Abstract
Background and Aims
Dutch tomato cultivars tend to have a greater yield than Japanese cultivars even if they are grown under the same conditions. Factors contributing to the increased yield of the Dutch cultivars were a greater light use efficiency and greater leaf photosynthetic rate. On the other hand, the relationship between tomato yields and anatomical traits is still unclear. The aim of this study is to identify the anatomical traits related to the difference in yield between Dutch and Japanese cultivars.
Methods
Anatomical properties were compared during different growth stages of Dutch and Japanese tomatoes. Hormone profiles and related gene expression in hypocotyls of Dutch and Japanese cultivars were compared in the hypocotyls of 3- and 4-week-old plants.
Key results
Dutch cultivars have a more developed secondary xylem than Japanese cultivars, which would allow for greater transport of water, mineral nutrients and phytohormones to the shoots. The areas and ratios of the xylem in the hypocotyls of 3- to 6-week-old plants were larger in the Dutch cultivars. In reciprocal grafts of the Japanese and Dutch cultivars, xylem development at the scion and rootstock depended on the scion cultivar, suggesting that some factors in the scion are responsible for the difference in xylem development. The cytokinin content, especially the level of N6-(Δ 2-isopentenyl) adenine (iP)-type cytokinin, was higher in the Dutch cultivars. This result was supported by the greater expression of Sl-IPT3 (a cytokinin biosynthesis gene) and Sl-RR16/17 (a cytokinin-responsive gene) in the Dutch cultivars.
Conclusions
These results suggest that iP-type cytokinins, which are locally synthesized in the hypocotyl, contribute to xylem development. The greater xylem development in Dutch cultivars might contribute to the high yield of the tomato.
Keywords: Adenosine phosphate-isopentenyltransferase, cytokinin, tomato, vascular tissues, xylem development, yield
INTRODUCTION
Tomatoes have been bred for many different traits, such as yield, fruit size and sugar content. With respect to yield, Higashide and Heuvelink (2009) found that the fresh fruit weights of Dutch cultivars were much greater than those of a Japanese cultivar. Factors contributing to the increased yield of the Dutch cultivars were a greater light use efficiency and greater leaf photosynthetic rate (Higashide and Heuvelink, 2009). The numbers of flowers and fruit, and the fruit set ratio in Japanese tomato were also lower than the values reported in the Dutch cultivars, though there was no significant difference in truss number (Higashide and Heuvelink, 2009). However, it is unclear whether there is a difference in the internal structures of the Japanese and Dutch tomatoes and if a difference in internal structures can explain the difference in tomato yields.
Vascular tissues consist of phloem, xylem and cambium. Phloem transports photosynthetic products and plant growth regulators (phytohormones) mainly from the shoots to the roots, while xylem transports water, mineral nutrients and phytohormones from the roots to the shoots. Xylem also provides mechanical support and stores water and mineral nutrients (Myburg and Sederoff, 2001; Matsumoto-Kitano et al., 2008; White, 2012). Phloem and xylem differentiate from cambium cells in the centrifugal and centripetal directions, respectively. On the xylem side of the cambium, the cells first undergo several stages of differentiation that involve cell division, expansion, maturation, lignification, secondary cell wall thickening and programmed cell death (Chaffey, 1999). The rate of cell production in the cambium is the primary determinant of stem and root thickening, which is important for biomass production (Larson, 1994; Jang et al., 2015).
Cytokinins have diverse functions in plant growth and development (Hwang et al., 2012). Generally, cytokinin signalling is considered to be an important regulator of cambium development (Matsumoto-Kitano et al., 2008; Jang et al., 2015). Cytokinins have one of several types of isoprene-derived side chain, such as N6-(Δ 2-isopentenyl) adenine (iP), trans-zeatin (tZ), cis-zeatin (cZ) and dihydrozeatin (DZ) (Sakakibara, 2006; Kiba et al., 2013). The first step in their biosynthesis is catalysed by adenosine phosphate-isopentenyltransferase (IPT). In Arabidopsis thaliana, knocking out four genes encoding cytokinin biosynthetic IPT by T-DNA insertions adversely affected cambium formation and reduced xylem differentiation in the root and shoot. The differentiation was restored by treating the mutant with exogenous cytokinin (Matsumoto-Kitano et al., 2008). Also in arabidopsis, cytokinin receptor loss-of-function mutants ahk2 and ahk3 (ARABIOPSIS HISTIDINE KINASE 2 and 3) and plants with reduced levels of endogenous cytokinins show defects in procambium proliferation and an absence of secondary growth (Hejátko et al., 2009). In poplar (Populus trichocarpa), overexpressing the cytokinin catabolic gene CYTOKININ OXIDASE 2 decreased the cytokinin concentration and reduced secondary growth (Nieminen et al., 2008). These findings strongly indicate that cytokinins are important regulators of vascular development.
To understand the reasons for the differences in yield between Dutch and Japanese tomatoes, we compared anatomical traits of two Dutch tomato cultivars and two Japanese tomato cultivars, all of which were different from those examined by Higashide and Heuvelink (2009). We found that the areas and ratios of the secondary xylem in the hypocotyls and the stems of young (e.g. 5-, 6- or 10-week-old) plants were larger in the Dutch cultivars. We then further compared the cultivars with respect to (1) the development of secondary vascular tissues in the hypocotyls, epicotyls and stems; (2) cytokinin levels in the hypocotyls; and (3) the expression of cytokinin-related genes. Our results suggest that cytokinins are important factors in the difference in xylem development between Dutch and Japanese cultivars.
MATERIALS AND METHODS
Plant materials and growth conditions
The tomato (Solanum lycopersicum L.) seeds used in this study were from F1 hybrid lines of the Dutch cultivars ‘Managua’ (MNG; Rijk Zwaan, de Lier, The Netherlands) and ‘Endeavor’ (ENV; Rijk Zwaan), and the Japanese cultivars ‘CF Momotaro York’ (MY; Takii Seeds, Kyoto, Japan) and ‘Reiyo’ (RYO; Sakata seed, Yokohama, Japan). The seeds were soaked in distilled water at 4 °C under dark conditions for 24 h. Water-swollen seeds were germinated in plugs filled with vermiculite. The plugs were covered with a plastic film and black cloth. The seeds were grown at 25 °C under a 14 h light/10 h dark photoperiod. The plastic film and black cloth were removed when the seeds germinated. A commercial nutrient solution (Otsuka-A; Otsuka AgriTechno, Tokyo, Japan) was diluted 20 times and was added to each plug every day after germination. Two-week-old seedlings were transplanted into rockwool squares (8 × 8 cm; Grodan BV, Roermond, The Netherlands).
Sectioning
Samples were cut 0.5 cm from the base of the hypocotyl and fixed with FAA fixative solution [5 % (v/v) formalin, 5 % acetic acid and 63 % ethanol]. Transverse sections (120 μm) were cut with a Plant Microtome MTH-1 (Nippon Medical & Chemical Instruments Co., Ltd, Osaka, Japan) according to the manufacturer’s instructions, and stained for xylem with 0.05 % toluidine blue O solution. Images were taken with a light microscope (DM5000 B; Leica Microsystems, Wetzlar, Germany) with a CCD camera (DFC310 FX; Leica Microsystems). The areas of the xylem and hypocotyl (or stem) were measured using ImageJ software (Version 1.49p). The ratio of xylem area was calculated by dividing the xylem area by the whole section area. To count the number of phloem vessels, sections from 4-week-old plants were stained with 0.5 % aniline blue solution, a fluorescent dye that binds to callose, which is present in the sieve plates of phloem vessels. Stained sections were viewed under a fluorescence microscope using UV light (DM5000; UV filter: excitation filter, Ex 360/40; dichroic mirror, DM-400; suppression filter, BP 470/40) with a CCD camera (DFC310 FX; Leica Microsystems).
Grafting
Using hypocotyls of 17-day-old plants, the rootstock of one cultivar was grafted to the scion of another cultivar. Grafted plants were incubated under high humidity for 7 d and then cultured for another 11 d under normal conditions (a total of 18 d after grafting). An approx. 5 mm section of the stem immediately above the grafting point was used for making sections. The rest of the scion was immediately weighed with a balance, transferred to an oven for 7 d and weighed again to obtain the dry weight.
Hormone measurement
Hypocotyls were cut 1.5 cm from the base, weighed, frozen in liquid nitrogen and transferred to a freeze drier (Freezone 1; Labconco Corp., Kansas City, MO, USA) for 48 h. Cytokinins, auxin [indole-3-acetic acid (IAA)] and abscisic acid (ABA) were extracted and identified as described previously (Kojima et al., 2009; Shinozaki et al., 2015).
Exogenous cytokinin application
Tomato seedlings were treated with cytokinins by spraying and immersion. After 2 weeks of growth, MY plants were sprayed with 10 μm 6-benzylaminopurine (6-BA) (Wako Pure Chemical Industries, Ltd, Japan) or 0.1 % dimethylsulfoxide (DMSO) solution (mock) every 2 d until the 3 week stage, and then used for RNA extraction. Three-week-old seedlings were immersed in a solution of 3 μg mL–1 kinetin, 100 μm 6-BA or 0.1 % DMSO solution (mock) for 1 h and allowed to stand for another 4 h. The hypocotyl was cut at 1.5 cm from the base and the lower part was used for RNA extraction.
RNA extraction and quantitative reverse transcription–PCR (qRT–PCR)
Total RNA was extracted from frozen tissues of hypocotyls at different growing stages using an RNeasy Plant Mini Kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s protocols. For qRT–PCR, 10 ng of the RNA was used as a template. Transcript levels were measured using a One Step SYBR Prime Script RT-PCR Kit II (Takara Bio Inc., Otsu, Japan) and a StepOnePlus Real-Time PCR System (Applied Biosystems, Foster City, CA, USA) according to the manufacturer’s protocols. A PCR fragment of each gene was purified and quantified, and then was used to draw standard curves for absolute quantification. Total RNA from four biological replicates was pooled for use as the qRT–PCR template. Primer sequences are shown in Supplementary data Table S1.
RESULTS
Difference in xylem development between Dutch and Japanese tomato cultivars
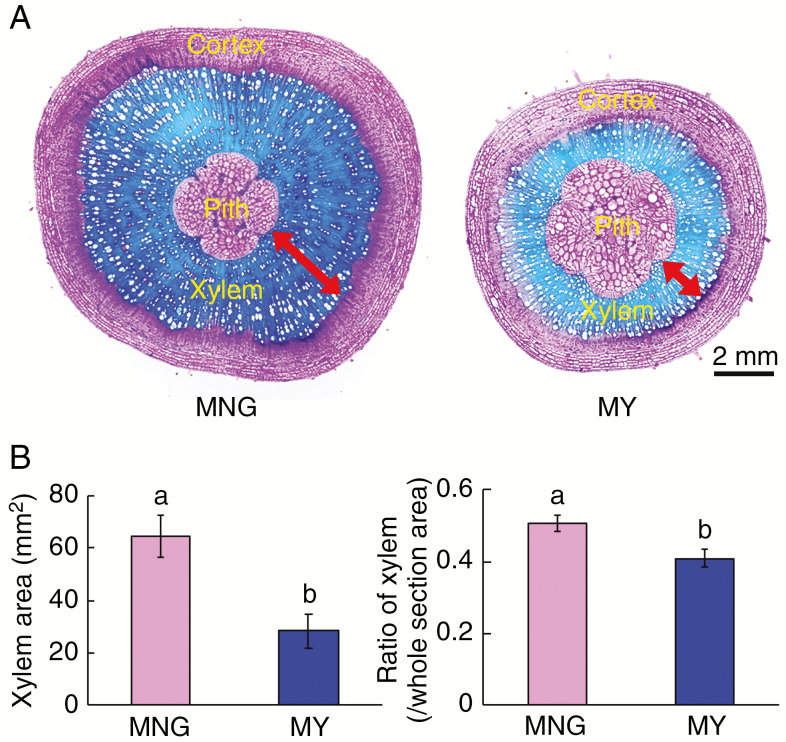
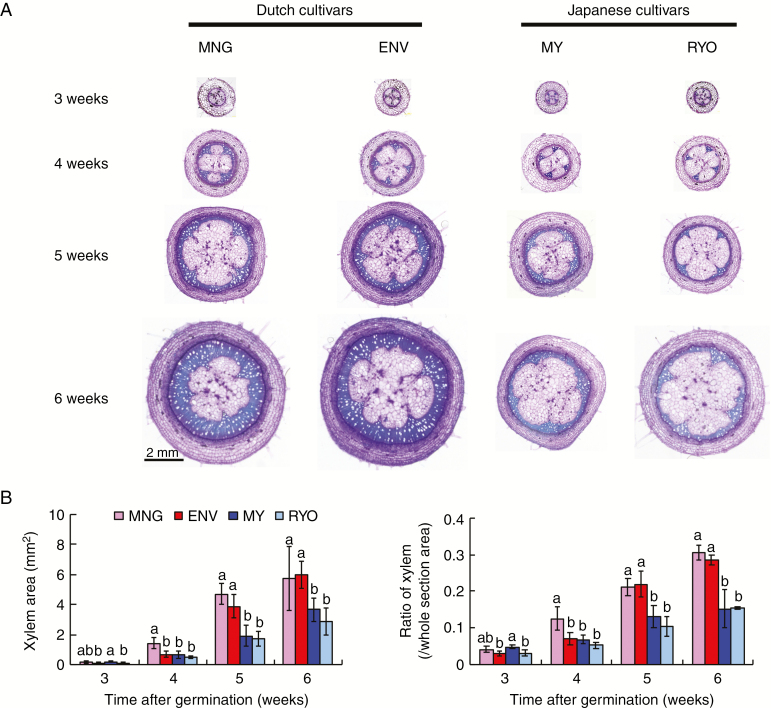
We compared cross-sections of hypocotyls and stems of 10-week-old plants of the Dutch cultivar MNG and the Japanese cultivar MY. The secondary xylem areas and their ratios in the hypocotyl were larger in MNG than in MY (Fig. 1). To confirm this observation, we compared xylem development in the hypocotyls of 3-, 4-, 5- and 6-week-old plants of MNG and another Dutch cultivar ENV, and of MY and another Japanese cultivar RYO. Xylem areas and their ratios were not different between the Dutch and Japanese cultivars 3 weeks after germination (Fig. 2). However, the area and ratio of xylem in MNG were larger than those of other cultivars at 4 weeks after germination. Xylem area was significantly larger in the Dutch cultivars (MNG and ENV) 5 weeks after germination. The xylem ratio was significantly larger in the Dutch cultivars (MNG and ENV) than in Japanese cultivars (MY and RYO) 5 and 6 weeks after germination (Fig. 2). In addition, the number of phloem vessels and the amount of shoot growth were not different between the Dutch and Japanese cultivars (Supplementary data Fig. S1). These findings suggest that secondary xylem development in the hypocotyls is slower and less extensive in the Japanese cultivars.
Fig. 1.
Comparison of xylem development at the base of 10-week-old Dutch (MNG) and Japanese (MY) tomato hypocotyls. (A) Cross-sections. (B) Xylem areas. (C) Ratio of xylem. Xylem area is indicated by red arrows. Values are means (n ≥ 5) ± s.d. Different lower case letters indicate a significant difference among all the conditions (P < 0.05, one-way ANOVA and then LSD test for multiple comparisons).
Fig. 2.
Xylem formation in hypocotyl of Dutch and Japanese tomato seedlings during 6 weeks. (A) Cross-sections at the base of hypocotyls of Managua (MNG), Endeavor (ENV), CF Momotaro York (MY) and Reiyo (RYO) from the 3- to 6-week-old stage. Scale bar = 2 mm. (B) Xylem area and ratio at the base hypocotyls of 3- to 6-week-old plants. Values are means (n ≥ 5) ± s.d. Dutch tomatoes are MNG and ENV; Japanese tomatoes are MY and RYO. Different lower case letters indicate a significant difference among all the conditions (P < 0.05, one-way ANOVA and then LSD test for multiple comparisons).
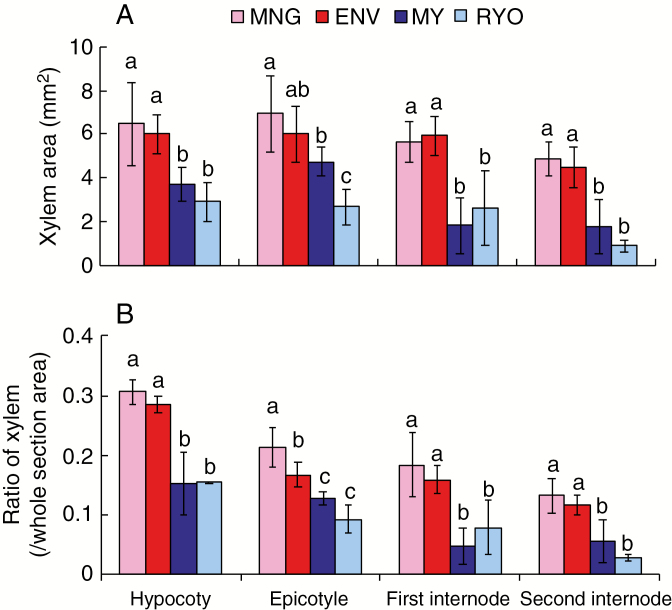
In 6-week-old plants, the Dutch cultivars also had larger xylem areas (Fig. 3A) and xylem ratios (Fig. 3B) at the epicotyl and first and second internodes than the Japanese cultivars (Fig. 3B). In all cultivars, the xylem ratio decreased with increasing stem position (Fig. 3B).
Fig. 3.
Xylem development in cross-sections at different positions along the stem of 6-week-old Dutch and Japanese tomato. (A) Xylem area. (B) Xylem ratio. Values are means (n ≥ 5) ± s.d. Different lower case letters indicate a significant difference among all the conditions (P < 0.05, one-way ANOVA and then LSD test for multiple comparisons).
Effect of reciprocal grafting on xylem development
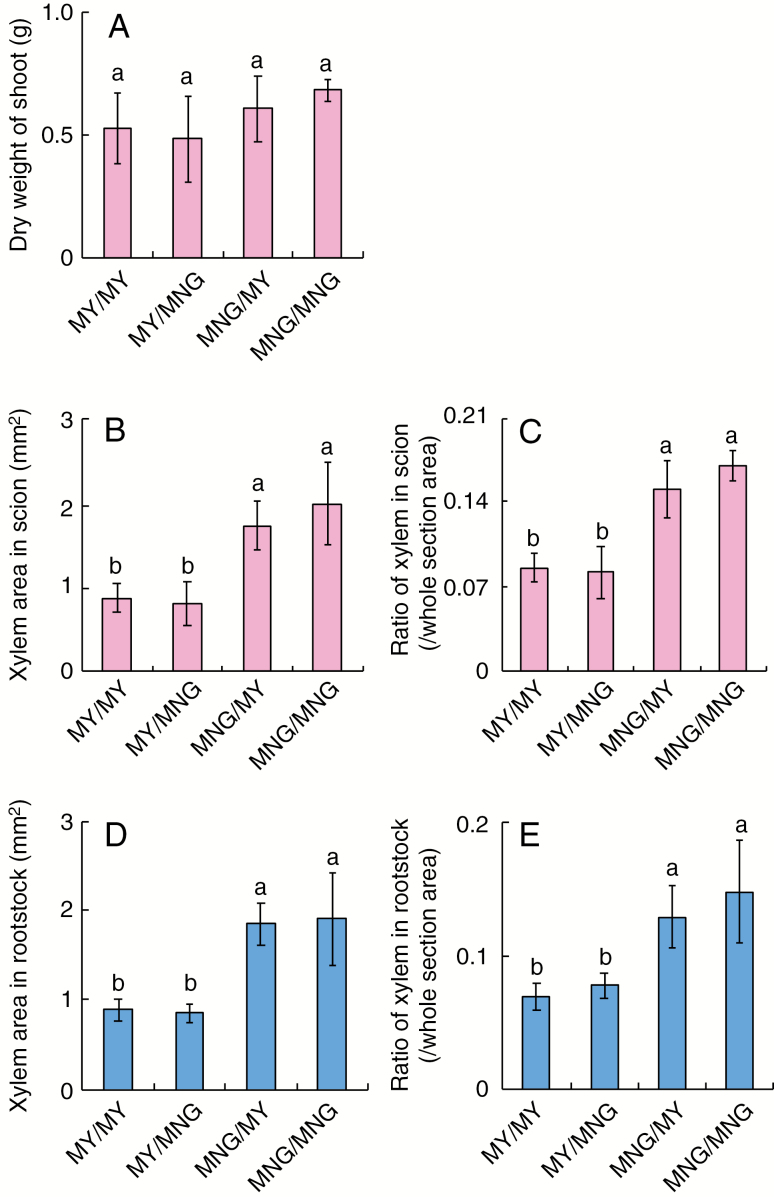
In the following, grafts are denoted as S/R, where S is the scion and R is the rootstock. MNG/MNG and MY/MY were used as controls. The shoot growths were similar among the grafted plants (Fig. 4A). Xylem areas (Fig. 4B) and xylem ratios (Fig. 4C) in the hypocotyl above the graft union were significantly larger in MNG/MY and MNG/MNG than in MY/MNG and MY/MY. In addition, xylem areas (Fig. 4D) and xylem ratios (Fig. 4E) in the hypocotyl below the graft union were significantly larger in MNG/MY and MNG/MNG than in MY/MNG and MY/MY, suggesting that development of the secondary xylem of both scion and rootstock is affected by the genotype of the scion. Three other grafting combinations between RYO and MNG, RYO and ENV, and MY and ENV also showed that the genotype of the scion affected the secondary xylem development of both scion and root stock (Supplementary data Figs S2 and S3). Taken together, these results suggest that the shoots determine xylem development in the scion and rootstock during the early stage of tomato development.
Fig. 4.
Effects of grafting between MNG and MY on the xylem development and shoot growth. The cultivars listed before and after the slash correspond to the scion and stock, respectively. Dry weight of shoot (A), xylem areas (B) and ratios of xylem (C) in the scion of grafted plants between MNG and MY. Xylem areas (D) and ratios of xylem (E) in rootstock of grafted plants between MNG and MY. Values are means (n ≥ 5) ± s.d. Different lower case letters indicate a significant difference among all the conditions (P < 0.05, one-way ANOVA and then LSD test for multiple comparisons).
Hypocotyls of Dutch tomato cultivars contain greater levels of iP-type cytokinin
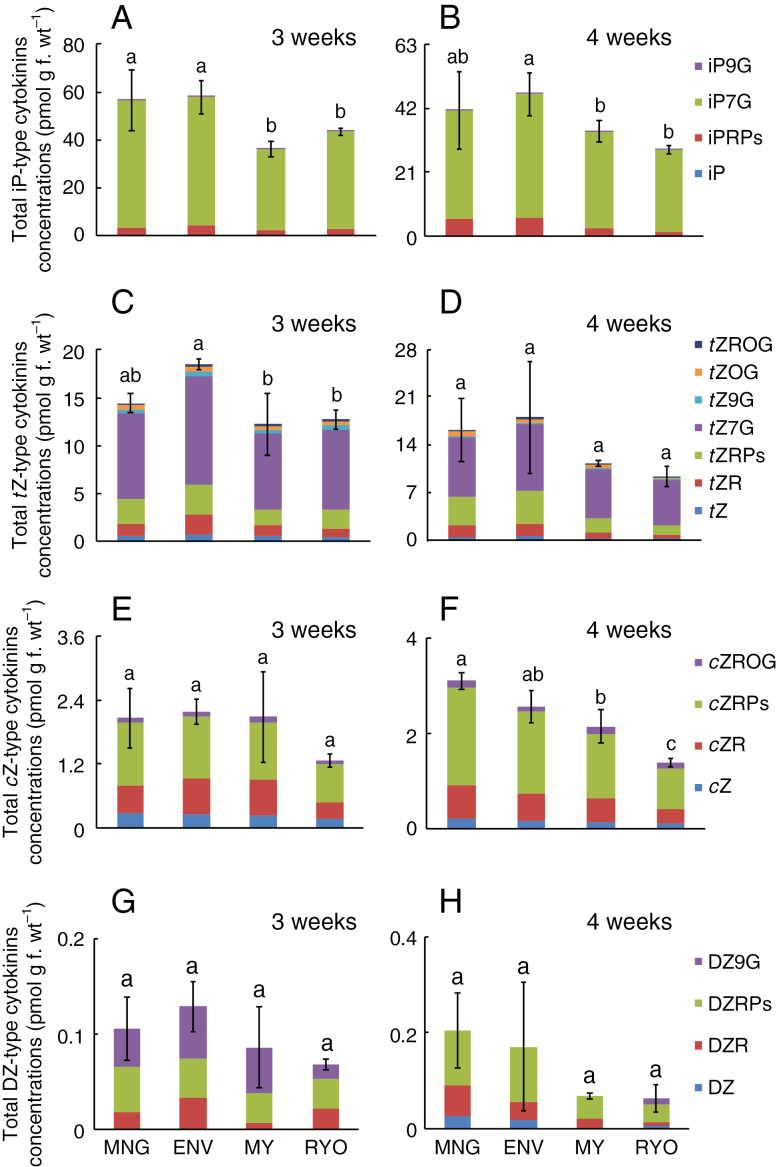
In hypocotyls of 3-week-old plants, the total concentration of DZ-type cytokinins (about 0.1 pmol g f. wt–1 in each cultivar) and the total concentration of cZ-type cytokinins (about 3 pmol g f. wt–1 in each cultivar) were much lower than those for iP and tZ cytokinins (about 50 and 15 pmol g f. wt–1, respectively) (Fig. 5). Similar results were obtained in hypocotyls of 4-week-old plants. The most abundant cytokinins in all four cultivars were iP-type cytokinins. In hypocotyls of 3-week-old plants, iP-type cytokinins were significantly more abundant in the Dutch cultivars than in the Japanese cultivars (Fig. 5A), but this was not the case in 4-week-old plants (Fig. 5B). iP7G, which accounted for >86 % of the iP-type cytokinins, was significantly more abundant in the Dutch cultivars than in the Japanese cultivars (Fig. 5A, B; Supplementary data Table S2). However, the concentrations of the other types of cytokinins (tZ, DZ and cZ) were similar between Dutch and Japanese cultivars (Fig. 5C–H; Supplementary data Tables S3–S5).
Fig. 5.
Cytokinin levels in hypocotyls of 3- and 4-week-old Dutch cultivars MNG and ENV and Japanese cultivars MY and RYO. Dutch and Japanese tomato cultivars were grown for 3–4 weeks, and hypocotyls were harvested for plant hormone analysis. iP-type (A, B), tZ-type (C, D), cZ-type (E, F) and DZ-type cytokinin (G, H) concentrations in 3- and 4-week-old tomatoes, respectively. Values are means (n ≥ 3) ± s.d. Different lower case letters indicate a significant difference among all the conditions (P < 0.05, one-way ANOVA and then LSD test for multiple comparisons).
The concentrations of salicylic acid (SA) and ABA were not significantly different between the Dutch and Japanese cultivars (Supplementary data Fig. S4). IAA concentrations were significantly greater in ENV than in RYO at 4 weeks, but not in other stages or cultivars (Supplementary data Fig. S4). The gibberellins GA44 and GA53 tended to be lower in the Japanese cultivars, while other GAs were not significantly different among the four cultivars (Supplementary data Fig. S4).
Expression of cytokinin biosynthesis genes and cytokinin-responsive genes
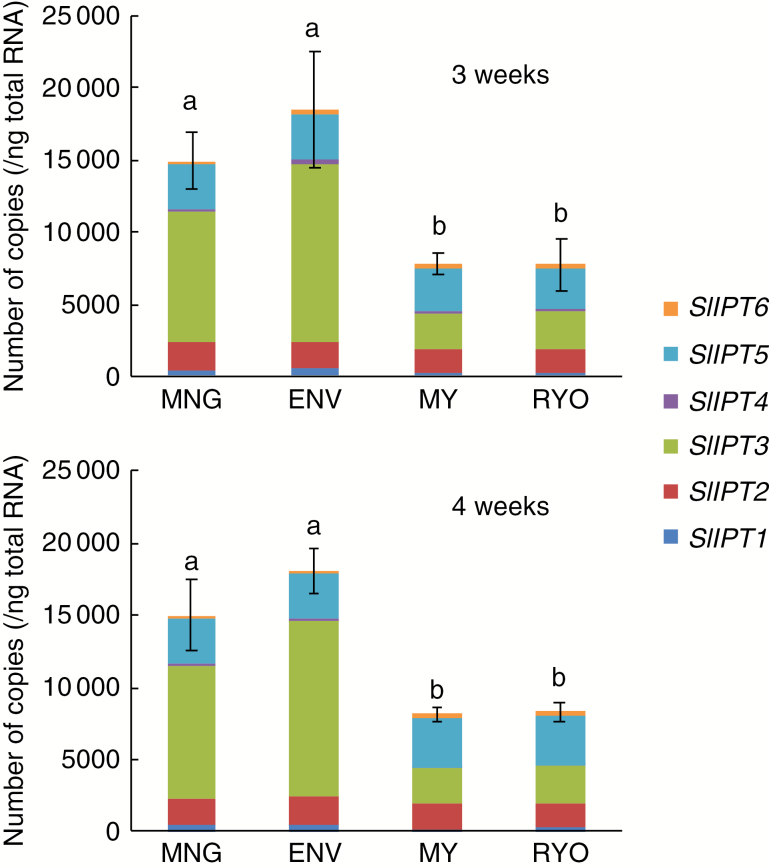
The first step in isoprenoid (iP) cytokinin biosynthesis is N-prenylation of adenosine 5-phosphates (AMP, ADP or ATP) at the N6-terminus with dimethylallyl diphosphate, which is catalysed by IPT (Sakakibara, 2006). Because the amounts of total iP and iP7G were greater in the Dutch cultivars (Supplementary data Table S2), we hypothesized that expression of IPT genes would also be greater in the Dutch cultivars. Six tomato IPT genes (Sl-IPT1– Sl-IPT6) were previously identified (Matsuo et al., 2012). Among the six Sl-IPT genes, Sl-IPT3 was expressed the most strongly. Sl-IPT3 was expressed significantly more strongly in the Dutch cultivars than in the Japanese cultivars both 3 and 4 weeks after germination, while Sl-IPT1 was expressed significantly more strongly in the Dutch cultivars only 3 weeks after germination (Fig. 6; Supplementary data Fig. S5).
Fig. 6.
Expression levels of total Sl-IPT genes in hypocotyls of 3- and 4-week-old plants of Dutch and Japanese tomato. Values are means (n = 4) ± s.d. Different lower case letters indicate a significant difference among all the conditions (P < 0.05, one-way ANOVA and then LSD test for multiple comparisons).
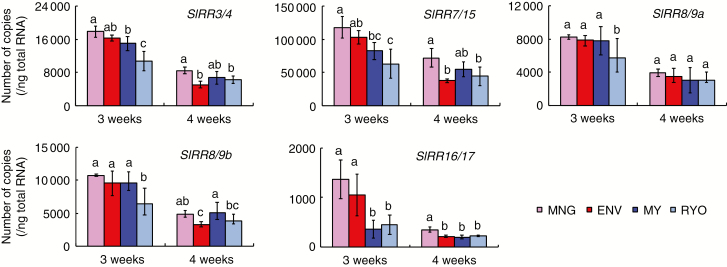
Tomato has five Responsive Regulator genes (Sl-RR3/4, Sl-RR7/15, Sl-RR8/9a, Sl-RR8/9b and Sl-RR16/17) that are markers of cytokinin signalling (Shani et al., 2010; Matsuo et al., 2012). Spraying the hypocotyls with 6-BA (Supplementary data Fig. S6A) or immersing them in 6-BA or kinetin solution (Supplementary data Fig. S6B) upregulated all five Sl-RR genes. Among the five Sl-RR genes, only Sl-RR16/17 was expressed more strongly in the Dutch cultivars than in the Japanese cultivars at 3 weeks (Fig. 7).
Fig. 7.
Expression levels of cytokinin response genes (Sl-RR genes) in hypocotyls of 3- and 4-week-old plants of Dutch and Japanese tomato. Values are means (n = 4) ± s.d. Different lower case letters indicate a significant difference among all the conditions (P < 0.05, one-way ANOVA and then LSD test for multiple comparisons).
DISCUSSION
The xylem conducts water and mineral nutrients from the roots to the shoots, and its development is related to the biomass production of plants (Larson, 1994). In tomato, the flow of sap in the xylem was reported to be faster in Dutch cultivars than in Japanese cultivars (Nakano et al., 2013). At first, we hypothesized that xylem development in the Dutch cultivars was faster (Figs 1–3) because of their greater water or nutrient uptake. However, the reciprocal grafting did not affect either shoot growth or xylem development in the scion (Fig. 4A–C; Supplementary data Fig. S2). On the other hand, xylem development in the rootstock was affected by which cultivar was used for the scion (Fig. 4D, E; Supplementary data Fig. S3), suggesting that factors related to the development of secondary xylem in Dutch cultivars were derived from the shoot.
Hormones such as auxin, GAs and cytokinins regulate vascular development (Sachs, 1981; Savidge, 1988; Eriksson et al., 2000; Mähönen et al., 2000). Cytokinins have been shown to be involved in the maintenance of shoot and cambial meristem activities, which determines the shoot or root size, in several plants, including tobacco (Nicotiana tabacum L., Werner et al., 2001, 2003), populus (Matsumoto-Kitano et al., 2008), arabidopsis (Nieminen et al., 2008) and radish (Raphanus sativus L., Jang et al., 2015). Our findings that the Dutch cultivars have higher concentrations of iP-type cytokinins (Fig. 5) and higher expression levels of Sl-IPT1 and Sl-IPT3 suggest that locally synthesized cytokinins contribute to the difference in the development of secondary xylem between Dutch and Japanese cultivars. So far, four types of active cytokinins have been identified in plants, but two of them (DZ and cZ) play minor roles in the growth of most plants (Matsumoto-Kitano et al., 2008). iP and tZ as well as their sugar conjugates are biologically important cytokinins. tZ-type cytokinins predominate in the xylem sap, whereas iP-type cytokinins predominate in the leaf exudates (Salama and Wareing, 1979; Kakimoto, 2003; Hirose et al., 2008). Recent reciprocal grafting of roots and shoots of cytokinin-deficient mutants revealed that tZ-type cytokinins are translocated from roots to shoots via the xylem to regulate shoot growth. iP-type cytokinins are major components of phloem cytokinins (Hirose et al., 2008). The amounts of iP and iP7G in the Dutch cultivars were greater than those in the Japanese cultivars 3 weeks after germination (Fig. 5A; Supplementary data Table S2). iP7G, which is an inactive form of cytokinin, had the highest concentration (Supplementary data Table S2). However, cytokinin N-glucosyltransferase can convert iP to the N-glucoside of iP7G. iP7G has been suggested to represent an inactive, but stable storage form of cytokinins (Frébort et al., 2011). Differences in the iP and iP7G levels between the Dutch and Japanese cultivars were consistent with their rates of xylem development. The greater level of iP7G and active iP in the Dutch cultivars might lead to an earlier and more rapid development of xylem. The accumulation of iP-type cytokinins (which predominate in the leaves) rather than tZ-type cytokinins (which predominate in the roots) implies that shoot-derived cytokinins rather than root-derived cytokinins are involved in the difference in xylem development between the Dutch and Japanese cultivars. This was supported by the grafting experiments, in which the cultivar used for the rootstock did not affect the xylem size of the scion (Fig. 4B, C; Supplementary data Fig. S2), but the cultivar used for the scion affected the xylem size of the rootstock (Fig. 4D, E; Supplementary data Fig. S3). This indicates that the genotype of the scion has a predominant role in xylem development in the shoot and rootstock. So, iP-type cytokinins might also be the shoot-to-root signal that determines xylem formation in the root. Additionally, the expression of Sl-IPT3 was greater in the hypocotyls of the Dutch cultivars (Fig. 6). The greater expression levels of the cytokinin biosynthesis gene Sl-IPT3 and the cytokinin-responsive gene Sl-RR16/17 (Fig. 7) support the differences in the iP and iP7G levels. These results suggest that local biosynthesis of an iP-type cytokinin contributed to the difference of xylem development between the Dutch and Japanese cultivars.
We did not detect any significant difference in other plant hormones (Supplementary data Fig. S4). Auxin was reported to be involved in the initiation and promotion of vascular development. In the woody plants Pinus sylvestris L. (Uggla et al., 1996) and hybrid aspen (Populus tremula × Populus tremuloides, Tuominen et al., 1997), the auxin level peaked in the cambial zone, and steeply decreased toward the mature xylem and phloem. In the Ptt iaa3m mutant (IAA3m is a key component in the auxin signalling pathway) of hybrid aspen, the radial and axial dimensions of xylem formation were reduced (Nilsson et al., 2008). However, we did not find a close relationship between IAA concentration and xylem development (Supplementary data Fig. S4). (One exception was the IAA concentration in hypocotyls of 4-week-old plants, which was significantly greater in ENV than in RYO.) GAs have also been reported to be involved in xylem development. Application of GA3 enhanced xylem development in the shoot of linseed and carrot (McKenzie and Deyholos, 2011; Wang et al., 2015). Overexpression of GIBBERELLIN 20-OXIDASE1 (GA20ox), a GA biosynthetic gene, in populus resulted in increased growth, biomass production and xylem fibre length (Eriksson et al., 2000). GA20ox mRNA and bioactive GAs have also been demonstrated to accumulate in the expansion zone of developing xylem (Israelsson et al., 2005). Moreover, decreasing the levels of GA precursor and reduced bioactive GA resulted in reduced secondary growth (Mauriat et al., 2011). The levels of GA44 and GA53 tended to be lower in the Japanese cultivars while the levels of the other GAs were not significantly different among the four cultivars (Supplementary data Fig S4), suggesting that other plant hormones did not contribute to the difference of xylem development between the Dutch and Japanese cultivars.
Conclusions
Our results provide evidence that the xylem in hypocotyls and stems of the Dutch cultivars were more developed than those of the Japanese cultivars at the early and late stages of tomato growth. We attributed this difference to the level of iP-type cytokinins, which are locally synthesized in the phloem by IPT. Although we found that xylem development was greater in the Dutch cultivars, further experiments are needed to clarify whether it also contributes to tomato growth and fruit production.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Table S1: the primers used for quantitative RT–PCR. Table S2: iP-type cytokinin concentrations in the hypocotyl of MNG, ENV, MY and RYO at the 3 and 4 week stages. Table S3: tZ-type cytokinin concentrations in the hypocotyl of MNG, ENV, MY and RYO at the 3 and 4 week stages. Table S4: cZ-type cytokinin concentrations in the hypocotyl of MNG, ENV, MY and RYO at the 3 and 4 week stages. Table S5: DZ-type cytokinin concentrations in the hypocotyl of MNG, ENV, MY and RYO at the 3 and 4 week stages. Figure S1: comparison of shoot growth and the number of phloem vessels in hypocotyl sections in Japanese and Dutch tomatoes. Figure S2: the effects of grafting on shoot growth and xylem formation of the scion in other combinations of tomato cultivars. Figure S3: the effects of grafting on xylem area and xylem ratio in rootstock under grafting. Figure S4: IAA, GA, ABA and SA levels in hypocotyls of 3- and 4-week-old plants of MNG, ENV, MY and RYO. Figure S5: the expression levels of six IPT genes in 3- and 4-week-old Dutch and Japanese tomato hypocotyls. Figure S6: the effects of cytokinin treatment on expression of Sl-RR genes in MY.
ACKNOWLEDGEMENTS
We thank Tadahisa Higashide, Akimasa Nakano and Akio Ohyama for stimulating discussions.
FUNDING
This work was supported by Cross-ministerial Strategic Innovation Promotion Program (SIP), ‘Technologies for creating next-generation agriculture, forestry and fisheries’.
LITERATURE CITED
- Chaffey N. 1999. Cambium: old challenges – new opportunities. Trees (Berlin) 13: 138–151. [Google Scholar]
- Eriksson ME, Israelsson M, Olsson O, Moritz T. 2000. Increased gibberellin biosynthesis in transgenic trees promotes growth, biomass production and xylem fiber length. Nature Biotechnology 18: 784–788. [DOI] [PubMed] [Google Scholar]
- Frébort I, Kowalska M, Hluska T, Frébortová J, Galuszka P. 2011. Evolution of cytokinin biosynthesis and degradation. Journal of Experimental Botany 62: 2431–2452. [DOI] [PubMed] [Google Scholar]
- Hejátko J, Ryu H, Kim GT, et al. 2009. The histidine kinases CYTOKININ-INDEPENDENT1 and ARABIDOPSIS HISTIDINE KINASE2 and 3 regulate vascular tissue development in Arabidopsis shoots. The Plant Cell 21: 2008–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashide T, Heuvelink E. 2009. Physiological and morphological changes over the past 50 years in yield components in tomato. Journal of the American Society for Horticultural Science 134, 460–465. [Google Scholar]
- Hirose N, Takei K, Kuroha T, Kamada-Nobusada T, Hayashi H, Sakakibara H. 2008. Regulation of cytokinin biosynthesis, compartmentalization and translocation. Journal of Experimental Botany 59: 75–83. [DOI] [PubMed] [Google Scholar]
- Hwang I, Sheen J, Müller B. 2012. Cytokinin signaling networks. Annual Review of Plant Biology 63: 353–380. [DOI] [PubMed] [Google Scholar]
- Israelsson M, Sundberg B, Moritz T. 2005. Tissue-specific localization of gibberellins and expression of gibberellin-biosynthetic and signaling genes in wood-forming tissues in aspen. The Plant Journal 44: 494–504. [DOI] [PubMed] [Google Scholar]
- Jang G, Lee JH, Rastogi K, Park S, Oh SH, Lee JY. 2015. Cytokinin-dependent secondary growth determines root biomass in radish (Raphanus sativus L.). Journal of Experimental Botany 66: 4607–4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakimoto T. 2003. Perception and signal transduction of cytokinins. Annual Review of Plant Biology 54: 605–627. [DOI] [PubMed] [Google Scholar]
- Kiba T, Takei K, Kojima M, Sakakibara H. 2013. Side-chain modification of cytokinins controls shoot growth in Arabidopsis. Developmental Cell 27: 452–461. [DOI] [PubMed] [Google Scholar]
- Kojima M, Kamada-Nobusada T, Komatsu H, et al. 2009. Highly sensitive and high-throughput analysis of plant hormones using MS-probe modification and liquid chromatography–tandem mass spectrometry: an application for hormone profiling in Oryza sativa. Plant & Cell Physiology 50: 1201–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson PR. 1994. The vascular cambium, development and structure. Berlin: Springer-Verlag. [Google Scholar]
- Mähönen AP, Bonke M, Kauppinen L, Riikonen M, Benfey PN, Helariutta Y. 2000. A novel two-component hybrid molecule regulates vascular morphogenesis of the Arabidopsis root. Genes & Development 14: 2938–2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto-Kitano M, Kusumoto T, Tarkowski P, et al. 2008. Cytokinins are central regulators of cambial activity. Proceedings of the National Academy of Sciences, USA 105: 20027–20031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo S, Kikuchi K, Fukuda M, Honda I, Imanishi S. 2012. Roles and regulation of cytokinins in tomato fruit development. Journal of Experimental Botany 63: 5569–5579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauriat M, Sandberg LG, Moritz T. 2011. Proper gibberellin localization in vascular tissue is required to control auxin-dependent leaf development and bud outgrowth in hybrid aspen. The Plant Journal 67: 805–816. [DOI] [PubMed] [Google Scholar]
- McKenzie RR, Deyholos MK. 2011. Effects of plant growth regulator treatments on stem vascular tissue development in linseed (Linum usitatissimum L.). Industrial Crops and Products 34, 1119–1127. [Google Scholar]
- Myburg AA, Sederoff RR. 2001. Xylem structure and function. Encyclopedia of Life Sciences doi: 0.1038/npg.els.0001302. [Google Scholar]
- Nakano A, Kaneko S, Yasuda KE, et al. 2013. Yield and root activity in tomatoes grown in a low-truss nutrient film technique under high-yielding conditions. Bulletin of the National Institute of Vegetable and Tea Science 12, 75–80. [Google Scholar]
- Nieminen K, Immanen J, Laxell M, et al. 2008. Cytokinin signaling regulates cambial development in poplar. Proceedings of the National Academy of Sciences, USA 105: 20032–20037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson J, Karlberg A, Antti H, et al. 2008. Dissecting the molecular basis of the regulation of wood formation by auxin in hybrid aspen. The Plant Cell 20: 843–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs T. 1981. The control of the patterned differentiation of vascular tissues. Advances in Botanical Research 9: 151–262. [Google Scholar]
- Sakakibara H. 2006. Cytokinins: activity, biosynthesis, and translocation. Annual Review of Plant Biology 57: 431–449. [DOI] [PubMed] [Google Scholar]
- Salama AM, Wareing PF. 1979. Effects of mineral nutrition on endogenous cytokinin responses in plants of sunflower (Helianthus annuus L.). Journal of Experimental Botany 30: 971–981. [Google Scholar]
- Savidge RA. 1988. Auxin and ethylene regulation of diameter growth in trees. Tree Physiology 4: 401–414. [DOI] [PubMed] [Google Scholar]
- Shani E, Ben-Gera H, Shleizer-Burko S, Burko Y, Weiss D, Ori N. 2010. Cytokinin regulates compound leaf development in tomato. The Plant Cell 22: 3206–3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinozaki Y, Hao S, Kojima M, et al. 2015. Ethylene suppresses tomato (Solanum lycopersicum) fruit set through modification of gibberellin metabolism. The Plant Journal 83: 237–251. [DOI] [PubMed] [Google Scholar]
- Tuominen H, Puech L, Fink S, Sundberg B. 1997. A radial concentration gradient of indole-3-acetic acid is related to secondary xylem development in hybrid aspen. Plant Physiology 115: 577–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uggla C, Moritz T, Sandberg G, Sundberg B. 1996. Auxin as a positional signal in pattern formation in plants. Proceedings of the National Academy of Sciences, USA 93: 9282–9286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GL, Que F, Xu ZS, Wang F, Xiong AS. 2015. Exogenous gibberellin altered morphology, anatomic and transcriptional regulatory networks of hormones in carrot root and shoot. BMC Plant Biology 15: 290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner T, Motyka V, Strnad M, Schmülling T. 2001. Regulation of plant growth by cytokinin. Proceedings of the National Academy of Sciences, USA 98: 10487–10492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner T, Motyka V, Laucou V, Smets R, Van Onckelen H, Schmülling T. 2003. Cytokinin-deficient transgenic Arabidopsis plants show multiple developmental alterations indicating opposite functions of cytokinins in the regulation of shoot and root meristem activity. The Plant Cell 15: 2532–2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White PJ. 2012. Long-distance transport in the xylem and phloem. In: Marschner P. ed. Mineral nutrition of higher plants, 3rd edn. London: Academic Press, 49–70. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.