Related Article, p 286
In this issue of Kidney Medicine, Karaboyas et al1 report on an interesting study that attempts to solidify the linkage between inflammation and erythropoiesis-stimulating agent (ESA) hyporesponsiveness in patients undergoing hemodialysis. This subject is important and deserves closer scrutiny. In this editorial, we explore this relationship and review how both inflammation and ESA hyporesponsiveness connect to adverse outcomes among patients undergoing hemodialysis. As reviewed, the subject is currently understood as a group of intertwined relationships that may ultimately increase patients’ risk for death.
Central to this subject is the clinical syndrome of ESA resistance or hyporesponsiveness. Though it has been defined in different numeric ways, we oversimplify by describing it as difficulty reaching hemoglobin targets despite treatment with higher ESA doses. It is a state that might be transient or chronic and could range from mild to severe on a spectrum. The development of ESA hyporesponsiveness may not be noticed by the nephrologist because in most dialysis facilities, ESA dose adjustments are managed by nurses using protocols supported by computerized dosing suggestions. This is an efficient process but it tends to distance the nephrologist from anemia management and may cause ESA hyporesponsiveness to go unrecognized.
There are 3 reasons why ESA hyporesponsiveness may be clinically relevant. The first is obvious, that inability to achieve target hemoglobin levels may result in unresolved symptoms such as fatigue. Second, the cause of ESA hyporesponsiveness (such as severe hyperparathyroidism, iron deficiency, or occult gastrointestinal blood loss) may require further intervention and/or treatment. Third, ESA hyporesponsiveness is strongly associated with increased risk for mortality.2
The cause of this mortality relationship is not clear. It may simply be that ESA hyporesponsiveness is identifying sicker patients, those with comorbid conditions or other disease processes that would cause an increased risk for death. Alternatively, ESA hyporesponsiveness may contribute as a causal factor for increased mortality risk by resulting in the need for higher ESA doses. There has been a clearly demonstrated association between higher ESA doses and increased risk for mortality. Zhang et al3 used US Renal Data System data to establish that higher ESA doses, independent of hemoglobin level, were associated with increased risk for death.
Szczech et al4 performed a reanalysis of the Correction of Hemoglobin and Outcomes in Renal Insufficiency (CHOIR) trial. This trial found that patients with non–dialysis-dependent chronic kidney disease randomly assigned to a higher compared with a lower hemoglobin target during epoetin alfa treatment experienced increased risk for death, myocardial infarction, congestive heart failure, or stroke. In their post hoc analysis, it was epoetin alfa dose, not hemoglobin level, that correlated best with increased risk for adverse outcomes.4
Similarly, Solomon et al5 reanalyzed the Trial to Reduce Cardiovascular Events With Aranesp Therapy (TREAT), which had found increased stroke risk in patients with type 2 diabetes and chronic kidney disease treated with darbepoetin to a target hemoglobin level of 13 g/dL compared with placebo. They found that patients with ESA hyporesponsiveness had higher rates of a composite cardiovascular end point or death.5
Taken together, these studies clearly link higher ESA dose and risk for cardiovascular events and death. Whether this relationship is causal is controversial and would be difficult to elucidate. Nonetheless, it can be seen that ESA hyporesponsiveness and resulting higher ESA doses are at least associated with adverse outcomes. Accordingly, a conservative approach to anemia management is warranted. The US Food and Drug Administration recommends that the lowest possible ESA doses necessary to avoid transfusions should be used.
The next set of relationships that we examine are those between inflammation and ESA hyporesponsiveness and between inflammation and mortality risk. Inflammation is frequently present in patients receiving maintenance hemodialysis. Various markers of systemic inflammation, including C-reactive protein (CRP), interleukin 1 (IL-1) receptor antagonist, IL-1β, and IL-6 levels, have been shown to be increased long-term in patients receiving maintenance dialysis.6 In patients with infections or smoldering wounds, the cause of inflammation is straightforward. However, in most other patients receiving hemodialysis, it is unclear why inflammation is present.
Central to the purpose of the study by Karaboyas et al1 is clarifying whether inflammation causes ESA hyporesponsiveness. As the authors note in their introduction section, the relationship has already been reported in previous cross-sectional or longitudinal observational studies. To their credit, the investigators use a clever methodology in the current study to more firmly establish the association. They began by specifically identifying patients with normal CRP levels for 3 months from among 12,389 hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study (DOPPS) from 2009 to 2018. In this way they selected patients who probably were free of clinically meaningful inflammation. From this group they identified 3,568 new episodes of inflammation, which they reasonably defined as the new onset of CRP level > 10 mg/L. Thus, this was a novel study cohort composed of patients who were initially stable but subsequently developed inflammation. By assessing the corresponding change in ESA responsiveness, the investigators were able to evaluate the effect of the development of inflammation on anemia and ESA responsiveness. The authors found that after the increase in CRP levels, hemoglobin levels declined rapidly and ESA dose requirements increased.1 These results clearly add to the previous literature and strengthen the observation of a relationship between inflammation and ESA hyporesponsiveness.
The relationship between inflammation and mortality risk among patients treated with hemodialysis has previously been reported. Elevated CRP,7 IL-6,8 IL-1, and tumor necrosis factor α6,9 levels have been found to independently predict all-cause mortality in patients receiving hemodialysis. The association between inflammation and increased mortality risk among hemodialysis patients could be mediated by an underlying disease process. For example, cardiovascular disease causes both inflammation and increased risk for death; the finding of increased levels of inflammatory biomarkers may simply reflect the underlying disease state. Alternatively, inflammation by itself could be harmful and might directly increase mortality risk. Furthermore, inflammation, by increasing ESA hyporesponsiveness as discussed in the preceding paragraph, could contribute to increased risk for mortality by leading to a requirement for higher ESA doses.
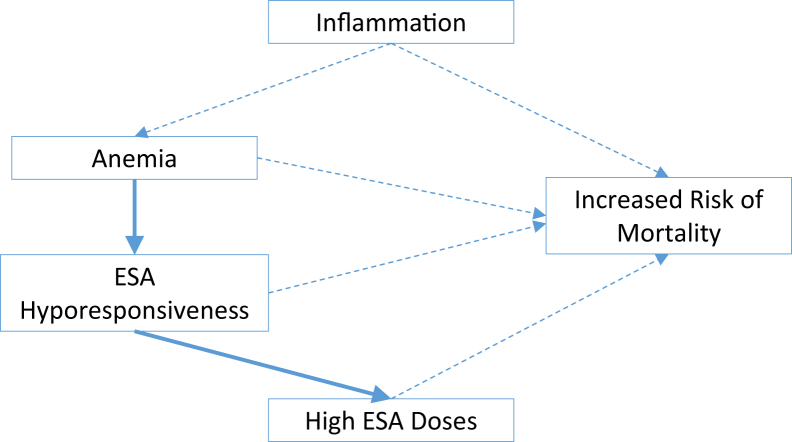
Among the relationships that might be important in improving patient outcomes, we have seen that inflammation, ESA hyporesponsiveness, and higher ESA doses are associated with increased mortality. The intertwining of these associations (Fig 1) and the fact that none are definitively causal creates interesting questions for the development of agents aiming to reduce the excessive mortality risk of hemodialysis patients. Possible targets could be any of the direct inflammatory mediators such as IL-6 or tumor necrosis factor α.
Figure 1.
Factors related to anemia that have been related to adverse outcomes. Most of these relationships are demonstrated associations but without proof of causality (represented by dashed lines with arrows). Solid lines are used to display causal relationships, anemia and erythropoiesis-stimulating agent (ESA) hyporesponse, and higher ESA doses.
A related target could be hepcidin or pathways that it affects. Hepcidin is a protein that has been identified as the master regulator of iron homeostasis.10 Beyond regulating iron stores, hepcidin also plays an important role in infection and inflammation. In these states, hepcidin levels increase and iron is blocked from entering the circulation.10 This is what causes anemia of infection and inflammation. Microorganisms often have a great need for iron as a growth factor. Teleologically it would appear that the body is responding to the threat of infection in its release of hepcidin. When a patient is infected, this positive adaptive response may enhance survival by limiting the microorganism’s access to iron. In contrast, with inflammation not caused by anemia, as is often the case in hemodialysis patients, increased hepcidin levels and the resulting iron restriction may be maladaptive, causing an unnecessary worsening of anemia. Drugs that block the hepcidin effect could increase iron availability and reduce ESA hyporesponsiveness. Hypoxia inducible factor-prolyl hydroxylase drugs are currently in development.11 They cause endogenous erythropoietin release and improved iron availability, while indirectly reducing hepcidin levels. Because they cause much lower increases in serum erythropoietin levels than ESAs, the possible toxicity of the latter agents might be avoided. At the time of this writing, the full spectrum of efficacy and safety of these drugs from phase 3 studies has not been published.
Irrespective of the target of drug development, a second question is the outcome measures following intervention. An inhibitor of the maladaptive inflammation present in hemodialysis patients could be studied as a way to improve hemoglobin levels or reduce ESA dose requirements, and those would be worthy outcomes. Alternatively, because the ultimate goal is to improve outcomes and the pathways that we have discussed converge at reduced mortality risk, it might be that a formal cardiovascular outcomes trial would be justified. Like most design questions, there is a major tradeoff between relevant outcome measures and the practicality and risk built into a study. This is no clear correct answer, but the potential for improved treatment and outcomes is exciting. Karaboyas et al1 have firmed the relationship between inflammation and anemia treatment through ESA response. It is now time for interventional studies.
Article Information
Authors’ Full Names and Academic Degrees
Hitesh H. Shah, MD, Nupur N. Uppal, MD, and Steven Fishbane, MD.
Support
None.
Financial Disclosure
Dr Fishbane has received research and consulting funds from Astra Zeneca, Akebia, Megapro, and Corvidia. The remaining authors declare that they have no relevant financial interests.
Peer Review
Received March 4, 2020, in response to an invitation from the journal. Direct editorial input by the Editor-in-Chief. Accepted in revised form March 30, 2020.
References
- 1.Karaboyas A., Morgenstern H., Fleischer N.L. Inflammation and erythropoiesis-stimulating agent response in hemodialysis patients: a self-matched longitudinal study of anemia management in the Dialysis Outcomes and Practice Patterns Study (DOPPS) Kidney Med. 2020;2(3):286–296. doi: 10.1016/j.xkme.2020.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kainz A., Mayer B., Kramar R., Oberbauer R. Association of ESA hypo-responsiveness and haemoglobin variability with mortality in haemodialysis patients. Nephrol Dial Transplant. 2010;25(11):3701–3706. doi: 10.1093/ndt/gfq287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang Y., Thamer M., Stefanik K., Kaufman J., Cotter D.J. Epoetin requirements predict mortality in the secondary analysis of the CHOIR trial epoetin-alpha dose and achieved hemoglobin outcomes. Am J Kidney Dis. 2004;44(5):866–876. [PubMed] [Google Scholar]
- 4.Szczech L.A., Barnhart H.X., Inrig J.K. Secondary analysis of the CHOIR trial epoetin-alpha dose and achieved hemoglobin outcomes. Kidney Int. 2008;74(6):791–798. doi: 10.1038/ki.2008.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Solomon S.D., Uno H., Lewis E.F. Erythropoietic response and outcomes in kidney disease and type 2 diabetes. Trial to Reduce Cardiovascular Events with Aranesp Therapy (TREAT) Investigators. N Engl J Med. 2010;363(12):1146–1155. doi: 10.1056/NEJMoa1005109. [DOI] [PubMed] [Google Scholar]
- 6.Nowak K.L., Chonchol M. Does inflammation affect outcomes in dialysis patients? Semin Dial. 2018;31(4):388–397. doi: 10.1111/sdi.12686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zimmermann J., Herrlinger S., Pruy A., Metzger T., Wanner C. Inflammation enhances cardiovascular risk and mortality in hemodialysis patients. Kidney Int. 1999;55:648-658. doi: 10.1046/j.1523-1755.1999.00273.x. [DOI] [PubMed] [Google Scholar]
- 8.Beberashvili I., Sinuani I., Azar A. IL-6 levels, nutritional status, and mortality in prevalent hemodialysis patients. Clin J Am Soc Nephrol. 2011;6:2253–2263. doi: 10.2215/CJN.01770211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kimmel P.L., Phillips T.M., Simmens S.J. Immunologic function and survival in hemodialysis patients. Kidney Int. 1998;54:236–244. doi: 10.1046/j.1523-1755.1998.00981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ganz T., Nemeth E. Hepcidin and disorders of iron metabolism. Annu Rev Med. 2011;62:347–360. doi: 10.1146/annurev-med-050109-142444. [DOI] [PubMed] [Google Scholar]
- 11.Locatelli F., Fishbane S., Block G.A., Macdougall I.C. Targeting hypoxia-inducible factors for the treatment of anemia in chronic kidney disease patients. Am J Nephrol. 2017;45(3):187–199. doi: 10.1159/000455166. [DOI] [PubMed] [Google Scholar]