Abstract
Diabetes is associated with substantially increased mortality. Classic risk factors explain a portion of the excess of mortality in type 2 diabetes. The aim of this study was to examine whether visit-to-visit variation in fasting glucose and haemoglobin A1c values in the Veteran Affairs Diabetes Trial were associated with all-cause mortality in patients with type 2 diabetes in addition to other comorbidity conditions, hypoglycaemic events and adverse lifestyle behaviours. The Veteran Affairs Diabetes Trial was a randomized trial that enrolled 1791 military veterans who had a suboptimal response to therapy for type 2 diabetes to receive either intensive or standard glucose control. During the Veteran Affairs Diabetes Trial, fasting glucose and haemoglobin A1c were measured quarterly for up to 84 months. Variability measures included coefficient of variation and average real variability. We found that variability measures (coefficient of variation and average real variability) of fasting glucose were predictors of all-cause mortality, even after adjusting for comorbidity index, mean fasting glucose and adverse lifestyle behaviour during the study. Accounting for severe hypoglycaemia did not weaken this association. Our analysis indicates that in the Veteran Affairs Diabetes Trial, longitudinal variation in fasting glucose was associated with all-cause mortality, even when accounting for standard measures of glucose control as well as comorbidity and lifestyle factors.
Keywords: Mortality, glycaemic control, long-term glycaemic variability, hypoglycaemia, type 2 diabetes
Introduction
Diabetes is associated with substantially increased mortality.1–4 Classic risk factors, for example, older age and pre-existing cardiovascular disease (CVD), explain a portion of the excess of mortality in type 2 diabetes (T2D).5–7 Although improving glycaemic control, usually assessed by mean levels of haemoglobin A1c (HbA1c) or fasting glucose, is a key recommended goal for clinicians to enhance diabetes care,8–10 the relationship of glucose control with mortality appears more complicated. Data from observational studies have shown J-shaped distributions for mortality and glycaemic control, with not only high HbA1c but also low HbA1c associated with mortality risk.11–13 Moreover, intensive efforts to lower glucose in more advanced T2D patients have failed to reduce, or even increased, mortality.14–16
Recently, several studies reported adverse effects of glycaemic variation on macro- and microvascular complications, as well as risk of hypoglycaemia.17–22 However, studies to examine the relationship of glycaemic instability with mortality in T2D patients have been limited in number and have varied widely in the patient population studied, the length of follow-up and in the source and nature of the data collected.23–26 An investigation using primary care medical record data from the United Kingdom among diabetes patients aged 70 years and older reported the association between glycaemic variability, as measured by variability in HbA1c over a 5-year period, and mortality.11 Among US military veterans with T2D, Prentice et al.4 used electronic medical records (EMR) to examine the relationship between HbA1c variability and adverse health outcomes including all-cause mortality. Using data from the only glucose lowering trial that has examined this issue, visit-to-visit glycaemic variability (HbA1c and blood glucose) was identified as a strong independent predictor of mortality for mildly hyperglycaemic T2D patients randomized to intensive glucose lowering therapy in the Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation (ADVANCE) trial.18 However, a critical concern in prior studies is whether unmeasured factors in underlying health or behaviour may confound the relationships of glycaemic variation with mortality. Whereas Prentice et al. adjusted for baseline comorbidity, this may be less completely captured in EMR. Moreover, no studies have adequately considered adverse lifestyle behaviours during follow-up.
Therefore, we used data collected during the Veteran Affairs Diabetes Trial (VADT) to examine the association of time-dependent glycaemic variability with mortality. As extensive data collection was possible during the frequent in person visits, the current analysis was able to more fully account for health status and adverse lifestyle behaviours when examining the association of glycaemic variability with all-cause mortality.
Methods
The VADT was a randomized trial that enrolled 1791 military veterans (mean age, 60.4 years) who had a suboptimal response to therapy for T2D (HbA1c > 7.5%) to receive either intensive or standard glucose control.5,27 HbA1c and fasting glucose were measured every 3 months up to a maximum of 84 months. We excluded observations from the first 6 months of the trial to eliminate the effect of rapid reduction (per protocol) in fasting glucose and HbA1c and excluded individuals with two or fewer measurements of fasting glucose or HbA1c. The primary outcome for this analysis was all-cause mortality.
Lifestyle data were extracted from baseline and quarterly follow-up questionnaires: (1) do patients currently smoke cigarettes: if ‘Yes’ code as 1, if ‘No’ code as ‘0’; (2) do patients exercise regularly: if ‘Yes’ code as ‘0’, if ‘No’ code as ‘1’; (3) do patients adhere to diet: if ‘Yes’ code as ‘0’, if ‘No’ code as ‘1’. To generate adverse lifestyle score, we counted the total number of adverse behaviours over the three questions at each visit. In order to study the contribution of lifestyle score to glycaemic variability, we first categorized visit-to-visit glycaemic variability into the lower 50% and upper 50% and used the lifestyle score in a generalized estimation equation model to estimate its relationship with glycaemic variability. We found that a cumulative (worse) lifestyle score was a modest but statistically significant predictor of increased glycaemic variability [odds ratio (confidence interval, CI), 1.021 (1.011, 1.031), p < 0.0001] and all-cause mortality [hazard ratio (HR) (CI), 1.027 (1.010, 1.044), p = 0.001].
We compared risks of cumulative mean, maximum and most recent fasting glucose or HbA1c values prior to the mortality event with measures of variability for both fasting glucose and HbA1c. Coefficient of variation (CV) and average real variability (ARV) are frequently used and distinct measures of glycaemic variability.4,18,21,28–30 As previously described,21 we normalized these by means of fasting glucose and HbA1c, respectively. Variables of glycaemic risk were calculated as continuous and time-dependent covariates in Cox proportional hazard models.17,31 We first examined quintiles of (CV)log-glucose and ARV-glucose (or similar variability measures using HbA1c) to compare the risks of mortality between high versus low variability groups in an age-adjusted model.21 Risk of continuous glycaemic variation measures were then modelled after adjusting for: Model 1: age only; Model 2: age and baseline covariates reflecting significant baseline differences in characteristics between those who did and did not die during the study (Table 1) including a modified updated Charlson comorbidity index to reflect diabetes-related comorbidity (Supplementary Table 1; similar results were obtained if using standard Charlson comorbidity index;32,33 Model 3: age, baseline covariates and cumulative mean of fasting glucose or HbA1c to clarify whether variability measures provided risk prediction beyond standard glucose measures; Model 4: Model 3 and a lifestyle score that was treated as a time-dependent covariate. Finally, we considered whether severe hypoglycaemia could be driving the relationship between glycaemic variability and mortality. We first added the variable cumulative severe hypoglycaemia to Model 4 and second, we repeated analysis after removing all patients with severe hypoglycaemia events.
Table 1.
Baseline characteristics by incident all-cause mortality event status.
| All-cause mortality |
p-value | ||
|---|---|---|---|
| No (n = 1493) | Yes (n = 166) | ||
| Age (years) | 59.71 (8.38) | 66.30 (8.18) | <0.001 |
| Treatment (n, %) | |||
| Standard | 752 (50.4) | 81 (48.8) | 0.762 |
| Intensive | 741 (49.6) | 85 (51.2) | |
| Sex (n, %) | |||
| Male | 1445 (96.8) | 165 (99.4) | 0.1 |
| Female | 48 (3.2) | 1 (0.6) | |
| NHW (n, %) | |||
| No | 580 (38.8) | 47 (28.3) | 0.01 |
| Yes | 913 (61.2) | 119 (71.7) | |
| Smoking status (n, %) | |||
| No | 1253 (83.9) | 133 (80.1) | 0.413 |
| Yes | 240 (16.1) | 31 (18.9) | |
| BMI (kg/m2) | 31.28 (4.44) | 31.11 (4.78) | 0.637 |
| Diabetes duration (years) | 11.41 (7.39) | 12.82 (8.42) | 0.023 |
| Prior CVD event (n, %) | |||
| No | 927 (62.1) | 57 (34.3) | <0.001 |
| Yes | 566 (37.9) | 109 (65.7) | |
| History of hypertension (n, %) | |||
| No | 415 (27.8) | 39 (23.5) | 0.414 |
| Yes | 1075 (72.0) | 127 (76.5) | |
| Missing | 3 (0.2) | 0 | |
| History of TZD (n, %) | |||
| No | 1205 (80.7) | 135 (81.3) | 0.931 |
| Yes | 288 (19.3) | 31 (18.7) | |
| Charlson Comorbidity Indexa | 1.43 (1.78) | 2.74 (2.65) | <0.001 |
| Glycated haemoglobin level (%) | 9.4 (1.5) | 9.4 (1.6) | 0.977 |
| DBP (mmHg) | 76 (10) | 72.7 (11) | <0.001 |
| SBP (mmHg) | 131 (16) | 133 (18) | 0.384 |
| HDL cholesterol (mg/dL) | 36 (10) | 34 (10) | 0.003 |
| LDL cholesterol (mg/dL) | 111 (63) | 108 (80) | 0.522 |
| Total cholesterol (mg/dL) | 184 (49) | 176 (39) | 0.046 |
| Triglycerides (mg/dL) | 215(295) | 211 (131) | 0.856 |
SD: standard deviation; NHW: non-Hispanic White; BMI: body mass index; CVD: cardiovascular diseases; TZD: thiazolidinediones; DBP: diastolic blood pressure; SBP: systolic blood pressure; HDL: high-density lipoprotein; LDL: low-density lipoprotein.
Data are number of participants and (percent) or means and (SD).
Updated Charlson Comorbidity Index.
All statistical analyses were performed using R version 3.4.4 (https://www.r-project.org). A two-sided p < 0.05 was considered statistically significant.
Results
A total of 1659 individuals who had at least two measurements of fasting glucose or HbA1c after the first 6 months were included in the analysis, of which 166 died during the study. The mean and median time to all-cause death was 48.5 and 48.4 months. There were on average 18.5 visit fasting glucose and HbA1c measures for individuals within the cohort, and a maximum of 26 measures. The cohort was 90% men and had a mean (SD) age of 64.4 (8.6) years. Several baseline risk factors were associated with mortality, including ethnicity [non-Hispanic White (NHW) or not], diabetes duration, prior CVD event, baseline diastolic blood pressure, baseline high-density lipoprotein (HDL) cholesterol, baseline total cholesterol and the updated Charlson comorbidity index (Table 1).
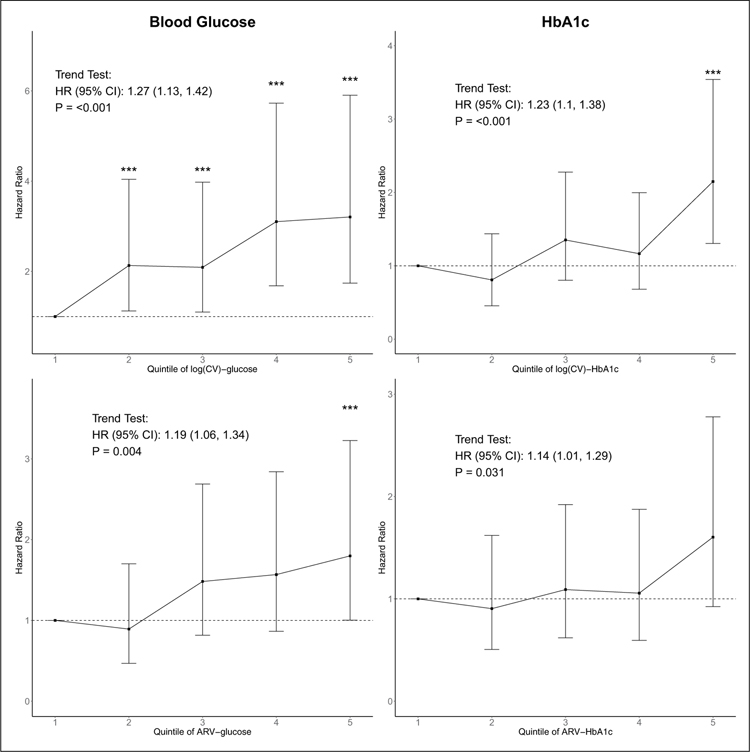
In an age-adjusted model, for fasting glucose and HbA1c, both log(CV) and ARV show significant trends for increasing risk of mortality with higher quintiles of glucose variability (Figure 1). Compared with quintile 1, individuals with fasting glucose variability in the upper quintiles (i.e. quintiles 2, 3, 4 and 5) had significantly higher risk of all-cause mortality. Although there was a significant trend for increasing mortality risk with HbA1c variability, HbA1c variability measures were generally weaker predictors of all-cause mortality than fasting glucose measures of variability.
Figure 1.
Hazard ratio (HR) estimates for quintiles of Log(CV)-glucose and ARV-glucose for mortality adjusted for age. Vertical bars shown are the 95% confidence interval (95% CI) associated with HR estimates. ***Estimated HR in the indicated variability quintile is significantly higher than the HR of lowest variability quintile (quintile 1). Trend test results are presented as the text annotation in the figures. CV: coefficient of variation; ARV: average real variability.
In Model 2 adjusting for multiple baseline risk factors, the variables’ cumulative mean fasting glucose, cumulative maximum fasting glucose, glucose measures (glucose and HbA1c) just prior to death, and log(CV) and ARV of fasting glucose were all significant risk factors (p < 0.05) for all-cause death (Table 2). After additionally adjusting for cumulative mean HbA1c or glucose (Model 3), both fasting glucose and HbA1c variability measures were still significant. Interestingly, variability measures, but not standard measures of glucose control, were significant predictors of all-cause mortality, after adjusting for age, baseline risk factors and cumulative mean HbA1c or glucose.
Table 2.
Cox proportional hazards model for all-cause mortality.
| Variables | All-cause mortality (n = 155) |
|||
|---|---|---|---|---|
| Model 1 |
Model 2 |
Model 3 |
Model 4 |
|
| HR (CI), p-value | HR (CI), p-value | HR (CI), p-value | HR (CI), p-value | |
| Blood glucose | ||||
| Cum-Mean glucose | 1.179 (1.033, 1.347), 0.015 | 1.139 (0.992, 1.308), 0.066 | – | – |
| Cum-Max glucose | 1.224 (1.082, 1.383), 0.002 | 1.165 (1.026, 1.322), 0.019 | 1.147 (0.945, 1.391), 0.165 | – |
| Prior glucose | 1.159 (1.013, 1.327), 0.032 | 1.162 (1.012, 1.334), 0.033 | 1.115 (0.934, 1.331), 0.228 | – |
| Log(CV) glucose | 1.407 (1.197, 1.655), <0.001 | 1.380 (1.160, 1.641), <0.001 | 1.362 (1.146, 1.619), <0.001 | 1.326 (1.115, 1.577), 0.002 |
| ARV glucose | 1.272 (1.115, 1.451), <0.001 | 1.232 (1.070, 1.418), 0.004 | 1.218 (1.058, 1.403), 0.006 | 1.186 (1.028, 1.369), 0.020 |
| HbA1c | ||||
| Cum-Mean HbA1c | 1.083 (0.947, 1.238), 0.243 | – | – | – |
| Cum-Max HbA1c | 1.128 (0.989, 1.288), 0.074 | – | – | – |
| Prior HbA1c | 0.951 (0.816, 1.108), 0.519 | – | – | – |
| Log(CV) HbA1c | 1.244 (1.069, 1.449), 0.005 | 1.213 (1.034, 1.424), 0.018 | 1.215 (1.016, 1.454), 0.033 | 1.194 (0.997, 1.430), 0.054 |
| ARV HbA1c | 1.211 (1.059, 1.384), 0.005 | 1.196 (1.038, 1.382), 0.014 | 1.201 (1.021, 1.421), 0.028 | 1.174 (0.996, 1.384), 0.056 |
NHW: non-Hispanic White; CVD: cardiovascular diseases; HDL: high-density lipoprotein; HbA1c: glycated haemoglobin; CV: coefficient of variation; ARV: average real variability; CI: confidence interval; HR: hazard ratio.
Data are HRs, 95% CI and p-values estimated by Cox proportional hazards model for all-cause mortality. Glycaemic control variables that were significant in age-adjusted models (Model 1) were further adjusted for ethnicity (NHW or not), diabetes duration, prior CVD event, baseline diastolic blood pressure, baseline HDL cholesterol, baseline total cholesterol and Charlson comorbidity index (Model 2). The remaining significant glycaemic variation variables in Model 2 were additionally adjusted for the cumulative mean of glucose or HbA1c, respectively, in Model 3. In Model 4, we additionally adjusted for cumulative adverse lifestyle factors. p-values in bold font show significant (p-value < 0.05) risk for the primary outcome.
Note that in Models 2, 3 and 4, additional adjustment for multiple risk factors leads to reduced sample size of 1610 participants and 155 all-cause deaths.
As adverse lifestyle behaviour may confound the association between glucose variation and mortality, we examined whether adverse behaviours contributed to glucose variability and whether this contribution explained the association of glucose variability with mortality. We found that a cumulative (worse) lifestyle score was a modest but statistically significant predictor of increased glycaemic variability (Supplementary Material). However, after additional adjustment for the effects of cumulative lifestyle factors (Model 4), fasting glucose variability remained a significant predictor of all-cause mortality and was weakened by only 2%–4%.
Additional adjustment for severe hypoglycaemia did not reduce the association of fasting glucose variability with all-cause mortality, but reduced significance of HbA1c variability. When excluding participants who experienced severe hypoglycaemia events (n = 268), we found no change in the relationships of log(CV) glucose and ARV glucose with all-cause mortality (Table 3).
Table 3.
Cox proportional hazards model for all-cause mortality, after accounting for severe hypoglycaemia.
| Variables | Analysis with additional cumulative severe hypoglycaemia adjustment |
Analysis excluding patients having severe hypoglycaemiaa |
|---|---|---|
| Model 4 + severe hypo |
Model 4 |
|
| HR (CI), p-value | HR (CI), p-value | |
| Blood glucose | ||
| Log(CV) glucose | 1.316 (1.104, 1.568), 0.003 | 1.451 (1.193, 1.766), <0.001 |
| ARV glucose | 1.177 (1.017, 1.361), 0.029 | 1.272 (1.080, 1.498), 0.004 |
| HbA1c | ||
| Log(CV) HbA1c | 1.188 (0.992, 1.423), 0.061 | 1.276 (1.049, 1.553), 0.015 |
| ARV HbA1c | 1.169 (0.991, 1.379), 0.064 | 1.251 (1.049, 1.492), 0.013 |
CV: coefficient of variation; ARV: average real variability; HbA1c: glycated haemoglobin; HR: hazard ratio; CI: confidence interval.
Data are HR, 95% CI and p-values estimated by Cox proportional hazards model for all-cause mortality. Left panel shows results by additionally adjusting for cumulative hypoglycaemia in Model 4, while right panel shows results when participants with severe hypoglycaemia were excluded.
p-values in bold font show significant (p-value <0.05) risk for the primary outcome. A severe hypoglycaemia episode was defined as ‘incomplete loss of consciousness that requires assistance’ or ‘complete loss of consciousness’ occurring since the last visit.
n = 268.
Discussion
Our findings show that during the VADT glucose lowering intervention phase, visit-to-visit variability measures were significantly associated with all-cause mortality. Adjustment for standard risk factors and standard measures of glucose control (e.g. fasting glucose) did not lessen the association. These data indicate that even these relatively simple measures of visit-to-visit variation may provide additional information regarding future mortality risk. Although the number of events was substantially lower, significant associations were found for both CVD specific (n = 57) and all other (n = 109) mortality endpoints in exploratory analyses (p < 0.05 for both fasting glucose log(CV) and ARV for using Model 4).
These findings are consistent with the growing body of work demonstrating that oscillation of plasma glucose can enhance oxidative stress generation and alter endothelial function more than stable elevated levels of glucose.28,34,35 This suggests that the pattern of glucose control, not just the absolute levels, may also be a determinant of disease risk.
The VADT was a large, carefully conducted trial that provides a more carefully defined and better characterized cohort than was examined in most previously reported studies.11,19 This allows us both confidence in the ‘fasting’ nature of blood draws and in the estimates for very important potential confounders such as hypoglycaemia, comorbidity and unhealthy lifestyle behaviour; factors that have infrequently been considered (and never altogether) in analyses. The persistence of glucose variation measures as predictors of all-cause mortality after accounting for these variables provides further support for their unique and clinical importance. In addition, there were many visits over the nearly 7 years of follow-up providing many glucose measures for a robust estimate of long-term visit-to-visit variation. As the VADT was a randomized study of glucose treatment intensity (not different medication classes), participants’ diabetes medications were quite similar overall, removing an important potential contributor to glucose variation and outcomes that were less readily addressed in prior observational studies. In contrast to the sub-analysis of ADVANCE,18 this analysis was not limited to the more intensively treated arm, providing a complementary whole cohort analysis that helps make these findings more generalizable.
Our study has several limitations. The typical participant in the VADT was older, predominantly male and at high CVD risk. These results do however support the findings reported from ADVANCE,18 which included a more diverse set of T2D participants. We were not able to estimate daily glucose variation as that requires more extensive collection of daily glucose measures than was conducted within the VADT. This within day glycaemic variation could add to, or perhaps account for, the effects of visit-to-visit variation. Finally, there are potentially other unmeasured variables, including other adverse lifestyle behaviours, that may account for some of the relationship between glucose variation and mortality.
In conclusion, our study finds a strong association between higher visit-to-visit glycaemic variability and increased risk of mortality during the VADT that is independent of other traditional risk factors. These associations persist even when accounting for the increased risk for severe hypoglycaemia that accompanies greater glucose variation. These results greatly strengthen the growing body of evidence supporting the importance of glycaemic variation in diabetes complications and suggest that efforts to improve glucose control in patients may need to consider how these strategies influence glucose fluctuation.
Supplementary Material
Acknowledgements
The contents of this article do not represent the views of the US Department of Veterans Affairs or the United States Government. The authors also acknowledge the contributions of the Hines VA Cooperative Studies Program Coordinating Center. J.J.Z. and P.R. conceived and designed the study, analysed and interpreted the data, and wrote the manuscript. G.B. advised on statistical analysis methods and acquired the data. P.R. was an executive committee member for the VADT. All authors reviewed and edited the manuscript, approved the final version and are accountable for all aspects of the work. J.J.Z. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Glycemic Control and Complications in Diabetes Mellitus Type 2 (VADT) is registered at ClinicalTrials.gov with identifier: NCT00032487.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Veterans Affairs Cooperative Studies Program, Department of Veterans Affairs Office of Research and Development. Additional support was received from the National Institutes of Health (R01-067690 and 5R01-094775 to P.R.) and the American Diabetes Association (to P.R.). J.J.Z. is supported by NIH grant (K01DK106116).
Footnotes
Data availability
The datasets used and/or analysed during this study are available from the corresponding author on reasonable request as permitted by VA guidance.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Supplemental material
Supplemental material for this article is available online.
References
- 1.Wang SL, Head J, Stevens L, et al. Excess mortality and its relation to hypertension and proteinuria in diabetic patients. Diabetes Care 1996; 19: 305–312. [DOI] [PubMed] [Google Scholar]
- 2.Wei M, Gaskill SP, Haffner SM, et al. Effects of diabetes and level of glycemia on all-cause and cardiovascular mortality. Diabetes Care 1998; 21: 1167–1172. [DOI] [PubMed] [Google Scholar]
- 3.de Marco R, Locatelli F, Zoppini G, et al. Cause-specific mortality in type 2 diabetes. Diabetes Care 1999; 22: 756–761. [DOI] [PubMed] [Google Scholar]
- 4.Prentice JC, Pizer SD and Conlin PR. Identifying the independent effect of HbA1c variability on adverse health outcomes in patients with type 2 diabetes. Diabet Med 2016; 33: 1640–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ford ES and DeStefano F. Risk factors for mortality from all causes and from coronary heart disease among persons with diabetes. Am J Epidemiol 1991; 133: 1220–1230. [DOI] [PubMed] [Google Scholar]
- 6.Gall MA, Borch-Johnsen K, Hougaard P, et al. Albuminuria and poor glycemic control predict mortality in NIDDM. Diabetes 1995; 44: 1303–1309. [DOI] [PubMed] [Google Scholar]
- 7.Laakso M, Lehto S, Penttila I, et al. Lipids and lipoproteins predicting coronary heart disease mortality and morbidity in patients with non-insulin-dependent diabetes. Circulation 1993; 88: 1421–1430. [DOI] [PubMed] [Google Scholar]
- 8.Nathan DM, Genuth S, Lachin J, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993; 329: 977–986. [DOI] [PubMed] [Google Scholar]
- 9.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998; 352: 837–853. [PubMed] [Google Scholar]
- 10.Stettler C, Allemann S, Juni P, et al. Glycemic control and macrovascular disease in types 1 and 2 diabetes mellitus: meta-analysis of randomized trials. Am Heart J 2006; 152: 27–38. [DOI] [PubMed] [Google Scholar]
- 11.Forbes A, Murrells T, Mulnier H, et al. Mean HbA1c, HbA1c variability, and mortality in people with diabetes aged 70 years and older: a retrospective cohort study. Lancet Diabetes Endocrinol 2018; 6: 476–486. [DOI] [PubMed] [Google Scholar]
- 12.Currie CJ, Peters JR, Tynan A, et al. Survival as a function of HbA(1c) in people with type 2 diabetes: a retrospective cohort study. Lancet 2010; 375: 481–489. [DOI] [PubMed] [Google Scholar]
- 13.Huang ES, Liu JY, Moffet HH, et al. Glycemic control, complications, and death in older diabetic patients: the diabetes and aging study. Diabetes Care 2011; 34: 1329–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Action to Control Cardiovascular Risk in Diabetes Study Group, Gerstein HC, Miller ME, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358: 2545–2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009; 360: 129–139. [DOI] [PubMed] [Google Scholar]
- 16.ADVANCE Collaborative Group, Patel A, MacMahon S, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358: 2560–2572. [DOI] [PubMed] [Google Scholar]
- 17.Lachin JM, Bebu I, Bergenstal RM, et al. Association of glycemic variability in type 1 diabetes with progression of microvascular outcomes in the diabetes control and complications trial. Diabetes Care 2017; 40: 777–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirakawa Y, Arima H, Zoungas S, et al. Impact of visit-to-visit glycemic variability on the risks of macrovascular and microvascular events and all-cause mortality in type 2 diabetes: the ADVANCE trial. Diabetes Care 2014; 37: 2359–2365. [DOI] [PubMed] [Google Scholar]
- 19.Gorst C, Kwok CS, Aslam S, et al. Long-term glycemic variability and risk of adverse outcomes: a systematic review and meta-analysis. Diabetes Care 2015; 38: 2354–2369. [DOI] [PubMed] [Google Scholar]
- 20.Zinman B, Marso SP, Poulter NR, et al. Day-to-day fasting glycaemic variability in DEVOTE: associations with severe hypoglycaemia and cardiovascular outcomes (DEVOTE 2). Diabetologia 2018; 61: 48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou JJ, Schwenke DC, Bahn G, et al. Glycemic variation and cardiovascular risk in the veterans affairs diabetes trial. Diabetes Care 2018; 41: 2187–2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodbard D Glucose variability: a review of clinical applications and research developments. Diabetes Technol Ther 2018; 20(S2): S25–S215. [DOI] [PubMed] [Google Scholar]
- 23.Egi M, Bellomo R, Stachowski E, et al. Variability of blood glucose concentration and short-term mortality in critically ill patients. Anesthesiology 2006; 105: 244–252. [DOI] [PubMed] [Google Scholar]
- 24.Krinsley JS. Glycemic variability: a strong independent predictor of mortality in critically ill patients. Crit Care Med 2008; 36: 3008–3013. [DOI] [PubMed] [Google Scholar]
- 25.Hermanides J, Vriesendorp TM, Bosman RJ, et al. Glucose variability is associated with intensive care unit mortality. Crit Care Med 2010; 38: 838–842. [DOI] [PubMed] [Google Scholar]
- 26.Timmons JG, Cunningham SG, Sainsbury CA, et al. Inpatient glycemic variability and long-term mortality in hospitalized patients with type 2 diabetes. J Diabetes Complications 2017; 31: 479–482. [DOI] [PubMed] [Google Scholar]
- 27.Hayward RA, Reaven PD, Wiitala WL, et al. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2015; 372: 2197–2206. [DOI] [PubMed] [Google Scholar]
- 28.Brownlee M and Hirsch IB. Glycemic variability: a hemoglobin A1c-independent risk factor for diabetic complications. JAMA 2006; 295: 1707–1708. [DOI] [PubMed] [Google Scholar]
- 29.Bolli GB. Glucose variability and complications. Diabetes Care 2006; 29: 1707–1709. [DOI] [PubMed] [Google Scholar]
- 30.Skriver MV, Sandbaek A, Kristensen JK, et al. Relationship of HbA1c variability, absolute changes in HbA1c, and all-cause mortality in type 2 diabetes: a Danish population-based prospective observational study. BMJ Open Diabetes Res Care 2015; 3: e000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin CC, Chen CC, Chen FN, et al. Risks of diabetic nephropathy with variation in hemoglobin A1c and fasting plasma glucose. Am J Med 2013; 126: 1017.e1–1017.e10. [DOI] [PubMed] [Google Scholar]
- 32.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40: 373–383. [DOI] [PubMed] [Google Scholar]
- 33.Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol 2011; 173: 676–682. [DOI] [PubMed] [Google Scholar]
- 34.Ceriello A, Esposito K, Piconi L, et al. Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes 2008; 57: 1349–1354. [DOI] [PubMed] [Google Scholar]
- 35.Monnier L, Mas E, Ginet C, et al. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA 2006; 295: 1681–1687. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.