Summary
Brachycephalic dog breeds have experienced a marked rise in popularity in recent years. While numerous people clearly desire this phenotype in their pets, many of these dogs unfortunately experience several concomitant sequelae, including major problems with respiration and thermoregulation, as well as gastrointestinal, ophthalmological, dermatological, reproductive and even dental problems. This mini review focuses on the anatomical and pathological changes associated with brachycephalic skull shape, including brachycephalic obstructive airway syndrome and other co-existent disorders. It then details the known genetic contributors to brachycephaly, and concludes with a brief discourse on the welfare of these animals.
Keywords: anatomy, brachycephalic obstructive airway syndrome, dog, gene
Introduction
Canine brachycephaly is a phenomenon created by man, the result of years of artificial selection. Canine skull shape ranges from brachycephalic (short and wide cranial proportions, i.e. ‘flat-faced’; breeds such as the bulldog, pug and boxer) to dolichocephalic (long muzzle, long and narrow cranial proportions; breeds such as the greyhound, Saluki and collie), with mesaticephalic (also known as mesocephalic; medium muzzle length and intermediate cranial proportions; breeds such as the Labrador retriever and beagle, as well as the wolf ancestor of the dog) as an intermediate phenotype (Fig. 1). A definitive list of brachycephalic breeds does not exist because there is variation in how ‘brachycephaly’ is defined (skull width-to-length ratio, craniofacial ratio, craniofacial angle, etc.); moreover, variation within some breeds means the term is best suited for individual dogs rather than breeds as a whole. Nevertheless, the most common brachycephalic breeds are the French bulldog, pug, bulldog, Boston terrier and boxer, with the first three generally considered more ‘extreme’ in their brachycephaly.
Fig. 1.
Canine cranioskeletal shape. (A) Shetland sheepdog (dolichocephalic); (B) Labrador retriever (mesaticephalic); (C) bulldog (brachycephalic); (D) French bulldog (brachycephalic).
The brachycephalic phenotype was likely originally selected as a potential advantage in fighting, under the assumption that this craniofacial conformation resulted in increased biting forces; conversely, many of today’s brachycephalic breeds are selected as companion dogs with appealing looks. The increasing global popularity of breeds such as bulldogs, French bulldogs and pugs has been attributed to the distinctive physical facial characteristics selected for in brachycephalic dogs: a paedomorphic face resulting from a rounded skull, shortened rostrum, large forehead, large protruding eyes and bulging cheeks. It has been suggested that these infantile facial features trigger the same attraction, nurture response and positive emotions in adults that are aroused by a human baby.
Anatomy of Brachycephaly
Canine brachycephaly is characterized by a variably shortened muzzle and a rounded, often massive, head. Compared with mesaticephalic dogs, there is medio-lateral widening of the skull together with rostrocaudal shortening of the muzzle (and its underlying premaxillary and maxillary bones). The shortening of the muzzle can be severe and the snout may be angled upward rostrally. Prognathism is frequently present, typically as an underbite (Fig. 1C). Some breeds may have markedly reduced or absent frontal sinuses. Nasal conchae (turbinates) may also be aberrant: they may be oversized and extend caudally into the nasopharynx from the choanae.
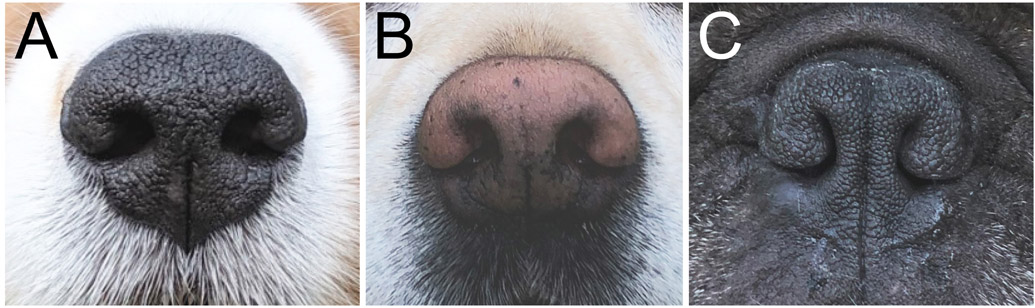
These skeletal changes also result in the compression of the nasal and pharyngeal air passages. The soft tissues of the upper respiratory tract, including the nostrils, nasal mucosa of the conchae, soft palate, tonsils and even the tongue, do not scale down proportionately with the midface skeletal reduction; this leads to incongruous dimensions and a narrower upper respiratory tract lumen due to a relative excess of soft tissue blocking the airflow. Soft tissue changes include: (1) stenotic nares (Fig. 2), where the wing of the nostril is deformed and the opening is abnormally narrow, sometimes reduced to just a vertical slit with nearly complete obstruction, forcing dogs to breathe in open-mouthed fashion almost continually; (2) soft palate displacement and hyperplasia, where the relatively posterior displacement (i.e. elongated soft palate) extends beyond the tip of the epiglottis, allowing the soft palate to flutter and its redundant tissue to obstruct the rima glotidis during inspiration (Fig. 3); this tissue subsequently progresses to further pathological thickening; and (3) relative macroglossia, where a thickened and overlong tongue further displaces the soft palate. The trachea may be congenitally hypoplastic, with tracheal cartilages touching or overlapping; while this can exacerbate respiratory clinical signs, it is not thought to be a major contributor to brachycephalic obstructive airway syndrome (BOAS). These respiratory soft tissue misconfigurations ultimately restrict/impede airflow, resulting in major impacts on respiratory function that can lead to secondary changes.
Fig. 2.
Nares. (A) Shetland sheepdog and (B) Labrador retriever: normal nares; (C) French bulldog with abnormal, stenotic nares. Note that nares can be even more stenotic (more severely malformed) than pictured here.
Fig. 3.
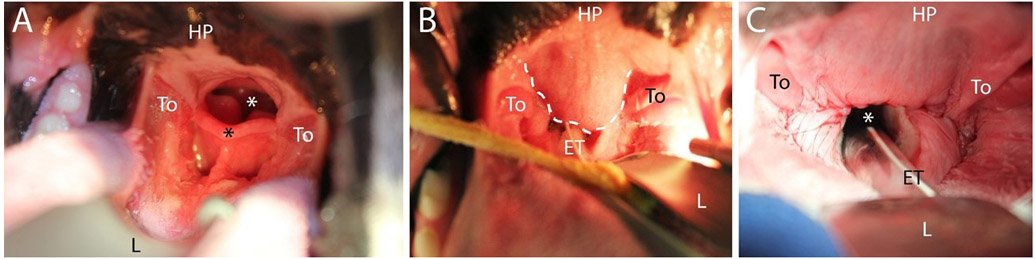
Palate and larynx. (A) Normal palate and laryngeal anatomy in a mesaticephalic dog (Briard); (B) and (C) Abnormal palate anatomy (bulldog) preoperatively (B), where white dashed line indicates excess soft palate obscuring larynx, and the same dog corrected postoperatively (C). All images taken with dog in sternal recumbency. HP, hard palate; To, tonsil; L, laryngoscope; ET, endotracheal tube; black asterisk, tip of epiglottis; white asterisk, rima glottidis.
Brachycephalic Obstructive Airway Syndrome
BOAS is characterized primarily by respiratory and thermoregulatory problems in a spectrum of disease involving partial or complete obstruction of the upper airways. It has been reported predominantly in ‘extreme’ brachycephalic breeds, with the risk of BOAS increasing significantly as the relative length of the muzzle decreases. BOAS is diagnosed typically in dogs 2–3 years of age, but can be severe even in young (<6-month-old) puppies. Clinically, BOAS consists of stertor, stridor, inspiratory dyspnoea, increased respiratory effort, chronic shortness of breath, exercise intolerance and tendency to overheat; these can progress to low blood oxygen levels with subsequent collapse, and even death.
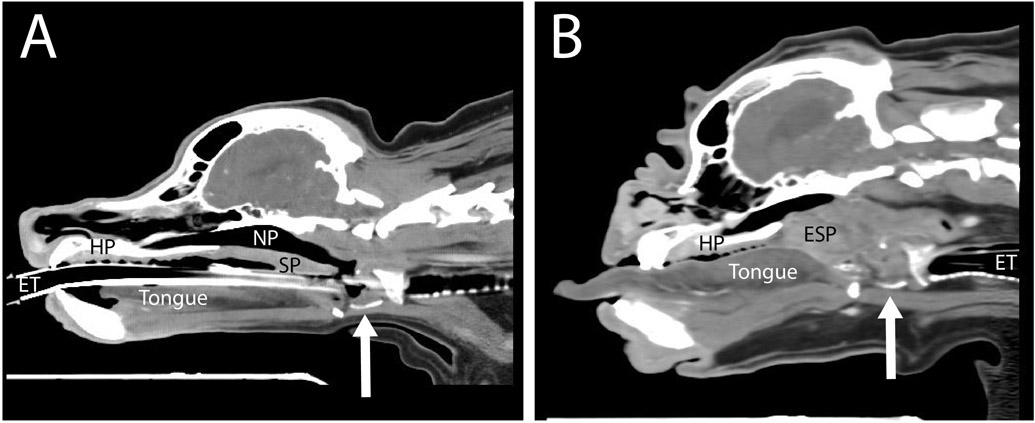
The primary anatomical malformations (described above) together cause airway resistance and an increase in the negative pressure within the upper airways during respiration. This increased airway resistance also increases the pressure gradient during inspiration, which, in turn, induces pathological changes such as oedema of the nasopharyngeal mucosa, exacerbation of the thickening and elongation of the soft palate (Fig. 4), general upper airway tissue inflammation and other secondary abnormalities, all of which create further obstruction to an already narrow lumen. Such secondary changes include everted laryngeal ventricles (tissue rostral to the vocal cords is pulled into the ventral glottis), everted tonsils (tonsils everting from their crypts secondary to increased negative pressure) and compromise of the cartilaginous larynx and trachea, which can subsequently collapse. Laryngeal collapse has been diagnosed in brachycephalic dogs younger than 6 months of age. It is not entirely clear which part of the obstructed airway (nasal cavity, nasopharynx or rima glottis) is most responsible for the clinical signs in BOAS. Regardless, severe obstructions can result in syncopal episodes or even death from suffocation.
Fig. 4.
Airway soft tissue configurations. Computed tomography images of (A) mesaticephalic (cocker spaniel) and (B) brachycephalic (bulldog) dogs, at a sagittal plane slightly off midline, capturing the laryngeal cartilages at a similar level through the cricoid cartilage (arrow indicates the ventral cut section of the thyroid cartilage). In (B) note the following differences compared with (A): the thickened and elongated soft palate (ESP), the lack of patency of the nasopharyngeal (NP) airway to the level of the larynx, macroglossia and narrowed nasal airway. HP, hard palate; SP, soft palate; ET, endotracheal tube.
Panting increases turbulent airflow and, therefore, obstruction, which can cause distress and potential overheating. In addition, since nasal ventilation is restricted, the specialized dissipation of heat through evaporation, via the moist nasal mucosa covering the nasal conchae, is lost, further impacting the ability of affected dogs to thermoregulate. Indeed, hyperthermia is common in affected dogs, as they are unable to cool efficiently, making them prone to heat stress and heat stroke, which can lead to death. Physiological arousal of any kind can aggravate these conditions and lead to respiratory crises. Therefore, it is important for brachycephalic dogs to avoid heat, humidity, excessive activity, excitement or stress. Dogs may adopt compensatory behaviours to assist breathing, including abducted elbows or, in more severe cases, generalized use of accessory abdominal musculature in breathing. Sleep patterns may be disturbed in these dogs; for example, sleep apnoea may be observed. Affected dogs often snore during sleep. They may develop adaptive behaviours in order to avoid airway obstruction during sleep, including sleeping in a sitting position or sleeping with a toy/ball between their teeth to keep the mouth open and airway patent, as a compensation for nasal obstruction.
Early diagnosis of BOAS is beneficial, as surgical interventions for improving airflow can minimize progression of secondary changes. Stenotic nares are easily diagnosed with visualization. Examination of the pharyngeal and laryngeal structures, often via endoscopy, is best performed under general anaesthesia and, since anaesthesia is higher risk in these dogs, such diagnostic procedures are typically combined with immediate obstruction-relieving surgeries. Various surgeries can be performed with the goal of reducing airway obstruction in order to provide adequate airflow. Rhinoplasty to widen the nostril can be performed in puppies as young as 3–4 months of age; when performed this early, progression to secondary changes can be avoided. Soft palate resection (staphylectomy or folded flap palatoplasty) can remove excess caudal palatal tissue (Fig. 3C) and everted laryngeal ventricles can be excised surgically. Laryngeal collapse may not need treatment if the more proximal airway obstructions are rectified; arytenoid lateralization can be attempted if sufficient mineralization remains in the cartilages. In advanced BOAS, when laryngeal cartilages lose integrity (chondromalacia), permanent tracheostomy may be required.
The prognosis for dogs suffering from BOAS varies. If treated early, the overall prognosis for significant postoperative improvement can be excellent. However, many dogs continue to experience postsurgical airway restrictions. Dogs that have progressed to a state of laryngeal collapse have a guarded prognosis. Finally, it is important to keep affected dogs from carrying extra weight, as obesity is a well-recognized risk factor for BOAS and is known to exacerbate the condition.
Additional Brachycephaly-Related Problems
The effects of BOAS are not limited to the respiratory and thermoregulatory systems; indeed, the chronic negative intrathoracic pressure generated by increased inspiratory effort is believed to be a major cause of gastro-oesophageal reflux. Associated regurgitation, gagging, ptyalism and vomiting then contribute to inflammation of the upper oesophagus, pharynx and larynx. There is likely a correlation between the severity of gastrointestinal signs and respiratory signs among dogs with BOAS. Surgical treatments, as described above for respiratory abnormalities, typically also help alleviate gastrointestinal clinical signs.
In addition, the shortened muzzle of these dogs throws the excessive skin into folds around the muzzle, eyes and ears (visible in Fig. 4B on the dorsal aspect of the skull and muzzle); these folds are predisposed to dermatitis (intertrigo) and infections. Brachycephalic skull morphology also results in eyes that look bigger; this is misleading, as the eyes are, in fact, of normal size. Rather, the orbit is too shallow, resulting in globes that bulge outward. This exophthalmia predisposes the globes to infections and corneal ulcers, lagophthalmia and increased risk of globe prolapse. These eyes are in further danger of trichiasis, specifically from hairs on the nasal skin fold at the medial canthus. Together these conditions can result in blindness or even loss of the eye.
The altered facial skeletal structure, with a normal number of teeth packed into a much smaller space, creates dental problems such as malocclusion, rotation of premolars and severe crowding of teeth. Rugae of the hard palate can have excessive depth between palatine folds, which traps food and hair resulting in palatitis and chronic ulcers. Dental disease is difficult to prevent; it is challenging to brush the teeth of these dogs because they need their mouths to breathe and it is harder to clean each tooth because of crowding. Dental surgery, specifically extractions (of both deciduous and permanent teeth) is often required.
Finally, brachycephalic breeds also experience reproductive issues, primarily dystocia. For example, over 85% of bulldogs in the UK are delivered via caesarean section. The high rate of dystocia in brachycephalic breeds is due to puppy heads being too large to pass through the birth canal.
Histopathology
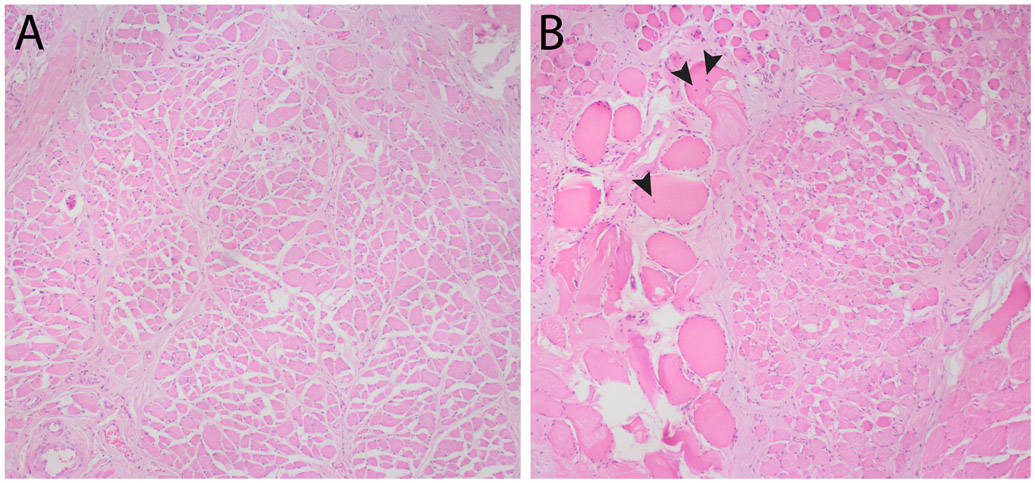
Histopathological examination of the thickened portions of the soft palate in brachycephalic dogs with severe BOAS has been performed, with equivalent tissue from mesaticephalic dogs collected post mortem serving as controls. BOAS-affected dogs had a marked increase in both acute and chronic muscle degeneration and necrosis (Fig. 5). The increased thickness of palatal tissue in BOAS-affected dogs was not due to muscle hypertrophy or fat deposition, but rather to increased stroma within the lamina propria, oedema and increased proportions of salivary tissue. In fact, the BOAS-affected dogs actually had a reduction in muscle mass.
Fig. 5.
Palatinus muscle histopathology. Photomicrographs of transverse sections of the central soft palate from (A) a mesaticephalic dog and (B) a brachycephalic dog with severe brachycephalic obstructive airway syndrome, demonstrating hypereosinophilic, swollen, degenerate skeletal muscle fibres, some with centralized nuclei (black arrowheads). Haematoxylin and eosin. x400.
Other work has shown that the caudal edge of the soft palate in brachycephalic dogs has histological features not observed in mesaticephalic dogs, such as thickened mucosal epithelium. These dogs were undergoing preventive surgery and were not yet considered clinically affected with BOAS. The palatal tissue of these dogs already exhibited hyperplasia and intra-cellular oedema of the mucosal lining, amplified myxoid matrix in the lamina propria, mucous gland hyperplasia and degenerate muscle fibres. In equivalent (post-mortem) tissue samples from mesaticephalic dogs, focal areas of muscular degeneration were observed rarely.
This myofibre degeneration throughout the palatine musculature is hypothesized to be the result of repetitive trauma, hypoxia or chronic overuse. The oedema is hypothesized to be secondary to trauma from turbulent airflow or irritation resulting from laryngopharyngeal reflux. The increase in palatine salivary gland proportion is hypothesized to be a response to the increased oral airflow (due to nasopharyngeal obstruction), which creates a drying effect, stimulating salivary tissue to increase in order to meet the lubrication needs of the caudal oral cavity. It is also possible that genetics may be driving some of these pathological changes (see below); much investigation remains to be done.
Interestingly, soft palates examined in neonatal brachycephalic dogs showed no signs of oedema or the other histopathological alterations seen typically (as described above) in tissues from adult brachycephalic dogs. For example, neonatal brachycephalic dogs did not have the extensive degenerative muscular lesions detected in adult brachycephalic dogs, nor did they have thickened superficial epithelium, broad oedema of the lamina propria or mucous gland hyperplasia. It is probable that such changes occur postnatally as a result of chronic airway turbulence and barotrauma.
Finally, since the tongue in brachycephalic dogs has been shown to be abnormally dense, glossal bi-opsies and histopathological analysis of this organ, comparing brachycephalic to mesaticephalic/dolichocephalic specimens, would be informative.
Genetics of Brachycephaly and BOAS
The genetic underpinnings of artificially selected canine skull shape have been investigated widely. Skull morphology has a complex genetic basis and is subject to multigenic control. Multiple different chromosomal regions, several of which have been described, are responsible for the cranioskeletal differences between brachycephalic and dolichocephalic breeds, based on single nucleotide polymorphism data and craniometric measurements. Airorhynchy, or dorsal bending of the snout, and midface length have been correlated with a repeat expansion in the RUNX2 gene, on canine chromosome 12 (CFA12). Additionally, a brachycephaly-associated CFA32 locus was narrowed to a specific missense variant in the BMP3 gene. Interestingly, carrier breeds (those with some degree of heterozygosity at the BMP3 locus) tend to fall between the ancestral wolf skull phenotype (mesaticephalic) and extreme brachycephalic breeds. Recently, a CFA1 LINE-1 insertion variant in the SMOC2 gene has been identified as the underlying mutation for ‘face length variation’ (reduction in the mandible and non-braincase skull; i.e. palatine, maxillary and incisive bones); this insertion mutation accounts for up to 36% of this face length variation. Almost 92% of brachycephalic dog chromosomes had the SMOC2 LINE-1 insertion, while it was found in only ~2% of non-brachycephalic dog chromosomes. Finally, it is possible that a CFA5 variant contributes to brachycephaly; a single base deletion in the DVL2 gene has been associated with the truncated, kinked tail (screw tail) in bulldogs, French bulldogs and Boston terriers. Previous genome-wide studies of brachycephalic skull shape have highlighted a neighbouring CFA5 locus, meaning that it is possible that the DVL2 variant also contributes to brachycephaly. Several other loci related to brachycephaly continue to be investigated, as existing studies have not exhaustively detected and described all contributing genetic loci.
Because BOAS is clinically heterogeneous within breeds (i.e. there are conformation-typical brachycephalic dogs that do not suffer even mild BOAS) it is possible that other genetic determinants contribute to BOAS susceptibility, independent of those driving skull formation. Such investigations are ongoing. The phenotype of BOAS is probably polygenic; however, another genetic variant merits mentioning here: a recently published ADAMTS3 missense variant (CFA13) was shown to be strongly associated with Norwich terrier upper airway syndrome (UAS). UAS has a strikingly similar pathological phenotype to BOAS, including elongated and thickened soft palates, oedema of the nasopharynx and everted laryngeal ventricles, despite Norwich terriers being a mesaticephalic breed. The same ADAMTS3 variant was also highly prevalent in French bulldogs and bulldogs (which were not phenotyped for BOAS), suggesting the enticing possibility that this locus may contribute to BOAS in these breeds. Further inquiry is needed to validate this allele in non-Norwich terriers with clinical BOAS.
Welfare
Collectively, the perturbations leading to BOAS have a severe impact on the wellbeing of affected individuals; affected dogs may have little or almost no activity because they are entirely occupied with breathing. Clearly there is a significant welfare concern for brachycephalic dogs experiencing respiratory distress, ocular trauma, heat stroke, skin fold dermatitis and other sequelae of this artificially selected skull form. Many dogs still experience airway restrictions and a compromised quality of life, even after surgical corrections. A recent UK study demonstrated that nearly 60% of owners of brachycephalic dogs recognized clinical signs of BOAS in their dogs, but dismissed them as ‘normal for the breed’, and despite reporting a high frequency and severity of clinical signs, also did not think their dog had a breathing problem. This is highly concerning because it suggests that many dogs are experiencing BOAS and not receiving appropriate veterinary attention. Veterinarians must not be desensitized to BOAS, accepting it as ‘normal for the breed’; rather, this community should lead the way in encouraging prevention, not just palliation, of BOAS. A laudable step was taken in 2017, when the British Veterinary Association announced that it would no longer use images of brachycephalic breeds in its advertising as a way of decreasing demand for these breeds and to avoid normalizing the associated health issues. Some stakeholders support changing breed standards as the ideal way to address these concerns; however, lack of phenotypic and genetic variability within a breed might then require out-crossing, which is, unsurprisingly, controversial. It is possible that if breeders and kennel clubs do not proactively address the situation, eventually there will be regulation and legal compulsion to do so.
Conclusions
While several aspects of the pathology and genetics remain to be solved, the widespread evidence documenting the link between extreme brachycephalic phenotypes and various chronic diseases means the compromise in welfare cannot be denied. Canine brachycephaly provides a fascinating study of genetic selection driving anatomical changes, with a spectrum of subsequent pathological and, ultimately, welfare concerns.
Acknowledgments
Dr. D. Dreger is thanked for assistance with the figures, as are Drs. C. Carlson and G. O’Sullivan for their thoughtful comments on the manuscript. KJ Ekenstedt was partially supported by the Office of the Director, National Institutes of Health (NIH) under award number K01-OD027051. The funders had no role in study design, data collection, analysis or interpretation, decision to publish or preparation of this manuscript.
Footnotes
Conflict of Interest Statement
The authors declare no conflict of interest with respect to publication of this manuscript.
Key References
- Arrighi S, Pichetto M, Roccabianca P, Romussi S (2011) The anatomy of the dog soft palate. I. Histological evaluation of the caudal soft palate in mesaticephalic breeds. The Anatomical Record, 294, 1261–1266. [DOI] [PubMed] [Google Scholar]
- Crosse KR, Bray JP, Orbell GMB, Preston CA (2015) Histological evaluation of the soft palate in dogs affected by brachycephalic obstructive airway syndrome. New Zealand Veterinary Journal, 63, 319–325. [DOI] [PubMed] [Google Scholar]
- Dupre G, Heindenreich D (2016) Brachycephalic syndrome. Veterinary Clinics of North America: Small Animal Practice, 46, 691–707. [DOI] [PubMed] [Google Scholar]
- Ibarra AMR, Legendre L (2019) Anatomy of the brachycephalic canine hard palate and treatment of acquired palatitis using CO2 laser. Journal of Veterinary Dentistry, 36, 186–197. [DOI] [PubMed] [Google Scholar]
- Jones BA, Stanley BJ, Nelson NC (2019) The impact of tongue dimension on air volume in brachycephalic dogs. Veterinary Surgery, 10.1111/vsu.13302. [DOI] [PubMed] [Google Scholar]
- Ladlow J, Liu NC, Kalmar L, Sargan D (2018) Brachycephalic obstructive airway syndrome. Veterinary Record, 182, 375–378. [DOI] [PubMed] [Google Scholar]
- Mansour TA, Lucot K, Konopelski SE, Dickinson PJ, Sturges BK et al. (2018) Whole genome variant association across 100 dogs identifies a frame shift mutation in DISHEVELLED2 which contributes to Robinow-like syndrome in bulldogs and related screw tail dog breeds. PLoS Genetics, 14, e1007850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant TW, Dietschi E, Rytz U, Schawalder P, Jagannathan V et al. (2019) An ADAMTS3 missense variant is associated with Norwich terrier upper airway syndrome. PLoS Genetics, 15, e1008102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant TW, Johnson EJ, McTeir L, Johnson CI, Gow A et al. (2017) Canine brachycephaly is associated with a retrotransposon-mediated missplicing of SMOC2. Current Biology, 27, 1573–1584 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packer RMA, O’Neill DG, Fletcher F, Farnworth MJ (2019) Great expectations, inconvenient truths, and the paradoxes of the dog-owner relationship for owners of brachycephalic dogs. PloS One, 14, e0219918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packer RMA, Tivers MS (2015) Strategies for the management and prevention of conformation-related respiratory disorders in brachycephalic dogs. Veterinary Medicine, 6, 219–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichetto M, Arrighi S, Gobbetti M, Romussi S (2015) The anatomy of the dog soft palate. III. Histological evaluation of the caudal soft palate in brachycephalic neonates. The Anatomical Record, 298, 618–623. [DOI] [PubMed] [Google Scholar]
- Pichetto M, Arrighi S, Roccabianca P, Romussi S (2011) The anatomy of the dog soft palate. II. Histological evaluation of the caudal soft palate in brachycephalic breeds with grade I brachycephalic airway obstructive syndrome. The Anatomical Record, 294, 1267–1272. [DOI] [PubMed] [Google Scholar]
- Schmidt-Nielsen K, Bretz WL, Taylor CR (1970) Panting in dogs: unidirectional air flow over evaporative surfaces. Science, 169, 1102–1104. [DOI] [PubMed] [Google Scholar]
- Schoenebeck JJ, Hutchinson SA, Byers A, Beale HC, Carrington B et al. (2012) Variation of BMP3 contributes to dog breed skull diversity. PLoS Genetics, 8, e1002849. [DOI] [PMC free article] [PubMed] [Google Scholar]