Abstract
Cancer complicates 1 in 1,000 pregnancies. Multidisciplinary consensus comprised of Gynecologic Oncology, Pathology, Neonatology, Radiology, Anesthesiology, Maternal Fetal Medicine, and Social Work should be convened. Pregnancy provides an opportunity for cervical cancer screening, with deliberate delays in treatment permissible for early stage carcinoma. Vaginal delivery is contraindicated in the presence of gross lesion(s) and radical hysterectomy with lymphadenectomy at cesarean delivery is recommended. Women with locally advanced and metastatic/recurrent disease should commence treatment at diagnosis with chemoradiation and systemic therapy, respectively; neoadjuvant chemotherapy to permit gestational advancement may be considered in select cases. Most adnexal masses are benign and resolve by the second trimester. Persistent, asymptomatic, benign-appearing masses can be managed conservatively; surgery, if indicated, is best deferred to 15–20 weeks, with laparoscopy preferable over laparotomy whenever possible. Benign and malignant germ cell tumors and borderline tumors are occasionally encountered, with unilateral adnexectomy and preservation of the uterus and contralateral ovary being the rule. Epithelial ovarian cancer is exceedingly rare. Ultrasonography and magnetic resonance imaging lack ionizing radiation and can be employed to evaluate disease extent. Tumor markers, including CA-125, AFP, LDH, inhibin-B, and even CEA and ßhCG may be informative. If required, chemotherapy can be administered following organogenesis during the second and third trimesters. Because platinum and other anti-neoplastic agents crosses the placenta, chemotherapy should be withheld after 34 weeks to avoid neonatal myelosuppression. Bevacizumab, immune checkpoint inhibitors, and PARP inhibitors should be avoided throughout pregnancy. Although antenatal glucocorticoids to facilitate fetal pulmonary maturation and amniotic fluid index assessment can be considered, there is no demonstrable benefit of tocolytics, antepartum fetal heart rate monitoring, and/or amniocentesis. Endometrial, vulvar, and vaginal cancer in pregnancy are curiosities, although leiomyosarcoma and the dreaded twin fetus/hydatidiform mole have been reported. For gynecologic malignancies, pregnancy does not impart aggressive clinical behavior and/or worse prognosis.
Introduction
Cancer complicates an estimated 1 in 1000 pregnancies.1 The birthrate for women greater than 30 years has steadily increased over the past few decades. Coupled with the fact that the incidence of many malignancies begins to rise during the fourth decade of life, the rare and challenging case of cancer in pregnancy is becoming relatively more common.2 Gynecologic malignancies, along with breast and hematologic malignancies, are of the most common cancers diagnosed in pregnancy.
Decisions regarding diagnosis and treatment of cancers in pregnancy must carefully weigh pregnancy physiology, dynamic anatomy, and fetal considerations. As the body of literature regarding cancer in pregnancy continues to grow, clinicians can find guidance in achieving excellent oncologic as well as safe obstetric outcomes. We will review the management, diagnosis, and treatment of cervical dysplasia, adnexal masses, and gynecologic malignancies in pregnancy.
Cervical Dysplasia
Cervical changes in pregnancy
Pregnancy changes the appearance of the cervix, both grossly and with the aid of colposcopy.3 Hyperestrogenism during pregnancy produces an increase in cervical volume. Hypervascularity produces a blue hue, which is exaggerated with acetic acid. Areas of fusion between columnar villa and immature metaplastic epithelium are prominent at the end of the first trimester. In the second and third trimesters, stromal edema, enlargement of glandular structures, inflammation, and stromal decidualization (all benign processes) may appear suspicious. The Arias-Stella reaction, described in 1954 by Dr. Javier Arias-Stella as an endometrial change in response to trophoblasts and hormonal fluctuations, may resemble clear cell carcinoma. The reaction is characterized by nuclear enlargement with prominent nucleoli, vacuolated or eosinophilic cytoplasm, and hyperchromasia.4 Although these cells originate in the uterine cavity, they may be misinterpreted as high grade cellular changes on cervical cytology or biopsy.
Screening recommendations
The peak incidence of CIN and childbearing occurs during the third decade.5 While there are no specific societal guidelines for cytologic screening in pregnancy, prenatal visits offer an opportunity for screening. For some patients, the prenatal visit may be the only opportunity to undergo screening. Abnormal cytology is common in pregnancy and can be found in up to 5%. HPV DNA testing for screening is typically not performed during pregnancy.
Management of Abnormal Cytology
The goal of evaluating abnormal cytology in pregnancy is to identify microinvasive carcinoma. CIN1–3 can be expectantly managed with treatment deferred following delivery.
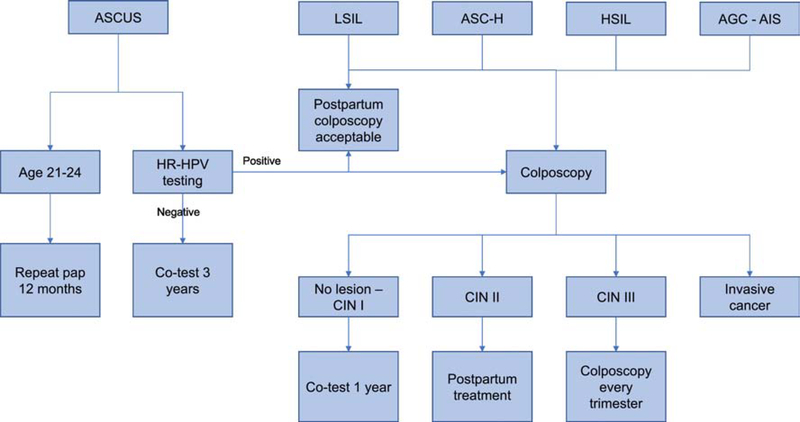
In 2012, the American Society for Colposcopy and Cervical Pathology (ASCCP) updated its recommendations for cervical cancer screening and management of dysplasia. Their algorithm is summarized in Figure 1.
Figure 1.
Algorithm for management of cervical dysplasia in pregnancy
In a multicenter retrospective study of >1,000 patients with abnormal cytology in pregnancy, 26% were ASCUS, 55%LSIL, 15% HSIL, and 4% ASC-H.6 Of patients who underwent biopsy, CIN 2–3was diagnosed 20% of the time when colposcopic impression was normal or CIN1, as compared to 55% of the time when colposcopic impression was CIN 2–3. The 20% occurrence of CIN 2–3 with colposcopic impression of CIN 1 or normal highlights that the physiologic changes of pregnancy may mask true pathology. Postpartum cytology reverted to normal in 64% of patients with ASC/LSIL and in 53% with HSIL.
Atypical Glandular Cells
Pregnant women with atypical glandular cells of unknown significance (AGUS) should undergo colposcopic evaluation with directed biopsies. Up to 40% of AGUS cytology indicates a significant tissue abnormality, with over half harboring squamous lesions.7 If adenocarcinoma in situ (AIS) is discovered on biopsy, excisional procedures should be reserved for cases where invasion is suspected. While cold knife conization (CKC) is preferred to large loop excision of the transformation zone (LLETZ) among non-pregnant patients due to lower rates of positive margins or residual disease, contemporary studies suggest that LLETZ may have comparable oncologic outcomes.8 The progression for AIS to invasive adenocarcinoma in pregnancy is unknown.
The risk of underlying malignancy can be 26% in non-pregnant populations with AGC-favor neoplasia.9 If no obvious lesion is noted on colposcopy, imaging with pelvic MRI without contrast or pelvic ultrasound can be considered, although the sensitivity and specificity of these tests in pregnancy is not known.
Colposcopy-directed Biopsy
As in non-pregnant colposcopy, the presence of punctations, mosaicism, atypical vessels or friable lesions should raise the suspicion for invasive cancer. Colposcopy has high diagnostic accuracy, and a complication rate of 0.6% (eg., hemorrhage, preterm labor, miscarriage, and infection).10,11 Because hypervascularization increases the risk of bleeding, biopsies should be limited to the most suspicious areas and random biopsies avoided.
Endocervical curettage is contraindicated in pregnancy, even with unsatisfactory colposcopy. We recommend coin biopsy (shallow cone) or even wedge excision to rule out invasive carcinoma during the early second trimester. Colposcopy can be repeated every trimester in the absence of severe lesions.
Natural progression
In immunocompetent women, CIN rarely progresses to microinvasive disease. Postpartum CIN regression rates after delivery range from 37–74%, with regression of CIN I >80%.12 Once the temporary immunosuppression of pregnancy resolves, cervical dysplasia may regresses. Higher rates of regression have been reported with vaginal delivery (60–66% vs 12% via Cesarean), possibly through debridement of dysplasia with vaginal birth traumna.13
Treatment
LLETZ is safe during the first trimester without significant hemorrhage, pregnancy loss, or preterm birth.14 Beyond the first trimester, there are significant maternal and fetal risks with excisional procedures including hemorrhage and preterm delivery.
Hemorrhage may occur in up to 14% of 3rd trimester CKCs. Blood transfusions may be required in 9.4%.15 Pregnancy loss in the first trimester (15–33%) and perinatal death (3–6%) may result from hemorrhage or previable or preterm birth.16 Excisional procedures should therefore be avoided in pregnancy unless there is high suspicion for invasive cancer. Low progression rates of CIN during pregnancy support deferring treatment to the postpartum period.
Cervical cancer
Early Stage
Microinvasive disease
As in the non-pregnant patient, staging of cervical cancer diagnosed during pregnancy is according to the International Federation of Obstetrics and Gynecology (FIGO).17 Excision is indicated in patients with microinvasive cervical cancer (MIC) (i.e., FIGO IA1–2) in order to determine the depth of stromal invasion. Patients with stage IA1 disease may safely continue pregnancy to term; those with stage IA2 or occult IB1 disease should consider delivery and treatment with fetal lung maturation.
Multiple case reports and series describe the outcomes after treatment delay of 1 to 32 weeks to allow for fetal maturity in women with Stage IA-IB1 cervical cancer (Table 1).18 Overall, disease progression was rare. For women with early stage disease, a deliberate delay in treatment to allow for fetal maturation may be considered.
Table 1.
Deliberate Delay of Definitive Treatment for Frankly Invasive Cervical Cancer
| Authors | Year | Stage | Patients (n) | Delay | Maternal Outcome |
|---|---|---|---|---|---|
| Prem et al. | 1966 | I | 4 | 6 wk | NED 5 yr |
| I | 5 | 11–17 wk | NED 3–5 yr | ||
| Dudan et al. | 1973 | IB | 2 | 2 and 6 mo | Progression |
| Lee et al. | 1981 | IB | 1 | 12 wk | NED 10 yr |
| IB | 2 | 11 wk | No progression | ||
| II | 5 | 1–11 wk | No progression | ||
| Nisker and Shubat | 1983 | IB | 1 | 24 wk | DOD |
| Greer et al. | 1989 | IB | 5 | 6–17 wk | NED 1–3 yr (n = 4), DOD (n = 1) |
| Monk and Montz | 1992 | IB | 4 | 10–16 wk | NED 3.5 yr |
| Hopkins and Morley | 1992 | IB | 5 | 12 wk | NED 5 yr (n = 40) |
| Mack et al. | 1981 | IB | 3 | 10–16 wk | NED |
| Duggan et al. | 1993 | IB1 | 5 | 7–24 wk | NED 3 yr |
| Sivanesaratnam et al | 1993 | IB | 2 | 2 and 4 wk | NED 5 yr |
| Allen et al. | 1995 | IB | 2 | 18–19 wk | NED 5 yr |
| Sorosky et al | 1995 | IB1 | 7 | 7–29 wk | NED 1.5–5.5 yr |
| Sood et al. | 1996 | IB | 3 | 3–32 wk | NED 1–30 yr |
| Tewari et al. | 1997 | IB2 | 1* | 11 wk | NED 2 yr |
| IIA | 1* | 18 wk | DOD 9 mo | ||
| van Vliet et al. | 1998 | IB | 5 | 2–10 wk | DOD (n = 1), NED 1.5–9 yr |
| IIA | 1 | 2 wk | NED 12 yr | ||
| Marana et al. | 2001 | IIB | 1* | 21 wk | DOD 18 mo* |
| Takushi et al. | 2002 | IB1 | 2 | 13 and 15 wk | NED 8 and 9 yr |
| IB2 | 1 | 6 wk | NED 7 yr | ||
| Germann et al. | 2005 | IB1 | 9 | 4 mo | NED 5 yr |
| Traen et al. | 2006 | IB1 | 1 | 19 wk | NED |
| Gonzalez Basquet et al. | 2008 | IB | 1 | 8 wk | NED 1 yr |
| Favero et al. | 2010 | IB1 | 10 | 11–28 wk | NED 5–102 mos |
| IB2 | 1 | 12 wk | NED 68 mos | ||
Modified from Tewari K, Cappuccini F, Gambino A, et al. Neoadjuvant chemotherapy in the treatment of locally advanced cervical carcinoma in pregnancy: a report of two cases and review of issues specific to the management of cervical carcinoma in pregnancy including planned delay of therapy. Cancer 1998;82:1529.
The use of CO2 laser conization for MIC is safe if the specimen length is <2 cm.19 Potassium titanyl phosphate laser conization and vaporization was reported in four women with MIC performed between 16–23 weeks of gestation, all of whom delivered at term; three had subsequent radical hysterectomy with lymphadenectomy and one underwent CKC.20 None have developed recurrence in a 2–13 year follow-up.
Patients with FIGO stage IA1 disease without lymphovascular space invasion (LVSI) and negative margins on excisional biopsy can be offered post-partum extrafascial hysterectomy. If future childbearing is desired, these patients may undergo surveillance with serial cytology and ECC.
Patients with MIC are not required to undergo cesarean section. However, a gross lesion is a contraindication to vaginal delivery due to risks for tumor dissemination with obstructed labor and episiotomy site recurrence. For those undergoing cesarean, a vertical uterine incision should be employed to maintain the integrity of the lower uterine segment for pathologic assessment and the placenta should also be sent to pathology. However, patients with FIGO stage IA1 with LVSI, IA2, or occult IB1 disease should undergo radical hysterectomy with bilateral pelvic lymphadenectomy at the time of cesarean or 6–8 weeks’ postpartum following vaginal delivery to mitigate blood loss.21
Radical hysterectomy and trachelectomy
For stage IA2-IB2 tumors, radical hysterectomy with bilateral pelvic lymphadenectomy is feasible in any trimester. Monk and Montz reported their institutional experience of gravid radical hysterectomy in 13 patients in the first and second trimester (before 24 weeks) (mean estimated blood loss (EBL) 1,750 cc) and in 7 patients who underwent cesarean delivery with radical hysterectomy from 23 weeks to 37 weeks (mean EBL 777 cc). There were no perioperative deaths and the most common morbidity was fever.
Both abdominal and vaginal radical trachelectomy with lymphadenectomy, have been associated with successful obstetric outcomes. Capilna et al reviewed 10 cases of vaginal and 11 cases of abdominal radical trachelectomy performed between 5 and 22 weeks’ gestation.22 Following vaginal radical trachelectomy, two spontaneous abortions occurred within the first week postoperatively and the other eight patients delivered between 29 and 37 weeks’. After abdominal radical trachelectomy, four spontaneous abortions occurred in the post-operative period, three of which were during the first trimester. The six remaining patients delivered between 36 and 39 weeks.
Locally Advanced and Metastatic Cervical Cancer
Chemoradiation
The recommended treatment for locally advanced cervical cancer is external beam radiation therapy with radiosensitizing weekly platinum-based chemotherapy, followed by high-dose-rate intracavitary brachytherapy. Treatment should not be delayed when the diagnosis occurs during the first or early second trimester. Short delays in the third trimester to allow for fetal lung maturation may be considered.23
A gravid uterus is not suitable for intracavitary radiation. Spontaneous abortion after initiation of chemoradiation occurs after 35 days in the first trimester and after 45 days in the second trimester. If spontaneous abortion does not occur by the end of external beam radiation treatment, the uterus can be evacuated by hysterotomy prior to brachytherapy application.
Nodal evaluation
Pelvic lymph node metastases are an important prognostic factor for cervical cancer. While imaging may identify suspiciously enlarged nodes, pathologic confirmation may be necessary. A retrospective study of 8 patients undergoing pelvic and/or para-aortic lymph node dissection spanning all trimesters of pregnancy for stage IB1 to IIIA cancers resulted in adequate sampling in all cases and resulted in only one spontaneous abortion in a patient who underwent concurrent vaginal trachelectomy.24 Laparoscopic or robotic lymph node dissection can be considered to evaluate areas suspicious for nodal metastasis.
Neoadjuvant Chemotherapy
Patients with locally advanced disease should undergo immediate therapy. For patients who desire pregnancy preservation, an approach using neoadjuvant chemotherapy, first reported by Tewari et al in 1998, can be considered. 25 Two women received vincristine (1mg/m2) and cisplatin (50 mg/m2) during the early second and third trimesters. Both experienced significant tumor regression and underwent Cesarean-radical hysterectomy at 32 and 34 weeks’ gestation. Both infants were born healthy. Multiple case reports and series have since been reported,26 the largest of which reported on 21 patients treated with three cycles of neoadjuvant platinum-based chemotherapy. Cesarean delivery between 30.4 and 36.5 weeks produced 22 healthy neonates without significant renal, hepatic, auditory, or hematopoietic impairment. Platinum concentrations in the amniotic fluid were demonstrably lower than in the umbilical cord blood (11–42% vs 23–65%), suggesting a placental filtration mechanism for platinum.
In a meta-analysis by Song et al, 88 pregnant women receiving neoadjuvant chemotherapy were identified.27 Most were diagnosed during the second trimester (84.8%) with stage I-IIA (87.5%) disease. All but two patients received cisplatin (55 single-agent; 31 combined with paclitaxel, vincristine, doxorubicin, 5-FU, or bleomycin). The majority had a complete (8.7%) or partial response (46.4%), 42.0% experienced stable disease, and only 2.9% progressed. All were delivered via cesarean (79% (n=65) by cesarean-radical hysterectomy), except for one patient who presented at 33.1 weeks with advanced cervical dilatation. Ultimately, 84 pregnancies resulted in 88 live-born infants, 71 of whom were completely healthy. Seventeen experienced respiratory difficulty (n=9), anemia (n=2), and there were 1 case each of mild elevation of serum creatinine, hypoglycemia, first degree intraventricular hemorrhage, bilateral hearing loss, erythema, and supraventricular tachycardia. Follow-up was noteworthy for one case of acute myeloid leukemia (AML) diagnosed at 22 months and rhabdomyosarcoma at 5 years. A relationship between alkylating agents and leukemia has been previously reported, but proof of direct causality remains elusive.. In this case the neonate lacked chromosomal translocations typical of secondary AML and the karyotypic abnormalities observed in treatment-related cancers.28
Metastatic Cervical Cancer
Very few pregnant women will present with stage IVB cervical cancer. Patients with distant metastases have a very poor prognosis. If diagnosed early in pregnancy, termination should be discussed and standard therapy should be recommended (i.e., platinum-based chemotherapy plus bevacizumab). If metastatic cervical cancer is diagnosed after viability or if the patient chooses to continue pregnancy, systemic chemotherapy should be initiated immediately without bevacizumab. Although carboplatin is non-inferior to cisplatin for this indication, subset analyses favor cisplatin among cisplatin-naïve patients.29 Topotecan and cisplatin can be considered in patients who cannot tolerate taxanes. Although pembrolizumab has activity in patients with PDL1+ tumors,31 immune checkpoint inhibitors are US FDA Category D as their use in animal models increased the risk of spontaneous abortions.30
Episiotomy site recurrence
Recurrence of cervical cancer at perineal laceration or episiotomy is a rare phenomenon with at least 20 cases reported since 1986.31 The perineum can be seeded via spread of occult cervical cancer cells during the vaginal birth. For patients with a gross lesion who undergo vaginal delivery (as well as those diagnosed postpartum following vaginal birth) inspection and palpation of episiotomy and laceration sites during surveillance is mandatory.
A matched case-control study of women diagnosed with cervical cancer antenatally or within 6 months postpartum found that vaginal delivery was the most significant predictor of recurrence.32
Adnexal masses and Ovarian cancer
Adnexal masses are detected in 2–5% of pregnancies but persist in only 0.7–1.4%.33 Most are asymptomatic, incidentally discovered on obstetric ultrasound. The 3–5 cm cystic corpus luteum arises from the dominant follicle after ovulation. In the case of pregnancy, the corpus luteum is rescued by secretion of human chorionic gonadotropin (hCG) and persists as a progesterone-secreting organ throughout the first trimester until placentation.34 Over 90% of functional cysts resolve during gestation. Size is inversely related to regression and directly related to complication rates. Only 2–5% of adnexal masses are found to be malignant.35
Workup
Ultrasonography is primarily recommended with MRI reserved for certain cases. Webb and associates reviewed 557 patients with adnexal masses in pregnancy. Complex masses (loculations, septa, cystic and solid features, papillary projections, or poorly defined borders), were malignant in 9%.36 Conversely, 1% of simple cysts were malignant.
Management
Symptomatic adnexal masses and those concerning for malignancy warrant surgical extirpation. The second trimester (15–20 weeks’) is the optimal time for surgical intervention due to the lower risk of spontaneous abortion, the remoteness from fetal viability, avoidance of disrupting the corpus luteum, avoidance of anesthetic agents during organogenesis, and allowance for resolution of many benign conditions. When possible, minimally invasive techniques are preferred.37
There is no consensus on management of adnexal masses simply based on size. In the general population, the American College of Obstetricians and Gynecologists (ACOG) identifies adnexal masses >10 cm as concerning for malignancy; however, simple and asymptomatic cysts can be expectantly managed.38 Historically, surgery was considered for asymptomatic masses >6 cm due to the risk of torsion and concern for malignancy.39 Koo et al studied 470 pregnant women and reported that masses between 6–10 cm are nearly three times more likely to cause torsion than those <6 cm. Masses >15 cm had a 12-fold higher risk of malignancy than those <6 cm, while masses 6–15 cm were not more likely to be malignant compared to those <6cm. If a patient with an adnexal mass is expectantly managed, they should be counseled on the risk of torsion (10%), rupture (2%), or undiagnosed malignancy (1–9%).36
Benign Ovarian Masses
Benign ovarian masses have a broad differential that is similar to non-pregnant individuals, but also include entities that are specific to pregnancy.
Mature teratomas are the most common germ cell tumor. On ultrasound, they are often unilocular with complex echo patterns representing fat, solid, and calcified components. Unlike other benign cysts, mature teratomas often persist throughout pregnancy but rarely enlarge.
Endometriomas appear as homogenous masses with low level echoes.40 They can be multiloculated, but lack other suspicious features (eg., internal vascularity or mural nodules). A case series of 53 endometriomas diagnosed in the first trimester found that on repeat ultrasound in the second trimester, 24% increased in size, 27% remained stable, 34% decreased in size, and 15% resolved.41 Only 10 (19%) required cystectomy in pregnancy.
If these benign masses are suspected in pregnancy, they can be expectantly managed, but may require intervention if they become symptomatic (eg., torsion, rupture).
Ovarian masses specific to pregnancy
First described in 1965, luteoma is a benign, hyperplastic reaction of theca lutein cells that can be as large as 15 cm and bilateral in 20%.42 They appear as multiple, well-circumscribed solid nodules. Grossly, they are brown yellow and black elements may manifest. Luteomas are associated with elevations in plasma testosterone and other androgens, which can cause maternal hirsutism or virilization during the latter half of pregnancy. Approximately half of female infants may experience virilization by affected mothers.43 Although they can appear suspicious, luteomas regress spontaneously after delivery, thus surgery is not necessary.44
Theca-lutein cysts appear as multiple, thin-walled cysts, and can occur when hCG levels are elevated (eg., multiple gestations, hydatidiform mole). They typically regress spontaneously postpartum and surgery is reserved only for acute complications.
Malignant Ovarian Masses
As for non-pregnant patients, staging of ovarian cancer in pregnancy is according to FIGO.45
Malignant Germ Cell Tumors
Malignant germ cell tumors (MGCT) are the most common type of ovarian malignancy diagnosed in pregnancy.46 Dysgerminoma is the most common MGCT in pregnancy, comprising approximately 38%, followed by yolk sac tumors (30.4%).47 MGCTs are characteristically rapidly growing and unilateral, although dysgerminoma is bilateral in 10% of cases.
Alpha-fetoprotein (AFP) and lactate dehydrogenase (LDH) are typically elevated in patients with MGCT. AFP is produced by the yolk sac, fetal liver and gastrointestinal tract. AFP is routinely screened in the second trimester for neural tube defects. Elevated values should also raise suspicion for malignant germ cell tumors or hepatocellular carcinomas.48 The isoenzymes 1 and 2 of lactate dehydrogenase (LDH) are specifically elevated in women with dysgerminoma.49 LDH levels typically remain stable and within normal range during pregnancy, although may be elevated in the case of pre-eclampsia.
The majority of MGCT are diagnosed at an early stage.47 Unilateral adnexectomy with preservation of the gravid uterus and contralateral ovary is recommended. Complete bilateral salpingo-ophorectomy is rarely necessary, even with bilateral gross involvement. Complete surgical staging, including pelvic and para-aortic lymphadenectomy may be performed for clinical Stage IA dysgerminoma or Stage IA-B grade 1–2 immature teratoma, as in the absence of gross and microscopic spread, these lesions historically do not require adjuvant chemotherapy. In all other histologies of malignant germ cell tumors, adjuvant chemotherapy is recommended, thus unilateral adnexectomy and removal of gross metastatic disease (if present) is sufficient. Adjuvant chemotherapy for Stage IC/Grade 3 immature teratoma and Stage IB-IC dysgerminoma is controversial with some data supporting close surveillance only.50,51
Chemotherapeutic regimens typically include bleomycin, etoposide, and cisplatin (BEP). Case reports of this combination regimen given in the third trimester resulted in no significant neonatal malformations, with the exception of a 28-week neonate born with ventriculomegaly and cerebral atrophy.52 However, other cases of pregnancies with earlier and more cycles of BEP reported no minor or major malformations.53 Conversely, MGCTs (excluding stage I dysgerminoma and stage I immature teratoma) are characterized by rapid growth and often recur when adjuvant chemotherapy is withheld, thus delays in initiating systemic therapy may affect maternal outcome.
Sex Cord Stromal Tumors
Sertoli-Leydig and granulosa cell tumors only account for 2–3% of all ovarian neoplasms and are rarely encountered in pregnancy.54 They behave similarly in non-pregnant women, presenting with early-stage disease and having a slow, low-grade, and indolent course.55
Inhibin, a glycoprotein produced by granulosa and leydig cells, is often elevated in granulosa cell tumors. Of the two isoforms, inhibin B is predominantly secreted by granulosa cells.56 While inhibin is typically stable and normal throughout pregnancy, inhibin A may be elevated in pre-eclampsia.57
Blake et al conducted a systematic literature search and identified 46 cases of sex cord stromal tumors diagnosed in pregnancy between 1955–2012.58 Granulosa cell tumors were the most common (22.0%) followed by thecoma (18.6%) and Sertoli-Leydig cell (8.5%). Virilization was experienced by 26.1% of patients. The majority of patients were diagnosed at stage I (76.1%) with tumors <15 cm (64.9%). The live birth rate was 78.3% with 60.9% were full term. Overall survival rates were similar in pregnant and non-pregnant patients. Maternal or fetal serious adverse events were noted in 41.3%, with hemoperitoneum resulting in shock in 6 patients (13.0%), severe hypertension in 4 patients (8.7%), and maternal death in 3 (6.5%). Surgery was completed during pregnancy in 36 of 45 cases with fetal conservation in 25 cases. The majority of cases were managed with unilateral salpingo-oophorectomy (80.4%); hysterectomy was performed in 13.6% and lymphadenectomy in 6.5%. Felt to have been unrelated to surgery, fetal loss occurred in one case; there were three cases of IUFD, one case of stillbirth, and one case of neonatal death.
Borderline Ovarian Tumors
Borderline tumors are one of the most frequent ovarian tumors diagnosed in pregnancy as one third of all borderline tumors are diagnosed in women less than 40 years of age. In the series (n=40) by Fauvet et al, of the 36 who underwent surgery during pregnancy, 22 were in the 2nd trimester and only 4 were at time of cesarean. Laparotomy was performed more than laparoscopy, and the majority underwent unilateral salpingo-oophorectomy. The median tumor size was 12.1 cm. The majority of the 21 patients who underwent reassessment surgery did so in the postpartum period (67%) or at time of cesarean section (24%), and 5 patients (24%) were upstaged as a result.
Histologically and clinically, borderline tumors in pregnancy commonly contain features concerning for aggressive behavior including peritoneal implants, microinvasion, intraepithelial carcinoma, and micropapillary features. Micropapillary features were found in 41% of serous borderline tumors in the series discussed earlier. Pathologic review of 10 cases at MD Anderson also found aggressive histologic features in borderline tumors diagnosed in pregnancy.59 However, for two patients who underwent restaging surgery postpartum, these aggressive histologic features had regressed, suggesting that they may be transient and related to pregnancy physiology.
Epithelial ovarian cancer
Epithelial ovarian cancer (EOC) is exceedingly rare in pregnancy. Blake et al conducted a systematic literature review and identified 105 reports of epithelial ovarian cancer in pregnancy between 1955 and 2013.60 Serous carcinomas comprised the largest histologic subtype (47.6%) followed by mucinous (27.6%) and endometrioid (10.5%). Nearly half were diagnosed during the first trimester (45.3%), and there were 78 live births (81.3%), 41 of which were at term (57.7%). Surgery was performed predominantly during the second trimester (43.0%), with unilateral adnexectomy alone most commonly performed (63.4%). Hysterectomy was performed during pregnancy in 16 (15.8%) and omentectomy in 21 (20.8%).
CA125 values are commonly elevated in the first trimester, normalize in the second trimester and remain low until delivery.61 Although uncommon, CA125 can be elevated during the third trimester in the absence of malignancy.62 CEA, a marker of mucinous adenocarcinomas, can rise in the third trimester, but often remains within normal range.62
Masses that appear suspicious on ultrasound or MRI require pathologic diagnosis, either by surgical exploration or, if present, sampling of ascitic fluid, pleural effusion, and/or metastatic deposits.
Once the diagnosis of epithelial ovarian cancer is made, appropriate therapy should not be withheld. A multidisciplinary approach should be employed, involving Gynecologic Oncology, Neonatology, Pathology, Anesthesiology and occasionally, Maternal Fetal Medicine. Primary cytoreductive surgery versus neoadjuvant chemotherapy, depends on the gestational age at diagnosis, desire for pregnancy continuation, clinical disease distribution, and maternal acuity.
The Society of Gynecologic Oncology (SGO) recommends that all women with a diagnosis of ovarian, fallopian tube, or primary peritoneal cancer should receive genetic counseling and offered germline genetic testing, regardless of family history. If germline testing is negative, somatic tumor testing for BRCA1/2 and homologous recombination deficiency should be pursued.63
Cytoreductive Surgery
For patients diagnosed with metastatic epithelial ovarian cancer in the first trimester, treatment should not be delayed. Surgical debunking, including gravid hysterectomy, should be recommended, followed by adjuvant chemotherapy.
When epithelial ovarian cancer is diagnosed in the second trimester or at the time of surgery for investigation of an adnexal mass, debulking of all visible disease should be undertaken with a hands-off approach to the uterus so as to minimize uterine irritability. Adjuvant (or neoadjuvant) platinum- and taxane-based chemotherapy can be given during the second and third trimester safely. Interval debulking can be performed at cesarean or during the postpartum period.64
Fertility-sparing surgery
While fertility-sparing surgery is not the standard of care for treatment of epithelial ovarian cancer, there is retrospective evidence to suggest it may be an option for select populations. Retrospective studies of women 40 or younger with stage IA or unilateral stage IC epithelial ovarian cancer with serous, mucinous, or clear cell histology who underwent unilateral salpingo-oophorectomy with preservation of contralateral ovary and uterus did not have an increased risk of death compared with patients who underwent comprehensive staging.65,66,67 These data should be interpreted with caution as there are no prospective studies investigating this approach, nor studies that include pregnant patients.
Neoadjuvant chemotherapy
SGO and the American Society of Clinical Oncology (ASCO) have provided guidelines for the use of neoadjuvant chemotherapy for advanced ovarian cancer.68 While explicit recommendations for pregnant women are not addressed, patients with high perioperative risk and/or low likelihood of achieving cytoreduction to <1 cm of residual disease should receive neoadjuvant chemotherapy. Accordingly, neoadjuvant chemotherapy can be considered in pregnant women during the second and third trimester. Response Evaluation Criteria in Solid Tumors (RECIST) guideline version 1.1 and serial CA125 should be used to track tumor response.
Chemotherapy for Epithelial Ovarian cancer
Chemotherapy is contraindicated in the first trimester as it may interfere with organogenesis. Zheng et al reviewed 13 patients treated with combination chemotherapy during the second and third trimester for advanced stage ovarian cancer. These included carboplatin plus paclitaxel (n=9), cisplatin plus paclitaxel (n=3), cisplatin plus docetaxel (n=1).69 13 healthy neonates (of 14) were born with an mean birthweight of 2,442 grams.
Bevacizumab and PARP inhibitors
In recent years, anti-angiogenesis agents and poly-ADP ribosome polymerase (PARP) inhibitors have been shown to significantly prolong progression-free survival in women with newly diagnosed and platinum-sensitive recurrent advanced EOC. The safety of bevacizumab, a humanized monoclonal antibody that targets vascular endothelial growth factor (VEGF) ligand and prevents binding to VEGF receptors, is unknown in human pregnancy. The VEGF pathway plays a role in maintaining the corpus luteum as well as amniotic fluid regulation.70 Interference with VEGF signaling can induce a preeclampsia-like syndrome of hypertension and proteinuria.71 Anti-angiogenesis agents should be avoided in pregnancy.
Similarly, there is no data regarding the use of PARP inhibitors in pregnancy. In murine models, PARP1 upregulation is crucial for embryo implantation.72 Interestingly, PARP inhibition has been shown to prevent the development of endothelial dysfunction and hypertension in rat models of pre-eclampsia.73 Nevertheless, due to the lack of data, PARP inhibitors should be avoided in pregnancy.
Uterine abnormalities
Uterine Sarcoma
Uterine leiomyoma are the most common gynecologic tumor in reproductive aged women and are found in 10–20% of pregnancies.74 Asymptomatic fibroids should be expectantly managed in pregnancy due to the risk of hemorrhage and fetal loss with surgery. Most fibroids remain do not enlarge significantly during pregnancy. Although the incidence of leiomyosarcoma in rapidly growing “fibroids” is only 0.27%, there is a lack of alternative, reliable preoperative diagnostics. MRI findings of ill-defined margins, lack of calcifications, or intra-lesional hemorrhage are suggestive, but not diagnostic. 75 A review of 15 uterine sarcomas in pregnancy found that the most common presenting symptoms were abnormal vaginal bleeding (40%), abdominal pain (33%), and an enlarging uterine mass (20%).76 The majority of patients underwent surgery during the 3rd trimester or postpartum (67%). The median survival for uterine sarcoma diagnosed in pregnancy has been estimated at 1.5 years.
Complete hydatidiform mole with coexisting fetus
The incidence of complete hydatidiform mole with coexisting fetus (CHCF) is estimated between 1 in 10,000 to 1 in 100,000.77 Presenting symptoms are similar to complete hydatidiform mole, including fundal height measuring size greater than dates and vaginal bleeding. A review of 130 cases of CHCF reported 33 livebirths (25%), incident risk of preeclampsia (30%), persistent GTN (33%), and metastatic GTN (22%).78 In comparing cases of CHCF that were evacuated before versus after viability, those evacuated before viability had significantly higher levels of pre-evacuation hCG, increased incidence of pre-eclampsia, and higher rates of persistent GTN.79 The risk of post-molar GTN following a partial mole and complete mole is 4% and 20%, respectively. In comparison, the risk of post-molar GTN is as high as 50–57% following CHCF.80 To mitigate the maternal risks of CHCF, continuation of pregnancy is acceptable in the case of normal fetal karyotype, normal fetal anatomy, absence of early pre-eclampsia, and declination in hCG. The concentration of hCG peaks at 8–10 weeks to 60,000–90,000 mIU/mL, then declines to an average of 12,000 mIU/mL at 20 weeks and stays relatively constant until term.81
Endometrial Cancer
Approximately 25 cases of endometrial cancer diagnosed during or after pregnancy have been reported, with 16 discovered following first trimester abortion, 9 diagnosed within 14 months of childbirth, and one found incidentally at hysterectomy for placenta accreta.82 Grade 1 and 2 predominated (n=23), and prognosis has been favorable in these cases. In the setting of an endometrial cancer diagnosis in a woman subsequently found to be pregnant, because the uterus itself is involved, definitive treatment with preservation of the pregnancy is impossible.
Vulvar and Vaginal Cancer
Fewer than 40 cases of vulvar cancer in pregnancy have appeared in the literature.83 Radical local excision with sentinel lymph node mapping for FIGO stage I lesions should be considered. Pregnancy-related increased vulvar blood flow can precipitate significant perioperative blood loss which can be mitigated with judicious use of electrocautery. Fetal exposure to locally injected technetium-99 (0.25 mCi, T1/2 6 hours) can be reduced by performing the procedure 2 hours after injection. Because technetium is captured in the lymph node, there is minimal systemic exposure. Additionally, nodal excision reduces exposure further. Both lymphoscintigraphy and lymphazurin blue should be omitted, the latter due to risk of anaphylaxis. Cesarean delivery is preferable to prevent vulvar wound dehiscence.
To date, there are only 12 reports of primary invasive vaginal cancer during pregnancy.
Evaluation and Therapeutic Modalities
Anesthesia
In December 2016, the FDA warned that repeated or lengthy use of general anesthetic and sedation drugs (inhalational or intravenous) during procedures or surgeries in children younger than three years or pregnant women during the third trimester may affect brain development.84 Inhaled anesthetics, NMDA antagonists, and propofol have been associated with anesthesia-induced neurotoxicity in preclinical studies and nonhuman primates, although the doses and duration of exposure exceed those used in clinical practice.85 ACOG and the American Society of Anesthesiologists released a joint committee opinion in 2017 emphasizing the lack of evidence that human in utero exposure to anesthetics or sedatives have any effect on the human brain.86 While complete avoidance is impossible, limiting the use of these agents is advised.
Surgery
As discussed earlier, surgical exploration during the second trimester between 15 and 20 weeks is preferable. Surgical intervention after beyond 23–24 weeks is challenging due to the large, gravid uterus and complicated by periviability decision-making, preterm birth, and neonatal morbidity/mortality.
When the clinical suspicion for malignancy is high, surgery should not be delayed. Minimally invasive surgery should be utilized over laparotomy when feasible. In a review of 2,233 laparoscopic and 2,491 open laparotomy cases, both methods increased risk of preterm delivery and low birthweight.87 Notably, there was no difference in fetal malformations or neonatal survival.
The 2017 Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) guidelines for laparoscopic surgery during pregnancy include utilizing the open Hasson entry, positioning in left lateral decubitus to minimize IVC compression, and avoiding the use of uterine manipulator.87 Intraoperative and postoperative pneumatic compression devices and early ambulation helps prevent deep vein thrombosis.
When laparotomy is required for known or suspected malignancy, a vertical, midline approach is recommended. This allows for optimal visualization and facilitates the hands-off approach to the uterus to minimize post-operative uterine irritability and contractions.
Peri-Operative Fetal Monitoring
Amniotic fluid index (normal range 8–18 from 20 to 35 weeks) should be assessed immediately prior to surgery with transabdominal ultrasonography. The ACOG Committee Opinion regarding non-obstetric surgery in pregnancy recommends that for previable pregnancies, fetal heart tones should be documented pre- and post-operatively.86 After viability (e.g., 23–24 weeks), continuous fetal heart rate monitoring can be considered when indicated with a clear plan for intervention in the case of fetal or maternal distress.
Perioperative prophylactic oncolytic are not recommended as there is no evidence to support their use.88 Oncolytic may be used to manage symptomatic contractions (e.g., terbutaline 0.25 mg subcutaneously every 4 hours), but there is no evidence that this intervention has a significant impact on preterm delivery.89
Diagnostic Radiology
When planning diagnostic imaging, gestational age and the dose of ionizing radiation to the fetus must be considered. In the preimplantation phase, radiation produces an allor-nothing effect, either destroying the fertilized egg or having no consequence. The most sensitive period of organogenesis for the fetus is day 18 through day 38. After 40 days, larger doses of radiation are required to impact the fetus.90
Ultrasound and MRI are safe in pregnancy as they utilize non-ionizing radiation. Although there are theoretical risks of acoustic damage and fetal heating with MRI, there is no increased risk of vision loss, hearing loss, stillbirth, or fetal anomaly associated with first trimester MRI.91 However, the use of gadolinium contrast increases fetal risk of any rheumatological, inflammatory, or infiltrative skin condition (adjusted HR 1.36, 95% CI 1.09 to 1.69) as well as stillbirth and neonatal death (adjusted RR 3.70, 95% CI 1.55 to 8.85). The use of gadolinium should be limited to scenarios where benefits outweigh risk.
Because conventional radiography, computed axial tomography, and nuclear medicine scans utilize ionizing radiation, gestational age and fetal dose should be considered when employing these imaging modalities. Imaging Wisely, a joint initiative of the American College of Radiology, Radiological Society of North America, American Society of Radiological Technologists, and the American Association of Physicists in Medicine recommends that if positron emission tomography is indicated in pregnancy, FDG dose reduction to 5 mCi should be used to minimize fetal exposure.92
Chemotherapy
The safety of chemotherapy during pregnancy depends on the gestational age, mechanism of action, dose, route of delivery.93
First trimester chemotherapy exposure increases the risk of significant malformations, spontaneous abortion, and fetal death.94 The developing fetus is maximally susceptible to teratogens during the fifth through tenth week of gestational age. During this time, teratogenic exposure may affect the heart, neural tube, and limb development, followed by palate, eye, and ear development.
The second and third trimesters are important for organ maturation, neurologic development, and fetal growth. Second and third trimester exposure to chemotherapy has been associated with intrauterine growth restriction and preterm birth.95,96 Data on long term effect of in utero chemotherapy exposure are lacking. Therefore, if chemotherapy is indicated during pregnancy, oncologists should consider enrolling their patients in available prospective databases.
There are no societal guidelines regarding fetal monitoring or surveillance for women receiving chemotherapy. Chemotherapy-induced fetal anemia may manifest with sinusoidal tracings on antepartum fetal heart rate monitoring when indicated for obstetric reasons.
The period of gestational advancement, irrespective of whether chemotherapy is being administered, from 24 to 34 weeks can be used to facilitate fetal lung maturation with glucocorticoids, although amniocentesis to document pulmonary maturity is rarely indicated and becoming obsolete. Chemotherapy should not be given after the 34th week or within 3 weeks of scheduled delivery to avoid the hematologic nadir period, both for mother and neonate. Transient neonatal myelosuppression has been documented when chemotherapeutics are administered in the weeks leading up to delivery among patients with hematologic malignancies.97
Breast Feeding
Antineoplastic drugs concentrate in breast milk and can affect the neonate. We do not recommend women receiving chemotherapy to breast feed their infants.
Conclusion
A multidisciplinary approach should be utilized in all cases of malignancies in pregnancy. Comprehensive risk-benefit counseling and shared decision-making that consider the most updated and relevant studies are integral to appropriate patientcentered care. Provided that patients are managed according to the recommendations described in this review, there is no evidence that pregnancy has a detrimental effect on the prognosis of women diagnosed with gynecologic malignancies.
Supplementary Material
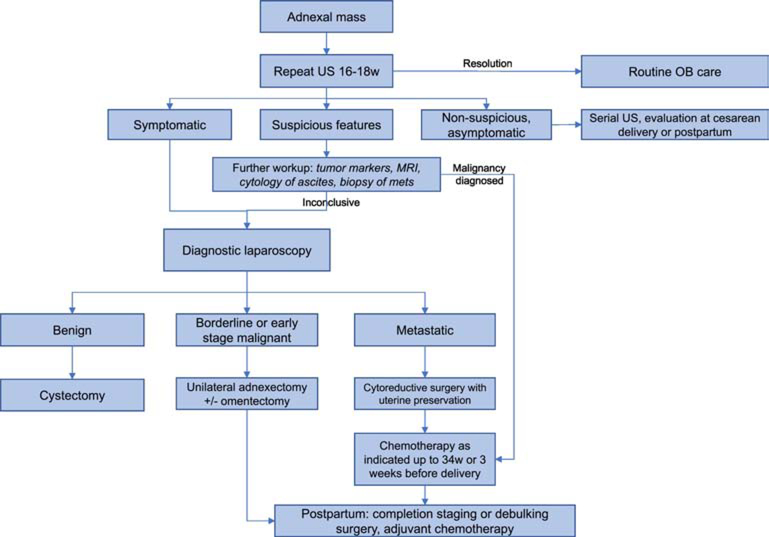
Figure 2.
Algorithm for management of adnexal mass
Table 2.
Tumor Markers in Pregnancy
| Tumor Marker | Malignancy | Normal range | Effect of pregnancy |
|---|---|---|---|
| CA-125 | Epithelial ovarian cancer | <35 U/mL | Elevated in first trimester |
| Carcinoembryonic antigen (CEA) | Mucinous cystadenocarcinoma of ovary, pseudomyxoma peritoneii, colorectal | <5 ng/mL | Rise in third trimester, but still remains normal |
| Alpha fetoprotein (AFP) | Malignant germ cell tumor (yolk sac tumor, immature teratoma, mixed germ cell) | <15 ng/mL | Peaks at 13 weeks |
| Lactate dehydrogenase | Dysgerminoma and mixed germ cell tumor | 45–90 U/L | May be elevated with pre-eclampsia |
| Inhibin | Granulosa cell tumor | 33–45 pg/mL | Inhibin A elevated with pre-eclampsia |
Highlights.
Cancer affects 1 in 1000 pregnancies
Diagnostic workup for cancer must be carefully selected and interpreted in pregnancy
Cervical cancer is the most common gynecologic malignancy diagnosed in pregnancy
Surgery and chemotherapy can be appropriately used with preservation of pregnancy
Footnotes
Conflict of Interest Statement
The authors have no relevant financial conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Smith LH, Danielsen B, Allen ME, Cress R. Cancer associated with obstetric delivery: results of linkage with the California cancer registry. Am J Obstet Gynecol. 2003;189(4):1128–1135. doi: 10.1067/S0002-9378(03)00537-4 [DOI] [PubMed] [Google Scholar]
- 2.Voulgaris E, Pentheroudakis G, Pavlidis N. Cancer and pregnancy: A comprehensive review. Surg Oncol. 2011;20(4):e175–e185. doi: 10.1016/j.suronc.2011.06.002 [DOI] [PubMed] [Google Scholar]
- 3.Michael CW, Esfahani FM. Pregnancy-related changes: a retrospective review of 278 cervical smears. Diagn Cytopathol. 1997;17(2):99–107. doi: [DOI] [PubMed] [Google Scholar]
- 4.Arias-Stella J The Arias-Stella reaction: facts and fancies four decades after. Adv Anat Pathol. 2002;9(1):12–23. doi: 10.1097/00125480-200201000-00003 [DOI] [PubMed] [Google Scholar]
- 5.Insinga RP, Glass AG, Rush BB. Diagnoses and outcomes in cervical cancer screening: A population-based study. Am J Obstet Gynecol. 2004;191(1):105–113. doi: 10.1016/j.ajog.2004.01.043 [DOI] [PubMed] [Google Scholar]
- 6.Fader AN, Alward EK, Niederhauser A, et al. Cervical dysplasia in pregnancy: a multi-institutional evaluation. Am J Obstet Gynecol. 2010;203(2):113.e1–113.e6. doi: 10.1016/j.ajog.2010.04.016 [DOI] [PubMed] [Google Scholar]
- 7.Tam KF, Cheung ANY, Szeto E, Ngan HYS. Atypical glandular cells diagnosed during pregnancy and the postpartum period: a retrospective analysis. Eur J Obstet Gynecol Reprod Biol. 2011;155(2):213–216. doi: 10.1016/j.ejogrb.2010.12.009 [DOI] [PubMed] [Google Scholar]
- 8.Bai H, Liu J, Wang Q, et al. Oncological and reproductive outcomes of adenocarcinoma in situ of the cervix managed with the loop electrosurgical excision procedure. BMC Cancer. 2018;18(1):461. doi: 10.1186/s12885-018-4386-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tam KF, Cheung ANY, Liu KL, et al. A retrospective review on atypical glandular cells of undetermined significance (agus) using the Bethesda 2001 classification. Gynecol Oncol. 2003;91(3):603–607. doi: 10.1016/j.ygyno.2003.08.029 [DOI] [PubMed] [Google Scholar]
- 10.Hacker NF, Berek JS, Lagasse LD, Charles EH, Savage EW, Moore JG. Carcinoma of the cervix associated with pregnancy. Obstet Gynecol 1982;59(6):735–746. [PubMed] [Google Scholar]
- 11.Economos K, Perez Veridiano N, Delke I, Collado ML, Tancer ML. Abnormal cervical cytology in pregnancy: a 17-year experience. Obstet Gynecol. 1993;81(6):915–918. [PubMed] [Google Scholar]
- 12.Mailath-Pokorny M, Schwameis R, Grimm C, Reinthaller A, Polterauer S. Natural history of cervical intraepithelial neoplasia in pregnancy: postpartum histo-pathologic outcome and review of the literature. BMC Pregnancy Childbirth. 2016;16(1):74. doi: 10.1186/s12884-016-0861-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siristatidis C, Vitoratos N, Michailidis E, et al. The role of the mode of delivery in the alteration of intrapartum pathological cervical cytologic findings during the postpartum period. Eur J Gynaecol Oncol 2002;23(4):358–360. [PubMed] [Google Scholar]
- 14.Siegler E, Lavie O, Amit A, et al. Should the Risk of Invasive Cancer in Pregnancy and the Safety of Loop Electrosurgical Excision Procedure During the First 15 Weeks Change Our Practice? J Low Genit Tract Dis 2017;21(4):299–303. doi: 10.1097/LGT.0000000000000346 [DOI] [PubMed] [Google Scholar]
- 15.Averette HE, Nasser N, Yankow SL, Little WA. Cervical conization in pregnancy: Analysis of 180 operations. Am J Obstet Gynecol. 1970;106(4):543–549. doi: 10.1016/00029378(70)90039-6 [DOI] [PubMed] [Google Scholar]
- 16.Rogers RS, Williams JH. The impact of the suspicious Papanicolaou smear on pregnancy: A study of nationwide attitudes and maternal and perinatal complications. Am J Obstet Gynecol. 1967;98(4):488–496. doi: 10.1016/0002-9378(67)90101-9 [DOI] [PubMed] [Google Scholar]
- 17.Bhatla N, Berek JS, Fredes MC, et al. Revised FIGO staging for carcinoma of the cervix uteri. Int J Gynecol Obstet. 2019;145(1):129–135. doi: 10.1002/ijgo.12749 [DOI] [PubMed] [Google Scholar]
- 18.Sorosky JI, Squatrito R, Ndubisi BU, et al. Stage I Squamous Cell Cervical Carcinoma in Pregnancy: Planned Delay in Therapy Awaiting Fetal Maturity. Gynecol Oncol. 1995;59(2):207–210. doi: 10.1006/gyno.1995.0009 [DOI] [PubMed] [Google Scholar]
- 19.Tsuritani M, Watanabe Y, Kotani Y, Kataoka T, Ueda H, Hoshiai H. Retrospective Evaluation of CO2 Laser Conization in Pregnant Women With Carcinoma In Situ or Microinvasive Carcinoma. Obstet Gynecol Surv 2010;65(1):24. doi: 10.1097/01.ogx.0000367512.95546.93 [DOI] [PubMed] [Google Scholar]
- 20.Yahata T, Numata M, Kashima K, et al. Conservative treatment of stage IA1 adenocarcinoma of the cervix during pregnancy. Gynecol Oncol. 2008;109(1):49–52. doi: 10.1016/j.ygyno.2008.01.016 [DOI] [PubMed] [Google Scholar]
- 21.Bigelow CA, Horowitz NS, Goodman A, Growdon WB, Del Carmen M, Kaimal AJ. Management and outcome of cervical cancer diagnosed in pregnancy. Am J Obstet Gynecol. 2017;216(3):276.e1–276.e6. doi: 10.1016/j.ajog.2016.10.034 [DOI] [PubMed] [Google Scholar]
- 22.Căpîlna ME, Szabo B, Becsi J, Ioanid N, Moldovan B. Radical Trachelectomy Performed During Pregnancy: A Review of the Literature. Int J Gynecol Cancer Off J Int Gynecol Cancer Soc. 2016;26(4):758–762. doi: 10.1097/IGC.0000000000000655 [DOI] [PubMed] [Google Scholar]
- 23.Sood AK, Sorosky JI, Mayr N, et al. Radiotherapeutic management of cervical carcinoma that complicates pregnancy. Cancer. 1997;80(6):1073–1078. doi: [DOI] [PubMed] [Google Scholar]
- 24.Alouini S, Rida K, Mathevet P. Cervical cancer complicating pregnancy: Implications of laparoscopic lymphadenectomy. Gynecol Oncol. 2008;108(3):472–477. doi: 10.1016/j.ygyno.2007.12.006 [DOI] [PubMed] [Google Scholar]
- 25.Tewari K, Cappuccini F, Gambino A, Kohler MF, Pecorelli S, DiSaia PJ. Neoadjuvant chemotherapy in the treatment of locally advanced cervical carcinoma in pregnancy: a report of two cases and review of issues specific to the management of cervical carcinoma in pregnancy including planned delay of therapy. Cancer. 1998;82(8):1529–1534. doi: [DOI] [PubMed] [Google Scholar]
- 26.Ricci C, Scambia G, Vincenzo RD. Locally Advanced Cervical Cancer in Pregnancy: Overcoming the Challenge. A Case Series and Review of the Literature. Int J Gynecol Cancer. 2016;26(8):1490–1496. doi: 10.1097/IGC.0000000000000795 [DOI] [PubMed] [Google Scholar]
- 27.Song Y, Liu Y, Lin M, Sheng B, Zhu X. Efficacy of neoadjuvant platinum-based chemotherapy during the second and third trimester of pregnancy in women with cervical cancer: an updated systematic review and meta-analysis. Drug Des Devel Ther. 2018;13:79–102. doi: 10.2147/DDDT.S186966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Masetti R, Vendemini F, Zama D, Biagi C, Pession A, Locatelli F. Acute Myeloid Leukemia in Infants: Biology and Treatment. Front Pediatr. 2015;3. doi: 10.3389/fped.2015.00037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kitagawa R, Katsumata N, Shibata T, et al. Paclitaxel Plus Carboplatin Versus Paclitaxel Plus Cisplatin in Metastatic or Recurrent Cervical Cancer: The Open-Label Randomized Phase III Trial JCOG0505. J Clin Oncol 2015;33(19):2129–2135. doi: 10.1200/JCO.2014.58.4391 [DOI] [PubMed] [Google Scholar]
- 30.Johnson DB, Sullivan RJ, Menzies AM. Immune checkpoint inhibitors in challenging populations. Cancer. 2017;123(11):1904–1911. doi: 10.1002/cncr.30642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carocha A, Pedroso C, Correia L, Gomes A, Jorge A. Glassy Cell Carcinoma of the Cervix and Metastasis in Episiotomy Scar: A Case Report. J Low Genit Tract Dis. 2015;19(2). doi: 10.1097/LGT.0000000000000054 [DOI] [PubMed] [Google Scholar]
- 32.Sood A, Sorosky J, Mayr N, Anderson B, Buller R, Niebyl J. Cervical cancer diagnosed shortly after pregnancy: Prognostic variables and delivery routes. Obstet Gynecol. 2000;95:832–838. doi: 10.1016/S0029-7844(00)00789-4 [DOI] [PubMed] [Google Scholar]
- 33.Goh W, Bohrer J, Zalud I. Management of the adnexal mass in pregnancy. Curr Opin Obstet Gynecol. 2014;26(2):49–53. doi: 10.1097/GCO.0000000000000048 [DOI] [PubMed] [Google Scholar]
- 34.Bonde AA, Korngold EK, Foster BR, et al. Radiological appearances of corpus luteum cysts and their imaging mimics. Abdom Radiol. 2016;41(11):2270–2282. doi: 10.1007/s00261-016-0780-1 [DOI] [PubMed] [Google Scholar]
- 35.Whitecar P, Turner S, Higby K. Adnexal masses in pregnancy: A review of 130 cases undergoing surgical management. Am J Obstet Gynecol. 1999;181(1):19–24. doi: 10.1016/S0002-9378(99)70429-1 [DOI] [PubMed] [Google Scholar]
- 36.Webb K, Sakhel K, Chauhan S, Abuhamad A. Adnexal Mass during Pregnancy: A Review. Am J Perinatol. 2015;32(11):1010–1016. doi: 10.1055/s-0035-1549216 [DOI] [PubMed] [Google Scholar]
- 37.Liu Y-X, Zhang Y, Huang J-F, Wang L. Meta-analysis comparing the safety of laparoscopic and open surgical approaches for suspected adnexal mass during the second trimester. Int J Gynecol Obstet. 2017;136(3):272–279. doi: 10.1002/ijgo.12069 [DOI] [PubMed] [Google Scholar]
- 38.Practice Bulletin No. 174: Evaluation and Management of Adnexal Masses. Obstet Gynecol. 2016;128(5). doi: 10.1097/AOG.0000000000001768 [DOI] [PubMed] [Google Scholar]
- 39.Sherard GB, Hodson CA, Williams HJ, Semer DA, Hadi HA, Tait DL. Adnexal masses and pregnancy: a 12-year experience. Am J Obstet Gynecol. 2003;189(2):358–362. doi: 10.1067/S0002-9378(03)00731-2 [DOI] [PubMed] [Google Scholar]
- 40.Perera D, Prabhakar H. Imaging of the Adnexal Mass. Clin Obstet Gynecol 2015;58(1):28–46. doi: 10.1097/GRF.0000000000000083 [DOI] [PubMed] [Google Scholar]
- 41.Bailleux M, Bernard JP, Benachi A, Deffieux X. Ovarian endometriosis during pregnancy: a series of 53 endometriomas. Eur J Obstet Gynecol Reprod Biol. 2017;209:100–104. doi: 10.1016/j.ejogrb.2015.09.037 [DOI] [PubMed] [Google Scholar]
- 42.Burandt E, Young R. Pregnancy Luteoma: A Study of 20 Cases on the Occasion of the 50th Anniversary of its Description by Dr William H. Sternberg, With an Emphasis on the Common Presence of Follicle-like Spaces and Their Diagnostic Implications. Am J Surg Pathol. 2014;38(2):239–244. doi: 10.1097/PAS.0000000000000100 [DOI] [PubMed] [Google Scholar]
- 43.Ugaki H, Enomoto T, Tokugawa Y, Kimura T. Luteoma-induced fetal virilization. J Obstet Gynaecol Res. 2009;35(5):991–993. doi: 10.1111/j.1447-0756.2009.01046.x [DOI] [PubMed] [Google Scholar]
- 44.Masarie K, Katz V, Balderston K. Pregnancy luteomas: clinical presentations and management strategies. Obstet Gynecol Surv. 2010;65(9):575–582. doi: 10.1097/OGX.0b013e3181f8c41d [DOI] [PubMed] [Google Scholar]
- 45.Prat J Staging classification for cancer of the ovary, fallopian tube, and peritoneum. Int J Gynecol Obstet 2014;124(1):1–5. doi: 10.1016/j.ijgo.2013.10.001 [DOI] [PubMed] [Google Scholar]
- 46.Leiserowitz GS, Xing G, Cress R, Brahmbhatt B, Dalrymple JL, Smith LH. Adnexal masses in pregnancy: How often are they malignant? Gynecol Oncol. 2006;101(2):315–321. doi: 10.1016/j.ygyno.2005.10.022 [DOI] [PubMed] [Google Scholar]
- 47.Kodama M, Grubbs BH, Blake EA, et al. Feto-maternal outcomes of pregnancy complicated by ovarian malignant germ cell tumor: a systematic review of literature. Eur J Obstet Gynecol Reprod Biol. 2014;181:145–156. doi: 10.1016/j.ejogrb.2014.07.047 [DOI] [PubMed] [Google Scholar]
- 48.Horbelt D, Delmore J, Meisel R, Cho S, Roberts D, Logan D. Mixed germ cell malignancy of the ovary concurrent with pregnancy. Obstet Gynecol. 1994;84(4 Pt 2):662–664. [PubMed] [Google Scholar]
- 49.Sheiko MC, Hart WR. Ovarian germinoma (dysgerminoma) with elevated serum lactic dehydrogenase: case report and review of literature. Cancer. 1982;49(5):994–998. doi: [DOI] [PubMed] [Google Scholar]
- 50.Ray-Coquard I, Morice P, Lorusso D, et al. Non-epithelial ovarian cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol Off J Eur Soc Med Oncol 2018;29(Suppl 4):iv1–iv18. doi: 10.1093/annonc/mdy001 [DOI] [PubMed] [Google Scholar]
- 51.Fonseca A, Frazier AL, Shaikh F. Germ Cell Tumors in Adolescents and Young Adults. J Oncol Pract. August 2019. doi: 10.1200/JOP.19.00190 [DOI] [PubMed] [Google Scholar]
- 52.Elit L, Bocking A, Kenyon C, Natale R. An Endodermal Sinus Tumor Diagnosed in Pregnancy: Case Report and Review of the Literature. Gynecol Oncol. 1999;72(1):123–127. doi: 10.1006/gyno.1998.5190 [DOI] [PubMed] [Google Scholar]
- 53.Han J-Y, Nava-Ocampo AA, Kim T-J, Shim J-U, Park C-T. Pregnancy outcome after prenatal exposure to bleomycin, etoposide and cisplatin for malignant ovarian germ cell tumors: report of 2 cases. Reprod Toxicol Elmsford N. 2005;19(4):557–561. doi: 10.1016/j.reprotox.2004.11.002 [DOI] [PubMed] [Google Scholar]
- 54.Torre LA, Trabert B, DeSantis CE, et al. Ovarian cancer statistics, 2018. CA Cancer J Clin 2018;68(4):284–296. doi: 10.3322/caac.21456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Young RH, Dudley AG, Scully RE. Granulosa cell, Sertoli-Leydig cell, and unclassified sex cord-stromal tumors associated with pregnancy: a clinicopathological analysis of thirty-six cases. Gynecol Oncol. 1984;18(2):181–205. doi: 10.1016/0090-8258(84)90026-x [DOI] [PubMed] [Google Scholar]
- 56.Mom CH, Engelen MJA, Willemse PHB, et al. Granulosa cell tumors of the ovary: The clinical value of serum inhibin A and B levels in a large single center cohort. Gynecol Oncol. 2007;105(2):365–372. doi: 10.1016/j.ygyno.2006.12.034 [DOI] [PubMed] [Google Scholar]
- 57.Silver HM, Lambert-Messerlian GM, Star JA, Hogan J, Canick JA. Comparison of maternal serum total activin A and inhibin A in normal, preeclamptic, and nonproteinuric gestationally hypertensive pregnancies. Am J Obstet Gynecol. 1999;180(5):1131–1137. doi: 10.1016/S0002-9378(99)70606-X [DOI] [PubMed] [Google Scholar]
- 58.Blake EA, Carter CM, Kashani BN, et al. Feto-maternal outcomes of pregnancy complicated by ovarian sex-cord stromal tumor: a systematic review of literature. Eur J Obstet Gynecol Reprod Biol. 2014;175:1–7. doi: 10.1016/j.ejogrb.2013.12.025 [DOI] [PubMed] [Google Scholar]
- 59.Mooney J, Silva E, Tornos C, Gershenson D. Unusual Features of Serous Neoplasms of Low Malignant Potential during Pregnancy. Gynecol Oncol. 1997;65(1):30–35. doi: 10.1006/gyno.1996.4592 [DOI] [PubMed] [Google Scholar]
- 60.Blake EA, Kodama M, Yunokawa M, et al. Feto-maternal outcomes of pregnancy complicated by epithelial ovarian cancer: a systematic review of literature. Eur J Obstet Gynecol Reprod Biol. 2015;186:97–105. doi: 10.1016/j.ejogrb.2015.01.010 [DOI] [PubMed] [Google Scholar]
- 61.Han SN, Lotgerink A, Gziri MM, Van Calsteren K, Hanssens M, Amant F. Physiologic variations of serum tumor markers in gynecological malignancies during pregnancy: a systematic review. BMC Med. 2012;10(1):86. doi: 10.1186/1741-7015-10-86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ercan Ş, Kaymaz Ö, Yücel N, Orçun A. Serum concentrations of CA 125, CA 15–3, CA 199 and CEA in normal pregnancy: a longitudinal study. Arch Gynecol Obstet. 2012;285(3):579–584. doi: 10.1007/s00404-011-2025-4 [DOI] [PubMed] [Google Scholar]
- 63.Randall LM, Pothuri B, Swisher EM, et al. Multi-disciplinary summit on genetics services for women with gynecologic cancers: A Society of Gynecologic Oncology White Paper. Gynecol Oncol. 2017;146(2):217–224. doi: 10.1016/j.ygyno.2017.06.002 [DOI] [PubMed] [Google Scholar]
- 64.Ferrandina G, Distefano M, Testa A, De Vincenzo R, Scambia G. Management of an advanced ovarian cancer at 15 weeks of gestation: Case report and literature review. Gynecol Oncol. 2005;97(2):693–696. doi: 10.1016/j.ygyno.2005.02.011 [DOI] [PubMed] [Google Scholar]
- 65.Melamed A, Rizzo AE, Nitecki R, et al. All-Cause Mortality After Fertility-Sparing Surgery for Stage I Epithelial Ovarian Cancer. Obstet Gynecol. 2017;130(1):71–79. doi: 10.1097/AOG.0000000000002102 [DOI] [PubMed] [Google Scholar]
- 66.Watanabe T, Soeda S, Nishiyama H, et al. Clinical and reproductive outcomes of fertility-sparing surgery in stage I epithelial ovarian cancer. Mol Clin Oncol. 2020;12(1):44–50. doi: 10.3892/mco.2019.1954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nasioudis D, Chapman-Davis E, Frey MK, Witkin SS, Holcomb K. Could fertility-sparing surgery be considered for women with early stage ovarian clear cell carcinoma? J Gynecol Oncol. 2017;28(6):e71. doi: 10.3802/jgo.2017.28.e71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wright AA, Bohlke K, Armstrong DK, et al. Neoadjuvant Chemotherapy for Newly Diagnosed, Advanced Ovarian Cancer: Society of Gynecologic Oncology and American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2016;34(28):3460–3473. doi: 10.1200/JCO.2016.68.6907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zheng X, Zhu Y, Zhao Y, Feng S, Zheng C. Taxanes in combination with platinum derivatives for the treatment of ovarian cancer during pregnancy: A literature review. Int J Clin Pharmacol Ther. 2017;55(9):753–760. doi: 10.5414/CP202995 [DOI] [PubMed] [Google Scholar]
- 70.Pauli SA, Tang H, Wang J, et al. The Vascular Endothelial Growth Factor (VEGF)/VEGF Receptor 2 Pathway Is Critical for Blood Vessel Survival in Corpora Lutea of Pregnancy in the Rodent. Endocrinology. 2005;146(3):1301–1311. doi: 10.1210/en.2004-0765 [DOI] [PubMed] [Google Scholar]
- 71.Cross SN, Ratner E, Rutherford TJ, Schwartz PE, Norwitz ER. Bevacizumab-Mediated Interference With VEGF Signaling Is Sufficient to Induce a Preeclampsia-Like Syndrome in Nonpregnant Women. Rev Obstet Gynecol. 2012;5(1):2–8. [PMC free article] [PubMed] [Google Scholar]
- 72.Joshi A, Mahfooz S, Maurya VK, et al. PARP1 during embryo implantation and its upregulation by oestradiol in mice. Reproduction. 2014;147(6):765–780. doi: 10.1530/REP-13-0588 [DOI] [PubMed] [Google Scholar]
- 73.Crocker IP, Kenny LC, Thornton WA, Szabo C, Baker PN. Excessive stimulation of poly(ADP-ribosyl)ation contributes to endothelial dysfunction in pre-eclampsia. Br J Pharmacol. 2005;144(6):772–780. doi: 10.1038/sj.bjp.0706055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Laughlin S, Baird D, Savitz D, Herring A, Hartmann K. Prevalence of Uterine Leiomyomas in the First Trimester of Pregnancy: An Ultrasound-Screening Study. Obstet Gynecol 2009;113(3):630–635. doi: 10.1097/AOG.0b013e318197bbaf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Van den Bosch T, Coosemans A, Morina M, Timmerman D, Amant F. Screening for uterine tumours. Best Pract Res Clin Obstet Gynaecol. 2012;26(2):257–266. doi: 10.1016/j.bpobgyn.2011.08.002 [DOI] [PubMed] [Google Scholar]
- 76.Matsuo K, Eno ML, Im DD, Rosenshein NB. Pregnancy and Genital Sarcoma: A Systematic Review of the Literature. Am J Perinatol. 2009;26(7):507–518. doi: 10.1055/s0029-1215428 [DOI] [PubMed] [Google Scholar]
- 77.Suzuki M, Matsunobu A, Wakita K, Nishijima M, Osanai K. Hydatidiform mole with a surviving coexisting fetus. Obstet Gynecol 1980;56(3):384–388. [PubMed] [Google Scholar]
- 78.Vaisbuch E, Ben-Arie A, Dgani R, Perlman S, Sokolovsky N, Hagay Z. Twin pregnancy consisting of a complete hydatidiform mole and co-existent fetus: Report of two cases and review of literature. Gynecol Oncol. 2005;98(1):19–23. doi: 10.1016/j.ygyno.2005.02.002 [DOI] [PubMed] [Google Scholar]
- 79.Bristow RE, Shumway JB, Khouzami AN, Witter FR. Complete hydatidiform mole and surviving coexistent twin. Obstet Gynecol Surv. 1996;51(12):705–709. doi: 10.1097/00006254-199612000-00002 [DOI] [PubMed] [Google Scholar]
- 80.Matsui H, Sekiya S, Hando T, Wake N, Tomoda Y. Hydatidiform mole coexistent with a twin live fetus: a national collaborative study in Japan. Hum Reprod. 2000;15(3):608–611. doi: 10.1093/humrep/15.3.608 [DOI] [PubMed] [Google Scholar]
- 81.Braunstein GD, Rasor J, Adler D, Danzer H, Wade ME. Serum human chorionic gonadotropin levels throughout normal pregnancy. Am J Obstet Gynecol. 1976;126(6):678681. doi: 10.1016/0002-9378(76)90518-4 [DOI] [PubMed] [Google Scholar]
- 82.Shiomi M, Matsuzaki S, Kobayashi E, et al. Endometrial carcinoma in a gravid uterus: a case report and literature review. BMC Pregnancy Childbirth. 2019;19(1):425. doi: 10.1186/s12884-019-2489-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Amant F, Berveiller P, Boere IA, et al. Gynecologic cancers in pregnancy: guidelines based on a third international consensus meeting. Ann Oncol Off J Eur Soc Med Oncol. 2019;30(10):1601–1612. doi: 10.1093/annonc/mdz228 [DOI] [PubMed] [Google Scholar]
- 84.Research C for DE and. FDA Drug Safety Communication: FDA review results in new warnings about using general anesthetics and sedation drugs in young children and pregnant women. FDA. June 2019. http://www.fda.gov/drugs/drug-safety-and-availability/fda-drugsafety-communication-fda-review-results-new-warnings-about-using-general-anestheticsand. Accessed November 25, 2019.
- 85.Creeley CE, Dikranian KT, Dissen GA, Back SA, Olney JW, Brambrink AM. Isoflurane-induced apoptosis of neurons and oligodendrocytes in the fetal rhesus macaque brain. Anesthesiology. 2014;120(3):626–638. doi: 10.1097/ALN.0000000000000037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nonobstetric Surgery During Pregnancy - ACOG. https://www.acog.org/Clinical-Guidance-and-Publications/Committee-Opinions/Committee-on-Obstetric-Practice/Nonobstetric-Surgery-During-Pregnancy?IsMobileSet=false. Accessed December 9, 2019.
- 87.Reedy MB, Källén B, Kuehl TJ. Laparoscopy during pregnancy: A study of five fetal outcome parameters with use of the Swedish Health Registry. Am J Obstet Gynecol. 1997;177(3):673–679. doi: 10.1016/S0002-9378(97)70163-7 [DOI] [PubMed] [Google Scholar]
- 88.Jackson H, Granger S, Price R, et al. Diagnosis and laparoscopic treatment of surgical diseases during pregnancy: an evidence-based review. Surg Endosc 2008;22(9):1917–1927. doi: 10.1007/s00464-008-9989-6 [DOI] [PubMed] [Google Scholar]
- 89.Berkman ND, Thorp JM, Lohr KN, et al. Tocolytic treatment for the management of preterm labor: A review of the evidence. Am J Obstet Gynecol. 2003;188(6):1648–1659. doi: 10.1067/mob.2003.356 [DOI] [PubMed] [Google Scholar]
- 90.Tremblay E, Thérasse E, Thomassin-Naggara I, Trop I. Quality Initiatives: Guidelines for Use of Medical Imaging during Pregnancy and Lactation. RadioGraphics. 2012;32(3):897–911. doi: 10.1148/rg.323115120 [DOI] [PubMed] [Google Scholar]
- 91.Ray JG, Vermeulen MJ, Bharatha A, Montanera WJ, Park AL. Association Between MRI Exposure During Pregnancy and Fetal and Childhood Outcomes. JAMA. 2016;316(9):952–961. doi: 10.1001/jama.2016.12126 [DOI] [PubMed] [Google Scholar]
- 92.Colletti PM. PET-CT in the pregnant patient. November 2012. http://www.imagewisely.org/imaging-modalities/nuclear-medicine/articles/pregnantpatient. Accessed December 1, 2019.
- 93.Esposito S, Tenconi R, Preti V, Groppali E, Principi N. Chemotherapy against cancer during pregnancy: A systematic review on neonatal outcomes. Medicine (Baltimore). 2016;95(38). doi: 10.1097/MD.0000000000004899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zemlickis D, Lishner M, Degendorfer P, Panzarella T, Sutcliffe SB, Koren G. Fetal Outcome After In Utero Exposure to Cancer Chemotherapy. Arch Intern Med. 1992;152(3):573–576. doi: 10.1001/archinte.1992.00400150093017 [DOI] [PubMed] [Google Scholar]
- 95.Abdel-Hady E-S, Hemida RA-H, Gamal A, El-Zafarany M, Toson E, El-Bayoumi MA. Cancer during pregnancy: perinatal outcome after in utero exposure to chemotherapy. Arch Gynecol Obstet. 2012;286(2):283–286. doi: 10.1007/s00404-012-2287-5 [DOI] [PubMed] [Google Scholar]
- 96.Cardonick E, Usmani A, Ghaffar S. Perinatal outcomes of a pregnancy complicated by cancer, including neonatal follow-up after in utero exposure to chemotherapy: results of an international registry. Am J Clin Oncol. 2010;33(3):221–228. doi: 10.1097/COC.0b013e3181a44ca9 [DOI] [PubMed] [Google Scholar]
- 97.Cate FEAU ten, Hove CH ten, Nix WMLE, Vries JIP de, Loosdrecht AA van de, Elburg RM van. Transient Neonatal Myelosuppression after Fetal Exposure to Maternal Chemotherapy. Neonatology. 2009;95(1):80–85. doi: 10.1159/000151759 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.