Abstract
Patients with chronic kidney disease (CKD) are at increased risk for infection, attributable to immune dysfunction, increased exposure to infectious agents, loss of cutaneous barriers, comorbid conditions, and treatment-related factors (eg, hemodialysis and immunosuppressant therapy). Because iron plays a vital role in pathogen reproduction and host immunity, it is biologically plausible that intravenous iron therapy and/or iron deficiency influence infection risk in CKD. Available data from preclinical experiments, observational studies, and randomized controlled trials are summarized to explore the interplay between intravenous iron and infection risk among patients with CKD, particularly those receiving maintenance hemodialysis. The current evidence base, including data from a recent randomized controlled trial, suggests that proactive judicious use of intravenous iron (in a manner that minimizes the accumulation of non–transferrin-bound iron) beneficially replaces iron stores while avoiding a clinically relevant effect on infection risk. In the absence of an urgent clinical need, intravenous iron therapy should be avoided in patients with active infection. Although serum ferritin concentration and transferrin saturation can help guide clinical decision making about intravenous iron therapy, definition of an optimal iron status and its precise determination in individual patients remain clinically challenging in CKD and warrant additional study.
Index Words: Chronic kidney disease, infection, intravenous iron, hemodialysis, safety, iron deficiency, immunity
Introduction
Iron is required for both pathogen reproduction and host immunity, and in healthy individuals, iron homeostasis is tightly regulated. In chronic kidney disease (CKD), iron homeostasis is disordered as a result of increased iron losses, reduced iron absorption, and disruptions in iron storage and mobilization.1 Systemic iron homeostasis can be further affected by the administration of intravenous (IV) iron, a common practice in the management of renal anemia. In the United States, more than three-quarters of hemodialysis (HD) patients have received IV iron in the prior 3 months.2 Although most patients with non–dialysis-dependent CKD demonstrate evidence of iron deficiency,3 IV iron use is infrequent (∼10%) among these patients.4
It is recognized that “imbalances of iron homeostasis can affect the risk for, and the outcome of, infections”5(p32) but the clinical impact of therapeutic iron on this risk remains unclear. Whereas administration of IV iron has been implicated as a potential risk factor contributing to increased infection risk in CKD by some sources,6,7 clinical evidence supporting such an association is currently lacking. At the Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference on Iron Management in CKD in 2014, participants noted that much of the available data were derived from observational trials and that evidence examining an association between IV iron use and infection was “conflicting” and “inconclusive.”5
To that end, an international multidisciplinary group of experts was convened to examine available evidence and provide practical guidance for the use of IV iron in CKD with regard to infection risk. The meeting was sponsored by Vifor Fresenius Medical Care Renal Pharma, which manufactures several iron replacement therapies. Based on discussions at the meeting, this review examines the interplay between IV iron and infection risk among patients with CKD, particularly those receiving maintenance HD. We review laboratory data on the impact of iron administration on pathogen infectivity/virulence/growth and immune cell function. We examine potential lessons from other disease states before summarizing data from observational and randomized controlled trials (RCTs) conducted in CKD populations. Finally, we discuss evidence gaps and practical considerations for today’s clinical practice.
Although the authors examined the published literature critically, we did not perform a formal systematic review or formally grade the quality of evidence. Whereas this article examines the potential association between iron and infection risk, the reader is reminded that appropriate use of IV iron requires a balanced consideration of all of the associated benefits (eg, impact on anemia, heart failure, and cardiovascular outcomes) and risks (eg, allergic reaction and iron accumulation).
Infection in CKD
Infections are a leading contributor of morbidity and mortality among patients with CKD. They are the second most common cause of death in patients with end-stage kidney disease, and compared with the general population, patients receiving maintenance HD are approximately 100-fold more likely to die of sepsis.8,9 Such findings likely result from increased susceptibility to infection and impaired recovery from established infections. The increased risk for infection associated with CKD is not limited to patients with more advanced disease; patients with relatively preserved kidney function (eg, CKD stage 2) experience significant increases in infection-related hospitalization.10,11 In addition to more frequent infection-related hospitalization, the average length of stay for such admissions is nearly 10-fold longer than for the general population.12 The hospitalization rate among HD patients in the United States has remained similar from 2006 through 2016.8
Beyond the direct effects of infection, data suggest an interplay between infection and cardiovascular events. As observed in non-CKD populations,13 infectious episodes predict increased risk for subsequent cardiovascular events in patients with advanced CKD (ie, estimated glomerular filtration rate of 15-45 mL/min/1.73 m2).14 In the Canadian Study of Prediction of Risk and Evolution to Dialysis, Death and Interim Cardiovascular Events Over Time (CanPREDDICT), infection (ie, positive culture, use of antibiotics, or hospitalization for infection) was associated with 80% and 220% increases in risk for subsequent cardiovascular ischemia and congestive heart failure, respectively (median follow-up, 3.5 years).14 Similarly, in the Proactive IV Iron Therapy in Haemodialysis Patients (PIVOTAL) trial, there was a strong association between a first cardiovascular event and an infectious episode in the month leading up to the event (hazard ratio [HR], 2.83; 95% confidence interval [CI], 2.04-3.92).15
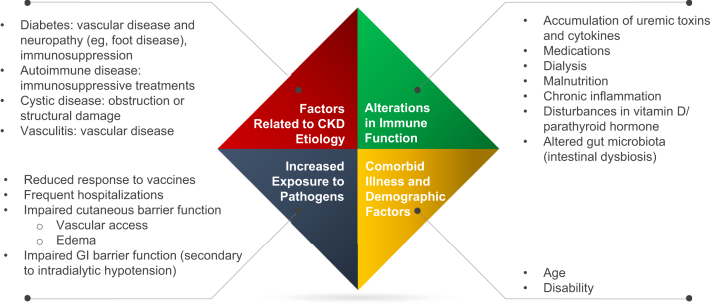
The association between CKD and increased infection risk is well established and appears to result from multiple contributing factors (Fig 1).6,7,16, 17, 18, 19, 20 Among patients with CKD not requiring dialysis, urinary tract infections and pneumonia represented the second and third most common causes of hospitalization, respectively, and were 59% and 49% higher in patients with CKD compared with patients without CKD.21 In contrast, in a retrospective review of patients receiving maintenance dialysis, vascular access device infections and skin and soft tissue infections (including below-the-knee infections) accounted for nearly 50% of infections.22 In the same study, cultures identified Gram-positive cocci (eg, staphylococci) and aerobic Gram-negative rods (eg, Pseudomonas aeruginosa and Escherichia coli) in 48% and 35% of infections, respectively. Patients with end-stage kidney disease are also at significantly increased risk for developing active tuberculosis.23 In addition, patients are at increased risk from viral and fungal infections. In one study, patients with CKD were more than 17 times more likely than the general population to be hospitalized for influenza.24
Figure 1.
Factors contributing to increased risk for infection and infection-related morbidity in chronic kidney disease (CKD).6,7,16, 17, 18, 19, 20 Abbreviation: GI, gastrointestinal.
Iron’s Vital Role in Cellular Function, Infection, and Immunity
The ability of iron to donate and accept electrons permits it to function in reactions that contribute to energy production, oxygen transport, and DNA synthesis.25,26 Inadequate iron can lead to impaired cellular growth and cell death.25 In contrast, an excess of iron can also lead to cell injury secondary to the formation of hydroxyl or lipid radicals.25,26 To control the amount of iron available to cells and prevent a toxic excess or deficit, iron is tightly regulated. Several proteins function as iron chaperones to prevent “free” or unbound iron from forming destructive radicals or being easily obtained by pathogens.27 These proteins are all considered acute-phase reactants and their concentrations are affected by infection and/or inflammation (Table 1).26, 27, 28, 29 Iron is released from transporting or storage cells (ie, enterocytes, macrophages, and hepatocytes) exclusively through the iron exporter ferroportin. Iron export is tightly regulated through modulation of production of the hormone hepcidin such that higher levels of hepcidin are associated with reduced iron export from storage cells.30 As explored next, blood hepcidin concentration is increased in the setting of inflammation, infection, and IV iron administration, thereby restricting iron export.1
Table 1.
Selected Protein Chaperones for Iron in Humans
| Chaperone Protein | Molecule Bound | Location | Function |
|---|---|---|---|
| Transferrin | Iron | Plasma and extracellular fluid | Iron transporter |
| Lactoferrin | Iron | Mucosal secretory fluids (tears, breast milk), phagocytes | Iron chelator |
| Ferritin | Iron | Intracellular and extracellular (secreted by hepatocytes and macrophages) | Iron storage |
| Haptoglobin | Hemoglobin | Plasma and extracellular fluid | Clear free hemoglobin |
| Hemopexin | Heme | Plasma and extracellular fluid | Clear free heme |
Iron is also required for growth of virtually all human pathogens.26,27 As such, many pathogens have evolved elegant mechanisms to evade our physiologic iron-withholding mechanisms and successfully “steal” iron. Some organisms have developed means for capturing free iron, whereas others can acquire protein-bound iron. Siderophores, secreted iron chelators, can bind iron with higher affinity than many chaperone proteins.26,27 Extracellular bacteria can also obtain iron through heme acquisition (ie, direct heme uptake systems and hemophore-dependent systems), endocytosis of transferrin or lactoferrin molecules through membrane-bound receptors, and ferric/ferrous iron transporters.26 Fungi can acquire iron through similar mechanisms, and some fungi even use ferric reductases to dissociate iron from chelators.26 Intracellular bacteria can acquire iron from host cells by using siderophores, by direct acquisition of host cytoplasmic iron, and/or by affecting host cell homeostasis to increase iron availability inside phagosomes.26 Finally, viruses also require iron for propagation and can acquire it by altering host-cell iron homeostasis, in some cases by altering cellular processing of iron, leading to relative iron loading within cells.31
Because iron is necessary for cellular growth and reproduction, strategies that restrict or limit pathogen access to iron can serve as an effective arm of innate immunity. This “nutritional immunity” relies on a number of host mechanisms to restrict iron availability.26,28 Infection triggers a rapid reduction in plasma iron concentration.32 Much of this hypoferremic response results from an increase in hepcidin level triggered by inflammatory cytokines, particularly interleukin 6 (IL-6).27,28 Whereas hepcidin is a major contributor to hypoferremia, non–hepcidin-dependent mechanisms have also been identified.28,33,34 Lactoferrin, found in milk, mucosal secretions, and sweat, binds iron at mucosal surfaces. It is also released within neutrophils and can bind to and retain iron in acidic environments, suggesting that it may be effective after a clinical infection has already started.26, 27, 28,34 Animal studies confirm the importance of lactoferrin in preventing skin infections by Staphylococcus aureus.35
As part of an immune strategy to limit iron acquisition by pathogens, lipocalin 2 (also referred to as siderocalin and neutrophil gelatinase–associated lipocalin) can be released by neutrophils, macrophages, and epithelial cells to bind and eliminate some microbial siderophores.26,28,34 Whereas hepcidin-induced retention of iron in macrophages is seemingly beneficial in preventing infections with extracellular pathogens,28 such a strategy could potentially be counterproductive for prevention of infections caused by Mycobacterium tuberculosis, an intracellular bacterium that reproduces in macrophage phagosomes. Alterations in cell membrane–bound transferrin receptors and ferroportin expression that may reduce iron availability have been documented in macrophages following infection with M tuberculosis.26,36,37 The immune system also uses iron to catalyze the generation of reactive oxygen species by phagocytes that can directly lead to oxidative damage of pathogens. Reactive oxygen species have also been implicated in a number of nonoxidative immune mechanisms.38
Laboratory Evidence Examining Iron Availability and Risk for Infection
Given that pathogen growth, host growth, and host immunity all require cellular access to iron, the net effect of increased iron availability can only be understood by examining the effects of “extra” iron on pathogen growth and the immune system. Experimental data have demonstrated that the growth of a number of pathogenic fungi, bacteria, viruses, and protozoa is stimulated by increased availability of iron.39,40 These findings extend to common causes of infectious disease (eg, Escherichia species, staphylococci, and mycobacteria) and pathogens less commonly encountered in clinical practice (eg, Vibrio species). In animal studies, IV administration of various forms of iron (eg, lysed red blood cells and iron dextran) has also been associated with increased virulence of many bacterial pathogens.41 However, the effect of iron on the virulence of bacterial species appears to vary by subtype/strain. This suggests an inconsistent evolutionary benefit of such iron-dependent growth strategies.42
Murine models have demonstrated that genetic ablation of hepcidin is associated with increased risk for infection, but the effect is largely limited to extracellular Gram-negative infections (Yersinia enterocolitica, Vibrio vulnificus, Klebsiella pneumoniae, and E coli).28,43, 44, 45, 46 In similar studies, hepcidin deficiency did not appear to increase the risk for infection associated with S aureus (Gram positive) or M tuberculosis (intracellular).44 In models of hepcidin deficiency, the increased risk for infection/bacterial growth observed with some Gram-negative bacteria appears to result from accumulation of non–transferrin-bound iron.28,44, 45, 46
The association between non–transferrin-bound iron levels and Gram-negative bacterial growth has been confirmed in several studies. Stefanova et al45 observed that the presence/absence of non–transferrin-bound iron in human plasma was a determinant of E coli growth. In an ex vivo experiment, Cross et al47 demonstrated that the growth of E coli, Y enterocolitica, and Salmonella typhimurium in serum collected after oral iron supplementation correlated with transferrin saturation (TSAT) levels.47 In that study, mean TSAT after iron administration was 75.7%47; non–transferrin-bound iron is generally detectable when TSAT is >75%.48 Finally, a strong linear relationship between non–transferrin-bound iron levels and the growth of a pathogenic strain of E coli in serum collected after transfusion of “older” red blood cells (ie, 40-42 days of storage) was observed among healthy volunteers.49 Data regarding the impact of supplemental iron on the growth of bacteria that spend at least part of their life cycle intracellularly (eg, M tuberculosis and S typhimurium) are conflicting.50
Given the variety of specialized immune cells, it is not surprising that experimental increases in iron concentration and availability appear to have differential effects on cells of the innate and adaptive immune systems. Evidence from in vitro and in vivo studies indicates that iron overload is preferentially associated with anomalies in T-lymphocyte function and the relative expansions of the 2 major T-cell populations (ie, CD8+ and CD4+).51,52 In return, selective T-lymphocyte defects influence the progression of iron overload.53,54 In rats, iron overload results in expansion of suppressor/cytotoxic T cells (CD8+) relative to T-helper (CD4+) cells.55 Similar imbalances in T-cell sets have been observed in patients with beta thalassemia major, such that increased numbers of CD8+ T lymphocytes correlate with increasing numbers of blood transfusions.56 Within the category of T-helper cells, the helper T cell type 1 subset, responsible for the production of interferon γ and tumor necrosis factor β, which activate macrophages, are more sensitive to changes in iron levels.34 In turn, as macrophage iron levels increase, there is inhibition of interferon γ–mediated pathways.34,41 The net result of these changes is a potential for impaired immunity against intracellular bacteria. Increased iron levels have also been associated with impaired neutrophil phagocytic function and M1 polarization of macrophages.57, 58, 59 Although a complete review of the immunologic effects of iron deficiency is beyond the scope of this review, iron deficiency appears to have immunosuppressive effects. Specifically, iron deficiency has been associated with impaired cell-mediated immune response and reduced CD4+ T-cell counts, reduced antibody concentrations, and reduced expression of some cytokines (eg, IL-2 and IL-6).60,61
KDIGO recommendations against the use of IV iron in patients with active systemic infection are supported by experimental data.5 IV iron administration in control animals resulted in oxidative stress, a modest inflammatory state, and no mortality, but the administration of iron in septic animals resulted in a mortality rate of ∼60%.62
Lessons from Inherited Iron Disorders
In HD patients, and less so among non–dialysis-dependent patients with CKD and peritoneal dialysis patients, IV iron is commonly administered on an ongoing basis, a practice that may place patients in a chronic state of positive iron balance.1 As such, we endeavored to examine the risk for infection in other conditions associated with increased total-body iron stores. Mutations in HFE, a gene encoding a major histocompatibility complex I–like protein that modulates hepcidin expression, account for most cases of hereditary hemochromatosis.63 Patients with HFE-associated hemochromatosis (Online Mendelian Inheritance in Man [OMIM] Type 1) exhibit increased tissue iron secondary to increased release of iron from intestinal cells and macrophages.64 Patients with the disorder, even those with unrecognized disease, are at increased risk for severe infections with V vulnificus and Y enterocolitica.28,65 HFE-associated hemochromatosis has also been associated with lymphocyte abnormalities, including reduced CD8+ T-lymphocyte numbers (driven by reductions in the most mature/differentiated effector memory T cells).66, 67, 68 It is worth noting that there is no evidence that these lymphocyte abnormalities influence infection risk, and at the population level, HFE-associated hemochromatosis does not affect survival.69 Increased risk for infection has also been observed in cases of transfusional iron overload, a potential consequence of repeated transfusions used to manage inherited conditions such as thalassemia major, sickle cell disease, and Diamond-Blackfan anemia.70 Infection is a major cause of death in this population, and this risk has been associated with increased availability of labile iron.70
Several parameters may limit the generalizability of these data to the use of IV iron in patients with CKD. Both the magnitude and duration of exposure to non–transferrin-bound iron likely affect the organ damage observed in hereditary conditions.71 HFE-associated hereditary hemochromatosis can be associated with long-term TSAT elevations (eg, >75%) and long-term exposure to non–transferrin-bound iron.64,72,73 Such findings are not generally observed with current IV iron use practices in the CKD population. Additionally, HFE-associated hereditary hemochromatosis results in a parenchymal deposition pattern of hepatic iron, not the reticuloendothelial macrophage deposition pattern observed with IV iron or transfusion.1,74 The classic form of ferroportin disease (OMIM Type 4A), a form of hereditary hemochromatosis in which a ferroportin loss-of-function mutation results in normal TSAT and a reticuloendothelial iron deposition pattern, may be a better model for IV iron use in CKD.1,75 Unfortunately, little is known about the impact of ferroportin mutations on infection risk, and no data exist regarding the epidemiology of infection among patients with ferroportin disease.76
Evidence suggesting that hepatic distribution of iron can affect clinical outcomes (including infection) was observed in the Hepatitis C Anti-Viral Long-Term Treatment to Prevent Cirrhosis (HALT-C) trial, in which the presence of stainable iron in macrophages or portal stromal cells was not associated with adverse clinical outcomes, whereas higher baseline iron levels in hepatocytes and portal triads was.77 Finally, differences in the timing and magnitude of iron loading among inherited diseases versus transfusional iron overload versus patients with CKD receiving IV iron may further limit the generalizability of findings from one disease state to another.
Examining the Risk for Infection Associated with IV Iron in Non-CKD States
IV iron is used in the management of anemia across therapeutic areas and clinical settings. As detailed in Table 2, the largest meta-analysis (which includes studies in renal and nonrenal fields) demonstrates 33% increased risk for all-cause infections following IV iron administration, whereas 3 smaller meta-analyses with a narrower scope indicate no association or even reduced perioperative infections in the setting of IV iron supplementation in patients undergoing orthopedic surgery.78, 79, 80, 81 Whereas the evidence surrounding the effect of iron administered for therapeutic purposes is mixed, iron deficiency has been consistently associated with increased risk for infection.82 For example, a large prospective population-based study from Norway demonstrated that TSAT ≤ 9% was associated with a 45% increase in the incidence of bloodstream infections (after adjustment for multiple demographic characteristics and comorbid conditions).83
Table 2.
Relationship Between IV Iron Use and Infection Risk in Non-CKD Populations in Meta-analyses
| Analysis | Studies and Populations Included | Comparison | Results |
|---|---|---|---|
| Shah78 (2019) | 6 RCTs conducted in adults admitted to surgical intensive care unit (4 studies) or mixed intensive care units (2 studies); N = 805 | Iron vs no iron (5 trials included an IV iron arm) | No difference in risk for in-hospital infection; risk ratio, 0.95 (95% CI, 0.79-1.19) |
| Shin79 (2019) | 12 clinical studies of patients undergoing orthopedic surgery; 4 RCTs (N = 616); 8 case-controlled studies (N = 1,253) | Perioperative IV iron vs no IV iron | IV iron was associated with lower risk for postoperative infection; risk ratio, 0.67 (95% CI, 0.49-0.91) |
| Shah80 (2018) | 2 RCTs conducted in adults undergoing hip fracture surgery; (N = 503) | IV iron vs control | No difference in risk for infection; risk ratio, 0.99 (95% CI, 0.55-1.80) |
| Litton81 (2013) | 72 RCTs conducted in renal (n = 19), obstetric (n = 19), surgical (n = 11), oncology/hematology (n = 11), cardiology (n = 4), gastroenterology (n = 4), and other (n = 7) settings; (total N = 10,605) | IV iron vs oral/no iron | In 24 studies with data, IV iron was associated with increased risk for all-cause infection; relative risk, 1.33 (95% CI, 1.10-1.64); no interaction between baseline ferritin, TSAT, iron dose, or ESA use and risk for infection |
Abbreviations: CI, confidence interval; CKD, chronic kidney disease; ESA, erythropoiesis-stimulating agent; IV, intravenous; RCT, randomized controlled trial; TSAT, transferrin saturation.
Observational Studies Examining the Risk for Infection Associated with IV Iron in CKD
To examine the relationship between IV iron administration and infection risk in the setting of CKD, we evaluated both observational studies and RCTs. Although observational studies are prone to treatment selection bias and residual confounding, thus limiting the ability to infer causality and magnitude of effect, these studies can be used for hypothesis generation. Potential biases include which patients are prescribed iron, the formulation of iron administered, dose of iron prescribed, and duration of therapy. Other sources of confounding include achieved iron status, hemoglobin levels, comorbid illness, concomitant anemia therapies, and potential center effects. When evaluating results of observational studies, it has been suggested that weaker associations (eg, relative risk < 4) may be attributable to confounding.84,85
Observational data examining a potential association between infection and IV iron are lacking in non–dialysis-dependent CKD populations, and we were unable to identify any relevant studies for this population. Data from populations undergoing peritoneal dialysis are very limited. In a single retrospective analysis of 61 patients who received 2 doses of 500 mg of IV iron dextran and/or iron sucrose, peritonitis rates were numerically (but not statistically) greater in the 6 months after IV iron administration compared with the 6 months before therapy.86
At the time of the KDIGO Controversies Conference on Iron Management in CKD,5 participants used the 2014 critical review by Ishida and Johansen87 as a starting point for their evidence base. That review concluded, “Overall, the current body of literature appears to favor an association between iron and infection in hemodialysis patients, though several limitations must be acknowledged and publication bias cannot be ruled out.”87(p34) Macdougall et al5 subsequently identified 4 additional observational studies for their assessment. Three of those studies88, 89, 90 found no increase in either infection-related mortality or hospitalization with IV iron use or higher ferritin concentrations, whereas 1 study91 demonstrated a significant risk. After the KDIGO Controversies Conference was held, Ishida et al92 published an observational study suggesting that in-hospital administration of IV iron was not associated with adverse outcomes among HD patients hospitalized for bacterial infection. In a 2018 meta-analysis of data from 8 observational trials (including many of those described), risk for infection was comparable between higher-dose IV iron (ie, >200 mg per month) and lower-dose IV iron/oral iron/no iron (HR, 1.13; 95% CI, 0.99-1.28).93
A large US cohort study (N = 13,249) demonstrated increased risk for infection-related events and infection-related mortality with more intense IV iron dosing strategies.94 It should be noted that as part of the reference strategy, patients received ≥25 mg of IV iron weekly, provided that TSAT was ≤50%, ferritin levels were ≤1,200 ng/mL, and all dosing strategies were more intensive than those assessed in the PIVOTAL study (discussed later).94,95 Additionally, a sizeable proportion of patients deviated from their index dosing strategy during follow-up.
Most recently, a retrospective case-crossover study in Taiwan found no evidence of increased infection risk associated with IV iron therapy (20.1% of the study cohort received IV iron; 8.6% of the study cohort received >300 mg of iron per month) among incident HD patients. The lack of an association was consistent across iron doses, catheter use, and comorbid conditions.96 These studies are summarized in Table 3.
Table 3.
Relationship Between IV Iron Use and/or Higher Ferritin Levels and Infection Risk in HD Populations (observational studies from 2014 and later)
| Study | Population Examined | Comparison | Results |
|---|---|---|---|
| Bailie88 (2015) | 32,435 patients receiving IV iron in 12 countries (median follow-up, 1.7 y) | 100-199 mg/mo (average) IV iron vs other dosing categories (ie, 0, 1-99, 200-299, 300-399, and ≥400 mg/mo [average]) | No significant differences in infection-related mortality (vs reference group) |
| Tangri89 (2015) | 9,544 incident HD patients in US (median follow-up, 23 mo) | 6-mo iron exposure of 1-900 mg vs other dosing categories (ie, 0, 901-2,100, and >2,100 mg/6 mo); 1- and 3-mo iron exposure analyses also conducted | No significant differences in infection-related hospitalization by dose across any of the IV iron exposure windows |
| Zitt90 (2014) | 235 incident dialysis patients (HD: n = 197; peritoneal dialysis: n = 38) in Austria (median follow-up, 34 mo) | Iron supplementation (IV, 81%; oral, 6%) vs no iron supplementation (13%) | Iron supplementation was associated with reduced sepsis-related mortality; HR, 0.31 (95% CI, 0.09-1.04) |
| Kuragano91 (2014) | 1,086 patients on maintenance HD in Japan (2-y follow-up) | High (≥50 mg/wk) or low IV iron (<50 mg/wk) vs no iron; ferritin consistently below the standard vs other ferritin categories (ie, high ferritin group, low-to-high ferritin group, high-to-low ferritin group, and fluctuating ferritin group) | Both IV iron cohorts were associated with increased risk for infection (vs no iron); HR, 1.78 (95% CI, 1.04-3.05) for low IV iron; HR, 5.22 (95% CI, 2.25-12.14) for high IV iron; high ferritin levels were associated with increased risk for infection (vs low ferritin); HR, 1.76 (95% CI, 1.29-2.4) |
| Ishida92 (2015) | 22,820 adults on HD and history of IV iron use hospitalized for bacterial infection | In-hospital IV iron vs no in-hospital IV iron | Receipt of in-hospital IV iron was not associated with adverse outcomes (ie, 30-d mortality, length of stay, or 30-d readmission) |
| Li94 (2019) | 13,249 HD patients in the US receiving IV iron with 1 of 5 prespecified administration strategies | 4 IV iron dosing strategies (ie, least intensive, less intensive, more intensive, and most intensive) vs reference dosing strategy | The “more” and “most” intensive strategies were associated with higher risk for infection-related events (ie, infection-related hospitalization or infection-related death); 60-d risk difference for “most” intensive strategy: 1.8% (95% CI, 1.2%-2.6%); 60-d risk difference for “more” intensive strategy: 0.8% (95% CI, 0.3%-1.3%) |
| Yen96 (2019) | 1,410 incident HD adults in Taiwan with any infectious disease requiring IV antibiotics | Iron use during the 4-wk case period vs iron use during 3 control periods for each patient | No significant difference in odds of receiving iron during the case period. Similar results in subgroup analyses for diabetes, heart failure, chronic lung disease, catheter use, and >300 mg/mo iron |
Abbreviations: CI, confidence interval; HD, hemodialysis; HR, hazard ratio; IV, intravenous; US, United States.
Observational studies have also examined the effects of dosing strategies and IV iron formulations on infection risk. In a large retrospective cohort, Brookhart et al97 found that HD patients receiving bolus dosing of IV iron (ie, ≥100 mg iron during ≥2 consecutive HD sessions) were at increased risk for infection-related hospitalization relative to patients receiving lower maintenance dosing of IV iron. In that analysis, patients receiving bolus dosing were administered a median of 700 mg per month of IV iron compared with 200 mg per month in the maintenance group. The risk for infection associated with maintenance dosing was similar to that observed among patients receiving no iron. There is a relative dearth of studies examining the effects of different iron formulations on infection risk, and the results of the few available studies are conflicting and therefore inconclusive.98, 99, 100
Clinical Trials Examining Infection Risk Associated with IV Iron in Patients with CKD
In 2008, a meta-analysis compared the safety and efficacy of IV iron relative to oral iron in non–dialysis-dependent patients with CKD, but no data were available regarding infections in any of the 6 included RCTs.101 Since that analysis, 3 RCTs with infection-related end points have been published.102, 103, 104 The study designs and (discordant) findings of these trials are summarized in Table 4. A meta-analysis of these 3 trials reveals similar risk for infection between patients receiving IV iron and those receiving oral iron (risk ratio, 1.31; 95% CI, 0.89-1.92).105 Of the 3 trials, the one that demonstrated an increased risk for infection with IV iron, REVOKE (Randomized Trial to Evaluate Intravenous and Oral Iron in CKD), included an 8-week treatment period and a 2-year follow-up period.103 In the first 3 months of the trial (ie, treatment plus 1 month of additional follow-up), the number of patients with infection was higher in the oral iron arm than in the IV iron arm (6 vs 3).
Table 4.
RCTs Examining the Infection-Related Safety of IV Iron in Non–Dialysis-Dependent CKD Populations (from 2008 and later)
| Study | Eligibility Criteria | N | Treatment Arms | Double-Blind Treatment Duration | Results |
|---|---|---|---|---|---|
| Qunibi102 (2011) | ≥12 y old with ND-CKD; eGFR ≤ 45 mL/min/1.73 m2; Hb ≤ 11 g/dL; TSAT ≤ 25%; ferritin ≤ 300 μg/L; fixed ESA dose (if applicable) | 255 | Up to 3 infusions of ferric carboxymaltose (up to 1,000 mg at d 1; 2 additional doses [up to 500 mg] if TSAT < 30% and ferritin < 500 ng/mL); oral ferrous sulfate, 325 mg, 3×/d | 8 wk | Similar rates of infection-related adverse events (including bronchitis, upper respiratory tract infection, and urinary tract infection) between treatment arms |
| Agarwal103 (REVOKE; 2015) | >18 y old with ND-CKD; eGFR > 20 and ≤60 mL/min/1.73 m2; Hb, 8-12 g/dL; ferritin < 100 μg/L or TSAT < 25% | 136 | IV iron sucrose, 200 mg, at wk 0, 2, 4, 6, and 8; oral ferrous sulfate, 325 mg, 3×/d × 8 wk | 8 wk (safety period of 24 mo) | Serious infection-related adverse events occurred more commonly in the IV iron group (adjusted IRR, 2.12 [95% CI, 1.24-3.64]); lung and skin infections 3- to 4-fold more common in IV iron arm |
| Macdougall104 (FIND-CKD; 2014) | ≥18 y old with ND-CKD; eGFR ≤ 60 mL/min/1.73 m2; Hb, 9-11 g/dL; ferritin < 100 μg/L (or <200 μg/L + TSAT ≤20%); ESA naive | 626 | High-dose ferric carboxymaltose (ferritin target, 400-600 μg/L); low-dose ferric carboxymaltose (ferritin target, 400-600 μg/L); oral ferrous sulfate, 100 mg, 2×/d | 56 wk | Similar rates of infection and serious infection between groups |
Abbreviations: CI, confidence interval; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; ESA, erythropoiesis-stimulating agent; FIND-CKD, Ferinject Assessment in Patients With Iron Deficiency Anaemia and Non-Dialysis-Dependent Chronic Kidney Disease; Hb, hemoglobin; IRR, incidence rate ratio; IV, intravenous; ND-CKD, non–dialysis-dependent chronic kidney disease; RCT, randomized controlled trial; REVOKE, Randomized Trial to Evaluate Intravenous and Oral Iron in Chronic Kidney Disease; TSAT, transferrin saturation.
Until 2018, RCT data examining the effects of IV iron on infection risk among patients on kidney replacement therapy were relatively scarce. A 2018 systematic review and meta-analysis examining the safety of IV iron in dialysis included infection data for only 4 studies (N = 743); 1 was conducted in patients receiving peritoneal dialysis and 3 examined HD populations. Overall, researchers determined that “higher doses” (the definition varied by study) of IV iron were not associated with increased risk for infection (HR, 1.02; 95% CI, 0.74-1.41).93 Although reassuring, results of the included studies and the meta-analysis should be viewed in light of numerous limitations. The study populations were generally small, with only 1 trial randomly assigning more than 100 patients.93,106 The definition of “higher doses” of IV iron varied greatly; in the study of (oral) ferric citrate by Lewis et al,106 “higher doses” of iron equated to a median weekly dose of ∼27 mg. The duration of follow-up across studies varied from 6 to 52 weeks, and each study used different formulations of IV iron.93 Most notably, each study targeted (or allowed) dissimilar TSAT and/or ferritin level cutoffs.
The PIVOTAL trial, conducted over nearly 5 years, was designed to compare the safety and efficacy of 2 iron sucrose dosing strategies in HD patients: a proactive higher-dose regimen and a reactive lower-dose regimen.95 Incident HD (<12 months) patients were eligible to participate if they had a ferritin concentration < 400 μg/L, had a TSAT < 30%, and were receiving treatment with an erythropoiesis-stimulating agent (ESA). Patients randomly assigned to the high-dose group (n = 1,093) received 400 mg of IV iron sucrose monthly (unless ferritin level was >700 μg/L or TSAT was ≥40%) and patients in the low-dose group (n = 1,048) received enough IV iron (up to 400 mg monthly) to maintain ferritin concentrations ≥ 200 μg/L and TSATs ≥ 20%. Iron was temporarily withheld if patients developed an active infection. Most patients were followed up for at least 2 years, and across the trial, median monthly doses of IV iron were 264 and 145 mg in the high- and low-dose groups, respectively. By month 12, patients in the high-dose group received ∼2 g more iron than patients in the control group.
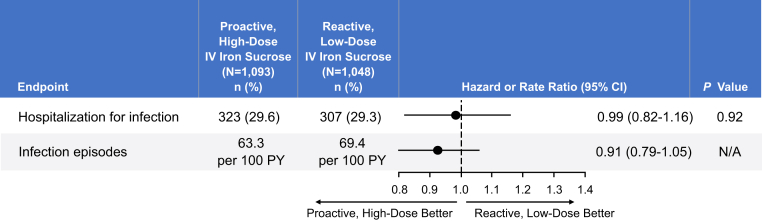
Although the primary end point in the PIVOTAL trial was cardiovascular, infection-related outcomes were included as safety end points.95 As detailed in Figure 2, random assignment to the proactive group with higher-dose IV iron did not lead to increased risk for infection or hospitalization for infection. Infection was identified as a cause of death in 4.3% of patients receiving high-dose iron and 3.9% of patients receiving low-dose iron. In a prespecified secondary analysis, no association between iron dose, serum ferritin level, or TSAT and infection risk was evident.15
Figure 2.
Infection end points in the Proactive IV Iron Therapy in Haemodialysis Patients (PIVOTAL) trial. Based on data in Macdougall et al.95 Abbreviations: CI, confidence interval; IV, intravenous; N/A, not available; PY, patient-years.
Clinical Implications and Areas of Uncertainty
The host and pathogens are engaged in a tug of war for iron; microbes expend considerable resources to secure iron and in turn, our immune system strives to restrict iron availability.107 Although there is a plausible connection between iron use and infection, available clinical data largely fail to demonstrate an association between IV iron use and infection risk among patients with CKD. Nonetheless, the decision to administer IV iron to a patient with CKD requires consideration of iron- and patient-related factors and should include an individualized assessment of risk versus benefit. In addition to considering the risks and benefits of iron therapy, one must consider the risks and benefits of not administering iron, such as the impact of persistent iron deficiency or anemia and risks associated with other therapies (eg, blood transfusions or higher ESA doses). For patients with chronic or latent infections, microbe-related factors should also be considered.
Some of the clinical benefits of IV iron in the CKD population are well established; it can help correct iron deficiency anemia, resulting in increased hemoglobin concentration while reducing the need for ESAs. In the HD population, the PIVOTAL trial demonstrated that a proactive approach to iron therapy (within the limits tested) resulted in significantly and meaningfully improved patient outcomes, as assessed by time to all-cause death, myocardial infarction, stroke, or hospitalization for heart failure.95 By contrast, in the non–dialysis-dependent CKD population, the impact of IV iron on “hard” outcomes remains ill-defined.
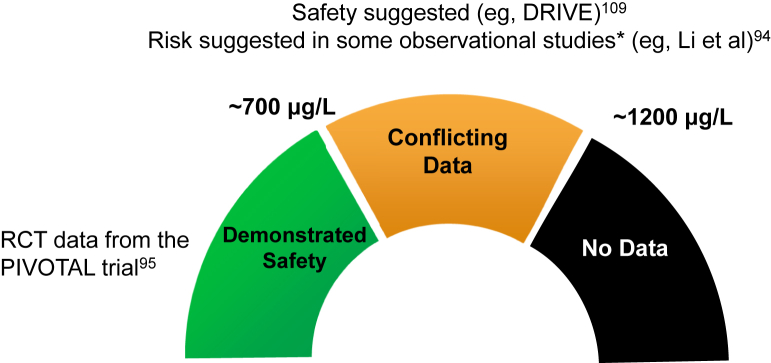
In an incident HD population with ferritin concentrations up to 700 μg/L, the high-dose IV iron regimen tested in the PIVOTAL trial did not demonstrate any evidence of harm related to infection.95 The mechanisms underlying these results have not been fully elucidated, and several hypotheses exist. Many patients in the low-dose group met criteria for iron deficiency and/or anemia, which may itself be associated with increased risk for infection.82,95 Patients in the low-dose arm also required significantly higher ESA doses, and recent data suggest that erythropoietin can enhance the growth of some bacteria (eg, E coli and S aureus).108 Although PIVOTAL has added greatly to the evidence base, several unanswered questions remain. For instance, it remains uncertain whether the infection-related results of the PIVOTAL trial can be generalized to: (1) non–dialysis-dependent CKD populations, (2) patients receiving peritoneal dialysis, (3) prevalent HD populations, (4) other iron formulations, (5) patients with ferritin levels > 700 μg/L, (6) patients not receiving ESAs, or (7) patients treated with hypoxia-inducible factor prolyl hydroxylase inhibitors. These issues notwithstanding, the available high-quality evidence supports an approach to IV iron dosing that permits, if not encourages, ferritin levels to reach ∼700 μg/L in HD patients. Such an approach appears to confer clinical benefit without increased risk for harm (eg, infections). Data examining infection risk among patients with greater iron stores (as assessed by ferritin levels of 700-1,200 μg/L) are varying,94,109 and there are no data examining the safety of IV iron administered to HD patients with ferritin levels > 1,200 μg/L (Fig 3). Beyond ferritin, TSAT should also be weighed into the decision to administer (or withhold) IV iron. Based on the PIVOTAL trial, IV iron can be safely administered to patients with TSATs < 40%. This is consistent with physiologic data suggesting that the risk for a resultant (after IV iron administration) TSAT level associated with more than transient non–transferrin-bound iron generation is low with baseline TSATs < 40%.
Figure 3.
Safety of administering intravenous (IV) iron to hemodialysis patients by ferritin concentration (based on available evidence). Eligibility criteria for each of the cited studies differ and may affect generalizability of the results to broader populations. ∗Results of observational studies should be viewed cautiously because weaker associations may be attributable to confounding. Abbreviations: DRIVE, Dialysis Patients' Response to IV Iron with Elevated Ferritin; PIVOTAL, Proactive IV Iron Therapy in Haemodialysis Patients; RCT, randomized controlled trial.
Consistent with prior recommendations, it remains prudent to avoid IV iron for the treatment of anemia in CKD in the setting of active systemic infection.110 The need to administer IV iron in the setting of anemia in CKD is rarely urgent and can generally be delayed until the infection subsides, avoiding the hypothetical risk for acute exacerbation of the infection. Additionally, in the setting of acute infection, the beneficial effects of IV iron are likely hindered by the effects of hepcidin and the sequestration of iron in storage cells. The optimal iron strategy for patients with CKD and chronic infections such as human immunodeficiency virus and hepatitis C, both of which can coexist and/or contribute to CKD, is less clear.111,112
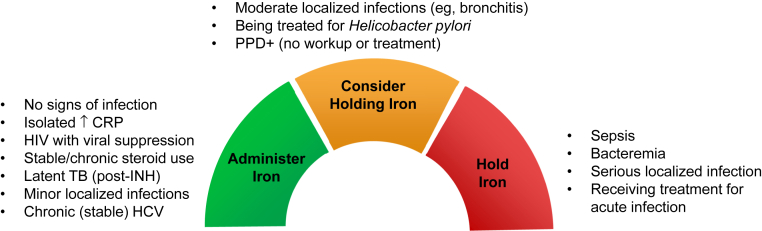
Last, using ferritin levels to guide IV iron dosing can be challenging during an infection because ferritin is an acute-phase reactant. In the absence of evidence-based guidance, there are a number of clinical scenarios for which the decision to administer iron should be carefully considered (Fig 4). Independent of the administration (or withholding) of IV iron, clinicians should continue efforts to reduce the risk for infection among patients with CKD, including evidence-based vaccination, minimization of catheter use, increased surveillance, and implementation of infection control procedures.
Figure 4.
Deciding whether to administer intravenous iron to hemodialysis patients with iron deficiency. Guidance based on expert opinion and assumes iron deficiency secondary to chronic kidney disease and noncritical hemoglobin concentration. Abbreviations: CRP, C-reactive protein; HCV, hepatitis C virus; HIV, human immunodeficiency virus; INH, isoniazid; PPD+, positive purified protein derivative skin test; TB, tuberculosis.
Conclusions
A multidisciplinary appraisal of the evidence confirms biological plausibility for an association between IV iron use and increased infection risk among patients with CKD. In the absence of an urgent need to treat anemia in CKD, there is little reason to administer IV iron to patients experiencing an acute infection. However, a growing evidence base of clinical data suggests that a judicious but proactive approach to the use of IV iron in CKD (similar to the 400–mg per month arm in the PIVOTAL trial) is not associated with a clinically meaningful increased risk for infection. Furthermore, the management of iron deficiency in patients with CKD appears to confer non–infection-related benefits that should be factored into clinical decision making.
Article Information
Authors’ Full Names and Academic Degrees
Tomas Ganz, MD, George R. Aronoff, MD, Carlo A.J.M. Gaillard, MD, Lawrence T. Goodnough, MD, Iain C. Macdougall, MD, Gert Mayer, MD, Graça Porto, MD, Wolfgang C. Winkelmayer, MD, ScD, and Jay B. Wish, MD.
Support
This article is based on discussions at an international advisory board meeting sponsored by Vifor Fresenius Medical Care Renal Pharma, which manufactures several iron replacement therapies. Vifor Fresenius Medical Care Renal Pharma provided funding for writing and editing services to assist with the preparation of this manuscript. The article sponsor had no role in the collection, analysis, and interpretation of data; writing the report; or the decision to submit the report for publication. The final decision on the main points to be communicated, including the conclusions drawn, was made by consensus of the authors.
Financial Disclosure
Dr Aronoff is consultant to Akebia, AstraZeneca, Bayer, Rockwell Medical, and Vifor; owner of Dosis, Inc; and employee of DaVita, Inc. Dr Gaillard received speakers’ fees, honoraria, and consultancy fees from Amgen, Corvidia, Keryx Pharma, Pharmacosmos, Roche, and Vifor Pharma. Dr Ganz is consultant to Akebia, Gilead, Keryx Pharma, La Jolla Pharmaceutical Company, and Vifor; and scientific founder, shareholder, and consultant to Intrinsic LifeSciences and Silarus Pharma. Dr Goodnough is advisory board member for American Reagent. Dr Macdougall received speakers’ fees, honoraria, and consultancy fees from Akebia, AMAG, Amgen, Astellas, Bayer, FibroGen, GlaxoSmithKline, Pharmacosmos, and Vifor Pharma. Dr Mayer is consultant to Amgen, Astellas, AstraZeneca, Boehringer Ingelheim, Gilead, and Vifor. Dr Porto received speakers’ fees and consultancy fees from Novartis, Silence Therapeutics, and Vifor Pharma. Dr Winkelmayer is consultant to Akebia, Amgen, AstraZeneca, Bayer, Daichii Sankyo, Janssen, Relypsa, and Vifor Fresenius Medical Care Renal Pharma. Dr Wish is consultant to Akebia, AstraZeneca, Otsuka, Rockwell Medical, and Vifor and on the speakers bureau for Akebia and AstraZeneca.
Acknowledgements
Assistance with the writing and editing of the manuscript was provided by Adam Perahia, MD, of NorthStar Strategic Consulting, LLC.
Peer Review
Received December 12, 2019. Evaluated by 3 external peer reviewers, with direct editorial input from the Editor-in-Chief. Accepted in revised form January 5, 2020.
Footnotes
Complete author and article information provided before references.
References
- 1.Wish J.B., Aronoff G.R., Bacon B.R. Positive iron balance in chronic kidney disease: how much is too much and how to tell? Am J Nephrol. 2018;47(2):72–83. doi: 10.1159/000486968. [DOI] [PubMed] [Google Scholar]
- 2.US-DOPPS (Dialysis Outcomes and Practice Patterns Study) Practice Monitor. IV iron use, last 3 months. October 2019. https://www.dopps.org/DPM/Files/maxIVIRON_use_c_overallTAB.htm
- 3.Fishbane S., Pollack S., Feldman H.I., Joffe M.M. Iron indices in chronic kidney disease in the National Health and Nutritional Examination Survey 1988-2004. Clin J Am Soc Nephrol. 2009;4(1):57–61. doi: 10.2215/CJN.01670408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.St Peter W.L., Guo H., Kabadi S. Prevalence, treatment patterns, and healthcare resource utilization in Medicare and commercially insured non-dialysis-dependent chronic kidney disease patients with and without anemia in the United States. BMC Nephrol. 2018;19(1):67. doi: 10.1186/s12882-018-0861-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Macdougall I.C., Bircher A.J., Eckardt K.U. Iron management in chronic kidney disease: conclusions from a “Kidney Disease: Improving Global Outcomes” (KDIGO) Controversies Conference. Kidney Int. 2016;89(1):28–39. doi: 10.1016/j.kint.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Syed-Ahmed M., Narayanan M. Immune dysfunction and risk of infection in chronic kidney disease. Adv Chronic Kidney Dis. 2019;26(1):8–15. doi: 10.1053/j.ackd.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 7.Dalrymple L.S., Go A.S. Epidemiology of acute infections among patients with chronic kidney disease. Clin J Am Soc Nephrol. 2008;3(5):1487–1493. doi: 10.2215/CJN.01290308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saran R., Robinson B., Abbott K.C. US Renal Data System 2018 Annual Data Report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2019;73(3 suppl 1) doi: 10.1053/j.ajkd.2019.01.001. Svii-Sxxii, S1-S772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sarnak M.J., Jaber B.L. Mortality caused by sepsis in patients with end-stage renal disease compared with the general population. Kidney Int. 2000;58(4):1758–1764. doi: 10.1111/j.1523-1755.2000.00337.x. [DOI] [PubMed] [Google Scholar]
- 10.Dalrymple L.S., Katz R., Kestenbaum B. The risk of infection-related hospitalization with decreased kidney function. Am J Kidney Dis. 2012;59(3):356–363. doi: 10.1053/j.ajkd.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishigami J., Grams M.E., Chang A.R., Carrero J.J., Coresh J., Matsushita K. CKD and risk for hospitalization with infection: the Atherosclerosis Risk in Communities (ARIC) study. Am J Kidney Dis. 2017;69(6):752–761. doi: 10.1053/j.ajkd.2016.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naqvi S.B., Collins A.J. Infectious complications in chronic kidney disease. Adv Chronic Kidney Dis. 2006;13(3):199–204. doi: 10.1053/j.ackd.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 13.Cowan L.T., Lutsey P.L., Pankow J.S., Matsushita K., Ishigami J., Lakshminarayan K. Inpatient and outpatient infection as a trigger of cardiovascular disease: the ARIC study. J Am Heart Assoc. 2018;7(22) doi: 10.1161/JAHA.118.009683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheikh Hassan H.I., Tang M., Djurdjev O., Langsford D., Sood M.M., Levin A. Infection in advanced chronic kidney disease leads to increased risk of cardiovascular events, end-stage kidney disease and mortality. Kidney Int. 2016;90(4):897–904. doi: 10.1016/j.kint.2016.07.013. [DOI] [PubMed] [Google Scholar]
- 15.Macdougall IC, Bhandari S, White C, et al. Intravenous iron dosing and infection risk in patients on hemodialysis: a prespecified secondary analysis of the PIVOTAL trial [published online ahead of print April 6, 2020]. J Am Soc Nephrol. 10.1681/ASN.2019090972. [DOI] [PMC free article] [PubMed]
- 16.Kato S., Chmielewski M., Honda H. Aspects of immune dysfunction in end-stage renal disease. Clin J Am Soc Nephrol. 2008;3(5):1526–1533. doi: 10.2215/CJN.00950208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Szeto C.C., McIntyre C.W., Li P.K. Circulating bacterial fragments as cardiovascular risk factors in CKD. J Am Soc Nephrol. 2018;29(6):1601–1608. doi: 10.1681/ASN.2018010068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kurts C., Panzer U., Anders H.J., Rees A.J. The immune system and kidney disease: basic concepts and clinical implications. Nat Rev Immunol. 2013;13(10):738–753. doi: 10.1038/nri3523. [DOI] [PubMed] [Google Scholar]
- 19.Lamarche C., Iliuta I.A., Kitzler T. Infectious disease risk in dialysis patients: a transdisciplinary approach. Can J Kidney Health Dis. 2019;6 doi: 10.1177/2054358119839080. 2054358119839080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.March D.S., Graham-Brown M.P., Stover C.M., Bishop N.C., Burton J.O. Intestinal barrier disturbances in haemodialysis patients: mechanisms, consequences, and therapeutic options. Biomed Res Int. 2017;2017:5765417. doi: 10.1155/2017/5765417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iwagami M., Caplin B., Smeeth L., Tomlinson L.A., Nitsch D. Chronic kidney disease and cause-specific hospitalisation: a matched cohort study using primary and secondary care patient data. Br J Gen Pract. 2018;68(673):e512–e523. doi: 10.3399/bjgp18X697973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berman S.J., Johnson E.W., Nakatsu C., Alkan M., Chen R., LeDuc J. Burden of infection in patients with end-stage renal disease requiring long-term dialysis. Clin Infect Dis. 2004;39(12):1747–1753. doi: 10.1086/424516. [DOI] [PubMed] [Google Scholar]
- 23.Al-Efraij K., Mota L., Lunny C., Schachter M., Cook V., Johnston J. Risk of active tuberculosis in chronic kidney disease: a systematic review and meta-analysis. Int J Tuberc Lung Dis. 2015;19(12):1493–1499. doi: 10.5588/ijtld.15.0081. [DOI] [PubMed] [Google Scholar]
- 24.Campbell C.N., Mytton O.T., McLean E.M. Hospitalization in two waves of pandemic influenza A(H1N1) in England. Epidemiol Infect. 2011;139(10):1560–1569. doi: 10.1017/S0950268810002657. [DOI] [PubMed] [Google Scholar]
- 25.Muckenthaler M.U., Lill R. Iron Physiology and Pathophysiology in Humans. Humana Press; New York, NY: 2012. Cellular iron physiology; pp. 27–50. [Google Scholar]
- 26.Cassat J.E., Skaar E.P. Iron in infection and immunity. Cell Host Microbe. 2013;13(5):509–519. doi: 10.1016/j.chom.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Drakesmith H., Prentice A.M. Hepcidin and the iron-infection axis. Science. 2012;338(6108):768–772. doi: 10.1126/science.1224577. [DOI] [PubMed] [Google Scholar]
- 28.Ganz T. Iron and infection. Int J Hematol. 2018;107(1):7–15. doi: 10.1007/s12185-017-2366-2. [DOI] [PubMed] [Google Scholar]
- 29.Soares M.P., Weiss G. The iron age of host-microbe interactions. EMBO Rep. 2015;16(11):1482–1500. doi: 10.15252/embr.201540558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ganz T. Hepcidin and its role in regulating systemic iron metabolism. Hematology Am Soc Hematol Educ Program. 2006 doi: 10.1182/asheducation-2006.1.29. 29-35, 507. [DOI] [PubMed] [Google Scholar]
- 31.Drakesmith H., Prentice A. Viral infection and iron metabolism. Nat Rev Microbiol. 2008;6(7):541–552. doi: 10.1038/nrmicro1930. [DOI] [PubMed] [Google Scholar]
- 32.Cartwright G.E., Lauritsen M.A., Humphreys S., Jones P.J., Merrill I.M., Wintrobe M.M. The anemia of infection. II. The experimental production of hypoferremia and anemia in dogs. J Clin Invest. 1946;25(1):81–86. doi: 10.1172/JCI101691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deschemin J.C., Vaulont S. Role of hepcidin in the setting of hypoferremia during acute inflammation. PLoS One. 2013;8(4) doi: 10.1371/journal.pone.0061050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weiss G. Modification of iron regulation by the inflammatory response. Best Pract Res Clin Haematol. 2005;18(2):183–201. doi: 10.1016/j.beha.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 35.Ward P.P., Mendoza-Meneses M., Park P.W., Conneely O.M. Stimulus-dependent impairment of the neutrophil oxidative burst response in lactoferrin-deficient mice. Am J Pathol. 2008;172(4):1019–1029. doi: 10.2353/ajpath.2008.061145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van Zandt K.E., Sow F.B., Florence W.C. The iron export protein ferroportin 1 is differentially expressed in mouse macrophage populations and is present in the mycobacterial-containing phagosome. J Leukoc Biol. 2008;84(3):689–700. doi: 10.1189/jlb.1107781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Olakanmi O., Schlesinger L.S., Ahmed A., Britigan B.E. Intraphagosomal Mycobacterium tuberculosis acquires iron from both extracellular transferrin and intracellular iron pools. Impact of interferon-gamma and hemochromatosis. J Biol Chem. 2002;277(51):49727–49734. doi: 10.1074/jbc.M209768200. [DOI] [PubMed] [Google Scholar]
- 38.Paiva C.N., Bozza M.T. Are reactive oxygen species always detrimental to pathogens? Antioxid Redox Signal. 2014;20(6):1000–1037. doi: 10.1089/ars.2013.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khan F.A., Fisher M.A., Khakoo R.A. Association of hemochromatosis with infectious diseases: expanding spectrum. Int J Infect Dis. 2007;11(6):482–487. doi: 10.1016/j.ijid.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 40.Weinberg E.D. Iron loading and disease surveillance. Emerg Infect Dis. 1999;5(3):346–352. doi: 10.3201/eid0503.990305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pieracci F.M., Barie P.S. Iron and the risk of infection. Surg Infect (Larchmt) 2005;6(suppl 1):S41–S46. doi: 10.1089/sur.2005.6.s1-41. [DOI] [PubMed] [Google Scholar]
- 42.Telang S., Vimr E., Mahoney J.R. Strain-specific iron-dependent virulence in Escherichia coli. J Infect Dis. 2001;184(2):159–165. doi: 10.1086/322017. [DOI] [PubMed] [Google Scholar]
- 43.Michels K.R., Zhang Z., Bettina A.M. Hepcidin-mediated iron sequestration protects against bacterial dissemination during pneumonia. JCI Insight. 2017;2(6) doi: 10.1172/jci.insight.92002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stefanova D., Raychev A., Arezes J. Endogenous hepcidin and its agonist mediate resistance to selected infections by clearing non-transferrin-bound iron. Blood. 2017;130(3):245–257. doi: 10.1182/blood-2017-03-772715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stefanova D., Raychev A., Deville J. Hepcidin protects against lethal Escherichia coli sepsis in mice inoculated with isolates from septic patients. Infect Immun. 2018;86(7):e00253–18. doi: 10.1128/IAI.00253-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arezes J., Jung G., Gabayan V. Hepcidin-induced hypoferremia is a critical host defense mechanism against the siderophilic bacterium Vibrio vulnificus. Cell Host Microbe. 2015;17(1):47–57. doi: 10.1016/j.chom.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cross J.H., Bradbury R.S., Fulford A.J. Oral iron acutely elevates bacterial growth in human serum. Sci Rep. 2015;5:16670. doi: 10.1038/srep16670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Le Lan C., Loréal O., Cohen T. Redox active plasma iron in C282Y/C282Y hemochromatosis. Blood. 2005;105(11):4527–4531. doi: 10.1182/blood-2004-09-3468. [DOI] [PubMed] [Google Scholar]
- 49.Hod E.A., Brittenham G.M., Billote G.B. Transfusion of human volunteers with older, stored red blood cells produces extravascular hemolysis and circulating non-transferrin-bound iron. Blood. 2011;118(25):6675–6682. doi: 10.1182/blood-2011-08-371849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nairz M., Weiss G. Infections associated with iron administration. Met Ions Life Sci. 2019;19 doi: 10.1515/9783110527872-011. [DOI] [PubMed] [Google Scholar]
- 51.de Sousa M., Reimão R., Porto G., Grady R.W., Hilgartner M.W., Giardina P. Iron and lymphocytes: reciprocal regulatory interactions. Curr Stud Hematol Blood Transfus. 1991;(58):171–177. doi: 10.1159/000419357. [DOI] [PubMed] [Google Scholar]
- 52.de Sousa M. Immune cell functions in iron overload. Clin Exp Immunol. 1989;75(1):1–6. [PMC free article] [PubMed] [Google Scholar]
- 53.Levy J.E., Montross L.K., Andrews N.C. Genes that modify the hemochromatosis phenotype in mice. J Clin Invest. 2000;105(9):1209–1216. doi: 10.1172/JCI9635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rodrigues P., Lopes C., Mascarenhas C., Arosio P., Porto G., De Sousa M. Comparative study between Hfe-/- and beta2m-/- mice: progression with age of iron status and liver pathology. Int J Exp Pathol. 2006;87(4):317–324. doi: 10.1111/j.1365-2613.2006.00491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Breedveld F.C., Dynesius-Trentham R., de Sousa M., Trentham D.E. Collagen arthritis in the rat is initiated by CD4+ T cells and can be amplified by iron. Cell Immunol. 1989;121(1):1–12. doi: 10.1016/0008-8749(89)90001-4. [DOI] [PubMed] [Google Scholar]
- 56.Grady R.W., Akbar A.N., Giardina P.J., Hilgartner M.W., de Sousa M. Disproportionate lymphoid cell subsets in thalassaemia major: the relative contributions of transfusion and splenectomy. Br J Haematol. 1985;59(4):713–724. doi: 10.1111/j.1365-2141.1985.tb07367.x. [DOI] [PubMed] [Google Scholar]
- 57.Handa P., Thomas S., Morgan-Stevenson V. Iron alters macrophage polarization status and leads to steatohepatitis and fibrogenesis. J Leukoc Biol. 2019;105(5):1015–1026. doi: 10.1002/JLB.3A0318-108R. [DOI] [PubMed] [Google Scholar]
- 58.Deicher R., Ziai F., Cohen G., Müllner M., Hörl W.H. High-dose parenteral iron sucrose depresses neutrophil intracellular killing capacity. Kidney Int. 2003;64(2):728–736. doi: 10.1046/j.1523-1755.2003.00125.x. [DOI] [PubMed] [Google Scholar]
- 59.Patruta S.I., Edlinger R., Sunder-Plassmann G., Horl W.H. Neutrophil impairment associated with iron therapy in hemodialysis patients with functional iron deficiency. J Am Soc Nephrol. 1998;9(4):655–663. doi: 10.1681/ASN.V94655. [DOI] [PubMed] [Google Scholar]
- 60.Hassan T.H., Badr M.A., Karam N.A. Impact of iron deficiency anemia on the function of the immune system in children. Medicine (Baltimore) 2016;95(47):e5395. doi: 10.1097/MD.0000000000005395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aly S.S., Fayed H.M., Ismail A.M., Abdel Hakeem G.L. Assessment of peripheral blood lymphocyte subsets in children with iron deficiency anemia. BMC Pediatr. 2018;18(1):49. doi: 10.1186/s12887-018-0990-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zager R.A., Johnson A.C., Hanson S.Y. Parenteral iron therapy exacerbates experimental sepsis. Kidney Int. 2004;65(6):2108–2112. doi: 10.1111/j.1523-1755.2004.00742.x. [DOI] [PubMed] [Google Scholar]
- 63.Barton J.C., Edwards C.Q., Acton R.T. HFE gene: structure, function, mutations, and associated iron abnormalities. Gene. 2015;574(2):179–192. doi: 10.1016/j.gene.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pietrangelo A. Hereditary hemochromatosis—a new look at an old disease. N Engl J Med. 2004;350(23):2383–2397. doi: 10.1056/NEJMra031573. [DOI] [PubMed] [Google Scholar]
- 65.Frank K.M., Schneewind O., Shieh W.J. Investigation of a researcher's death due to septicemic plague. N Engl J Med. 2011;364(26):2563–2564. doi: 10.1056/NEJMc1010939. [DOI] [PubMed] [Google Scholar]
- 66.Costa M., Cruz E., Barton J.C. Effects of highly conserved major histocompatibility complex (MHC) extended haplotypes on iron and low CD8+ T lymphocyte phenotypes in HFE C282Y homozygous hemochromatosis patients from three geographically distant areas. PLoS One. 2013;8(11) doi: 10.1371/journal.pone.0079990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cruz E., Melo G., Lacerda R., Almeida S., Porto G. The CD8+ T-lymphocyte profile as a modifier of iron overload in HFE hemochromatosis: an update of clinical and immunological data from 70 C282Y homozygous subjects. Blood Cells Mol Dis. 2006;37(1):33–39. doi: 10.1016/j.bcmd.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 68.Macedo M.F., Porto G., Costa M., Vieira C.P., Rocha B., Cruz E. Low numbers of CD8+ T lymphocytes in hereditary haemochromatosis are explained by a decrease of the most mature CD8+ effector memory T cells. Clin Exp Immunol. 2010;159(3):363–371. doi: 10.1111/j.1365-2249.2009.04066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Powell L.W., Seckington R.C., Deugnier Y. Haemochromatosis. Lancet. 2016;388(10045):706–716. doi: 10.1016/S0140-6736(15)01315-X. [DOI] [PubMed] [Google Scholar]
- 70.Porter J.B., Garbowski M. The pathophysiology of transfusional iron overload. Hematol Oncol Clin North Am. 2014;28(4):683–701. doi: 10.1016/j.hoc.2014.04.003. vi. [DOI] [PubMed] [Google Scholar]
- 71.Wood J.C. Estimating tissue iron burden: current status and future prospects. Br J Haematol. 2015;170(1):15–28. doi: 10.1111/bjh.13374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pietrangelo A. The ferroportin disease. Blood Cells Mol Dis. 2004;32(1):131–138. doi: 10.1016/j.bcmd.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 73.Pietrangelo A. Hemochromatosis: an endocrine liver disease. Hepatology. 2007;46(4):1291–1301. doi: 10.1002/hep.21886. [DOI] [PubMed] [Google Scholar]
- 74.Milic S., Mikolasevic I., Orlic L. The role of iron and iron overload in chronic liver disease. Med Sci Monit. 2016;22:2144–2151. doi: 10.12659/MSM.896494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pietrangelo A. Ferroportin disease: pathogenesis, diagnosis and treatment. Haematologica. 2017;102(12):1972–1984. doi: 10.3324/haematol.2017.170720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kasvosve I. Effect of ferroportin polymorphism on iron homeostasis and infection. Clin Chim Acta. 2013;416:20–25. doi: 10.1016/j.cca.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 77.Lambrecht R.W., Sterling R.K., Naishadham D. Iron levels in hepatocytes and portal tract cells predict progression and outcomes of patients with advanced chronic hepatitis C. Gastroenterology. 2011;140(5):1490–1500.e3. doi: 10.1053/j.gastro.2011.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shah A., Fisher S.A., Wong H. Safety and efficacy of iron therapy on reducing red blood cell transfusion requirements and treating anaemia in critically ill adults: a systematic review with meta-analysis and trial sequential analysis. J Crit Care. 2019;49:162–171. doi: 10.1016/j.jcrc.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 79.Shin H.W., Park J.J., Kim H.J., You H.S., Choi S.U., Lee M.J. Efficacy of perioperative intravenous iron therapy for transfusion in orthopedic surgery: a systematic review and meta-analysis. PLoS One. 2019;14(5) doi: 10.1371/journal.pone.0215427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shah A., Palmer A.J.R., Fisher S.A. What is the effect of perioperative intravenous iron therapy in patients undergoing non-elective surgery? A systematic review with meta-analysis and trial sequential analysis. Perioper Med (Lond) 2018;7:30. doi: 10.1186/s13741-018-0109-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Litton E., Xiao J., Ho K.M. Safety and efficacy of intravenous iron therapy in reducing requirement for allogeneic blood transfusion: systematic review and meta-analysis of randomised clinical trials. BMJ. 2013;347:f4822. doi: 10.1136/bmj.f4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tansarli G.S., Karageorgopoulos D.E., Kapaskelis A., Gkegkes I., Falagas M.E. Iron deficiency and susceptibility to infections: evaluation of the clinical evidence. Eur J Clin Microbiol Infect Dis. 2013;32(10):1253–1258. doi: 10.1007/s10096-013-1877-x. [DOI] [PubMed] [Google Scholar]
- 83.Mohus R.M., Paulsen J., Gustad L. Association of iron status with the risk of bloodstream infections: results from the prospective population-based HUNT study in Norway. Intensive Care Med. 2018;44(8):1276–1283. doi: 10.1007/s00134-018-5320-8. [DOI] [PubMed] [Google Scholar]
- 84.Grimes D.A., Schulz K.F. False alarms and pseudo-epidemics: the limitations of observational epidemiology. Obstet Gynecol. 2012;120(4):920–927. doi: 10.1097/AOG.0b013e31826af61a. [DOI] [PubMed] [Google Scholar]
- 85.Gerstein H.C., McMurray J., Holman R.R. Real-world studies no substitute for RCTs in establishing efficacy. Lancet. 2019;393(10168):210–211. doi: 10.1016/S0140-6736(18)32840-X. [DOI] [PubMed] [Google Scholar]
- 86.Prakash S., Walele A., Dimkovic N., Bargman J., Vas S., Oreopoulos D. Experience with a large dose (500 mg) of intravenous iron dextran and iron saccharate in peritoneal dialysis patients. Perit Dial Int. 2001;21(3):290–295. [PubMed] [Google Scholar]
- 87.Ishida J.H., Johansen K.L. Iron and infection in hemodialysis patients. Semin Dial. 2014;27(1):26–36. doi: 10.1111/sdi.12168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bailie G.R., Larkina M., Goodkin D.A. Data from the Dialysis Outcomes and Practice Patterns Study validate an association between high intravenous iron doses and mortality. Kidney Int. 2015;87(1):162–168. doi: 10.1038/ki.2014.275. [DOI] [PubMed] [Google Scholar]
- 89.Tangri N., Miskulin D.C., Zhou J., for DEcIDE Network Patient Outcomes in End-Stage Renal Disease Study Investigators Effect of intravenous iron use on hospitalizations in patients undergoing hemodialysis: a comparative effectiveness analysis from the DEcIDE-ESRD study. Nephrol Dial Transplant. 2015;30(4):667–675. doi: 10.1093/ndt/gfu349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zitt E., Sturm G., Kronenberg F. Iron supplementation and mortality in incident dialysis patients: an observational study. PLoS One. 2014;9(12) doi: 10.1371/journal.pone.0114144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kuragano T., Matsumura O., Matsuda A. Association between hemoglobin variability, serum ferritin levels, and adverse events/mortality in maintenance hemodialysis patients. Kidney Int. 2014;86(4):845–854. doi: 10.1038/ki.2014.114. [DOI] [PubMed] [Google Scholar]
- 92.Ishida J.H., Marafino B.J., McCulloch C.E. Receipt of intravenous iron and clinical outcomes among hemodialysis patients hospitalized for infection. Clin J Am Soc Nephrol. 2015;10(10):1799–1805. doi: 10.2215/CJN.01090115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hougen I., Collister D., Bourrier M. Safety of intravenous iron in dialysis: a systematic review and meta-analysis. Clin J Am Soc Nephrol. 2018;13(3):457–467. doi: 10.2215/CJN.05390517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li X., Cole S.R., Kshirsagar A.V., Fine J.P., Sturmer T., Brookhart M.A. Safety of dynamic intravenous iron administration strategies in hemodialysis patients. Clin J Am Soc Nephrol. 2019;14(5):728–737. doi: 10.2215/CJN.03970318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Macdougall I.C., White C., Anker S.D., for PIVOTAL Investigators and Committees Intravenous iron in patients undergoing maintenance hemodialysis. N Engl J Med. 2019;380(5):447–458. doi: 10.1056/NEJMoa1810742. [DOI] [PubMed] [Google Scholar]
- 96.Yen C.L., Lin Y.S., Lu Y.A. Intravenous iron supplementation does not increase infectious disease risk in hemodialysis patients: a nationwide cohort-based case-crossover study. BMC Nephrol. 2019;20(1):327. doi: 10.1186/s12882-019-1495-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Brookhart M.A., Freburger J.K., Ellis A.R., Wang L., Winkelmayer W.C., Kshirsagar A.V. Infection risk with bolus versus maintenance iron supplementation in hemodialysis patients. J Am Soc Nephrol. 2013;24(7):1151–1158. doi: 10.1681/ASN.2012121164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Winkelmayer W.C., Goldstein B.A., Mitani A.A. Safety of intravenous iron in hemodialysis: longer-term comparisons of iron sucrose versus sodium ferric gluconate complex. Am J Kidney Dis. 2017;69(6):771–779. doi: 10.1053/j.ajkd.2016.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Brookhart M.A., Freburger J.K., Ellis A.R., Winkelmayer W.C., Wang L., Kshirsagar A.V. Comparative short-term safety of sodium ferric gluconate versus iron sucrose in hemodialysis patients. Am J Kidney Dis. 2016;67(1):119–127. doi: 10.1053/j.ajkd.2015.07.026. [DOI] [PubMed] [Google Scholar]
- 100.Sirken G., Raja R., Rizkala A.R. Association of different intravenous iron preparations with risk of bacteremia in maintenance hemodialysis patients. Clin Nephrol. 2006;66(5):348–356. doi: 10.5414/cnp66348. [DOI] [PubMed] [Google Scholar]
- 101.Rozen-Zvi B., Gafter-Gvili A., Paul M., Leibovici L., Shpilberg O., Gafter U. Intravenous versus oral iron supplementation for the treatment of anemia in CKD: systematic review and meta-analysis. Am J Kidney Dis. 2008;52(5):897–906. doi: 10.1053/j.ajkd.2008.05.033. [DOI] [PubMed] [Google Scholar]
- 102.Qunibi W.Y., Martinez C., Smith M., Benjamin J., Mangione A., Roger S.D. A randomized controlled trial comparing intravenous ferric carboxymaltose with oral iron for treatment of iron deficiency anaemia of non-dialysis-dependent chronic kidney disease patients. Nephrol Dial Transplant. 2011;26(5):1599–1607. doi: 10.1093/ndt/gfq613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Agarwal R., Kusek J.W., Pappas M.K. A randomized trial of intravenous and oral iron in chronic kidney disease. Kidney Int. 2015;88(4):905–914. doi: 10.1038/ki.2015.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Macdougall I.C., Bock A.H., Carrera F., for FIND-CKD Study Investigators FIND-CKD: a randomized trial of intravenous ferric carboxymaltose versus oral iron in patients with chronic kidney disease and iron deficiency anaemia. Nephrol Dial Transplant. 2014;29(11):2075–2084. doi: 10.1093/ndt/gfu201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shepshelovich D., Rozen-Zvi B., Avni T., Gafter U., Gafter-Gvili A. Intravenous versus oral iron supplementation for the treatment of anemia in CKD: an updated systematic review and meta-analysis. Am J Kidney Dis. 2016;68(5):677–690. doi: 10.1053/j.ajkd.2016.04.018. [DOI] [PubMed] [Google Scholar]
- 106.Lewis J.B., Sika M., Koury M.J., for Collaborative Study Group Ferric citrate controls phosphorus and delivers iron in patients on dialysis. J Am Soc Nephrol. 2015;26(2):493–503. doi: 10.1681/ASN.2014020212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Golonka R., Yeoh B.S., Vijay-Kumar M. The iron tug-of-war between bacterial siderophores and innate immunity. J Innate Immun. 2019;11(3):249–262. doi: 10.1159/000494627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mittal A., Singh V., Chowdhary S. The effect of recombinant human erythropoietin on bacterial growth: a dual-edged sword. Kidney Dis (Basel) 2019;5(2):81–90. doi: 10.1159/000493684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Coyne D.W., Kapoian T., Suki W., and DRIVE Study Group Ferric gluconate is highly efficacious in anemic hemodialysis patients with high serum ferritin and low transferrin saturation: results of the Dialysis Patients' Response to IV Iron with Elevated Ferritin (DRIVE) Study. J Am Soc Nephrol. 2007;18(3):975–984. doi: 10.1681/ASN.2006091034. [DOI] [PubMed] [Google Scholar]
- 110.Kidney Disease: Improving Global Outcomes (KDIGO) Anemia Work Group KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int Suppl. 2012;2(4):279–335. [Google Scholar]
- 111.Jadoul M., Berenguer M.C., Doss W. Executive summary of the 2018 KDIGO Hepatitis C in CKD Guideline: welcoming advances in evaluation and management. Kidney Int. 2018;94(4):663–673. doi: 10.1016/j.kint.2018.06.011. [DOI] [PubMed] [Google Scholar]
- 112.Campos P., Ortiz A., Soto K. HIV and kidney diseases: 35 years of history and consequences. Clin Kidney J. 2016;9(6):772–781. doi: 10.1093/ckj/sfw104. [DOI] [PMC free article] [PubMed] [Google Scholar]