Abstract
Background
We lack reliable methods for predicting myocardial infarction in patients with established coronary artery disease. Coronary 18F-sodium fluoride (18F-NaF) positron emission tomography (PET) provides an assessment of atherosclerosis activity.
Objectives
We assessed whether 18F-NaF PET predicts myocardial infarction and provides additional prognostic information to current methods of risk stratification.
Methods
Patients with known coronary artery disease underwent 18F-NaF PET computed tomography and were followed-up for fatal or non-fatal myocardial infarction over 42 [31–49] months. Total coronary 18F-NaF uptake was determined using coronary microcalcification activity (CMA).
Results
In a post-hoc analysis of data collected for prospective observational studies we studied 293 study participants (65±9 years; 84% male), of whom 203 (69%) showed increased coronary 18F-NaF activity (CMA>0). Fatal or non-fatal myocardial infarction occurred only in patients with increased coronary 18F-NaF activity (20/203 CMA>0 versus 0/90 CMA=0; p<0.001). On receiver operator-curve analysis, fatal or non-fatal myocardial infarction prediction was highest for 18F-NaF CMA, outperforming coronary calcium scoring, modified Duke coronary artery disease index, REACH and SMART risk scores (areas under curve: 0.76 versus 0.54, 0.62, 0.52 and 0.54; p<0.001 for all). Patients with CMA>1.56 had >7-fold increase in fatal or non-fatal myocardial infarction (hazard ratio 7.1, 95% confidence interval 2.2 to 25.1; p=0.003) independent of age, gender, risk factors, segment involvement and coronary calcium scores, presence of coronary stents, coronary stenosis, REACH and SMART scores, the Duke coronary artery disease index and recent myocardial infarction.
Conclusion
In patients with established coronary artery disease, 18F-NaF PET provides powerful independent prediction of fatal or non-fatal myocardial infarction.
Keywords: 18F-NaF PET, coronary computed tomography, coronary artery disease, myocardial infarction, coronary event risk prediction
Condensed abstract
We assessed whether 18F-NaF PET predicts myocardial infarction and provides additional prognostic information to current methods of risk stratification. Patients with known coronary artery disease underwent contrast-enhanced 18F-NaF PET computed tomography and were followed-up for myocardial infarction over 42 [31–49] months. Among 293 study participants myocardial infarction occurred only in patients with increased coronary 18F-NaF activity. Patients with increased 18F-NaF uptake had >7-fold increase in myocardial infarction independent of age, gender, cardiovascular risk factors, segment involvement scores, presence of coronary stents, number of vessels with significant stenosis, coronary calcium scoring, REACH and SMART scores, the Duke index, initial patients presentation (acute coronary syndrome or stable) and the study in which individuals were initially recruited.
Introduction
Despite improvements in therapies for atherosclerotic disease, myocardial infarction remains a leading cause of death worldwide. Robust tools to identify patients at risk of myocardial infarction would be extremely valuable as they could facilitate the targeted application of novel or intensive therapies to patients at the highest risk of events or down escalation of therapy in patients at low risk. However, to date, risk prediction in patients with established coronary artery disease has proven challenging. Current approaches are based around clinical risk scores, anatomic assessments of coronary artery calcification and the severity of obstructive coronary stenoses (1). These approaches have shown limited predictive value in patients with established coronary artery disease and there is growing interest in novel risk stratification methods, including assessments of atherosclerotic disease activity (2), that might be used to target expensive yet effective new treatments to patients at highest risk.
Advanced positron emission tomography (PET) imaging can provide assessment of disease activity in the coronary arteries to complement the anatomic plaque imaging provided by computed tomography (CT). The PET tracer 18F-sodium fluoride (18F-NaF) is a marker of developing microcalcification and calcification activity across multiple different cardiovascular disease states (3). In coronary and carotid atherosclerosis, 18F-NaF localizes to culprit plaques following myocardial infarction and stroke as well as to plaques with multiple adverse characteristic in patients with stable disease (4–6). Moreover, coronary 18F-NaF uptake has demonstrated its ability to predict disease progression and change in coronary calcium score, similar to results in other cardiovascular conditions (7–9). While coronary 18F-NaF uptake appears to provide a marker of atherosclerosis disease activity, the prognostic significance of increased coronary 18F-NaF activity is unknown.
In this study, we investigated whether coronary 18F-NaF PET uptake predicts future myocardial infarction and MACE in patients with established coronary artery disease, and whether it can provide additional prognostic information over and above current methods of risk stratification including clinical risk scores, coronary calcium scoring and the severity of obstructive coronary artery disease.
Methods
Study Design and Participants
Patients with established coronary artery disease undergoing hybrid coronary 18F-NaF PET and contrast CT angiography at the Edinburgh Heart Centre and Cedars-Sinai Medical Center within prospective observational research studies were included in the current post-hoc analysis (NCT01749254, NCT02110303, NCT02607748) (4,10). The study cohort comprised patients with recent myocardial infarction or established stable angina pectoris undergoing elective invasive coronary angiography (inclusion and exclusion criteria have been presented in the Supplemental Appendix). All patients underwent a comprehensive baseline clinical assessment including evaluation of their cardiovascular risk factor profile. In particular, REACH [Reduction of Atherothrombosis for Continued Health] and SMART [Secondary Manifestations of Arterial Disease] risk scores were calculated (Supplemental Appendix). Both these scores were created specifically to predict risk in patients with established coronary artery disease (1,11). Patients also underwent hybrid 18F-NaF PET imaging alongside coronary CT calcium scoring and coronary CT angiography. Studies were conducted with the approval of the local research ethics committee, in accordance with the Declaration of Helsinki, and with the written informed consent of each participant.
18F-Sodium Fluoride and CT imaging
Acquisition and reconstruction
All patients underwent 18F-NaF PET on hybrid PET/CT scanners (128-slice Biograph mCT, Siemens Medical Systems, Knoxville, USA or Discovery 710 GE Healthcare, Milwaukee, WI, USA) using harmonized imaging protocols 60 min following intravenous 18F-NaF administration. During a single imaging session, we acquired a non-contrast CT attenuation correction scan followed by a 30-min PET emission scan in list mode. The electrocardiogram (ECG)-gated list mode dataset was reconstructed using a standard ordered expectation maximization algorithm with time-of-flight, and point-spread-function correction. Using 4 cardiac gates, the data were reconstructed on a 256×256 matrix (with 75 or 47 slices using 2 iterations, 21 subsets and 5-mm Gaussian smoothing or 4 iterations, 24 subsets and 5-mm gaussian smoothing for Biograph and Discovery respectively). Immediately after the PET scan, a low dose non-contrast ECG-gated CT for calculation of the coronary calcium score was performed. Subsequently, a contrast-enhanced, ECG-gated coronary CT angiogram was obtained in mid-diastole on the same PET/CT system without repositioning the patient. To compensate for coronary motion associated with heart contraction, we performed cardiac motion correction of the PET/CT images (Supplemental Appendix) (12,13).
Image analysis
Computed Tomography
The coronary artery calcium score was measured in Agatston units (AU) using clinical software (NetraMD, ScImage, Los Altos, CA, USA). The presence of coronary atherosclerosis, and the extent and severity of obstructive coronary artery disease, was evaluated on contrast-enhanced CT angiography by defining the segment involvement score; the number of vessels with >50% luminal stenosis; and the modified Duke coronary artery disease index (combining the extent, severity, and location of coronary stenoses) (14). Multivessel coronary artery disease was defined as at least 2 major epicardial vessels with any combination of either >50% stenosis, or previous revascularization.
18F-Sodium Fluoride
We used a dedicated software package for coronary PET image analysis (FusionQuant, Cedars-Sinai Medical Center, Los Angeles). PET and CT angiography reconstructions were reoriented, fused and systematically co-registered in 3 orthogonal planes (15). We used two methods to evaluate coronary 18F-NaF activity: the maximum target to background (TBR) approach (standard quantification) which relies on visual detection of lesions with increased tracer uptake; and the newly developed whole-coronary total microcalcification activity method (novel quantification) (4,16).
Target to Background Ratio quantification
On co-registered PET and CT angiography images, for a signal to be co-localized to a coronary artery, an atherosclerotic plaque had to be present on the CT angiogram and the increased pattern of radiotracer had to arise from the coronary artery and follow its course in three dimensions on 3-orthogonal views (3). In all plaques meeting these criteria, maximum standardized uptake values (SUVmax) were measured within manually drawn regions of interest. TBR values were calculated by dividing the coronary SUVmax by the blood pool activity measured in the right atrium (mean SUV in cylindrical volumes of interest at the level of the right coronary artery ostium: radius 10 mm and thickness 5 mm).
Blood clearance correction
To offset for variation in the delay between tracer injection and scanning, which has a major impact on blood pool activity, we used a recently validated correction factor to harmonize the background activity to a reference 60-minute injection-to-acquisition interval (Supplemental Appendix) (17).
Coronary microcalcification activity (CMA) quantification
We used a recently described measure of coronary 18F-NaF uptake, that quantifies PET activity across the entire coronary vasculature based upon analysis widely employed in oncology and cardiac sarcoidosis (16,18,19). First, we automatically extracted whole-vessel tubular and tortuous 3D volumes of interest from CT angiography datasets (Central Illustration, Supplemental Appendix). These encompass all the main epicardial coronary vessels and their immediate surroundings (4-mm radius) facilitating per-vessel and per-patient uptake quantification. Within such volumes of interest, we measured the coronary microcalcification activity (CMA)—representing the overall disease activity in the vessel and based upon both the volume and intensity of 18F-NaF PET activity within it (similar in principle to the Agatston score used for CT calcium scoring). CMA was defined as the integrated activity in SUV units exceeding the corrected background blood-pool mean SUV + 2 standard deviations (right atrium activity). The per-patient CMA was defined as the sum of the per-vessel CMA values.
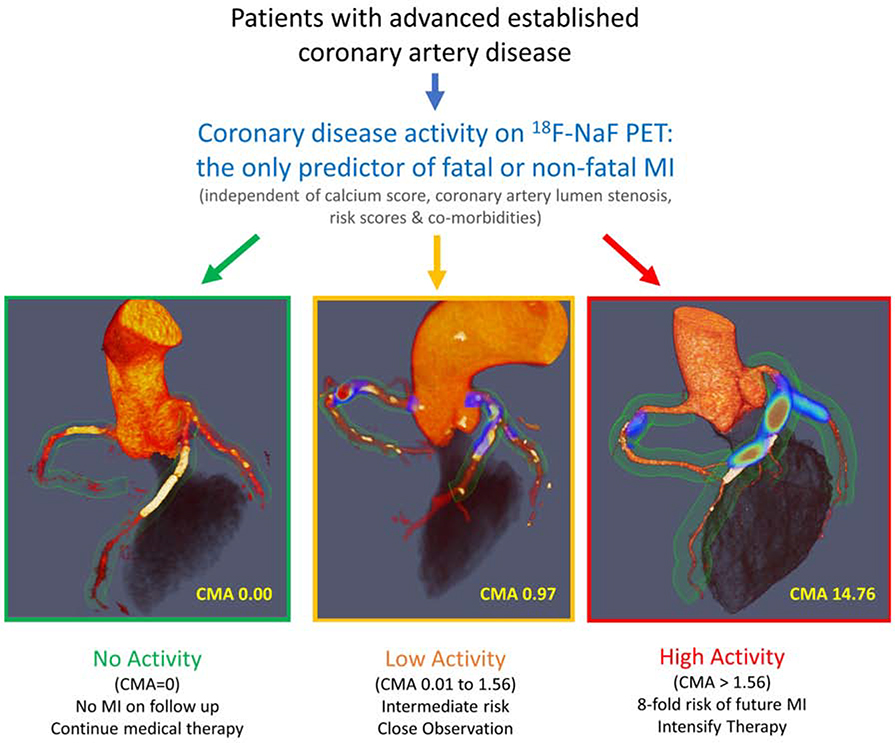
Central Illustration. 18F-sodium fluoride positron emission tomography as a marker of disease activity in the coronary arteries is a predictor of fatal or non-fatal myocardial infarction (MI) in patients with established coronary artery disease.
18F-fluoride PET can be used to measure disease activity across the coronary vasculature and to stratify patients into those with no, low and high disease activity. Patients with high disease activity (coronary microcalcification activity (CMA) >1.56) demonstrate a >7-fold risk of myocardial infarction. These patients might therefore be suitable for advanced medical therapies including PCSK9 or interleukin 1-beta inhibition, with 18F-fluoride PET used for targeting these expensive drugs to patients at greatest risk. Patients without coronary 18F-NaF uptake (CMA=0) have an excellent prognosis with no myocardial infarctions observed during follow-up despite advanced coronary artery disease. In these patients with dormant coronary artery disease (a third of the population studied), further intensification of medical therapy might not be warranted, nor might they benefit on prognostic grounds from complex revascularization such as multivessel percutaneous intervention or coronary artery bypass grafting.
Clinical Follow-up
The primary endpoint of the study was fatal or non-fatal myocardial infarction. The secondary endpoint was major adverse cardiovascular events (MACE), defined as myocardial infarction, stroke, delayed revascularization (more than 6 months after PET/CT) and cardiovascular death. Outcome information including invasive coronary angiography and coronary revascularization (percutaneous coronary intervention or coronary artery bypass graft surgery) were obtained from the local and national healthcare record systems that integrates primary and secondary health care records. Categorization of these outcomes was performed blinded to the coronary PET or other study data. Outcome data were collected in July 2019.
Statistical analysis
We assessed the distribution of data with the Shapiro-Wilk test. Continuous parametric variables were expressed as mean (SD) and compared using Student’s t tests. Non-parametric data were presented as median [Q1-Q3] and compared using Mann-Whitney U test. Fisher’s exact test or chi-squared test was used for analysis of categorical variables. We used the receiver-operating characteristic (ROC) analysis and pairwise comparisons according to DeLong et al to compare areas under the curves. Kaplan-Meier curves were used to elucidate the survival distributions with regard to myocardial infarction and MACE. Differences in the outcome of patients with and without 18F-NaF coronary activity exceeding the threshold derived from the ROC using Youden’s index were assessed using the log-rank test. A Cox proportional hazard regression with adjustment for potential confounders was performed to determine the predictors of worse outcome. Statistical analysis was performed with SPSS version 24 (IBM SPSS Statistics for Windows, Version 24.0. Armonk, NY: IBM Corp). A two-sided p<0.05 was considered statistically significant.
Results
Patients
The study population comprised 293 patients (84% males, mean age: 65±9 years). All participants had established coronary artery disease, the majority (n=232) had stable disease and the remaining 61 individuals were recruited and imaged (14 [10–19)] days) following recent myocardial infarction (Supplemental Appendix). Patients had advanced coronary atherosclerosis with a high burden of cardiovascular risk factors (hypertension 60%, hyperlipidemia 88%, tobacco use 67%, REACH clinical risk scores of 13 [11–15], SMART clinical risk scores of 18 [13–26]), widespread utilization of secondary preventative therapies (statin 90%, anti-platelet therapy 92%, ACE inhibitor or angiotensin receptor blockers 67%) and high rates of prior revascularization (n=237, 81%). None of the patients were taking PCSK9 inhibitor or interleukin 1-beta inhibitor therapy. On invasive angiography, 87 (30%) individuals had single vessel obstructive disease, 191 (65%) had multi-vessel obstructive coronary artery disease, and 18 (6%) had left main stem involvement.
Computed Tomography
Patients had advanced coronary artery disease on CT. The median CT calcium score was 334 [76–804], 59 (20%) subjects had a calcium score > 1000, 133 (45%) patients had a score > 400, and only 84 (29%) presented with a score <100. On coronary CT angiography, the overall median segment involvement score was 5 [3–7] with three-quarters of patients (n=218, 74%) having at least 4 segments involved (Supplemental Appendix). The median modified Duke index was 4 [3–5].
Positron Emission Tomography
On visual analysis of coronary PET, we identified increased tracer activity in 208 (70.9%) patients. Across the entire cohort, we found a median TBR of 1.22 [1.10–1.42]. Compared to those without uptake, patients with increased coronary 18F-NaF uptake had higher SMART risk scores (17 [13–23] vs 19 [13–27], p=0.029), and higher coronary calcium scores (184 [50–528] vs 371 [102–974] AU, p=0.0031), but there was no difference in the presence or severity of obstructive coronary stenoses (all p>0.10).
Assessing whole vessel microcalcification activity, 203 (69.3%) patients presented with CMA>0. The median CMA value was 0.66 [0–2.84]. Again, we observed that patients with a CMA>0 had higher SMART risk scores (17 [13–23] vs 19 [13–27], p=0.028) and increased coronary calcium scores (378 [103–993] vs 179 [48–529], p=0.003) than subjects with CMA=0, but there was no difference in the presence or severity of obstructive coronary stenoses (all p>0.10; Supplemental Appendix).
Clinical Outcomes
Over the 42 [31–49] months of follow-up, 20 subjects experienced a fatal (n=3) or non-fatal (n=17) myocardial infarction. Seven of these occurred in patients imaged following an acute coronary syndrome who had a median time from PET/CT to recurrent myocardial infarction of 12 (6–15) months. During follow-up a total of 40 patients suffered a MACE event (20 myocardial infarctions, 12 strokes, 3 cardiovascular deaths and 5 cases of delayed revascularization)
Primary endpoint: fatal or non-fatal myocardial infarction
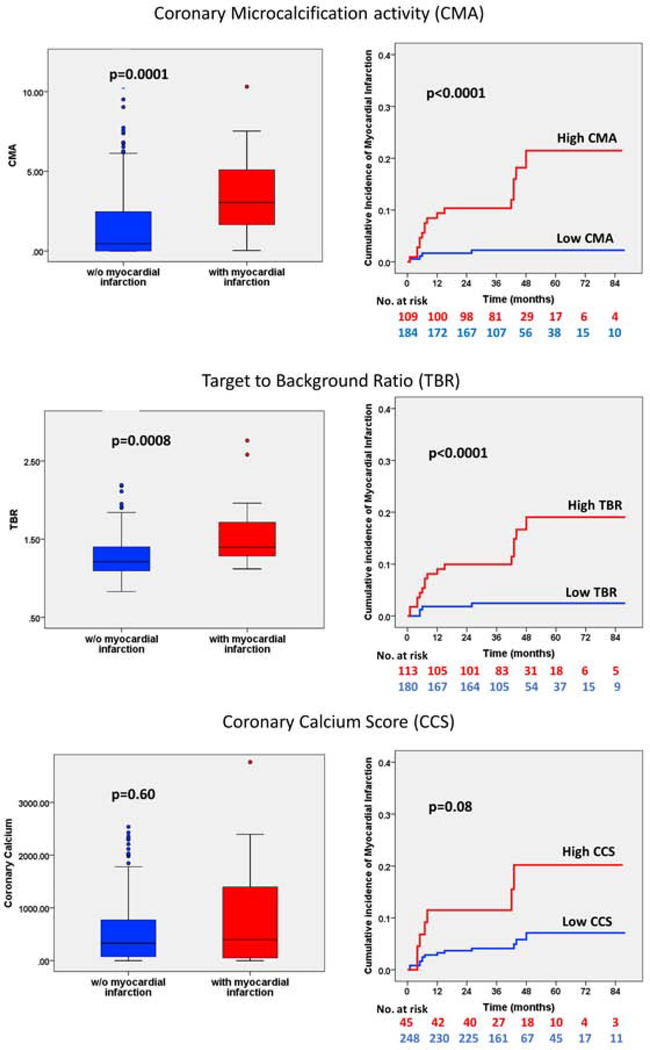
Patients who experienced myocardial infarction during follow-up had higher TBR values than those who did not (1.40 [1.28–1.77] versus 1.21 [1.09–1.40], p=0.006) and CMA (3.05 [1.62–5.25] versus 0.46 [0–2.47], p=0.002; Figure 1). Indeed, all the patients who had an infarct had increased coronary 18F-NaF PET uptake at baseline (CMA > 0). Interestingly, patients who experienced a fatal or non-fatal myocardial infarction did not have increased clinical risk scores (REACH: 13 [11–15] versus 13 [11–15], p=0.79; SMART 20 [13–28] versus 18 [13–26], p=0.52) nor coronary calcium scores (397 [39–1456] versus 331 [76–775] AU, p=0.60) compared to patients who did not have an infarct. Moreover, they did not have an increased prevalence of obstructive coronary artery disease (segment involvement score 6 [4–8] versus 5 [3–7], p=0.25), multivessel coronary disease (70% versus 65%, p=0.64) nor previous coronary stents (75% versus 74%, p=1.00). In patients who had a fatal or non-fatal myocardial infarction, 30% had a coronary calcium score <100 AU, 20% were within the 100–399 AU range, 20% were within the 400–999 AU range and 30% had a coronary calcium score >1000 AU (Figures 2 & 3). Only 12% (7/59) of patients with coronary calcium score >1000 AU experienced myocardial infarction (Supplemental Appendix).
Figure 1. Coronary disease activity and plaque burden in patients with and without future myocardial infarction.
Coronary microcalcification activity (CMA, top row), maximum target to background ratios (TBR, middle row) and the coronary calcium scores (CCS, bottom row) in patients with and without myocardial infarction during follow-up. For the Kaplan-Meier curves patients were dichotomized according to thresholds derived from receiver operator curves using the Youden’s index: CMA=1.56, TBR=1.28 and coronary calcium score = 1199 Agatston-units.
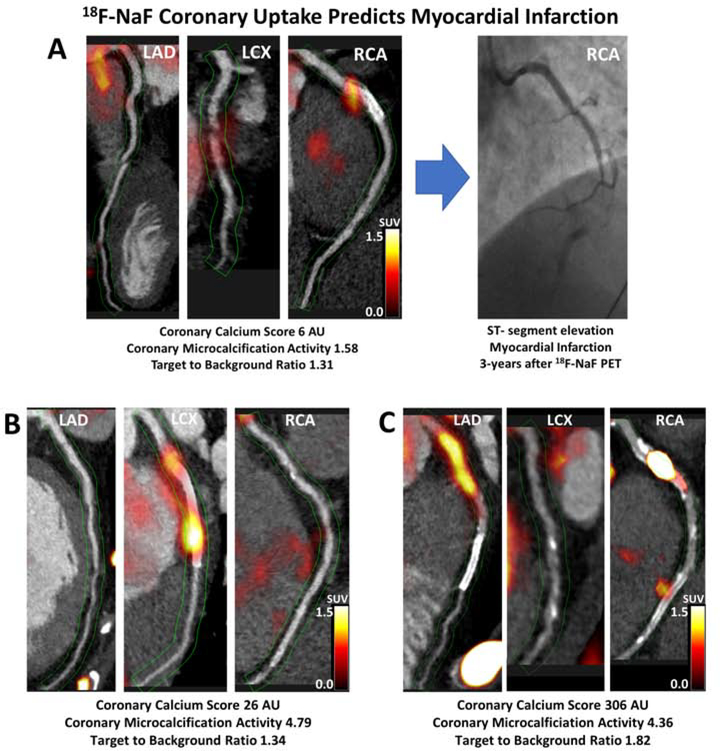
Figure 2. Case examples of 18F-sodium fluoride positron emission tomography in patients with established coronary artery disease and myocardial infarction during follow-up.
Hybrid CT angiography and 18F-NaF positron emission tomography of coronary arteries in: (A) a 56-year-old male who demonstrated increased 18F-NaF uptake in the RCA at baseline and presented with an inferior ST-segment elevation myocardial infarction and occlusion of the RCA during follow-up; (B) a 52-year-old male who demonstrated increased 18F-NaF uptake in the LCx at baseline and presented with a lateral non-ST-segment elevation myocardial infarction during follow-up; (C) a 60-year-old female who showed increased 18F-NaF uptake in the proximal RCA and presented with an inferior non-ST-segment elevation myocardial infarction during follow-up. LAD–left anterior descending, LCx–left circumflex, RCA–right coronary artery.
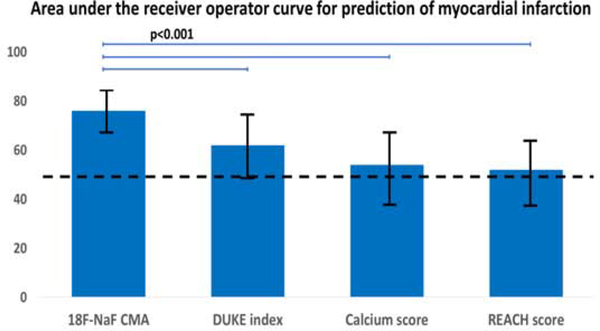
Figure 3. 18F-sodium fluoride positron emission tomography in the prediction of myocardial infarction in patients with established coronary artery disease.
In patients with established atherosclerosis the coronary microcalcification activity (as a marker of 18F-NaF activity across the coronary vasculature) had a significantly larger area under the receiver operator curve than the coronary calcium score (non-contrast CT), the modified Duke index (contrast CT angiography) or the REACH score (patient clinical data).AU-Agatston units, CMA–coronary microcalcification activity, REACH-Reduction of Atherothrombosis for Continued Health
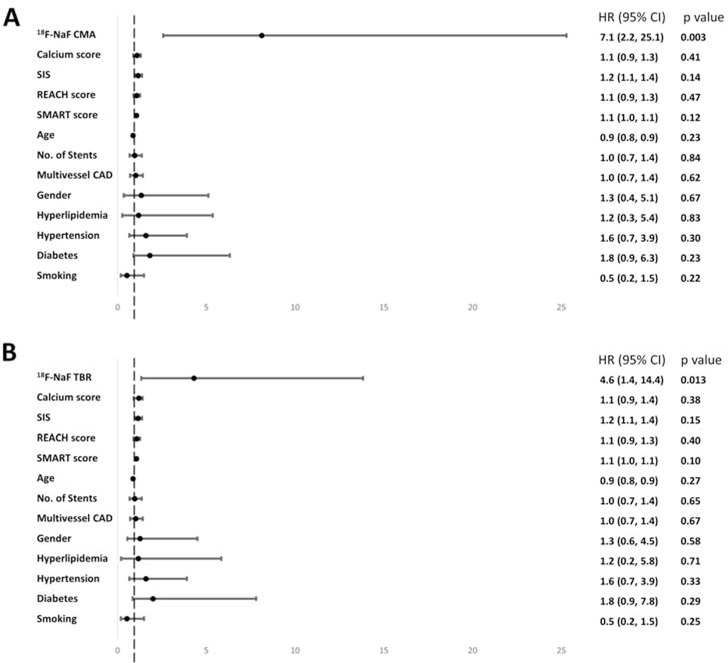
On ROC analysis, both CMA and TBR showed a greater area under the curve for the prediction of myocardial infarction than coronary calcium scores, or the REACH and SMART clinical risk scores (Supplemental Appendix). In order to generate distinct clinical risk groups, we dichotomized the population according to their coronary 18F-NaF uptake and derived the optimal TBR and CMA cutoffs for event prediction using the Youden’s index. A threshold of 1.56 for CMA achieved a specificity and sensitivity of 66% and 80% for the primary endpoint. A threshold of 1.28 for TBR achieved a specificity of 63% and sensitivity of 80% (Table 1). On univariable Cox proportional regression, both CMA >1.56 (hazard ratio (HR) 7.30, 95% confidence interval (CI) 2.44–21.84; p<0.001) and TBR >1.28 (HR 6.16, 95% CI 1.06–18.42; p=0.001) emerged as predictors of fatal or non-fatal myocardial infarction. Importantly, these associations persisted on multivariable analysis after adjustments for gender, comorbidities (presence of hypertension, hyperlipidemia, diabetes, smoking), the segment involvement score, number of coronary stents, multivessel coronary artery disease, coronary calcium score, SMART and REACH risk scores, initial patients presentation (acute coronary syndrome or stable coronary artery disease) and the study in which individuals were initially recruited (Figure 4). Indeed patients with CMA>1.56 had an adjusted hazard ratio of 7.1 (95% CI 2.2 to 25.1; p=0.003) for the primary end point, whilst patients with a TBR >1.28 had an adjusted hazard ratio of 4.6 (95% CI 1.4 to 14.4, p=0.013; Table 2). Similar results were observed when both CMA and TBR were considered as continuous variables, with both again emerging as the only independent predictors of fatal or non-fatal myocardial infarction on Cox modelling (Supplemental Appendix). In contrast, the number of stenosed vessels, the modified Duke index, age, and the SMART and REACH risk scores did not emerge as predictors of fatal non-fatal myocardial infarction on univariable Cox modelling (all p>0.1, Supplemental Appendix). Coronary calcium score was a predictor of events on univariable but not multivariable analysis (Table 2). Despite low statistical power when patients with acute myocardial infarction and stable subjects were considered separately, the AUCs on receiver-operator-characteristic curve analyses remained numerically similar (Supplemental Appendix).
Table 1.
Baseline Characteristics of Study Participants. Comparison of patients with coronary microcalcification activity (CMA) ≥1.56 vs <1.56, with Target to Background ratio (TBR) ≥1.28 vs <1.28 and coronary calcium score ≥1199 vs <1199.
| CMA | TBR | CCS | |||||||
|---|---|---|---|---|---|---|---|---|---|
| ≥ 1.56 (n=109) | < 1.56 (n=184) | P | ≥1.28 (n=113) | <1.28 (n=180) | P | ≥1199 (n=45) | <1199 (n=248) | P | |
| Age in years, mean (SD) | 67 (8) | 64 (9) | 0.0047 | 67 (8) | 63 (9) | 0.0001 | 68 (8) | 64 (9) | 0.006 |
| Men, n (%) | 97 (89%) | 148 (80%) | 0.071 | 103 (91%) | 142 (79%) | 0.006 | 44 (98%) | 201 (81%) | 0.004 |
| Body-mass index (kg/m2), mean (SD) | 28 (5) | 30 (5) | 0.024 | 29 (6) | 29 (5) | 1.00 | 30 (5) | 29 (5) | 0.22 |
| Systolic blood pressure (mm Hg), mean (SD) | 142 (21) | 141 (20) | 0.68 | 142 (20) | 141 (20) | 0.68 | 143 (15) | 141 (21) | 0.54 |
| Diastolic blood pressure (mm Hg), mean (SD) | 79 (12) | 80 (11) | 0.46 | 78 (11) | 80 (12) | 0.15 | 78 (11) | 80 (11) | 0.26 |
| Cardiovascular history, n (%) | |||||||||
| H of ACS | 53 (48.6%) | 108 (58.7%) | 0.11 | 58 (51.3%) | 103 (57.2%) | 0.34 | 25 (55.6%) | 136 (54.8%) | 1.00 |
| H of PCI | 64 (58.7%) | 119 (64.7%) | 0.32 | 69 (61.1%) | 114 (93.3%) | 0.71 | 23 (51.1%) | 160 (64.5%) | 0.10 |
| H of CABG | 20 (18.3%) | 28 (15.2%) | 0.52 | 21 (18.6%) | 27 (15.0%) | 0.44 | 23 (51.1%) | 25 (10.1%) | 0.0001 |
| H of angina | 60 (55.0%) | 76 (41.3%) | 0.029 | 57 (50.4%) | 79 (43.9%) | 0.28 | 30 (66.7%) | 106 (42.7%) | 0.003 |
| CVA/TIA | 3 (2.8%) | 6 (3.3%) | 1.000 | 3 (2.7%) | 6 (3.3%) | 1.00 | 1 (2.2%) | 8 (3.2%) | 1.00 |
| Comorbidities/risk factors, n (%) | |||||||||
| HTN | 71 (65.1%) | 103 (55.9%) | 0.14 | 76 (67.3%) | 98 (54.4%) | 0.038 | 30 (66.7%) | 144 (58.1%) | 0.32 |
| HPL | 97 (89.0%) | 160 (86.9%) | 0.71 | 101 (89.3%) | 156 (86.7%) | 0.58 | 40 (88.9%) | 217 (87.5%) | 1.00 |
| DM | 26 (23.9%) | 35 (19.0%) | 0.37 | 26 (23.0%) | 35 (19.4%) | 0.46 | 13 (28.9%) | 48 (19.4%) | 0.16 |
| Current smoking | 20 (18.3%) | 38 (21.1%) | 0.65 | 21 (18.6%) | 37 (20.6%) | 0.76 | 8 (17.8%) | 50 (20.2%) | 0.84 |
| Ex-smoker | 44 (40.3%) | 93 (51.7%) | 0.12 | 51 (45.1%) | 86 (47.8%) | 0.72 | 19 (42.2%) | 118 (47.6%) | 0.52 |
| Atrial fibrillation | 4 (3.7%) | 6 (3.3%) | 1.00 | 5 (4.4%) | 5 (2.8%) | 0.52 | 2 (4.4%) | 8 (3.2%) | 0.65 |
| Peripheral vascular disease | 4 (3.7%) | 12 (6.5%) | 0.43 | 4 (3.5%) | 12 (6.7%) | 0.30 | 8 (17.8%) | 8 (3.2%) | 0.0008 |
| Medications, n (%) * | |||||||||
| Aspirin | 101 (92.7%) | 167 (90.7%) | 0.67 | 107 (94.7%) | 161 (89.4%) | 0.14 | 41 (91.1%) | 227 (91.5%) | 1.00 |
| PY12 antagonist | 19 (17.4%) | 26 (14.1%) | 0.50 | 21 (18.6%) | 24 (13.3%) | 0.25 | 5 (11.1%) | 40 (16.1%) | 0.50 |
| Statin | 102 (93.6%) | 160 (86.9%) | 0.08 | 103 (91.2%) | 159 (88.3%) | 0.56 | 42 (93.3%) | 220 (88.7%) | 0.44 |
| Beta Blocker | 72 (66.1%) | 124 (67.4%) | 0.90 | 77 (68.1%) | 119 (66.1%) | 0.80 | 32 (71.1%) | 164 (66.1%) | 0.61 |
| ACEI/ARB | 76 (69.7%) | 121 (65.7%) | 0.61 | 81 (71.7%) | 116 (64.4%) | 0.20 | 38 (84.4%) | 159 (64.1%) | 0.009 |
| Insulin | 1 (0.9%) | 3 (1.6%) | 1.00 | 1 (0.9%) | 3 (1.7%) | 1.00 | 0 | 4 (1.4%) | 1.00 |
| Oral diabetic medications | 17 (15.6%) | 31 (16.8%) | 0.87 | 19 (16.8%) | 29 (1.6%) | 0.87 | 8 (17.8%) | 40 (16.1%) | 0.83 |
| CCB | 23 (21.1%) | 40 (21.7%) | 1.00 | 27 (23.9%) | 36 (20.0%) | 0.47 | 12 (26.7%) | 51 (20.6%) | 0.43 |
| Diuretics | 7 (6.4%) | 31 (16.8%) | 0.028 | 7 (6.2%) | 31 (17.2%) | 0.007 | 7 (15.6%) | 31 (12.5%) | 0.63 |
| Biomarkers, median (IQR) | |||||||||
| Total Cholesterol (mmol/L) | 4.0 (3.5–4.7) | 4.1 (3.6–4.8) | 0.41 | 4.0 (3.5–4.8) | 4.1 (3.6–4.7) | 0.63 | 4.2 (3.5–4.9) | 3.8 (4.1–4.7) | 0.53 |
| LDL (mmol/L) | 1.9 (1.3–2.5) | 1.9 (1.2–2.4) | 0.75 | 1.7 (1.2–2.5) | 2.1 (1.4–2.4) | 0.21 | 2.2 (1.2–2.7) | 1.9 (1.2–2.4) | 0.12 |
| HDL (mmol/L) | 1.2 (1.0–1.7) | 1.2 (1.0–1.8) | 0.76 | 1.2(1.0–1.7) | 1.2 (1.0–1.7) | 0.69 | 1.2 (1.0–1.7) | 1.2 (1.0 –1.7) | 0.74 |
| TAG (mmol/L) | 1.5 (1.1–2.5) | 1.6 (1.1–2.2) | 0.87 | 1.5(1.0–2.5) | 1.5 (1.1–2.2) | 0.87 | 1.4 (1.2–2.0) | 1.6 (1.1–2.4) | 0.37 |
| Creatinine (μmol/L) | 80 (70–94) | 77 (70–89) | 0.42 | 78(70–88) | 78(70–92) | 0.95 | 80 (70–91) | 77 (70–90) | 0.48 |
| CAD, n (%) | |||||||||
| Non-obstructive disease (<50%) | 5 (4.6%) | 10 (5.4%) | 0.79 | 2 (1.8%) | 13 (7.2%) | 0.05 | 1 (2.2%) | 14 (5.6%) | 0.48 |
| Single vessel disease | 31 (28.4%) | 56 (30.4%) | 1.00 | 29 (25.7%) | 58 (32.2%) | 0.24 | 8 (17.8%) | 79 (31.9%) | 0.08 |
| Two vessel disease | 37 (33.9%) | 73 (39.7%) | 0.38 | 44 (38.9%) | 66 (36.7%) | 0.71 | 12 (26.7%) | 98 (39.5%) | 0.13 |
| Three vessel disease | 36 (33.0%) | 45 (24.5%) | 0.10 | 38 (33.6%) | 43 (23.9%) | 0.081 | 24 (53.3%) | 57 (23.0%) | 0.0001 |
| LMS involvement | 7 (6.4%) | 7 (3.8%) | 0.40 | 7 (6.2%) | 7 (3.9%) | 0.41 | 7 (15.6%) | 7 (2.8%) | 0.002 |
| Coronary Stent, n (%) | 83 (76.1%) | 135 (73.4%) | 0.68 | 85 (75.2%) | 133 (73.9%) | 0.89 | 24 (53.3%) | 194 (78.2%) | 0.0013 |
| Segment involvement score. Median (IQR) | 6 (4–8) | 5 (3–7) | 0.008 | 4 (6–8) | 5(3–7) | 0.002 | 7 (6–9) | 5 (3–7) | <0.0001 |
| SIS breakdown, n (%) | |||||||||
| 0–1 | 4 (3.7%) | 16 (8.7%) | 0.15 | 3 (2.7%) | 17 (9.4%) | 0.030 | 1 (2.2%) | 19 (7.7%) | 0.33 |
| 2–3 | 19 (16.4%) | 36 (19.6%) | 0.76 | 14 (12.3%) | 41 (22.8%) | 0.031 | 0 | 55 (22.2%) | 0.0001 |
| 4–5 | 23 (21.1%) | 50 (27.2%) | 0.27 | 29 (25.6%) | 44 (24.4%) | 0.89 | 5 (11.1%) | 68 (27.4%) | 0.02 |
| >5 | 63 (57.8%) | 82 (44.6%) | 0.016 | 67 (59.3%) | 78 (43.3%) | 0.009 | 39 (86.7%) | 106 (42.7%) | 0.0001 |
| CCS, median (IQR) | 544 (184–1157) | 201 (64–541) | <0.0001 | 498 (188–1089) | 201 (59–558) | <0.0001 | N/A | N/A | N/A |
| CCS, n (%) | |||||||||
| 0–99 | 21 (19.3%) | 63 (34.2%) | 0.007 | 20 (17.7%) | 64 (35.6%) | 0.0009 | |||
| 100–399 | 24 (22.0%) | 52 (28.3%) | 0.27 | 25 (22.1%) | 51 (28.3%) | 0.2742 | |||
| 400–999 | 27 (24.8%) | 47 (25.5%) | 1.00 | 33 (29.2%) | 41 (22.8%) | 0.2691 | |||
| >1000 | 37 (33.9%) | 22 (12.0%) | 0.0001 | 35 (31.0%) | 24 (13.3%) | 0.0003 | |||
| TBR, median (IQR) | 1.45 (1.31–1.62) | 1.13 (1.05–1.22) | <0.001 | 1.45 (1.35–1.62) | N/A | N/A | 1.4 (1.23–1.62) | 1.2 (1.1–1.4) | <0.0001 |
| TBR≥1.28 | 86 (78.9%) | 27 (14.7%) | 0.0001 | N/A | N/A | N/A | 26 (57.8%) | 87 (35.1%) | 0.005 |
| CCS>1199 | 27 (24.8%) | 19 (10.3%) | 0.0015 | 26 (23.0%) | 20 (11.1%) | 0.0082 | N/A | N/A | N/A |
| Risk scores | |||||||||
| REACH score (IQR) CV event | 13 (11–15) | 13 (11–15) | 0.075 | 14 (12–16) | 12 (10–15) | 0.0039 | 15 (13–17) | 13 (11–15) | <0.0001 |
| 20-month risk of next CV event, % (IQR) | 6.3 (4.7–8.5) | 6.3 (4.7–8.5) | 0.13 | 7.3 (4.7–9.2) | 5.4 (4.0–8.5) | 0.0074 | 8.5 (6.3–11.0) | 5.4 (4.7–8.5) | <0.0001 |
| REACH score (IQR) CV death | 11 (10–13) | 11 (9–13) | 0.015 | 11 (10–13) | 11 (9–12) | 0.0012 | 12 (11–14) | 11 (9–12) | <0.0001 |
| 20-month cardiovascular death, % (IQR) | 1.8 (1.4–3.0) | 1.8 (1.1–2.8) | 0.014 | 1.8 (1.4–3.0) | 1.5 (1.0–2.0) | 0.0011 | 2.3 (1.8–3.8) | 1.8 (1.1 –2.3) | <0.0001 |
| Duke score | 4 (3–5) | 4 (3–5) | 0.28 | 4 (3–5) | 4 (3–5) | 0.0324 | 5 (4–5) | 4 (3–5) | 0.0024 |
| SMART risk score | 21 (15–27) | 17 (12–24) | 0.0050 | 20 (14–28) | 17 (13–24) | 0.0414 | 24 (18–32) | 17 (13–24) | 0.0002 |
| Outcomes | |||||||||
| Myocardial infarction | 16 (14.7%) | 4 (2.2%) | 0.0001 | 16 (14.2%) | 4 (2.2%) | 0.0002 | 13 (15.6%) | 7 (5.2%) | 0.08 |
| MACE | 23 (21.1%) | 17 (9.2%) | 0.008 | 23 (20.4) | 17 (9.4%) | 0.0078 | 10 (22.2%) | 30 (12.1%) | 0.10 |
| Stroke | 3 | 9 | 0.045 | 3 | 9 | 0.045 | 1 | 11 | 0.003 |
| Cardiovascular death | 2 | 1 | N/A | 2 | 1 | N/A | 0 | 3 | N/A |
| Delayed revascularization | 2 | 3 | N/A | 2 | 3 | N/A | 0 | 5 | N/A |
ACEI/ARB – angiotensin converting enzyme inhibitor/angiotensin receptor blocker, ACS – acute coronary syndrome, CABG – coronary artery bypass graft, CAD – coronary artery disease, CMA – coronary microcalcification activity, CVA – Cerebrovascular accident, MACE – major adverse cardiovascular event, PCI – percutaneous coronary intervention, REACH - Reduction of Atherothrombosis for Continued Health, SMART Secondary Manifestations of Arterial Disease, SIS – segment involvement score, TAG - triacylglycerides, TBR – target to background ratio, TIA - transient ischemic attack
medications at the time of scan
Figure 4. Predictors of myocardial infarction on Cox proportional hazards modelling.
Forest plots of hazard ratios derived from multivariable modelling with 95% confidence intervals for the coronary microcalcification activity (CMA) (A) and the target to background ratio values (B) along with covariates: coronary calcium scores, SIS, REACH score, SMART score, total number of implanted coronary stents, presence of multivessel coronary artery disease, age, gender, hyperlipidemia, hypertension, diabetes, smoking. CMA–coronary microcalcification activity, REACH-Reduction of Atherothrombosis for Continued Health, SMART - Secondary Manifestations of Arterial Disease, SIS–segment involvement score, TAG-triacylglycerides, TBR–target to background ratio
Table 2.
Uni- and multivariable Cox proportional regression models for prediction of myocardial infarction during follow-up.
| Coronary Microcalcification Activity >1.56 | Target to background ratio >1.28 | Coronary Calcium Score > 1199 | ||||
|---|---|---|---|---|---|---|
| Hazard ratio (95% CI) | p-value | Hazard ratio (95% CI) | p-value | Hazard ratio (95% CI) | p-value | |
| Model 1 | 7.30 (2.44–21.84) | <0.001 | 6.16 (1.06–18.42) | 0.001 | 3.24 (1.29–8.11) | 0.012 |
| Model 2 | 7.20 (2.36–21.95) | 0.001 | 5.94 (1.94–18.10) | 0.002 | - | |
| Model 3 | 6.66 (2.19–20.25) | 0.001 | 5.57 (1.80–17.00) | 0.003 | 2.65 (0.93–7.56) | 0.069 |
| Model 4 | 8.73 (2.44–31.29) | 0.001 | 4.80 (1.54–14.93) | 0.007 | 2.72 (0.90–8.21) | 0.075 |
| Model 5 | 8.91 (2.47–32.16) | 0.001 | 4.83 (1.54–15.20) | 0.007 | - | |
| Model 6 | 8.12 (2.57–25.28) | p<0.001 | 4.30 (1.34–13.82) | 0.014 | ||
| Model 7 | 7.10 (2.2–25.1) | 0.003 | 4.6 (1.4–14.4) | 0.013 | ||
Model 1 – unadjusted; Model 2 – adjusted for Coronary Calcium Score; Model 3 – adjusted for segment involvement score, number of coronary stents, multivessel coronary artery disease; Model 4 – adjusted for segment involvement score, number of coronary stents, multivessel coronary artery disease, age, gender, hyperlipidaemia, hypertension, diabetes, smoking; Model 5 – similar to Model 4 and additionally adjusted for coronary calcium scoring; Model 6 – similar to Model 5 and additionally adjusted for REACH and SMART risk scores. Model 7 – similar to model 6 and additionally adjusted for initial patient’s presentation (stable vs acute myocardial infarction) and the study in to which the patient was initially recruited.
Secondary Endpoint: Major Adverse Cardiovascular Events
Patients with MACE had higher CMA (1.9 [1.65–4.76] versus 0.51 [0–2.42], p=0.0098) and an apparent trend for higher TBR values (1.34 [1.13–1.54] versus 1.22 [1.10–1.40], p=0.073) than patients without MACE. There were no differences in the extent of obstructive coronary artery disease on CT angiography (the segment involvement score, the modified Duke index, presence of multivessel disease or coronary stents) nor cardiovascular risk scores and co-morbidities in patients with and without MACE (Supplemental Appendix). Similarly, there was no difference in coronary calcium scores 195 [50–1126] versus 344 [81–801] AU, p=0.50). Only 17% (10/59) of patients with a coronary calcium score >1000 AU experienced MACE.
On univariable Cox proportional regression, both CMA>1.56 and TBR>1.28 were predictors of MACE (HR 2.3, 95% CI 1.2–4.3, p=0.01 and HR 2.1, 95% CI 1.1–3.9, p=0.02). On multivariable analysis after adjustments for age, gender, comorbidities (presence of hypertension, hyperlipidemia, diabetes, smoking), the segment involvement score, number of coronary stents multivessel coronary artery disease, coronary calcium score and the REACH and SMART risk scores, CMA remained the only independent predictor of MACE (HR 2.1, 95% CI 1.1–4.1, p=0.030; Figure 4). When CMA and TBR were considered as continuous variables, these two measurements emerged as the only predictors of MACE on Cox modelling (Supplemental Appendix).
In contrast, coronary calcium score exceeding 1199 AU (HR 1.9, 95% CI 0.9–4.0, p=0.07), the modified Duke index (HR 1.2, 95% CI 0.9–1.6, p=0.14), the REACH (HR 1.7, 95% CI 0.5–5.5, p=0.38) and SMART (HR 1.5, 95% CI 0.8–2.8, p=0.23) risk scores were not predictors of MACE on univariable analysis.
Discussion
In this two-center multimodality imaging study, we have demonstrated for the first time that coronary 18F-NaF PET is a powerful prognostic tool for predicting myocardial infarction in patients with advanced established coronary artery disease. In a comprehensive analysis, we show that both 18F-NaF TBR values and whole vessel CMA emerge as powerful independent predictors of myocardial infarction outperforming all other established predictors including the presence of co-morbidities, the REACH and SMART risk scores, coronary calcium scoring and the presence, severity and extent of coronary artery disease. Our data therefore highlight the added prognostic value that assessments of disease activity can provide and confirm the potential of 18F-NaF PET to improve the risk stratification of patients with established CAD, a group in whom prediction of events has previously proved challenging.
18F-NaF PET provides an assessment of calcification activity across multiple different cardiovascular disease states including aortic stenosis, mitral annular calcification, abdominal aortic aneurysm, erectile dysfunction and bioprosthetic valve degeneration (7,20). In each condition, it is associated with vascular injury, disease activity and future disease progression. This is also the case in coronary atherosclerosis. Increased 18F-NaF uptake is associated with culprit coronary plaques in patients with myocardial infarction and adverse plaque features in patients with apparently stable disease (4). Moreover, similar to other cardiovascular conditions, baseline coronary 18F-NaF activity predicts the future progression of coronary calcium scores, confirming its status as a marker of disease activity (5,6). While there is major interest in using markers of atherosclerotic disease activity to improve patient assessment and risk stratification, this is the first study to demonstrate that increased 18F-NaF activity provides powerful prediction of future myocardial infarction. Indeed, this technique outperformed all the other commonly used predictors of events in patients with established coronary artery disease including two established clinical risk scores designed for this patient population, co-morbidities, coronary calcium scoring, and the presence and severity of obstructive coronary artery disease. 18F-NaF might therefore provide an important clinical tool in a patient population in whom risk stratification is currently suboptimal. A CMA >1.56 was associated with a >7-fold risk of myocardial infarction. This was despite almost universal prescription of aspirin, statins and other secondary preventative therapies. These patients might therefore be suitable for advanced medical therapies including PCSK9 or interleukin 1-beta inhibition, with 18F-fluoride PET providing the risk stratification tool that many have advocated for as a means of targeting these expensive drugs to those patients at greatest risk. In the wake of the ISCHEMIA trial this approach might also help select patients who would benefit from revascularization (21). Of equal importance, patients without coronary 18F-NaF uptake and a CMA=0 had an excellent prognosis with no myocardial infarctions observed in this group despite their advanced coronary artery disease. In these patients with dormant coronary artery disease (a third of the population studied), further intensification of medical therapy might not be warranted, nor might they benefit on prognostic grounds from complex revascularization such as multivessel percutaneous intervention or coronary artery bypass grafting. Further research is required to investigate these important clinical questions.
Our data demonstrating the modest predictive value of cardiovascular risk scores, coronary calcium scoring and obstructive coronary artery disease in patients with advanced established coronary artery disease is consistent with the recent literature. The diagnostic performance of the REACH and SMART risk scores was poor in several recent studies (C-statistic of 0.53 and 0.54 respectively (1,22). While coronary calcium scoring provides powerful prognostic information in asymptomatic individuals and those presenting with chest pain, its prognostic capability has been disappointing in other studies of patients with established advanced coronary artery disease (23,24). In line with recent literature, the presence and extent of obstructive coronary artery disease was also not a marker of adverse events in our study (25,26).
Our study has notable strengths. We have focused our analysis on patients with advanced established coronary artery disease for whom we lack robust methods for risk stratification and showed that 18F-NaF PET has the potential to fulfill this unmet clinical need. We utilized state-of-the-art 18F-NaF PET imaging, employing the latest advances in image acquisition and motion correction (14). We also employed a novel quantification technique, CMA, that measures 18F-NaF uptake along the course of the entire coronary vasculature and therefore provides a more complete summative assessment of disease activity than the TBR values derived from visually defined hot spot assessments (16). While both standard TBR values and CMA emerged as independent predictors of myocardial infarction, CMA demonstrated a superior hazard ratio for this endpoint, and was also the only independent predictor of MACE. CMA would therefore appear to hold advantages as a method for quantifying overall coronary 18F-NaF uptake and disease activity.
Limitations
Our study has some limitations. It is a post-hoc analysis of data collected for prospective observational studies. While all the subjects had advanced established coronary artery disease, we have included patients with both stable and unstable coronary artery disease thereby increasing the heterogeneity of the analyzed cohort. Similar results were, however, observed when patients with unstable coronary artery disease were excluded from the analysis (Supplemental Appendix). Our data therefore require confirmation in large prospective studies. Indeed, we are currently completing recruitment for the Prediction of Recurrent Events With 18F-Fluoride (PREFFIR) study which will prospectively investigate the ability of 18F-NaF coronary PET to predict recurrent events in patients with multi-vessel disease and recent myocardial infarction. While performing a CT angiogram alongside the 18F-NaF PET scan incurs a modest additional dose of radiation, this is currently essential for accurate image co-registration, interpretation and analysis (15). Although we have shown that delayed 18F-NaF imaging may improve image quality, in this study participants underwent PET imaging 1 h after tracer injection (27). The potential prognostic benefits of delaying image acquisition therefore remain to be evaluated.
Conclusions
18F-NaF PET is a determinant of disease activity in the coronary arteries and a powerful prognostic technique to predict myocardial infarction in patients with advanced established coronary artery disease. Further studies are required to confirm our findings and to investigate how best to use this technique to improve patient risk stratification and to guide the use of advanced therapeutic interventions.
Supplementary Material
Clinical Perspective.
Competency in Patient Care and Procedural Skills: Positron emission tomography (PET imaging) using 18F-NaF, a calcification tracer, identifies disease activity in patients with coronary atherosclerosis and the degree of uptake is an independent predictor of myocardial infarction.
Funding and Acknowledgements
This research was supported in part by grants R01HL135557 from the National Heart, Lung, and Blood Institute/National Institute of Health (NHLBI/NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
ET was supported by a grant from Dr. Miriam and Sheldon G. Adelson Medical research Foundation. EVB is supported by Scottish Imaging Network, a Platform of Scientific Excellence (SINAPSE; www.sinapse.ac.uk). DEN (CH/09/002, RE/13/3/30183, RG/16/10/32375), MRD (FS/14/78/31020) and MCW (FS/11/014, CH/09/002) are supported by the British Heart Foundation. DEN is the recipient of a Wellcome Trust Senior Investigator Award (WT103782AIA) and MRD of Sir Jules Thorn Award for Biomedical Research Award (2015).
Abbreviations and acronyms
- CMA
Coronary Microcalcification Activity
- CT
Computed Tomography
- MACE
Myocardial Adverse Cardiovascular Events
- PET
Positron Emission Tomography
- SUV
Standard Uptake Value
- TBR
Maximum Target to Background Ratio
- 18F-NaF
18F-sodium Fluoride
Footnotes
Disclosures
The authors declare that they have no relevant or material financial interests that relate to the research described in this paper.
Translational Outlook: Additional research is necessary to assess the utility of 18F-NaF PET to guide the type and intensity of therapy for patients with coronary atherosclerosis.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dorresteijn JA, Visseren FL, Wassink AM et al. Development and validation of a prediction rule for recurrent vascular events based on a cohort study of patients with arterial disease:the SMART risk score. Heart 2013;99:866–72. [DOI] [PubMed] [Google Scholar]
- 2.Arbab-Zadeh A, Fuster V. From Detecting the Vulnerable Plaque to Managing the Vulnerable Patient: JACC State-of-the-Art Review. J Am Coll Cardiol 2019;74:1582–1593. [DOI] [PubMed] [Google Scholar]
- 3.Dweck MR, Chow MW, Joshi NV et al. Coronary arterial 18F-sodium fluoride uptake: a novel marker of plaque biology. J Am Coll Cardiol 2012;59:1539–48. [DOI] [PubMed] [Google Scholar]
- 4.Joshi NV, Vesey AT,Williams MC et al. 18F-fluoride positron emission tomography for identification of ruptured and high-risk coronary atherosclerotic plaques: a prospective clinical trial. Lancet 2014;383:705–13. [DOI] [PubMed] [Google Scholar]
- 5.Lee JM, Bang JI, Koo BK et al. Clinical Relevance of (18)F-Sodium-Fluoride Positron-Emission Tomography in Noninvasive Identification of High-Risk-Plaque in Patients With Coronary Artery Disease. Circ Cardiovasc Imaging 2017;10. [DOI] [PubMed] [Google Scholar]
- 6.Kitagawa T, Yamamoto H, Nakamoto Y et al. Predictive Value of (18)F-Sodium Fluoride Positron Emission Tomography in Detecting High-Risk Coronary Artery Disease in Combination With Computed Tomography. J Am Heart Assoc 2018;7:e010224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dweck MR, Jenkins WS, Vesey AT et al. 18F-sodium fluoride uptake is a marker of active calcification and disease progression in patients with aortic stenosis. Circ Cardiovasc Imaging 2014;7:371–8. [DOI] [PubMed] [Google Scholar]
- 8.Delgado V, Saraste A, Dweck M, Bucciarelli-Ducci C, Bax JJ. Multimodality imaging:Bird’s eye view from the European Society of Cardiology Congress 2019 Paris,August 31st-September 4th, 2019. J Nucl Cardiol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doris M, Moss AJ, Andrews JPM et al. 172Coronary 18F-sodium fluoride uptake predicts progression of coronary arterial calcification.European Heart Journal 2019;40. [Google Scholar]
- 10.Moss AJ, Dweck MR, Doris MK et al. Ticagrelor to Reduce Myocardial Injury in Patients With High-Risk Coronary Artery Plaque. JACC Cardiovasc Imaging 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson PW, D’Agostino R Sr.,Bhatt DL et al. An international model to predict recurrent cardiovascular disease. Am J Med 2012;125:695–703 e1. [DOI] [PubMed] [Google Scholar]
- 12.Rubeaux M, Joshi NV,Dweck MR et al. Motion Correction of 18F-NaF PET for Imaging Coronary Atherosclerotic Plaques. J Nucl Med 2016;57:54–9. [DOI] [PubMed] [Google Scholar]
- 13.Massera D, Doris MK,Cadet S et al. Analytical quantification of aortic valve 18F-sodium fluoride PET uptake.J Nucl Cardiol 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Min JK, Shaw LJ, Devereux RB et al. Prognostic value of multidetector coronary computed tomographic angiography for prediction of all-cause mortality. J Am Coll Cardiol 2007;50:1161–70. [DOI] [PubMed] [Google Scholar]
- 15.Kwiecinski J, Adamson PD, Lassen ML et al. Feasibility of Coronary (18)F-Sodium Fluoride Positron-Emission Tomography Assessment With the Utilization of Previously Acquired Computed Tomography Angiography. Circ Cardiovasc Imaging 2018;11:e008325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kwiecinski J, Cadet S, Dey D et al. Whole-vessel coronary 18F-sodium fluoride PET for assessment of the global coronary microcalcification burden. Eur J Nucl Med Mol Imaging. 2020. doi: 10.1007/s00259-019-04667-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lassen ML, Kwiecinski J, Dey D et al. Triple-gated motion and blood pool clearance corrections improve reproducibility of coronary (18)F-NaF PET. Eur J Nucl Med Mol Imaging 2019;46:2610–2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larson SM, Erdi Y, Akhurst T et al. Tumor Treatment Response Based on Visual and Quantitative Changes in Global Tumor Glycolysis Using PET-FDG Imaging. The Visual Response Score andChange in Total Lesion Glycolysis. Clin Positron Imaging 1999;2:159–171. [DOI] [PubMed] [Google Scholar]
- 19.Ahmadian A, Brogan A,Berman J et al. Quantitative interpretation of FDG PET/CT with myocardial perfusion imaging increases diagnostic information in the evaluation of cardiac sarcoidosis.J Nucl Cardiol 2014;21:925–39. [DOI] [PubMed] [Google Scholar]
- 20.Cartlidge TRG, Doris MK, Sellers SL et al. Detection and Prediction of Bioprosthetic Aortic Valve Degeneration. J Am Coll Cardiol 2019;73:1107–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maron DJ, Harrington RA,Hochman JS. Planning and Conducting the ISCHEMIA Trial. Circulation 2018;138:1384–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaasenbrood L, Bhatt DL,Dorresteijn JAN et al. Estimated Life Expectancy Without Recurrent Cardiovascular Events in Patients With Vascular Disease:The SMART-REACH Model. J Am Heart Assoc 2018;7:e009217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Budoff MJ, Young R, Burke G et al. Ten-year association of coronary artery calcium with atherosclerotic cardiovascular disease (ASCVD) events: the multi-ethnic study of atherosclerosis (MESA). Eur Heart J 2018;39:2401–2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Erbel R, Mohlenkamp S, Moebus S et al. Coronary risk stratification, discrimination, and reclassification improvement based on quantification of subclinical coronary atherosclerosis: the Heinz Nixdorf Recall study. J Am Coll Cardiol 2010;56:1397–406. [DOI] [PubMed] [Google Scholar]
- 25.Hoffmann U, Ferencik M,Udelson JE et al. Prognostic Value of Noninvasive Cardiovascular Testing in Patients With Stable Chest Pain: Insights From the PROMISE Trial.Circulation 2017;135:2320–2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang HJ, Lin FY, Lee SE et al. Coronary Atherosclerotic Precursors of Acute Coronary Syndromes. J Am Coll Cardiol 2018;71:2511–2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kwiecinski J, Berman DS, Lee SE et al. 2018 Three-hour delayed imaging improves assessment of coronary 18F-sodium fluoride PET. J Nucl Med 2019; 60(4):530–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.