Abstract
Rationale & Objective
Health-related quality of life (HRQoL) has been recognized as a strong predictor of mortality among hemodialysis patients. However, differences in the association of HRQoL with survival across diverse racial/ethnic groups have not been well studied in this population.
Study Design
Observational cohort study.
Setting & Participants
We examined the relationship between HRQoL and mortality in a prospective cohort of racially/ethnically diverse hemodialysis patients recruited from 18 outpatient dialysis units during 2011 to 2016.
Exposure
Using the 36-Item Short Form Health Survey (SF-36) administered every 6 months, HRQoL was ascertained by 36 questions summarized as 2 Physical and Mental Component and 8 subscale scores.
Outcome
All-cause mortality.
Analytical Approach
Associations of time-varying SF-36 scores with mortality were estimated using Cox models in the overall cohort and within racial/ethnic subgroups.
Results
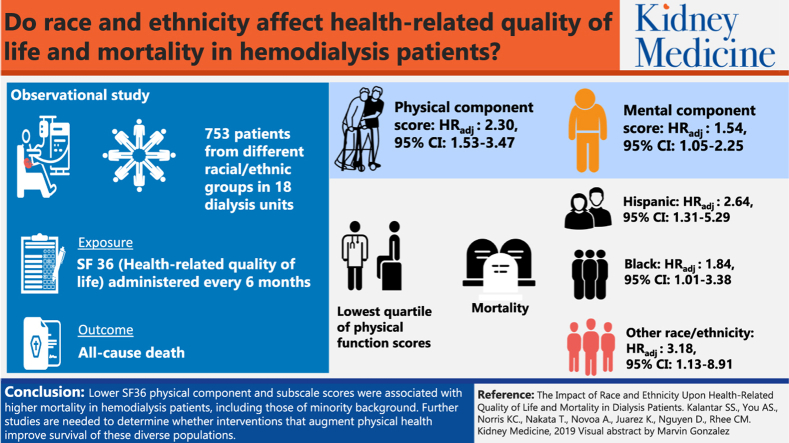
Among 753 hemodialysis patients who met eligibility criteria, expanded case-mix analyses showed that the lowest quartiles of time-varying Physical and Mental Component scores were associated with higher mortality in the overall cohort (reference: highest quartile): adjusted HRs, 2.30 (95% CI, 1.53-3.47) and 1.54 (95% CI, 1.05-2.25), respectively. In analyses stratified by race/ethnicity, the lowest quartile of Physical Component scores was significantly associated with higher mortality across all groups: adjusted HRs, 2.64 (95% CI, 1.31-5.29), 1.84 (95% CI, 1.01-3.38), and 3.18 (95% CI, 1.13-8.91) for Hispanic, African American, and other race/ethnicity patients, respectively. The lowest quartile of time-varying physical functioning, role limitations due to physical health, role limitations due to emotional problems, social functioning, and pain subscale scores were associated with higher mortality in the overall cohort and particularly in Hispanics and blacks.
Limitations
Residual confounding cannot be excluded.
Conclusions
Lower SF-36 Physical Component and subscale scores were associated with higher mortality in hemodialysis patients, including those of minority background. Further studies are needed to determine whether interventions that augment physical health might improve the survival of these diverse populations.
Index Words: Health-related quality of life, race, ethnicity, mortality, dialysis
Graphical abstract
Editorial, p. 232
Multiple studies have shown that impaired health-related quality of life (HRQoL) is a strong prognostic factor for mortality and hospitalization risk in the hemodialysis population.1, 2, 3, 4, 5, 6, 7 In addition, HRQoL assessment may reflect patient perspectives of the impact of the treatment of end-stage renal disease (ESRD), including selection of optimal dialysis modality, adjustment of the dialysis prescription (ie, frequency and time), anemia correction, and symptom management.1 Consequently, routine monitoring of hemodialysis patients’ HRQoL using validated instruments is mandated by regulatory bodies (eg, Centers for Medicare & Medicaid Services)8, 9 and recommended by clinical practice guidelines (eg, Kidney Disease: Improving Global Outcomes [KDIGO]).10
Among the half-million patients receiving maintenance hemodialysis in the United States, approximately one-third are African American, with Hispanics comprising one-fifth of the population.11 Although numerous health care disparities have been documented among minority dialysis patients (eg, poorer dialysis performance measures,12, 13 less pre-ESRD care,14 lower kidney transplantation rates,15, 16 and younger age at dialysis initiation17), population-based studies have shown that African American and Hispanic patients with ESRD have a paradoxically lower mortality risk compared with non-Hispanic whites.12, 15, 18, 19, 20, 21, 22 Despite the diversity of the US hemodialysis population and growing recognition of the importance of patient-reported outcomes in research and clinical practice,10 there remain substantial knowledge gaps regarding the impact of HRQoL on the health and survival of hemodialysis patients of varying racial and ethnic backgrounds.
Race and ethnicity are important considerations in the interpretation of HRQoL measurements because varying traditions, values, and expectations across distinct cultures are likely to exert a strong influence on patients’ self-reported physical and mental well-being.5 To date, several seminal studies have explored the differential relationships between HRQoL and survival across race/ethnicity in hemodialysis patients,2, 5 but interpretation of these data is limited by their lack of longitudinal measurements of HRQoL over time (ie, baseline HRQoL assessment only)2, 5 or examination of populations lacking substantial representation from certain minority groups such as Hispanic patients.2 We thus sought to conduct a study examining HRQoL and mortality risk in a diverse prospective cohort of hemodialysis patients recruited across 18 outpatient dialysis units in Southern California. Using validated self-administered questionnaires, namely the 36-Item Short Form Health Survey (SF-36), administered every 6 months, we granularly examined HRQoL defined by 2 main summary scores (ie, Physical and Mental Component scores) and 8 major subscales and their associations with survival across patients of different racial/ethnic backgrounds.
Methods
Study Population
The study population was a subcohort of adult hemodialysis patients from the Malnutrition, Diet and Racial Disparities in Chronic Kidney Disease (MADRAD) Study (ClinicalTrials.gov NCT01415570), an ongoing prospective cohort study in which the impact of dietary and nutritional intake patterns on outcomes across different racial/ethnic groups is being examined.23, 24, 25, 26, 27 In the MADRAD Study, participants are asked to complete questionnaires collecting information on sociodemographic backgrounds, comorbid conditions, dialysis treatment characteristics, and patient-centered outcomes (eg, HRQoL) every 6 months (ie, every semester).
In this substudy, we sought to examine the association of HRQoL, ascertained using the SF-36, and all-cause mortality risk across racial/ethnic groups using data from hemodialysis patients recruited across 18 outpatient dialysis facilities in Southern California during October 2011 to August 2016. Patients were included provided that they completed at least 1 or more SF-36 assessment, were at least 18 years of age at the time of study entry (ie, time of baseline SF-36 assessment), had been receiving thrice-weekly in-center hemodialysis for at least 4 consecutive weeks, and signed an Institutional Review Board–approved consent form. Patients were excluded if they were actively receiving peritoneal dialysis, had a life expectancy of less than 6 months (defined as having a terminal disease such as metastatic cancer or enrollment in hospice while concurrently receiving dialysis), or were unable to provide consent without a proxy. In the event that a patient had an incomplete SF-36 at 1 time point (defined as having ≥50% of SF-36 components unanswered or incomplete data for any of the SF-36 subscale scores) but had complete data at another time point, the patient was retained in the study cohort while incomplete survey data were excluded.
The study was approved by the Institutional Review Board committee at the University of California Irvine (research ethics committee approval number HS#2012-9045).
Exposure Ascertainment
The primary exposure of interest was HRQoL measured using the SF-36. The SF-36 assessment is comprised of 2 main summary scores, specifically the Physical and Mental Component scores, as well as 8 subscales that include physical functioning, role limitations due to physical health (role functioning/physical), role limitations due to emotional problems (role functioning/emotional), energy/fatigue, emotional well-being, social functioning, pain, and general health (ie, higher scores indicate a better state of health).24 In the current study, we opted to use the SF-36 questionnaire as a generic survey of HRQoL instead of disease-specific instruments (eg, Kidney Disease Quality of Life survey).28 Although the latter instruments have greater sensitivity in hemodialysis patients, we purposefully administered generic measures to allow for comparison across other populations in future studies.
In primary and secondary analyses, we sought to examine the association between time-varying HRQoL Physical and Mental Component scores, respectively, and all-cause mortality, in which repeated measurements of SF-36 data were considered to account for changes in HRQoL summary score over time.29 In secondary analyses, we also examined the association between time-varying HRQoL subscale scores with all-cause mortality risk.
Race and Ethnicity Ascertainment
Given our objective of examining differences in the association of HRQoL and mortality across racial/ethnic groups, we also examined patient race and ethnicity data. Information for primary race and ethnicity was self-reported by patients using sociodemographic questionnaires administered by MADRAD research coordinators at the time of study entry. Patients’ race/ethnicity was classified as Hispanic white, African American, or other race/ethnicity. Due to the lower prevalence of non-Hispanic white (N = 66; 9% of the cohort) and Asian/Pacific Islander (N = 75; 10% of the cohort) patients, these groups were included within the other race/ethnicity category. In the rest of this report, the former 3 groups are referred to as Hispanic, African American, and other patients, respectively.
Outcome Ascertainment
The primary outcome of interest was all-cause mortality. At-risk time began the day after the baseline SF-36 measurement, and patients were censored for kidney transplantation, transfer to a nonaffiliated outpatient dialysis unit or peritoneal dialysis, or at the end of the study (November 15, 2017). Each semester, information regarding mortality, censoring events, and associated dates from the preceding 6 months was collected from event forms completed by the research coordinators and reviewed by the MADRAD Study nephrologist (C.M.R.).23, 25, 26, 27
Statistical Analyses
Baseline characteristics between exposure groups were compared using χ2, analysis of variance, and Kruskal-Wallis tests according to variable type. We then examined the associations of time-varying SF-36 Physical Component score categorized as quartiles and mortality using time-varying Cox proportional hazard models. In the time-varying Cox models, quartile limits were defined according to the baseline distribution of SF-36 Physical Component scores. Cox models were analyzed using 3 incremental levels of covariate adjustment: (1) unadjusted model: included the SF-36 Physical Component score as the primary exposure of interest; (2) case-mix–adjusted model: adjusted for age, sex, race, ethnicity, diabetes, and dialysis vintage; and (3) expanded case-mix–adjusted model: adjusted for covariates in the case-mix model, as well as marital status, primary insurance, and vascular access type.
Our a priori primary model of interest was the expanded case-mix model. We also examined: (4) expanded case-mix + laboratory–adjusted models that adjusted for covariates in the expanded case-mix model, as well as potential body composition and laboratory confounders that included body mass index (BMI), serum albumin level, serum creatinine level, normalized protein catabolic rate, and hemoglobin level; and (5) expanded case-mix + laboratory + center–adjusted models that adjusted for covariates in the expanded case-mix + laboratory–adjusted model, as well as each dialysis center.
While case-mix and expanded case-mix covariates had no missing values (eg, age, sex, race, ethnicity, diabetes, marital status, and primary insurance) or <5% missing values (eg, dialysis vintage and vascular access type), there were higher proportions of missing data for BMI (10%) and laboratory covariates (ie, serum albumin [19%], serum creatinine [20%], normalized protein catabolic rate [19%], and hemoglobin [17%]). Hence, further adjustment for potential confounders in expanded case-mix + laboratory models (which have the potential for inherent selection bias due to missing data) were conducted as sensitivity analyses. In these analyses, missing data were handled using multiple imputation. Analogous analyses were conducted in which we examined the SF-36 Mental Component score and subscale scores categorized as quartiles and mortality using Cox proportional hazard models.
To determine whether race/ethnicity was a modifier of HRQoL scores and mortality risk, we also conducted subgroup analyses across racial/ethnic groups. The proportional hazards assumption was confirmed graphically and by Schoenfeld residual testing. The analyses and figures were generated using SAS, version 9.4 (SAS Institute Inc); Stata, version 13.1 (Stata Corp); and SigmaPlot, version 12.5 (Systat Software).
Results
Baseline Characteristics
After applying the eligibility criteria, the final study cohort was composed of 753 patients who underwent a total of 2,461 repeated SF-36 measurements over time (Fig S1). Among these patients, N = 368 (49%) were Hispanic, N = 239 (32%) were African American, and N = 146 (19%) were classified as being of other race/ethnicity. Among those of other race/ethnicity, N = 66 (9%) were non-Hispanic white, N = 75 (10%) were Asian/Pacific Islander, and N = 5 (<1%) were of another race/ethnicity.
Examination of median (25th, 75th percentiles) SF-36 scores across racial/ethnic groups showed that Hispanic, African American, and other race/ethnicity patients had similar distributions of Physical and Mental Component scores (Table S1). With respect to subscale scores, Hispanics had higher social functioning and pain scores yet lower emotional well-being and general health scores compared with African American and other race/ethnicity patients.
Baseline characteristics of the cohort stratified by baseline Physical Component score quartiles are shown in Table 1. Compared with patients in the highest (fourth) Physical Component score quartile, those in the lowest (first) quartile were more likely to be older, women, or African American; less likely to be Hispanic; more likely to have Medicare as their primary insurance, underlying diabetes, and a central venous catheter as the primary vascular access; and more likely to have higher BMI, lower serum albumin levels, and lower serum creatinine levels.
Table 1.
Baseline Characteristics According to Quartiles of Baseline 36-Item Short Form Health Survey Physical Component Scores
| Physical Component Score |
||||||
|---|---|---|---|---|---|---|
| Overall | Q1 | Q2 | Q3 | Q4 | P for Trend | |
| N | 753 | 189 | 187 | 189 | 188 | NA |
| Physical Component score range | 28.5-80.7 | 28.5-<42.8 | 42.8-<49.1 | 49.1-<56.1 | 56.1-80.7 | NA |
| Age, y | 55 ± 14 | 60 ± 13 | 54 ± 13 | 54 ± 14 | 50 ± 15 | <0.001 |
| Female sex | 336 (45%) | 98 (52%) | 81 (43%) | 83 (44%) | 74 (39%) | 0.02 |
| Race/ethnicity | ||||||
| Hispanic | 368 (49%) | 90 (48%) | 98 (52%) | 76 (40%) | 104 (55%) | 0.50 |
| African American | 239 (32%) | 65 (34%) | 59 (32%) | 69 (37%) | 46 (25%) | 0.10 |
| Other | 146 (19%) | 34 (18%) | 30 (16%) | 44 (23%) | 38 (20%) | 0.28 |
| Marital status | 0.81 | |||||
| Married | 341 (45%) | 84 (44%) | 84 (45%) | 88 (47%) | 85 (45%) | |
| Nonmarried | 412 (55%) | 105 (56%) | 103 (55%) | 101 (53%) | 103 (55%) | |
| Primary insurance | ||||||
| Medicare/Medicaid | 581 (77%) | 155 (82%) | 153 (82%) | 136 (72%) | 137 (73%) | 0.006 |
| Private | 82 (11%) | 15 (8%) | 19 (10%) | 29 (15%) | 19 (10%) | 0.25 |
| Other/unknown | 90 (12%) | 19 (10%) | 15 (8%) | 24 (13%) | 32 (17%) | 0.02 |
| Diabetes | 405 (54%) | 128 (68%) | 110 (59%) | 92 (49%) | 75 (40%) | <0.001 |
| Dialysis vintage, mo | 35 (14, 70) | 35 (16, 71) | 34 (13, 66) | 34 (11, 72) | 33 (16, 68) | 0.87 |
| Vascular access | 0.30 | |||||
| AVF/AVG | 612 (84%) | 149 (84%) | 148 (82%) | 153 (83%) | 162 (88%) | |
| Catheter | 115 (16%) | 29 (16%) | 32 (18%) | 31 (17%) | 23 (12%) | |
| BMI, kg/m2 | 26.6 (23.5, 31.8) | 27.8 (24.0, 34.1) | 26.5 (22.8, 31.4) | 26.8 (23.6, 32.8) | 25.9 (22.9, 29.5) | 0.01 |
| Laboratory results | ||||||
| Serum albumin, g/dL | 4.0 (3.8, 4.2) | 3.9 (3.7, 4.1) | 3.9 (3.7, 4.2) | 4.0 (3.9, 4.3) | 4.1 (3.9, 4.3) | <0.001 |
| Serum creatinine, g/dL | 9.5 (7.6, 11.7) | 8.7 (7.1, 10.6) | 9.4 (7.0, 11.6) | 9.8 (7.8, 11.5) | 10.7 (8.5, 12.6) | <0.001 |
| nPCR, g/kg/d | 1.0 (0.9, 1.2) | 1.0 (0.8, 1.2) | 1.0 (0.9, 1.1) | 1.0 (0.8, 1.2) | 1.1 (0.9, 1.3) | 0.002 |
| Hemoglobin, g/dL | 10.7 (10.1, 11.3) | 10.7 (10.1, 11.2) | 10.6 (10.0, 11.2) | 10.7 (10.2, 11.4) | 10.7 (10.2, 11.2) | 0.57 |
Note: Values expressed as N (percent), mean ± standard deviation, or median (25th, 75th percentiles).
Abbreviations: AVF, arteriovenous fistula; AVG, arteriovenous graft; BMI, body mass index; NA, not applicable; nPCR, normalized protein catabolic rate; Q, quartile.
Table S2 shows baseline characteristics of the cohort stratified by baseline Mental Component score quartiles. Compared with the highest (fourth) Mental Component score quartile, those in the lowest (first) quartile were more likely to be younger, women, and Hispanic; less likely to be African American, be married, or have private insurance as the primary insurance; and more likely to have a shorter dialysis vintage and a central venous catheter as the primary vascular access.
Clinical Characteristics Associated With Physical and Mental Component and Subscale Scores
We examined clinical characteristics associated with the lowest (first) quartile of Physical Component scores. In expanded case-mix logistic regression analyses, patients who were older and diabetic had a higher likelihood of low (ie, lowest quartile) Physical Component scores, whereas those with higher serum albumin levels had a lower likelihood (Table 2). We then examined clinical characteristics associated with the lowest (first) quartile of Mental Component scores and found that patients with an arteriovenous fistula or arteriovenous graft were less likely to have low scores in expanded case-mix logistic regression analyses (Table S3).
Table 2.
Clinical Characteristics Associated With The Lowest Quartile (Q1) of Baseline Physical Component Scores in Unadjusted and Adjusted Models
| Unadjusted |
Case-Mix |
Expanded Case-Mix |
Expanded Case-Mix + Laboratory |
|
|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Age at dialysis initiation (Δ10 y) | 1.47 (1.30-1.67)a | 1.41 (1.23-1.61)a | 1.41 (1.22-1.62)a | 1.40 (1.20-1.64)a |
| Female (vs male) sex | 1.48 (1.06-2.05)a | 1.42 (1.00-2.00)a | 1.40 (0.99-1.99) | 1.33 (0.91-1.93) |
| Race/ethnicity (vs Hispanic) | ||||
| African American | 1.15 (0.80-1.67) | 1.12 (0.75-1.65) | 1.08 (0.71-1.62) | 1.03 (0.67-1.60) |
| Other | 0.94 (0.60-1.47) | 0.86 (0.53-1.39) | 0.85 (0.52-1.38) | 0.90 (0.55-1.48) |
| Diabetes (vs nondiabetes) | 2.17 (1.54-3.08)a | 1.77 (1.21-2.59)a | 1.73 (1.18-2.54)a | 1.59 (1.07-2.37)a |
| Vintage (Δ12 mo) | 1.01 (0.97-1.06) | 1.03 (0.99-1.08) | 1.03 (0.98-1.08) | 1.03 (0.98-1.08) |
| Married (vs nonmarried) | 0.96 (0.69-1.33) | 0.81 (0.57-1.17) | 0.83 (0.58-1.19) | 0.86 (0.59-1.24) |
| Primary insurance (vs Medicare/Medicaid) | ||||
| Private | 0.62 (0.34-1.11) | 0.69 (0.37-1.28) | 0.71 (0.38-1.31) | 0.69 (0.37-1.28) |
| Other/unknown | 0.74 (0.43-1.26) | 0.99 (0.56-1.76) | 0.97 (0.55-1.73) | 1.00 (0.56-1.80) |
| AVF/AVG (vs CVC) | 0.95 (0.60-1.51) | 0.80 (0.49-1.31) | 0.83 (0.50-1.35) | 0.88 (0.53-1.46) |
| BMI (Δ5 kg/m2) | 1.16 (1.03-1.30)a | 1.13 (0.99-1.28) | 1.12 (0.98-1.27) | 1.10 (0.96-1.25) |
| Serum albumin (Δ0.5 mg/dL) | 0.55 (0.42-0.71)a | 0.64 (0.48-0.84)a | 0.64 (0.48-0.85)a | 0.64 (0.48-0.86)a |
| Serum creatinine (Δ1.0 g/dL) | 0.90 (0.84-0.96)a | 0.98 (0.91-1.07) | 0.98 (0.91-1.07) | 1.00 (0.92-1.09) |
| Normalized protein catabolic rate (Δ0.2 g/kg/d) | 0.93 (0.81-1.06) | 0.95 (0.82-1.10) | 0.96 (0.83-1.11) | 1.01 (0.87-1.18) |
| Hemoglobin (Δ1 g/dL) | 0.99 (0.84-1.17) | 1.02 (0.85-1.22) | 1.02 (0.85-1.23) | 1.03 (0.86-1.24) |
Note: Reference quartiles: Q2-Q4.
Abbreviations: AVF, arteriovenous fistula; AVG, arteriovenous graft; BMI, body mass index; CI, confidence interval; CVC, central venous catheter; OR, odds ratio; Q, quartile.
Significant estimates.
We also examined clinical characteristics associated with the lowest (first) quartile of each of the SF-36 subscale scores using expanded case-mix logistic regression models and found that age, comorbid condition status, and nutritional parameters were associated with various subscale scores (Table S4). For example, patients with diabetes had a higher likelihood of having the lowest quartile of physical functioning, energy/fatigue, emotional well-being, social functioning, pain, and general health scores. Older age was associated with a higher likelihood of having the lowest quartile of physical functioning and role functioning/physical scores, yet lower likelihood of having the lowest quartile of general health scores.
Higher serum albumin level was also associated with a lower likelihood of having the lowest quartile of physical functioning, role functioning/physical, energy/fatigue, social functioning, and pain scores. In addition, women had a higher likelihood of the lowest quartile of pain scores; those with a higher BMI had a higher likelihood of the lowest quartile of role functioning/physical and pain scores; and patients with a higher hemoglobin level had a higher likelihood of the lowest quartile of emotional well-being scores.
Physical and Mental Component Scores and Mortality Risk
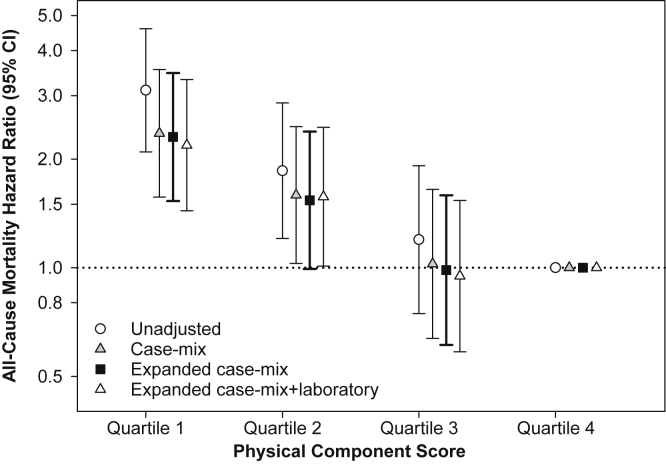
In primary analyses examining time-varying Physical Component scores in the overall cohort, expanded case-mix analyses showed that the lowest quartile of scores was associated with higher mortality risk (reference: highest quartile): adjusted hazard ratio (HR), 2.30 (95% confidence interval [CI], 1.53-3.47; Fig 1; Table S5). In secondary analyses examining Mental Component scores in the overall cohort, expanded case-mix analyses showed that the lowest quartile of scores was associated with higher mortality (reference: highest quartile): adjusted HR, 1.54 (95% CI, 1.05-2.25; Fig S2; Table S5). In expanded case-mix analyses that examined time-varying Physical and Mental Component scores in continuous 10-point increments, increasingly higher Physical Component scores were associated with lower mortality risk, whereas estimates for Mental Component scores did not achieve statistical significance (Table S6). Similar findings were observed in analyses adjusted for expanded case-mix + laboratory and expanded case-mix + laboratory + center covariates (Tables S5 and S6).
Figure 1.
Association between 36-Item Short Form Health Survey Physical Component scores and all-cause mortality in the overall cohort. Abbreviation: CI, confidence interval.
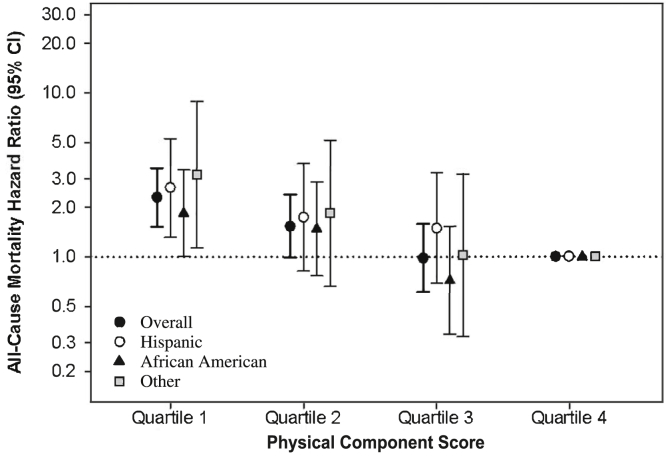
In analyses stratified by race/ethnicity, expanded case-mix analyses showed that the lowest time-varying Physical Component score quartile was associated with higher death risk in Hispanic, African American, and other race/ethnicity patients (reference: highest quartile): adjusted HRs, 2.64 (95% CI, 1.31-5.29), 1.84 (95% CI, 1.01-3.38), and 3.18 (95% CI, 1.13-8.91), respectively (P interaction for race/ethnicity = 0.86; Fig 2; Table S5).
Figure 2.
Association between 36-Item Short Form Health Survey Physical Component scores and mortality across racial/ethnic groups. Analyses adjusted for expanded case-mix covariates, which included age, sex, race, ethnicity, diabetes, dialysis vintage, marital status, primary insurance, and vascular access type. Abbreviation: CI, confidence interval.
Similar findings were observed with adjustment for expanded case-mix + laboratory + center covariates, although estimates for African American patients did not reach statistical significance. With respect to time-varying Mental Component scores, among Hispanic, African American, and other race/ethnicity patients, point estimates of the lowest time-varying quartile suggested higher death risk but did not reach statistical significance (P interaction for race/ethnicity = 0.37; Fig S2; Table S5). Similar findings were observed with adjustment for expanded case-mix + laboratory + center covariates.
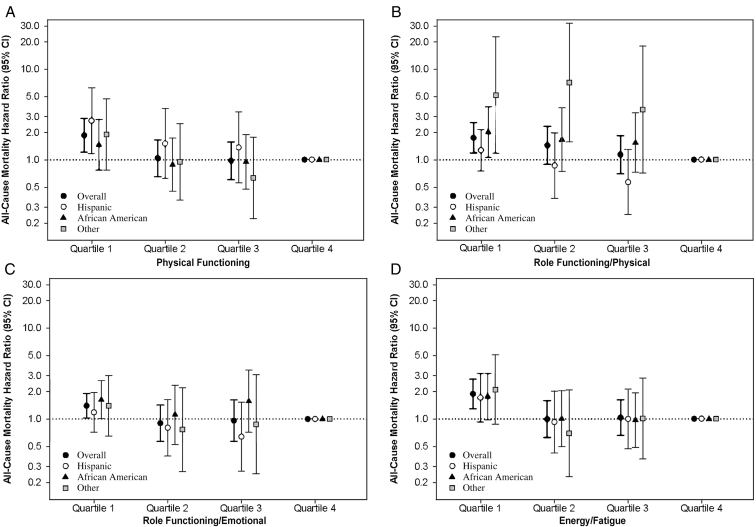
SF-36 Subscale Scores and Mortality Risk
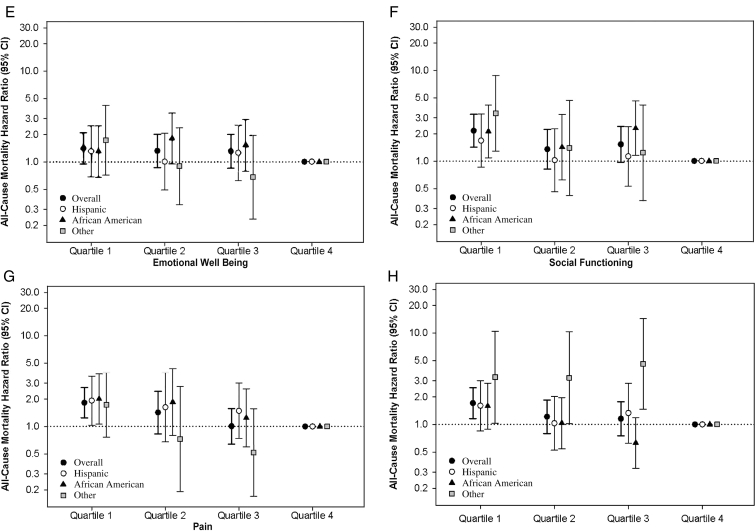
In secondary analyses examining time-varying subscale scores in the overall cohort, expanded case-mix analyses showed that the lowest quartile of each of the subscale scores except for emotional well-being were associated with higher mortality (reference: highest quartile; Fig 3; Table S7). In expanded case-mix analyses that examined time-varying subscale scores in 10-point increments, increasingly higher scores of all subscales except emotional well-being were associated with lower death risk (Table S6). Similar findings were observed with adjustment for expanded case-mix + laboratory + center covariates (Tables S6 and S7).
Figure 3.
Association between 36-Item Short Form Health Survey subscale scores and mortality across racial/ethnic groups: (A) Physical Functioning, (B) Role Functioning/Physical, (C) Role Functioning/Emotional, (D) Energy/Fatigue, (E) Emotional Well-Being, (F) Social Functioning, (G) Pain, and (H) General Health. Analyses adjusted for expanded case-mix covariates, which included age, sex, race, ethnicity, diabetes, dialysis vintage, marital status, primary insurance, and vascular access type. Abbreviation: CI, confidence interval.
In expanded case-mix analyses stratified by race/ethnicity, HRs for the lowest quartile of all time-varying subscale scores were >1, although associations were only statistically significant in the following racial/ethnic subgroups and time-varying subscale scores: Hispanics (physical functioning and pain), African Americans (role functioning/physical, role functioning/emotional, social functioning, and pain), and other race/ethnicity (role functioning/physical, social functioning, and general health; Fig 3; Table S7).
Discussion
In a racially/ethnically diverse, multicenter, and well-characterized prospective cohort of hemodialysis patients who underwent protocolized SF-36 assessments every 6 months, lower (worse) Physical and Mental Component scores were associated with higher mortality risk in the overall cohort. Lower (worse) scores across all SF-36 subscales except for emotional well-being were also associated with higher death risk in the overall cohort. Upon examining SF-36 scores across racial/ethnic subgroups, the lowest quartile of Physical Component scores was associated with higher mortality among Hispanic, African American, and other race/ethnicity patients. Similarly, point estimates of the lowest quartile of Mental Component scores trended toward higher mortality in Hispanic, African American, and other race/ethnicity patients, although estimates did not reach statistical significance.
Few studies have specifically compared the relationship between HRQoL and survival among hemodialysis patients of distinct racial/ethnic backgrounds.2, 5 In one of the earlier studies conducted to date, Lopes et al5 examined baseline Kidney Disease Quality of Life Short Form scores and survival among hemodialysis patients from the US Dialysis Outcomes and Practice Patterns Study (DOPPS) cohort. Similar to our observations, there was a robust association between lower Physical Component scores and worse survival across non-Hispanic whites, non-Hispanic African Americans, and Hispanics, whereas lower Mental Component scores were linked with higher death risk among non-Hispanic whites and non-Hispanic African Americans only.
In a subsequent analysis by Feroze et al,2 baseline SF-36 score–mortality associations were compared among African American versus non–African American hemodialysis patients. Although lower Physical and Mental Component scores were associated with higher death risk in non–African American patients, among African American patients, only lower Mental Component scores were associated with worse survival.
Among patients with non–dialysis-dependent chronic kidney disease, several rigorous studies have examined baseline and time-varying HRQoL scores among minorities. For example, in a secondary analysis of the African-American Study of Kidney Disease and Hypertension (AASK) cohort by Porter et al,6 baseline, time-varying, and cumulative Physical and Mental Component scores were associated with higher cardiovascular death risk among African American patients with non–dialysis-dependent chronic kidney disease.6
Most recently, in an analysis of non–dialysis-dependent patients with chronic kidney disease from the Chronic Renal Insufficiency Cohort (CRIC) and Hispanic CRIC Studies who underwent baseline Kidney Disease Quality of Life 36 assessment, Physical and Mental Component scores tended to be higher in non-Hispanic whites compared with non-Hispanic African Americans and Hispanics. Although analyses of HRQoL and mortality were not stratified by race/ethnicity, lower Physical and Mental Component scores were associated with higher death risk in the overall cohort.7
The present study builds on the existing literature in several ways. First, whereas the mentioned hemodialysis studies have examined baseline HRQoL scores only, ours is the first to consider repeated measurements of the SF-36 (ie, thereby taking into account changes in HRQoL over time), analogous to clinical practice in which HRQoL is routinely (ie, annually) monitored.1, 8, 9, 10
Second, our study leverages data from a prospective cohort dedicated to examining racial/ethnic disparities with a particularly high prevalence of minorities compared with previous investigations (ie, ∼50% vs 10% of patients of Hispanic background in our study vs the Lopes et al5 study, respectively). Notably, although population-based studies have reported a paradoxical survival advantage among minority hemodialysis patients,12, 15, 18, 19, 20, 21, 22 our study observed lower levels of self-reported HRQoL, as well as potent associations of lower SF-36 scores with mortality risk particularly in Hispanics (ie, for Physical Component and physical functioning and pain subscale scores) and African Americans (ie, for Physical Component and role functioning/physical, role functioning/emotional, social functioning, and pain subscale scores).
Potential reasons as to why statistically significant associations were more frequently observed in Hispanic and African American patients include their dominant representation in the diverse MADRAD cohort (ie, resulting in greater power to detect significant associations); differential responses to the SF-36 across race/ethnicity (ie, similar decrements in SF-36 scores may represent greater HRQoL impairments in Hispanic and African American patients); and more detrimental impact of impaired physical health on survival in these subgroups (ie, impaired SF-36 scores in the Hispanic and African American patients were largely centered on physical health). Thus, our findings highlight the critical importance of comprehensive HRQoL assessment in minority patients with ESRD and an urgent need to identify interventions that can improve their physical and mental well-being.
In addition, our study adds new knowledge to the field regarding potential modifiable and nonmodifiable factors linked with worse SF-36 scores in hemodialysis patients. Notably, we observed that markers of better nutritional status (ie, serum albumin) were associated with higher (better) SF-36 scores particularly related to physical health. In contrast, older age and the presence of diabetes were associated with lower (worse) SF-36 scores centered on physical health. In addition, we found that arteriovenous fistula/graft use was associated with better Mental Component scores versus central venous catheter use (which may be due to reduced infection, inflammation, and downstream impairments in HRQoL). Hence, further studies are needed to determine whether nutritional interventions and implementation of best dialysis practices can improve the HRQoL of hemodialysis patients.
The strengths of our study include its diverse cohort of hemodialysis patients who underwent protocolized HRQoL assessments every 6 months, rigorous use of an HRQoL instrument that has been validated in the hemodialysis and other chronic disease populations, and comprehensive availability of detailed patient-level data on sociodemographics, comorbid conditions, dialysis treatment characteristics, and vital status collected in the outpatient setting.
However, several limitations of our study bear mention. First, it is possible that patients who agreed to participate in the MADRAD Study may be healthier than those who were not recruited and may have thus had higher (better) HRQoL scores compared with the broader hemodialysis population.
Second, although we had the opportunity to examine a well-characterized prospective cohort, the limited sample size of our study population likely precluded the ability to detect significant interactions on the basis of race/ethnicity (ie, much larger sample size needed to estimate an interaction than to estimate a main effect).
Third, given that our recruitment was restricted to 18 outpatient dialysis units in Southern California, our findings may not be generalizable to other geographic regions in which patients’ case-mix characteristics and dialysis practice patterns may differ.
Fourth, because patients with ESRD receiving home modalities have been observed to have higher HRQoL scores versus those receiving in-center hemodialysis, our observations may not be relevant to the former population.30
Finally, as with all observational studies, we cannot exclude the possibility of residual confounding.
In conclusion, impaired HRQoL scores, defined by lower SF-36 Physical and Mental Component and subscale scores, were associated with higher mortality risk in hemodialysis patients, including those of minority background. Further studies are needed to identify interventions that can effectively improve the HRQoL and downstream survival of the diverse US hemodialysis population.
Article Information
Authors’ Full Names and Academic Degrees
Sara S. Kalantar, Amy S. You, MS, Keith C. Norris, MD, PhD, Tracy Nakata, BA, Alejandra Novoa, BS, Kimberly Juarez, BS, Danh V. Nguyen, MS, PhD, and Connie M. Rhee, MD, MSc.
Authors’ Contributions
Research idea and study design: SSK, CMR; data acquisition: CMR; data analysis/interpretation: SSK, ASY, KCN, TN, AN, KJ, DVN, CMR; statistical analysis: ASY; supervision or mentorship: CMR. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Support
The authors are supported by research grants from the National Institutes of Health (NIH)/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), including K23-DK102903 (Dr Rhee), R03-DK114642 (Dr Rhee), and R01-DK122767 (Dr Rhee). Resources for this study have also been provided by NIH/NIDDK grant K24-DK091419. Funders of this study did not have any role in the study design; collection, analysis, and interpretation of data; writing of the report; or the decision to submit the report for publication.
Financial Disclosure
The authors declare that they have no relevant financial interests.
Prior Presentation
Portions of these data have been presented as an oral abstract at the International Congress of Renal Nutrition and Metabolism, June 26-30, 2018, Genoa, Italy, and as an abstract at the American Society of Nephrology Kidney Week Meeting, October 23-28, 2018, San Diego, CA.
Peer Review
Received January 30, 2019. Evaluated by 2 external peer reviewers, with direct editorial input from the Statistical Editor, an Associate Editor, and the Editor-in-Chief. Accepted in revised form July 12, 2019.
Footnotes
Complete author and article information provided before references.
Figure S1: Study cohort creation.
Figure S2: Association between Short Form 36 Physical Component scores and mortality across racial/ethnic groups.
Table S1: Distribution of baseline Short Form 36 scores in the overall cohort and across race/ethnicity.
Table S2: Baseline characteristics according to quartiles (Q) of baseline Short Form 36 Mental Component scores.
Table S3: Clinical characteristics associated with the lowest quartile (Q1) of baseline Mental Component scores (reference: quartiles 2-4, Q2-4) in unadjusted, case-mix–, expanded case-mix–, and expanded case-mix + laboratory–adjusted models.
Table S4: Clinical characteristics associated with the lowest quartile (Q1) of each of the 8 baseline Short-Form 36 subscale scores (reference: quartiles 2-4, Q2-4) in expanded case-mix–adjusted models.
Table S5: Association of Short Form 36 time-varying Physical Component and Mental Component scores categorized as quartiles (Q) with all-cause mortality risk in the overall cohort and across race/ethnicity. Cox regression analyses adjusted for expanded case-mix covariates.
Table S6: Association of Short Form 36 time-varying Physical Component and Mental Component scores and 8 subscales in 10-point increments with all-cause mortality risk in the overall cohort.
Table S7: Association of the 8 Short Form 36 time-varying subscales categorized as quartiles (Q) with all-cause mortality risk in the overall cohort and across race/ethnicity. Cox regression analyses adjusted for expanded case-mix covariates.
Supplementary Material
. Figures S1 and S2; Tables S1-S7.
References
- 1.Chen S.S., Al Mawed S., Unruh M. Health-related quality of life in end-stage renal disease patients: how often should we ask and what do we do with the answer? Blood Purif. 2016;41(1-3):218–224. doi: 10.1159/000441462. [DOI] [PubMed] [Google Scholar]
- 2.Feroze U., Noori N., Kovesdy C.P. Quality-of-life and mortality in hemodialysis patients: roles of race and nutritional status. Clin J Am Soc Nephrol. 2011;6(5):1100–1111. doi: 10.2215/CJN.07690910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalantar-Zadeh K., Kopple J.D., Block G., Humphreys M.H. Association among SF36 quality of life measures and nutrition, hospitalization, and mortality in hemodialysis. J Am Soc Nephrol. 2001;12(12):2797–2806. doi: 10.1681/ASN.V12122797. [DOI] [PubMed] [Google Scholar]
- 4.Kimmel P.L., Peterson R.A., Weihs K.L. Aspects of quality of life in hemodialysis patients. J Am Soc Nephrol. 1995;6(5):1418–1426. doi: 10.1681/ASN.V651418. [DOI] [PubMed] [Google Scholar]
- 5.Lopes A.A., Bragg-Gresham J.L., Satayathum S. Health-related quality of life and associated outcomes among hemodialysis patients of different ethnicities in the United States: the Dialysis Outcomes and Practice Patterns Study (DOPPS) Am J Kidney Dis. 2003;41(3):605–615. doi: 10.1053/ajkd.2003.50122. [DOI] [PubMed] [Google Scholar]
- 6.Porter A., Fischer M.J., Wang X. Quality of life and outcomes in African Americans with CKD. J Am Soc Nephrol. 2014;25(8):1849–1855. doi: 10.1681/ASN.2013080835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Porter A.C., Lash J.P., Xie D. Predictors and outcomes of health-related quality of life in adults with CKD. Clin J Am Soc Nephrol. 2016;11(7):1154–1162. doi: 10.2215/CJN.09990915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beck R.W., Riddlesworth T.D., Ruedy K. Continuous glucose monitoring versus usual care in patients with type 2 diabetes receiving multiple daily insulin injections: a randomized trial. Ann Intern Med. 2017;167(6):365–374. doi: 10.7326/M16-2855. [DOI] [PubMed] [Google Scholar]
- 9.Finkelstein F.O., Wuerth D., Finkelstein S.H. Health related quality of life and the CKD patient: challenges for the nephrology community. Kidney Int. 2009;76(9):946–952. doi: 10.1038/ki.2009.307. [DOI] [PubMed] [Google Scholar]
- 10.Peipert J.D., Hays R.D. Using patient-reported measures in dialysis clinics. Clin J Am Soc Nephrol. 2017;12(11):1889–1891. doi: 10.2215/CJN.02250217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Polonsky W.H., Peters A.L., Hessler D. The impact of real-time continuous glucose monitoring in patients 65 years and older. J Diabetes Sci Technol. 2016;10(4):892–897. doi: 10.1177/1932296816643542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Owen W.F., Jr., Chertow G.M., Lazarus J.M., Lowrie E.G. Dose of hemodialysis and survival: differences by race and sex. JAMA. 1998;280(20):1764–1768. doi: 10.1001/jama.280.20.1764. [DOI] [PubMed] [Google Scholar]
- 13.Wasse H., Hopson S.D., McClellan W. Racial and gender differences in arteriovenous fistula use among incident hemodialysis patients. Am J Nephrol. 2010;32(3):234–241. doi: 10.1159/000318152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kausz A.T., Obrador G.T., Arora P., Ruthazer R., Levey A.S., Pereira B.J. Late initiation of dialysis among women and ethnic minorities in the United States. J Am Soc Nephrol. 2000;11(12):2351–2357. doi: 10.1681/ASN.V11122351. [DOI] [PubMed] [Google Scholar]
- 15.Rhee C.M., Lertdumrongluk P., Streja E. Impact of age, race and ethnicity on dialysis patient survival and kidney transplantation disparities. Am J Nephrol. 2014;39(3):183–194. doi: 10.1159/000358497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sequist T.D., Narva A.S., Stiles S.K., Karp S.K., Cass A., Ayanian J.Z. Access to renal transplantation among American Indians and Hispanics. Am J Kidney Dis. 2004;44(2):344–352. doi: 10.1053/j.ajkd.2004.04.039. [DOI] [PubMed] [Google Scholar]
- 17.Kanji Z., Powe C.E., Wenger J.B. Genetic variation in APOL1 associates with younger age at hemodialysis initiation. J Am Soc Nephrol. 2011;22(11):2091–2097. doi: 10.1681/ASN.2010121234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agodoa L., Eggers P. Racial and ethnic disparities in end-stage kidney failure-survival paradoxes in African-Americans. Semin Dial. 2007;20(6):577–585. doi: 10.1111/j.1525-139X.2007.00350.x. [DOI] [PubMed] [Google Scholar]
- 19.Arce C.M., Goldstein B.A., Mitani A.A., Winkelmayer W.C. Trends in relative mortality between Hispanic and non-Hispanic whites initiating dialysis: a retrospective study of the US Renal Data System. Am J Kidney Dis. 2013;62(2):312–321. doi: 10.1053/j.ajkd.2013.02.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kalantar-Zadeh K., Kovesdy C.P., Derose S.F., Horwich T.B., Fonarow G.C. Racial and survival paradoxes in chronic kidney disease. Nat Clin Pract Nephrol. 2007;3(9):493–506. doi: 10.1038/ncpneph0570. [DOI] [PubMed] [Google Scholar]
- 21.Kucirka L.M., Grams M.E., Lessler J. Association of race and age with survival among patients undergoing dialysis. JAMA. 2011;306(6):620–626. doi: 10.1001/jama.2011.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yan G., Norris K.C., Yu A.J. The relationship of age, race, and ethnicity with survival in dialysis patients. Clin J Am Soc Nephrol. 2013;8(6):953–961. doi: 10.2215/CJN.09180912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferrey A., You A.S., Kovesdy C.P. Dialysate potassium and mortality in a prospective hemodialysis cohort. Am J Nephrol. 2018;47(6):415–423. doi: 10.1159/000489961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rhee C.M., Chen Y., You A.S. Thyroid status, quality of life, and mental health in patients on hemodialysis. Clin J Am Soc Nephrol. 2017;12(8):1274–1283. doi: 10.2215/CJN.13211216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rhee C.M., Nguyen D.V., Moradi H. Association of adiponectin with body composition and mortality in hemodialysis patients. Am J Kidney Dis. 2015;66(2):313–321. doi: 10.1053/j.ajkd.2015.02.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rhee C.M., You A.S., Nguyen D.V. Thyroid status and mortality in a prospective hemodialysis cohort. J Clin Endocrinol Metab. 2017;102(5):1568–1577. doi: 10.1210/jc.2016-3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.You A.S., Kalantar-Zadeh K., Lerner L. Association of growth differentiation factor 15 with mortality in a prospective hemodialysis cohort. Cardiorenal Med. 2017;7(2):158–168. doi: 10.1159/000455907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kalantar-Zadeh K., Unruh M. Health related quality of life in patients with chronic kidney disease. Int Urol Nephrol. 2005;37(2):367–378. doi: 10.1007/s11255-004-0012-4. [DOI] [PubMed] [Google Scholar]
- 29.Dekker F.W., de Mutsert R., van Dijk P.C., Zoccali C., Jager K.J. Survival analysis: time-dependent effects and time-varying risk factors. Kidney Int. 2008;74(8):994–997. doi: 10.1038/ki.2008.328. [DOI] [PubMed] [Google Scholar]
- 30.Eneanya N.D., Maddux D.W., Reviriego-Mendoza M.M. Longitudinal patterns of health-related quality of life and dialysis modality: a national cohort study. BMC Nephrol. 2019;20(1):7. doi: 10.1186/s12882-018-1198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
. Figures S1 and S2; Tables S1-S7.