Minutolo, MD, PhD). Accepted in revised form April 15, 2019. The involvement of an Acting Editor-in-Chief to handle the peer-review and decision-making processes was to comply with Kidney Medicine’s procedures for potential conflicts of interest for editors, described in the Information for Authors & Journal Policies.
Footnotes
D.S. and L.F. contributed equally to the work.
Complete author and article information provided before references.
Table S1: Analytical measurements characteristics in the Guangzhou PD Study.
Table S2: Baseline demographics of excluded patients.
Table S3: Correlations between clearances and filtration markers in the combined, development, and internal validation cohort of the Guangzhou PD Study.
Table S4: Correlations between clearances and filtration markers and partial correlations between filtration markers adjusted for clearances in the Guangzhou PD Study and NECOSAD.
Table S5: Comparison of root-mean-square error (RMSE) of equations in the Guangzhou PD Study development data set during equation development (n = 552).
Table S6: Comparison of root-mean-square error (RMSE) of equations in the Guangzhou PD Study internal validation data set during equation validation (n = 271).
Table S7: Performance of equations in the Guangzhou PD Study combined data set (n = 823).
Table S8: Intercept and coefficients for the estimating equations developed in the Guangzhou PD Study combined data set compared to similar equations from Shafi et al.10
Table S9: Performance of estimating equations published by Shafi et al10 in the Guangzhou PD Study combined data set.
Table S10: Diagnostic accuracy of estimating equations to identify mClUN < 2 mL/min and mClUn-cr < 2.5 mL/min/1.73 m2 BSA in total NECOSAD, hemodialysis, and peritoneal dialysis subcohort.
Figure S1: Flow chart for patient inclusion in the Guangzhou PD Study.
Supplementary Material
Table S1. Analytical measurements characteristics in the Guangzhou PD Study.
Table S2. Baseline demographics of excluded patients.
Table S3. Correlations between clearances and filtration markers in the combined, development, and internal validation cohort of the Guangzhou PD Study.
Table S4. Correlations between clearances and filtration markers and partial correlations between filtration markers adjusted for clearances in the Guangzhou PD Study and NECOSAD.
Table S5. Comparison of root-mean-square error (RMSE) of equations in the Guangzhou PD Study development data set during equation development (n = 552).
Table S6. Comparison of root-mean-square error (RMSE) of equations in the Guangzhou PD Study internal validation data set during equation validation (n = 271).
Table S7. Performance of equations in the Guangzhou PD Study combined data set (n = 823).
Table S8. Intercept and coefficients for the estimating equations developed in the Guangzhou PD Study combined data set compared to similar equations from Shafi et al.10
Table S9. Performance of estimating equations published by Shafi et al10 in the Guangzhou PD Study combined data set.
Table S10. Diagnostic accuracy of estimating equations to identify mClUN < 2 mL/min and mClUn-cr < 2.5 mL/min/1.73 m2 BSA in total NECOSAD, hemodialysis, and peritoneal dialysis subcohort.
Figure S1. Flow chart for patient inclusion in the Guangzhou PD Study.
References
- 1.Bargman J.M., Thorpe K.E., Churchill D.N. Relative contribution of residual renal function and peritoneal clearance to adequacy of dialysis: a reanalysis of the CANUSA Study. J Am Soc Nephrol. 2001;12(10):2158–2162. doi: 10.1681/ASN.V12102158. [DOI] [PubMed] [Google Scholar]
- 2.Termorshuizen F., Korevaar J.C., Dekker F.W., van Manen J.G., Boeschoten E.W., Krediet R.T. The relative importance of residual renal function compared with peritoneal clearance for patient survival and quality of life: an analysis of the Netherlands Cooperative Study on the Adequacy of Dialysis (NECOSAD )-2. Am J Kidney Dis. 2003;41(6):1293–1302. doi: 10.1016/s0272-6386(03)00362-7. [DOI] [PubMed] [Google Scholar]
- 3.Paniagua R., Amato D., Vonesh E. Effects of increased peritoneal clearances on mortality rates in peritoneal dialysis: ADEMEX, a prospective, randomized, controlled trial. J Am Soc Nephrol. 2002;13(5):1307–1320. doi: 10.1681/ASN.V1351307. [DOI] [PubMed] [Google Scholar]
- 4.van der Wal W.M., Noordzij M., Dekker F.W. Full loss of residual renal function causes higher mortality in dialysis patients; findings from a marginal structural model. Nephrol Dial Transplant. 2011;26(9):2978–2983. doi: 10.1093/ndt/gfq856. [DOI] [PubMed] [Google Scholar]
- 5.Obi Y., Rhee C.M., Mathew A.T. Residual kidney function decline and mortality in incident hemodialysis patients. J Am Soc Nephrol. 2016;27(12):3758–3768. doi: 10.1681/ASN.2015101142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lo W.K., Bargman J.M., Burkart J. Guideline on targets for solute and fluid removal in adult patients on chronic peritoneal dialysis. Perit Dial Int. 2006;26(5):520–522. [PubMed] [Google Scholar]
- 7.Shafi T., Levey A.S. Measurement and estimation of residual kidney function in patients on dialysis. Adv Chronic Kidney Dis. 2018;25(1):93–104. doi: 10.1053/j.ackd.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang K., Kestenbaum B. Proximal tubular secretory clearance: a neglected partner of kidney function. Clin J Am Soc Nephrol. 2018;13(8):1291–1296. doi: 10.2215/CJN.12001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inker L.A., Schmid C.H., Tighiouart H. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367(1):20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shafi T., Michels W.M., Levey A.S. Estimating residual kidney function in dialysis patients without urine collection. Kidney Int. 2016;89(5):1099–1110. doi: 10.1016/j.kint.2015.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gerhardt T., Poge U., Stoffel-Wagner B. Serum levels of beta-trace protein and its association to diuresis in haemodialysis patients. Nephrol Dial Transplant. 2008;23(1):309–314. doi: 10.1093/ndt/gfm510. [DOI] [PubMed] [Google Scholar]
- 12.van Craenenbroeck A.H., Bragfors-Helin A.C., Qureshi A.R. Plasma beta-trace protein as a marker of residual renal function: the effect of different hemodialysis modalities and intra-individual variability over time. Kidney Blood Press Res. 2017;42(5):877–885. doi: 10.1159/000484537. [DOI] [PubMed] [Google Scholar]
- 13.Lindstrom V., Grubb A., Alquist Hegbrant M., Christensson A. Different elimination patterns of beta-trace protein, beta2-microglobulin and cystatin C in haemodialysis, haemodiafiltration and haemofiltration. Scand J Clin Lab Invest. 2008;68(8):685–691. doi: 10.1080/00365510802047693. [DOI] [PubMed] [Google Scholar]
- 14.Testa A., Gentilhomme H., Le Carrer D., Orsonneau J.L. In vivo removal of high- and low-molecular-weight compounds in hemodiafiltration with on-line regeneration of ultrafiltrate. Nephron Clin Pract. 2006;104(1):c55–c60. doi: 10.1159/000093671. [DOI] [PubMed] [Google Scholar]
- 15.Steubl D., Hettwer S., Dahinden P. C-Terminal agrin fragment (CAF) as a serum biomarker for residual renal function in peritoneal dialysis patients. Int Urol Nephrol. 2015;47(2):391–396. doi: 10.1007/s11255-014-0852-5. [DOI] [PubMed] [Google Scholar]
- 16.Steubl D., Roos M., Hettwer S. Comparison of peritoneal low-molecular-weight-protein-removal in CCPD and CAPD patients based on C-terminal agrin fragment clearance. Kidney Blood Press Res. 2016;41(2):175–185. doi: 10.1159/000443419. [DOI] [PubMed] [Google Scholar]
- 17.Huang S.H., Tirona R.G., Reid-Wilkinson F. The kinetics of cystatin C removal by hemodialysis. Am J Kidney Dis. 2015;65(1):174–175. doi: 10.1053/j.ajkd.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 18.Lysaght M.J., Pollock C.A., Moran J.E., Ibels L.S., Farrell P.C. Beta-2 microglobulin removal during continuous ambulatory peritoneal dialysis (CAPD) Perit Dial Int. 1989;9(1):29–35. [PubMed] [Google Scholar]
- 19.Kabanda A., Goffin E., Bernard A., Lauwerys R., van Ypersele de Strihou C. Factors influencing serum levels and peritoneal clearances of low molecular weight proteins in continuous ambulatory peritoneal dialysis. Kidney Int. 1995;48(6):1946–1952. doi: 10.1038/ki.1995.495. [DOI] [PubMed] [Google Scholar]
- 20.Wong J., Sridharan S., Berdeprado J. Predicting residual kidney function in hemodialysis patients using serum beta-trace protein and beta2-microglobulin. Kidney Int. 2016;89(5):1090–1098. doi: 10.1016/j.kint.2015.12.042. [DOI] [PubMed] [Google Scholar]
- 21.Hoek F.J., Korevaar J.C., Dekker F.W., Boeschoten E.W., Krediet R.T. Estimation of residual glomerular filtration rate in dialysis patients from the plasma cystatin C level. Nephrol Dial Transplant. 2007;22(6):1633–1638. doi: 10.1093/ndt/gfm027. [DOI] [PubMed] [Google Scholar]
- 22.Yang Q., Li R., Zhong Z. Is cystatin C a better marker than creatinine for evaluating residual renal function in patients on continuous ambulatory peritoneal dialysis? Nephrol Dial Transplant. 2011;26(10):3358–3365. doi: 10.1093/ndt/gfr045. [DOI] [PubMed] [Google Scholar]
- 23.Vilar E., Boltiador C., Wong J. Plasma levels of middle molecules to estimate residual kidney function in haemodialysis without urine collection. PLoS One. 2015;10(12) doi: 10.1371/journal.pone.0143813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blake P.G., Wilkie M. Peritoneal dialysis in China: a story of growth and innovation. Perit Dial Int. 2014;34(suppl 2):S27–S28. doi: 10.3747/pdi.2014.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shafi T., Levey A.S., Inker L.A. Plasma iohexol clearance for assessing residual kidney function in dialysis patients. Am J Kidney Dis. 2015;66(4):728–730. doi: 10.1053/j.ajkd.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Termorshuizen F., Dekker F.W., van Manen J.G., Korevaar J.C., Boeschoten E.W., Krediet R.T. Relative contribution of residual renal function and different measures of adequacy to survival in hemodialysis patients: an analysis of the Netherlands Cooperative Study on the Adequacy of Dialysis (NECOSAD)-2. J Am Soc Nephrol. 2004;15(4):1061–1070. doi: 10.1097/01.asn.0000117976.29592.93. [DOI] [PubMed] [Google Scholar]
- 27.Jansen M.A., Hart A.A., Korevaar J.C., Dekker F.W., Boeschoten E.W., Krediet R.T. Predictors of the rate of decline of residual renal function in incident dialysis patients. Kidney Int. 2002;62(3):1046–1053. doi: 10.1046/j.1523-1755.2002.00505.x. [DOI] [PubMed] [Google Scholar]
- 28.Inker L.A., Tighiouart H., Coresh J. GFR estimation using beta-trace protein and beta2-microglobulin in CKD. Am J Kidney Dis. 2016;67(1):40–48. doi: 10.1053/j.ajkd.2015.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levey A.S., Stevens L.A., Schmid C.H. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kulesa A., Krzywinski M., Blainey P., Altman N. Sampling distributions and the bootstrap. Nat Methods. 2015;12(6):477–478. doi: 10.1038/nmeth.3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.National Kidney Foundation KDOQI clinical practice guideline for hemodialysis adequacy: 2015 update. Am J Kidney Dis. 2015;66(5):884–930. doi: 10.1053/j.ajkd.2015.07.015. [DOI] [PubMed] [Google Scholar]
- 32.Fluss R., Faraggi D., Reiser B. Estimation of the Youden Index and its associated cutoff point. Biom J. 2005;47(4):458–472. doi: 10.1002/bimj.200410135. [DOI] [PubMed] [Google Scholar]
- 33.KDIGO 2012 clinical practice guidelines for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150. doi: 10.1038/ki.2013.243. [DOI] [PubMed] [Google Scholar]
- 34.White C.A., Ghazan-Shahi S., Adams M.A. beta-Trace protein: a marker of GFR and other biological pathways. Am J Kidney Dis. 2015;65(1):131–146. doi: 10.1053/j.ajkd.2014.06.038. [DOI] [PubMed] [Google Scholar]
- 35.Chakraborty D., Akbari A., Knoll G.A. Serum BTP concentrations are not affected by hepatic dysfunction. BMC Nephrol. 2018;19(1):87. doi: 10.1186/s12882-018-0881-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Donadio C., Tognotti D., Caponi L., Paolicchi A. Beta-trace protein is highly removed during haemodialysis with high-flux and super high-flux membranes. BMC Nephrol. 2017;18(1):68. doi: 10.1186/s12882-017-0489-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abrahamson M., Olafsson I., Palsdottir A. Structure and expression of the human cystatin C gene. Biochem J. 1990;268(2):287–294. doi: 10.1042/bj2680287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Argyropoulos C.P., Chen S.S., Ng Y.H. Rediscovering beta-2 microglobulin as a biomarker across the spectrum of kidney diseases. Front Med. 2017;4:73. doi: 10.3389/fmed.2017.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meert N., Eloot S., Schepers E. Comparison of removal capacity of two consecutive generations of high-flux dialysers during different treatment modalities. Nephrol Dial Transplant. 2011;26(8):2624–2630. doi: 10.1093/ndt/gfq803. [DOI] [PubMed] [Google Scholar]
- 40.Park J.S., Kim G.H., Kang C.M., Lee C.H. Application of cystatin C reduction ratio to high-flux hemodialysis as an alternative indicator of the clearance of middle molecules. Korean J Intern Med. 2010;25(1):77–81. doi: 10.3904/kjim.2010.25.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eloot S., Vanholder R., Dequidt C., Van Biesen W. Removal of different classes of uremic toxins in APD vs CAPD: a randomized cross-over study. Perit Dial Int. 2015;35(4):436–442. doi: 10.3747/pdi.2013.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Inker L.A., Levey A.S., Coresh J. Estimated glomerular filtration rate from a panel of filtration markers-hope for increased accuracy beyond measured glomerular filtration rate? Adv Chronic Kidney Dis. 2018;25(1):67–75. doi: 10.1053/j.ackd.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 43.Liu X., Foster M.C., Tighiouart H. Non-GFR determinants of low-molecular-weight serum protein filtration markers in CKD. Am J Kidney Dis. 2016;68(6):892–900. doi: 10.1053/j.ajkd.2016.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Foster M.C., Levey A.S., Inker L.A. Non-GFR determinants of low-molecular-weight serum protein filtration markers in the elderly: AGES-Kidney and MESA-Kidney. Am J Kidney Dis. 2017;70(3):406–414. doi: 10.1053/j.ajkd.2017.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peritoneal Dialysis Adequacy 2006 Work Group Clinical practice guidelines for peritoneal adequacy, update 2006. Am J Kidney Dis. 2006;48(suppl 1):S91–S97. doi: 10.1053/j.ajkd.2006.05.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Analytical measurements characteristics in the Guangzhou PD Study.
Table S2. Baseline demographics of excluded patients.
Table S3. Correlations between clearances and filtration markers in the combined, development, and internal validation cohort of the Guangzhou PD Study.
Table S4. Correlations between clearances and filtration markers and partial correlations between filtration markers adjusted for clearances in the Guangzhou PD Study and NECOSAD.
Table S5. Comparison of root-mean-square error (RMSE) of equations in the Guangzhou PD Study development data set during equation development (n = 552).
Table S6. Comparison of root-mean-square error (RMSE) of equations in the Guangzhou PD Study internal validation data set during equation validation (n = 271).
Table S7. Performance of equations in the Guangzhou PD Study combined data set (n = 823).
Table S8. Intercept and coefficients for the estimating equations developed in the Guangzhou PD Study combined data set compared to similar equations from Shafi et al.10
Table S9. Performance of estimating equations published by Shafi et al10 in the Guangzhou PD Study combined data set.
Table S10. Diagnostic accuracy of estimating equations to identify mClUN < 2 mL/min and mClUn-cr < 2.5 mL/min/1.73 m2 BSA in total NECOSAD, hemodialysis, and peritoneal dialysis subcohort.
Figure S1. Flow chart for patient inclusion in the Guangzhou PD Study.