Abstract
Pancreatic ductal adenocarcinoma is most frequently detected at an advanced stage. Such late detection restricts treatment options and contributes to a dismal 5-year survival rate of 3–15%. Pancreatic ductal adenocarcinoma is relatively uncommon and screening of the asymptomatic adult population is not feasible or recommended with current modalities. However, screening of individuals in high-risk groups is recommended. Here, we review groups at high risk for pancreatic ductal adenocarcinoma, including individuals with inherited predisposition and patients with pancreatic cystic lesions. We discuss studies aimed at finding ways of identifying pancreatic ductal adenocarcinoma in high-risk groups, such as among individuals with new-onset diabetes mellitus and people attending primary and secondary care practices with symptoms that suggest this cancer. We review early detection biomarkers, explore the potential of using social media for detection, appraise prediction models developed using electronic health records and research data, and examine the application of artificial intelligence to medical imaging for the purposes of early detection.
Introduction
Pancreatic ductal adenocarcinoma remains an intractable condition and a leading cause of deaths from cancer.1 Despite advances in the diagnosis and treatment of other gastrointestinal malignancies, such as colorectal and gastric cancers,2 the 5-year survival rates remain as low as 3–15%.3,4 These dismal figures are attributed to late-stage and incurable-stage diagnosis as well as high tumour chemoresistance.5 An incurable-stage diagnosis makes most treatment options ineffective.
The need for prompt diagnosis is recognised globally,6 and a shift toward early detection has been recommended by several health-care organisations.7 Numerous observations support the benefits of early detection of pancreatic ductal adenocarcinoma. Patients diagnosed with stage 1 disease experience longer survival times than patients diagnosed with disease at more advanced stages.8 Similarly, incidentally diagnosed pancreatic ductal adenocarcinoma (ie, discovered serendipitously while screening or checking for other diseases) is associated with longer median survival than pancreatic ductal adenocarcinoma that is diagnosed when patients have symptoms.8 Survival rates are also substantially better for patients diagnosed at an operable rather than an inoperable stage.9 Unfortunately, up to 85% of patients have cancers that are not surgically resectable when identified, and over half of patients in the UK are diagnosed following a non-specific disease course leading to an emergency hospital admission.10–13
Although identifying pancreatic ductal adenocarcinoma as early as possible is essential,14,15 there are several challenges. Despite its high mortality, pancreatic ductal adenocarcinoma is relatively uncommon, with an incidence of 8–12 per 100 000 per year and a 1·3% lifetime risk of developing the disease.3 The low disease prevalence makes screening of the asymptomatic adult population unfeasible. Existing diagnostic methods would incur unacceptably high rates of false-positive findings,16 and in the context of pancreatic ductal adenocarcinoma, false positives carry considerable ramifications. In some cases, the definitive diagnosis of pancreatic ductal adenocarcinoma is only possible following surgery (when there is sufficient tumour material to make the histological diagnosis), which carries the risk of substantial morbidity and mortality.
Screening individuals in high-risk groups increases the rate of detection and reduces false-positive results.8 Several groups at high risk of pancreatic ductal adenocarcinoma have been identified, including individuals in families with an inherited risk,17,18 people with cystic lesions of the pancreas,19–21 and people older than 50 years, newly diagnosed with type 2 diabetes.22,23 The following discussion focuses on the practicality of screening for pancreatic ductal adenocarcinoma, and appraises current clinical studies aimed at facilitating its early detection.
High-risk groups
Familial or inherited risk
Two indications for an inherited risk of pancreatic ductal adenocarcinoma are established. A family history of the disease is a risk factor for development of pancreatic ductal adenocarcinoma,24,25 and germline mutations in specific genes are associated with developing the condition.26–28
Although multiple genetic mutations have been shown to be associated with pancreatic ductal adenocarcinoma, the mutations are almost as frequent in sporadic disease as in individuals with a family history.29 Moreover, families with a strong history of pancreatic ductal adenocarcinoma mostly do not have mutations in the genes known to be associated with the disease.26,27,29,30 It is probable that the genetic risk is multigenic for most people; however, it is difficult to explain families with multiple cases affecting several generations without assuming a single mutation or tightly linked mutations at a single locus. Registries of such high-risk families indicate a roughly 50% lifetime risk, regardless of whether a causative mutation is known,31 consistent with a single highly penetrant germline mutation. When the causative mutation is known,26 it segregates with the disease. However, the absence of a family history in individuals with the same specific mutations indicates that these mutations are context-specific.29
One such context is the predisposition to other cancer types. Some hereditary cancer syndromes—including hereditary breast-ovarian cancer syndrome (HBOC), Lynch syndrome 2, familial atypical multiple mole melanoma (FAMMM), Peutz-Jegher’s syndrome, and Li-Fraumeni syndrome—are associated with pancreatic ductal adenocarcinoma. As described, some families in which a mutation linked with hereditary pancreatic cancer is prevalent do not have an increased incidence of pancreatic ductal adenocarcinoma. In some of these families with hereditary pancreatic cancer mutations but no increased rate of pancreatic ductal adenocarcinoma, other forms of cancer are prevalent. For example, HBOC is characterised as an autosomal dominant predisposition for breast or ovarian cancer, and many families with HBOC have mutations in BRCA2; BRCA2 mutations are also associated with pancreatic ductal adenocarcinoma. Some families affected by BRCA2 mutations have an increased incidence of pancreatic ductal adenocarcinoma and breast or ovarian cancer. These families are classified as having both HBOC and hereditary pancreatic cancer.33 A spectrum of different BRCA2 mutations are associated with HBOC. This spectrum seems to be similar in families affected by HBOC, whether or not they have an increased incidence of pancreatic ductal adenocarcinoma.34 Even in families affected by HBOC with no BRCA2 (or BRCA1) mutation, the risk of pancreatic ductal adenocarcinoma is greatly elevated.35 Similarly, Lynch syndrome (ie, hereditary non-polyposis colorectal cancer) is associated with mismatch repair mutations that have been linked to pancreatic ductal adenocarcinoma. Not all families with Lynch syndrome have an established high risk for pancreatic ductal adenocarcinoma. Only families affected by Lynch syndrome 1 have an elevated risk of colorectal cancer, whereas Lynch syndrome 2 is associated with other cancer types, such as pancreatic ductal adenocarcinoma. Both Lynch syndrome 1 and Lynch syndrome 2 include families with no identified causative mutation. A third example of a syndrome that is seen in families with and without causative mutations associated with pancreatic ductal adenocarcinoma is FAMMM. Many members of families with FAMMM have mutations in the CDKN2A gene (encoding the p16 tumour suppressor), and some families have cases of pancreatic ductal adenocarcinoma, defining a sub-syndrome called FAMMM pancreatic cancer. This subsyndrome can be clinically defined in families without a known causative mutation. While HBOC, Lynch syndrome 2, FAMMM-pancreatic cancer, Peutz-Jegher’s syndrome, and Li-Fraumeni syndrome are associated with pancreatic ductal adenocarcinoma, none of these syndromes are characterised by multiple occurrences of pancreatic ductal adenocarcinoma. In contrast, familial pancreatic cancer is a syndrome that is defined as having multiple first-degree relatives with pancreatic ductal adenocarcinoma, exclusive of other cancer syndromes.36 Familial pancreatic cancer can be associated with mutations in BRCA2 and possibly CDKN2A or even PALB2 (a breast cancer susceptibility gene with a protein product that enables BRCA2 anchorage to nuclear structures), but in most cases the causative mutation is unknown. It is only in families with autosomal dominant predisposition, with or without a known causative mutation, that screening can be justified. In the absence of autosomal dominance, risk for an individual might be high, but such a risk is difficult or impossible to prospectively predict. Identifying autosomal dominance requires careful examination of family history, ideally with identification of germline mutations.37
Cystic lesions
Pancreatic cysts are found in approximately 8% of individuals older than 70 years.38 They are of interest because two of the three precursors to pancreatic ductal adenocarcinoma, intraductal papillary mucinous neoplasms and mucinous cystic neoplasms, are pancreatic cysts. Intraductal papillary mucinous neoplasms and mucinous cystic neoplasms are collectively referred to as mucinous cystic lesions. In contrast to the third precursor lesion, pancreatic intraepithelial neoplasia, which can only be identified on surgical histopathology, pancreatic cysts are easy to detect and are incidentally found in 3% of individuals undergoing a CT scan. Identification of mucinous cystic lesions therefore offers the potential for early detection of pancreatic ductal adenocarcinoma. Two issues complicate this simple concept. First, not all pancreatic cystic lesions are intraductal papillary mucinous neoplasms and mucinous cystic neoplasms. Many are cystic lesions with no risk of malignant transformation and thus do not require surveillance. It is estimated that a cyst seen incidentally on MRI has a 10 in 100 000 chance of being a mucinous invasive malignancy, and a 17 in 100 000 chance of being a ductal cancer.21 Second, most intraductal papillary mucinous neoplasms and mucinous cystic neoplasms do not progress to pancreatic ductal adenocarcinoma. Currently available clinical tools are imperfect at differentiating between benign cysts that are safe to discharge, mucinous cystic lesions that harbour high-grade dysplasia or pancreatic ductal adenocarcinoma and require surgical resection, and mucinous cystic lesions that have low-grade dysplasia and are safe to watch. This limitation is a considerable problem, as highlighted by the fact that 25% of patients who have a presumed mucinous cystic lesion removed are ultimately found to have a cyst with no malignant potential,39 and up to 78% of individuals with branch duct intraductal papillary mucinous neoplasms that undergo surgical resection do not have high-grade dysplasia or pancreatic ductal adenocarcinoma and in hindsight did not require surgery.40 Progress has been made in overcoming these challenges. We now know that different types of pancreatic cysts have specific mutational profiles that can be used to identify the type of cysts with a high level of confidence. For example, serous cystadenomas, one of the most common types of cysts with no malignant potential, are associated with mutations in VHL. Mucinous cystic lesions can have mutations in KRAS, whereas GNAS mutations are highly specific. Overall, almost two-thirds of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms harbour a mutation in KRAS or GNAS in the cyst fluid.41 A novel approach to the management of patients with cysts is to combine the most specific clinical features with molecular markers. A study of 862 individuals undergoing surgery for pancreatic cysts found that a combined molecular and clinical marker panel was more accurate than current clinical features alone for classifying patients who required surgery, or routine monitoring, or did not require surveillance; use of these combined markers would have decreased the number of unnecessary operations by 60% compared with the use of the conventional clinical and imaging criteria alone.41
Progress has been made towards identifying those mucinous cystic lesions that harbour high-grade dysplasia or early invasive pancreatic ductal adenocarcinoma. There is increasing knowledge about the progression of intraductal papillary mucinous neoplasms from a genetic perspective, which suggests that early lesions are heterogeneous, whereas lesions with high-grade dysplasia have a smaller number of homogeneous driver genes.42 Preliminary studies evaluating the presence of mutations in PIK3CA, SMAD4, and TP53 in cyst fluid were promising, as they identified almost 80% of intraductal papillary mucinous neoplasms with high-grade dysplasia or cancer.43 Advances also occurred in endoscopy, with the development of confocal endomicroscopes that can be passed into the cyst, providing in-vivo real-time imaging of the cyst lining. Studies have shown good sensitivity with excellent specificity for differentiating between benign cysts that require no follow-up and intraductal papillary mucinous neoplasms.44–46 Further work is needed to differentiate the small number of individuals with mucinous cystic lesions who have the highest risk of progression to pancreatic ductal adenocarcinoma and require intensive surveillance from the vast majority of individuals who have intraductal papillary mucinous neoplasms and mucinous cystic neoplasms that will never progress.
New-onset diabetes mellitus
The relationship between pancreatic ductal adenocarcinoma and diabetes is multifaceted. People with long-standing type 2 diabetes (more than 5 years) have a 1–1·5-fold increased risk of pancreatic ductal adenocarcinoma compared with the general population. However, in individuals who have had type 2 diabetes for less than 1 year, the relative risk of pancreatic ductal adenocarcinoma increases to 5·4-fold,47 with substantial research pointing towards pancreatic ductal adenocarcinoma-induced hyperglycaemia and diabetes.48 When diagnosed with pancreatic ductal adenocarcinoma, around 80% of patients have abnormal fasting glucose or glucose intolerance.49,50 This concern does not apply to other common cancers, where the prevalence of diabetes is similar to that in people without cancer.51 The diabetes experienced by most patients with pancreatic ductal adenocarcinoma is of recent onset (diagnosed less than 24–36 months before diagnosis of pancreatic ductal adenocarcinoma), and is improved by surgical resection of the tumour.48 Thus, new-onset diabetes can be considered an early warning sign of pancreatic ductal adenocarcinoma,22 and individuals with new-onset diabetes are the highest risk group for sporadic pancreatic ductal adenocarcinoma.
Approximately 0·8–1% of individuals aged over 50 years with new-onset diabetes have diabetes secondary to pancreatic ductal adenocarcinoma. However, distinguishing pancreatic ductal adenocarcinoma-associated diabetes from the more prevalent type 2 diabetes is challenging, and improved strategies are urgently required to better identify those with pancreatic ductal adenocarcinoma in the new-onset diabetes group. The opportunities that new-onset diabetes presents for early detection of pancreatic ductal adenocarcinoma have been reviewed elsewhere.52
Practicalities of screening
Who should be screened?
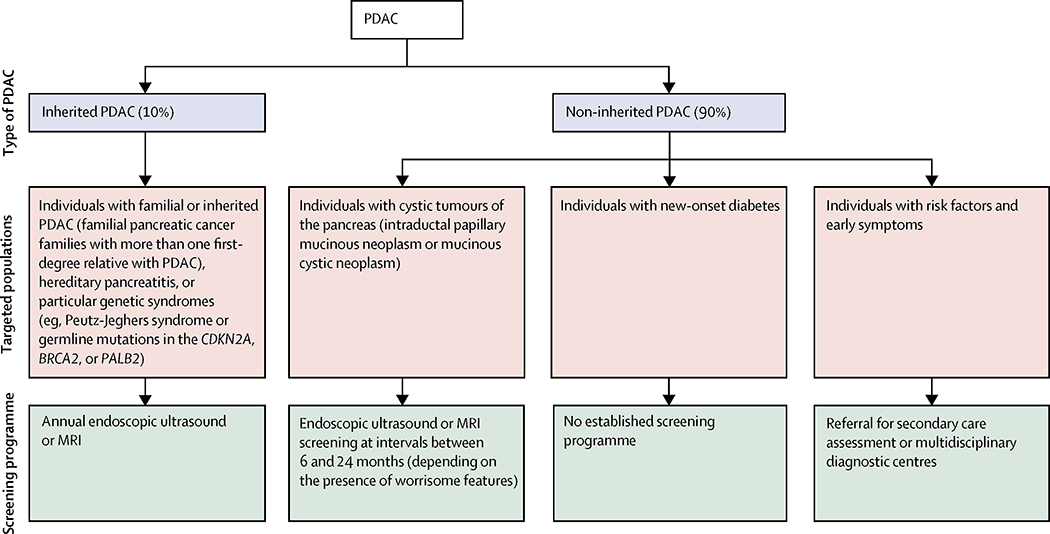
The US Preventive Services Task Force and other guidelines recommend against screening for pancreatic ductal adenocarcinoma in asymptomatic adults at average risk.6,53,54 Screening of the general population carries risk, and there is currently no evidence that it reduces mortality or is cost-effective.54,55 However, international guidelines and a white paper on the early detection of pancreatic ductal adenocarcinoma recommend targeted screening of individuals whose lifetime risk of developing pancreatic ductal adenocarcinoma is higher than 5% (figure 1).6,54 This target includes individuals with at least two first-degree relatives with pancreatic ductal adenocarcinoma, patients with hereditary pancreatitis or particular genetic syndromes (eg, Peutz-Jeghers syndrome, or germline mutations in the CDKN2A, BRCA2, or PALB2),6,54 and individuals with mucinous cystic lesions of the pancreas.19–21
Figure 1: Overview of screening programmes for pancreatic ductal adenocarcinoma.
PDAC=pancreatic ductal adenocarcinoma.
Other high-risk groups have been identified, such as individuals with new-onset diabetes (as discussed above), modifiable risk factors (eg, smoking, obesity), non-modifiable risk factors (eg, age), specific comorbidities (eg, obesity, chronic pancreatitis), or early symptoms (some of which are present for more than 1 year before diagnosis).10,12,13,56 With the exception of jaundice, weight loss, and new-onset diabetes, which indicate a considerably increased risk, most individual risk factors or symptoms (eg, dyspepsia, bloating, change in bowel habit) only provide a modest (1·5-fold to 3-fold) increased risk of developing pancreatic ductal adenocarcinoma.10,54 However, by combining these factors through cancer decision support tools, the 5% risk threshold can be reached in some individuals, defining a further group suitable for screening.10,12,13
How should individuals be screened?
Successful early detection should enable treatments that improve patient survival and wellbeing without solely increasing the time between diagnosis and death, or lead time. All screening programmes for pancreatic ductal adenocarcinoma aim to detect and treat T1N0M0 cancers or high-grade dysplastic premalignant lesions (ie, pancreatic intraepithelial neoplasia 3 or mucinous cystic tumours with high-grade dysplasia).6,54
Established screening programmes exist, including the pan-European EUROPAC registry,36 North American National Familial Pancreatic Tumor Registry,57 and the German National Case Collection for familial pancreatic cancer.58 The protocols of these prospective registry studies vary between the programmes, but they generally include cross-sectional imaging and blood tests (with tumour markers) at registration, and then annual non-radiating forms of imaging by MRI or endoscopic ultrasound. If a suspicious lesion is identified, further investigations and treatment are arranged as clinically necessary.
Approximately 8% of all pancreatic ductal adenocarcinomas are believed to arise from premalignant mucinous cystic lesions of the pancreas.59 Guidelines recommend immediate surgical resection for any lesion with high-risk stigmata, and regular surveillance with interval imaging (MRI or endoscopic ultrasound) for all mucinous or indeterminate lesions.21,60 Surveillance protocols vary between the different guidelines, but typically recommend cross-sectional imaging once every 6 months in the first year, and annually thereafter if no changes are detected. More frequent surveillance is advocated for larger lesions or lesions with worrisome features.21,60
For the newly identified high-risk groups (such as patients with new-onset diabetes), there are no established guidelines or screening programmes. However, studies underway in the USA61 and UK62 are actively investigating how best to detect pancreatic ductal adenocarcinoma in individuals with new-onset diabetes. In 2013, cancer decision support tools were incorporated into software systems of general practitioner practices in the UK.12 Because of the low incidence of pancreatic ductal adenocarcinoma, primary care doctors might see only one patient with the disease every 5 years. Although patients often show symptoms that suggest pancreatic ductal adenocarcinoma at repeated primary care consultations before diagnosis,12,13,63 they often encounter delays in the workup process.64 To address this delay, primary care doctors in the UK are gaining greater access to diagnostic investigations, including CT or rapid referral clinics (such as the newly developed multidisciplinary diagnostic centres),65 which enable the rapid assessment of patients with concerning but non-specific symptoms. Referrals can be triggered by the risk defined by cancer decision support tools or by the doctor’s clinical assessment. The usefulness and outcomes of the current programme are subject to ongoing assessment via the Accelerate, Coordinate, Evaluate Programme, an initiative supported by Cancer Research UK and Macmillan Cancer Support.65
Role for biomarkers in early detection of pancreatic ductal adenocarcinoma
Challenges and opportunities
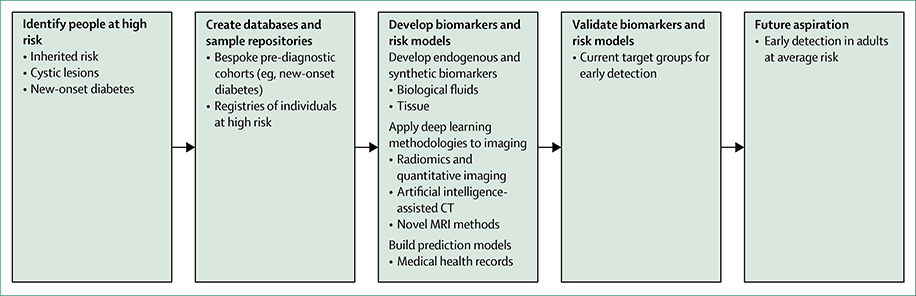
Biomarkers could play an important role in early detection of pancreatic ductal adenocarcinoma by enriching for individuals in high-risk groups who have the highest chance of a cancer diagnosis, thereby helping clinicians prioritise individuals for screening (figure 2). However, despite thousands of published papers, no single candidate biomarker has translated to clinical use for the early detection of pancreatic ductal adenocarcinoma. Indeed, biomarker development for this disease faces unique challenges. The low incidence of pancreatic ductal adenocarcinoma means that acquiring the quantity of samples necessary for biomarker development is not easy, and large national and international collaborations are required. Pancreatic tumours are highly heterogeneous, both within and between individuals.66,67 Consequently, single biomarkers will probably not have high sensitivity for detection of pancreatic ductal adenocarcinoma, and robust panels of biomarkers will be required.
Figure 2: Early detection strategies for pancreatic ductal adenocarcinoma, including identifying high-risk groups, creating resources, developing biomarkers, and constructing prediction models from medical health records and research databases.
Early detection efforts for pancreatic cancer (from top left, counterclockwise) include biospecimens from multiple locations (mouth, blood, electronic medical record, urine, stool, tissue) and deep learning applied to diagnostic imaging. Interpretation and integration of these sources of data, and other emerging areas such as synthetic biomarkers, will be key areas of research in the future.
Understanding and accounting for potential confounding factors is an important component of pancreatic ductal adenocarcinoma biomarker development.68 Most major studies now incorporate control samples from patients with different diseases, such as chronic pancreatitis.68 Moreover, awareness that the presence of obstructive jaundice can lead to false-positive biomarker findings is increasing.69–71 Currently, biomarker studies do not account well for the finding that a high proportion of patients with pancreatic ductal adenocarcinoma have diabetes;50 thus, emerging biomarkers could have an association with diabetes rather than with pancreatic ductal adenocarcinoma. Finally, a pronounced deficit in early detection studies for pancreatic ductal adenocarcinoma has been the absence of bespoke pre-diagnostic cohorts. Although large population-based cohorts that have recruited healthy individuals (such as UKCTOCS72 and EPIC73) contain patient samples that precede diagnosis of pancreatic ductal adenocarcinoma, crucial information—such as family history and comorbidity data, including the presence of chronic pancreatitis or diabetes—are not consistently available.
When calculating the cost versus benefit of a biomarker-assisted screening programme, the costs of both the initial screening and subsequent tests required to verify the diagnosis should be considered. As both true-positive and false-positive tests require further investigation, high specificity biomarkers are required. Ghatnekar and colleagues74 developed a framework for modelling cost and quality-adjusted life-years of serum biomarker-mediated early detection of pancreatic ductal adenocarcinoma in individuals with new-onset diabetes. This study, which was done within the Swedish health-care system, concluded that biomarker-mediated screening is highly desirable.
Current promising biomarkers for early detection
Using samples from patients already diagnosed with pancreatic ductal adenocarcinoma in biomarker development might result in biomarkers that are indicative of symptomatic disease. An alternative strategy is to use samples from mouse models, early-stage pancreatic ductal adenocarcinoma (stage 1 or 2), and precursor lesions (ie, pancreatic intraepithelial neoplasia, intraductal papillary mucinous neoplasm, mucinous cystic neoplasm) in biomarker development. Research into the molecular changes occurring during the sequential progression from lesion, through early-stage pancreatic ductal adenocarcinoma, and finally to advanced disease is generating a greater understanding of development and progression of pancreatic ductal adenocarcinoma. This understanding is providing a basis for designing rational strategies to develop biomarkers for early detection. Markers generated through this process represent aberrant changes at the genetic, transcriptomic, metabolomic, and proteomic level that are associated with the earliest histological stages of development of pancreatic ductal adenocarcinoma (table 1). Moreover, the use of high-risk groups as controls and combining molecular markers with clinical features is an important consideration when identifying biomarkers capable of selecting populations at highest risk of development of pancreatic ductal adenocarcinoma for screening.75
Table 1.
Biomarkers representative of early pancreatic ductal adenocarcinoma development
| Biomarkers | |
|---|---|
| Proteomic | CA19-9, CEA, CEMIP, TSP-1, TSP-2, VNN1 (downstream markers), MUC1, MUC2 |
| Metabolomic | M2-pyruvate kinase, palmitic acid, inositol, proline, ceramide, phosphatidyl choline, isocitrate |
| Genetic | KRAS, GNAS, SMAD4, TP53 |
| Transcriptomic | miR-486·5p, –16, –24, –27a, –30a.5p, –323·3p, –20a, –25, –29c, –483·5p |
CA19-9=carbohydrate antigen 19-9. CEA=carcinoembryonic antigen.
Currently, there are no biomarkers validated for early detection of pancreatic ductal adenocarcinoma. However, several published biomarkers show potential for future evaluation. Elevated concentrations of carbohydrate antigen 19–9 (CA19–9), the only biomarker routinely used in the management of pancreatic ductal adenocarcinoma, have been shown in samples taken before diagnosis.76 However, using elevated CA19–9 for screening is not recommended for screening. CA19–9 is not expressed in individuals with a Lewis-negative genotype, only 65% of patients with resectable pancreatic ductal adenocarcinoma have elevated serum levels of CA19–9,77 and the marker is increased in other benign and malignant diseases. The diagnostic value of CA19–9 could, in the future, be improved by measuring the antigen on individual proteins or using it in combination with additional markers. Aiming to find a way to distinguish pancreatic ductal adenocarcinoma from benign disease and healthy controls, Lee and colleagues78 reported an improved area under receiver operating characteristic curve (AUC) using a combination of CA19–9 and cell migration-inducing hyaluronan binding protein (CEMIP; a protein involved in the degradation of hyaluronan) compared with using CA19–9 alone (AUC=0·94 vs 0·89). Importantly, CEMIP correctly diagnosed 68 of 79 patients with pancreatic ductal adenocarcinoma whose CA19–9 concentrations were in the normal range (below 37 U/mL).
While a comprehensive review of candidate biomarkers for early detection is beyond the scope of this review, we have highlighted several novel biomarkers resulting from studies designed with early detection in mind, summarised in table 2.
Table 2.
Selected protein, DNA, and miRNA biomarkers highlighted for early detection of pancreatic ductal adenocarcinoma, reported between 2016 and 2019
| Marker type | Sample | Technique | AUC | Reference | |
|---|---|---|---|---|---|
| TSP-2 and CA19-9 | Protein | Blood plasma | ELISA and MS | 0·96 (PDAC all stages vs HC) | 79 |
| TSP-1 and CA19-9 | Protein | Blood serum | MS | 0·86 (pre-diagnostic PDAC vs HC) | 80 |
| MUC1 and MUC2 | Protein | Pancreatic juice | IHC | 0·85 (malignant vs benign IPMN) | 81 |
| LYVE-1, REG1A, and TFF1 | Protein | Urine | ELISA | 0·93 (PDAC stage 1 and 2 vs HC) | 82 |
| miR-16, –24, –27a, –30a.5p, –323·3p, –20a, –25, –29c, –483·5p and CA19–9 | miRNA and protein | Blood serum | microRNA array | 0·93 (PDAC stage 1 and 2 vs HC) | 83 |
| Cysteamine or GSH or PPAR-γ (VNN1) | Protein | Blood serum | HPLC or ELISA | 0·84, 0·86, 0·82 (PDAC vs HC) | 84 |
| Neutrophil-to-lymphocyte ratio and clinical features | Protein | Blood serum | Clinical measurement | 0·89 (non-invasive vs invasive IPMN) | 85 |
| CEMIP | Protein | Blood serum | ELISA | 0·94 (PDAC including Lewis-negative individuals vs benign disease and HC) | 86 |
| 29-protein biomarker panel | Protein | Blood serum | Antibody microarray | 0·96 (PDAC stage 1 and 2 vs HC) | 87 |
| CancerSEEK | Mutant cell-free DNA and eight circulating proteins | Blood plasma | Multianalyte test | Sensitivity over 70% when specificity is over 99% (PDAC vs HC) | 88 |
AUC=area under (receiver operating characteristic) curve. CA19-9=carbohydrate antigen 19-9. ELISA=enzyme-linked immunosorbent assay. HC=healthy controls. PLC=high-performance chromatography. IHC=immunohistochemistry. IPMN=intraductal papillary mucinous neoplasm. MS=mass spectrometry. PDAC=pancreatic ductal adenocarcinoma.
Future validation of these candidate biomarkers and others should provide sufficient evidence to support their application in early detection through the use of pre-diagnostic human samples and appropriate controls (eg, samples from established high-risk groups and individuals with benign diseases). Ultimately, the most promising early detection strategy will probably come from a discrete panel of biomarkers used in combination with clinical features.
Synthetic biomarkers
An emerging area of research and development involves the engineering of probes that are activated by tumour cells or by the stromal cells within the tumour microenvironment. The activated probes can be detected in different ways, including in the blood, in urine, or by imaging methods. These synthetic biomarkers have advantages over endogenous biomarkers (eg, CA19–9). Because signals from normal tissues are reduced or eliminated, the signals can be engineered to be highly specific for cancer, and the method can be tailored for highly sensitive detection tools. Proof of concept has been shown in several settings, including early detection of ovarian cancer in mouse models through use of cleavable substrates by upregulated proteases in the tumour microenvironment,89 sequential activation by tumour acidity and hypoxia for ultrasensitive imaging detection of tumours as small as 1 mm in mice,90 and identification of residual cancer after surgery in humans through a protease-activated fluorescent probe.91 Development of synthetic biomarkers for pancreatic ductal adenocarcinoma could substantially impact early detection efforts and other clinical applications in the future.
Ongoing trials and studies for early detection
The shortage of tools for early diagnosis of pancreatic disease (both chronic pancreatitis and pancreatic ductal adenocarcinoma) has triggered several initiatives from the National Institutes of Health in the USA, aiming for improvements in outcome for patients with these diseases. These initiatives were reinforced by the Recalcitrant Cancer Act that was passed by the US Congress in 2012 and signed in 2013. These actions were greatly facilitated by several partners, including the National Pancreas Foundation, the Kenner Family Research Fund, and the Pancreatic Cancer Action Network. A key outcome of these efforts was the creation and funding of the Chronic Pancreatis, Diabetes, and Pancreatic Cancer (CPDPC) Consortium.92 The main goals of the CPDPC Consortium are to establish large prospective cohorts of patients with carefully assessed phenotypes (both paediatric and adult), and to collect radiological data and biospecimens for diagnosis and monitoring disease status. The new-onset diabetes cohort of CPDPC is designed to recruit 10 000 patients with new onset of diabetes after age 50 years, to assess their phenotype, and to collect biospecimens.61 Onset of diabetes after age 50 was chosen as the best clinical marker available for early detection of pancreatic ductal adenocarcinoma, although the incidence of pancreatic ductal adenocarcinoma in this group is predicted to be only 1–2% over a 3-year period. The collected biospecimens and clinical data will be available for validation of promising biomarkers for the early detection of pancreatic ductal adenocarcinoma.61
Similarly, in the UK, Cancer Research UK is funding the UK Early Detection Initiative (UK-EDI)62 to recruit 2500 individuals aged over 50 years who were diagnosed with new-onset diabetes in the previous 6 months (UK new-onset diabetes cohort). This cohort study is designed to recruit from both primary and secondary care centres, and to collect questionnaire and clinical data alongside longitudinal biosamples, over 3 years. As with the new-onset diabetes cohort of the CPDPC, data and biospecimens will be made available for research on early detection of pancreatic ductal adenocarcinoma, including validation of existing biomarkers that have shown promise for early detection, and to support new discovery programmes. Working across both US and UK cohorts (with a combined assessment of both groups), researchers will assess sensitivity and specificity for detection of pancreatic ductal adenocarcinoma by analysis of biomarkers combined with epidemiological or clinical features. Finally, the cost-effectiveness of diagnosing pancreatic ductal adenocarcinoma earlier in the setting of new-onset diabetes will be assessed.
With appropriate EU funding directed towards early detection of pancreatic ductal adenocarcinoma, similar studies could be done more widely in Europe. This expansion would ensure that future biomarker-driven screening of pancreatic ductal adenocarcinoma is relevant to other countries and health-care systems within Europe.
Accelerated Diagnosis of neuroendocrine and Pancreatic TumourS (ADEPTS) is a multicentre diagnostic accuracy study, funded by Pancreatic Cancer UK, that aims to improve diagnostic pathways in pancreatic cancer.93 The overall objective of the study is to develop a diagnostic tool that combines refined cancer decision support tools with a minimally invasive blood test for circulating biomarkers to be used for screening in selected high-risk patient cohorts. The health economic implications of implementing a tool that will allow prioritisation of urgent investigations in patients with a raised combined risk score are also being studied. As part of this study, a large, multicentre, prospective sample collection of liquid and tissue biopsies from healthy and symptomatic individuals, as well as from those known to have a genetic association or high-risk cystic lesions of the pancreas, is underway.
A small pilot study suggested that pancreatic ductal adenocarcinoma-associated diabetes might be distinguished from the more prevalent type 2 diabetes by a blunted pancreatic polypeptide response to a mixed meal.94 An ongoing study called DETECT (Evaluation of a Mixed Meal Test for Diagnosis and Characterization of Pancreatogenic Diabetes Secondary to Pancreatic Cancer and Chronic Pancreatitis), which is supported by the CPDPC Consortium, aims to validate this observation.95 Additionally, this study will comprehensively examine differences in glucose homoeostasis between these subtypes of diabetes, including differences in insulin secretion, beta cell function, glucagon response, and incretin hormone response. These results will provide the opportunity to further refine our approach to early detection of pancreatic ductal adenocarcinoma in adults with new-onset diabetes.
The European Registry of Hereditary Pancreatitis and Familial Pancreatic Cancer (EUROPAC) was established in 1997 in Liverpool, UK, as a registry of patients at high risk of pancreatic ductal adenocarcinoma. Such a registry is invaluable in developing secondary screening techniques for the identification of early cancer.31,96 EUROPAC quantified that the lifetime risk of pancreatic ductal adenocarcinoma in hereditary pancreatitis is approximately 40%.97 They further characterised this risk in terms of different genotypes.98 EUROPAC has also shown that the age of onset of pancreatic ductal adenocarcinoma becomes progressively earlier with each generation in familial pancreatic cancer,99 and that diabetes predisposes for cancer in hereditary pancreatitis.100 On this basis, EUROPAC has proceeded with the development of a screening programme based on blood tests and imaging.
Advances in artificial intelligence and deep learning methodologies in relation to early detection
Using social media
Social media has forever changed how society communicates. There are 3 billion social media users worldwide,101 and this big data resource presents a unique opportunity to learn about the lives of non-experimental patients outside the walls of health-care facilities. Because patients with pancreatic ductal adenocarcinoma first experience subtle, vague symptoms that can precede the diagnosis by years, medical researchers need to learn how to use the power of social media for identifying online signals of early pancreatic ductal adenocarcinoma.
Use of social netnography—a type of ethnography that analyses perceptions and behaviours of individuals online102—might enable the development of social media and online behaviour pattern recognition algorithms for diagnosing pancreatic ductal adenocarcinoma at an early stage. To achieve this, researchers can partner with social media mining services to identify individuals with a self-reported diagnosis of pancreatic ductal adenocarcinoma online and to collect all of their previous de-identified and publicly available online posts, many of which will have been posted before their cancer diagnosis. By conducting topic modelling and thematic analysis on the corpus of posts, researchers can identify themes and online behaviour signals that could be predictive of early pancreatic ductal adenocarcinoma.
Scientists can also use prospective methods that track users who opt in across social and other online platforms. For example, researchers can use programmatic display adverts and other digital channels to find online users who precisely match the attributes of a previously identified pancreatic ductal adenocarcinoma segment. After the opt-in group has been built, vast and highly granular online insights can be prospectively collected and analysed in real time. These online insights can cover many data categories, such as demographic, behavioural, contextual, and diagnosis of pancreatic ductal adenocarcinoma, among many others. These data could then lead to robust pattern recognition algorithms—informed by social media behaviour, online behaviour, and commercial data—which might signal early pancreatic ductal adenocarcinoma and facilitate earlier diagnosis and treatment.
Models based on electronic health records
Electronic health records offer opportunities to use longitudinal and cumulative health-care data to build prediction models. Parametric models that can identify high risk for pancreatic ductal adenocarcinoma among individuals with new-onset diabetes, or new-onset pre-diabetes, have been developed, with varying predictive performances.103–106 A model based on patients with new-onset diabetes residing in Minnesota, USA, incorporated age at onset of diabetes and changes in weight and blood glucose to identify people with a 4·5% 3-year predicted risk of developing pancreatic ductal adenocarcinoma, with 78% sensitivity and 80% specificity (AUC 0·87).103 Another electronic health record model, developed using a UK population with new-onset diabetes, incorporated age, BMI change, smoking, diabetes medications, proton-pump-inhibitors, changes in haemoglobin A1c, total cholesterol, creatinine, and alkaline phosphatase. Among individuals with new-onset diabetes, this model identifies a population with 5% 3-year predicted risk of pancreatic ductal adenocarcinoma, with 11% sensitivity and 99·7% specificity (AUC 0·82).104 The same model applied to individuals with pre-diabetes showed lower accuracy (AUC 0·71), but consistent direction of association with pancreatic ductal adenocarcinoma.105 A model based on Medicare claims among individuals with new-onset diabetes incorporated multiple health indicators, including pancreatitis, dyspepsia, depression, abdominal pain, weight jaundice, and nausea or vomiting. This model has been applied to an older population (≥68 years) with new-onset diabetes, and identified people at 3·5% predicted risk of pancreatic ductal adenocarcinoma over 1 year (AUC 0·73).106 Further models based on insurance claims have been developed, with poor diagnostic performance (AUC 0·73).106,107 These models incorporate parametric106 or machine-learning methods,107 and include diagnoses such as pancreatitis, dyspepsia, abdominal pain, weight loss, and jaundice. Reliance on coded diagnoses without laboratory parameters limited the performance of these models. Data derived from routine health examination in South Korea have been used to develop a time-to-event prediction model incorporating age, sex, height, BMI, smoking, alcohol consumption, blood, and urine glucose.108 This model performed as well (AUC=0·81) as the UK model,105 but had low positive predictive value (0·3–0·4% risk over 10 years) because it was evaluated in the general unselected population aged 40 years and older.108 A consistent performance characteristic of electronic health record models is high specificity and low sensitivity, leading to high false-negative rates. Thus, these models might have insufficient value as the first tool to identify people who need further work-up for pancreatic ductal adenocarcinoma risk. Beyond previously mentioned studies, development of models incorporating both parametric and machine-learning methods on selected patients are underway at large US health systems, such as the Kaiser Permanente Southern California and the VA Health System (PRO-TECT study).
Models based on research data
Data specifically collected for research purposes or as part of population-based surveys have been valuable for identifying populations at high risk for pancreatic ductal adenocarcinoma.109–111 Data from the US National Health Interview Survey and the Prostate, Lung, Colon, and Ovarian Trial were used to develop a prediction model for pancreatic ductal adenocarcinoma based on artificial neural networks. This model reached an AUC of 0·85 and incorporated data on age, sex, race and ethnicity, diabetes, emphysema, asthma, stroke, cardiovascular diseases, ulcers, other cancer, hypertension, smoking status, physical activity, alcohol consumption, and family history of pancreatic ductal adenocarcinoma. Other models based on research interviews have not reached similar levels of performance, but have consistently shown that age, sex, smoking, diabetes, family history of pancreatic ductal adenocarcinoma, abdominal symptoms, and blood group point to an increased risk of pancreatic ductal adenocarcinoma. Because information on smoking or drinking intensity and blood type are not readily available in electronic health records, implementation of models based on research data poses some challenges for the general population.
Deep learning methodologies applied to abdominal imaging
The role of imaging for early detection of pancreatic ductal adenocarcinoma was reviewed in 2019.52 Here, we focus on the emergence of artificial intelligence in this context, in terms of its application to imaging. Notably, artificial intelligence could play an important role in early detection of pancreatic ductal adenocarcinoma by identifying not only the physical location of the primary tumour, but also its secondary effects on the body. For identification of the primary tumour, Fishman and colleagues have described a radiomics-based machine learning algorithm to differentiate pancreatic ductal adenocarcinoma from benign situations (ie, healthy pancreas and pancreatitis), with high specificity and sensitivity.112 Other applications of radiomics and quantitative imaging approaches have shown that the enhancement and morphology of the primary tumours have biological underpinnings and clinical relevance,113–115 suggesting that quantitative imaging and further application of artificial intelligence to these imaging features can provide non-invasive insight into the disease. This insight could have relevance to early detection by improving stratification and personalised approaches to screening in individuals at high risk.
In terms of secondary effects of pancreatic cancer on the body, weight loss has been validated as one of three key factors to predict early stage disease in patients with new-onset diabetes,103 and exocrine insufficiency appears to be a contributing cause.116 Indeed, sarcopenia and fat loss are known to be part of a wasting syndrome of pancreatic ductal adenocarcinoma and might have prognostic implications.117,118 The non-invasive measurement of different body compartments has already been fully automated and applied to pancreatic ductal adenocarcinoma through artificial intelligence methodologies, and this application could be easily incorporated into the evaluation of routine diagnostic imaging.119 For patients undergoing screening or imaging for reasons other than the pancreas, the measurement of body compartments—especially over time—could provide important metrics in the earlier detection of pancreatic cancer.
Artificial intelligence-assisted CT methods and novel MRI methods
A considerable concern in the field is that current imaging methods might not be adequate to identify tumours in the pancreas at a stage when treatment would be optimal. A retrospective review of CT scans done for other indications showed no evidence of a pancreatic mass in most patients 6 months or earlier before the diagnosis of pancreatic ductal adenocarcinoma.120,121 Thus, there is a need for advanced external body imaging techniques to improve detection of pancreatic ductal adenocarcinoma at an earlier stage than is presently possible. The advances in methods for external body imaging should be accompanied by endoscopic advancements in imaging to obtain tissue for validating the diagnosis.
Medical imaging (such as CT and MRI) plays an essential role in diagnosis of pancreatic ductal adenocarcinoma by allowing a comprehensive evaluation of the morphological and biological changes in parenchyma and duct of the pancreas. The ability to detect precancerous tissue changes in the pancreas using medical imaging among individuals at high risk has also been shown.110,122–125 However, visual assessment by imaging physicians is qualitative, subjective, and prone to errors and intra-observer and inter-observer variabilities. More importantly, many distinguishing features of images are hidden from human observers.
Artificial intelligence is a powerful analysis tool based on the human brain’s neural structure.126 Roffman and colleagues127 used artificial intelligence to predict non-melanoma skin cancer by using personal health data (eg, sex, race, Hispanic ethnicity, hypertension, heart disease, exercise habits, history of stroke) commonly available in electronic medical record systems. Muhammad and colleagues used artificial intelligence to analyse available personal health data to calculate risk for pancreatic ductal adenocarcinoma in the general population and to identify high-risk individuals.109 Imaging represents more sensitive and specific information for parenchyma and duct of the pancreas than personal health data. Radiomic analysis of medical images using deep learning might allow identification of unique image features in pre-diagnostic images to allow accurate prediction of pancreatic ductal adenocarcinoma in the near future.
Summary
A study of 3·9 million patients with cancer in seven countries (Australia, Canada, Denmark, Ireland, New Zealand, Norway, and the UK) examining seven sites of cancer (oesophagus, stomach, colon, rectum, pancreas, lung, and ovary) found that pancreatic ductal adenocarcinoma had the lowest 5-year survival rates among the investigated cancer types, ranging from 7·9% in the UK to 14·6% in Australia.4 Early diagnosis will undoubtedly play an important role in improving these figures, and, as our review points out, progress has been made. The establishment of new tailor-made cohorts (of individuals with new-onset diabetes or with symptoms) provides unique pre-diagnostic resources for biological and epidemiological marker discovery and validation. Careful and ethical use of existing data—whether through social media or electronic health records—has the power to facilitate prediction models, whereas artificial intelligence applied to imaging offers the possibility of detecting earlier lesions. With respect to mucinous cysts, identifying the few individuals with mucinous cystic lesions at the highest risk of progression to pancreatic ductal adenocarcinoma is still a key knowledge gap. Much work remains to be done to improve the early detection of pancreatic ductal adenocarcinoma, including the ongoing studies reviewed here. The cohort studies underway are important in many respects, not least because they serve to increase awareness among health-care providers and patients of the symptoms of pancreatic ductal adenocarcinoma and its link with new-onset diabetes. Advances in early detection will go together with improvements in treatment to extend survival for people with pancreatic ductal adenocarcinoma.
Search strategy and selection criteria.
References for this review were identified through searches of PubMed for papers published in English with the search terms “early detection”, “pancreatic cancer”, “high-risk”, “screening”, “artificial intelligence”, and “biomarker” from Jan 1, 1993, until Aug 31, 2019. Articles were also identified through searches of the authors’ own files. The final reference list was generated on the basis of relevance to the scope of this review, with a focus on the most recently published papers.
Acknowledgments
SPP is supported by the UCLH/UCL Comprehensive Biomedical Centre, which receives a proportion of funding from the Department of Health’s National Institute for Health Research (NIHR) Biomedical Research Centres funding scheme. PAH is funded by the National Cancer Institute and National Institute of Diabetes and Digestive and Kidney Diseases (U01DK108327). CYJ (R21CA220073, R01CA230442, U01DK108314) and SJP (U01 DK108314) are supported by the US National Institutes of Health. AML is supported by the Lustgarten Foundation for Pancreatic Cancer Research, The Sol Goldman Center for Pancreatic Cancer Research, The Benjamin Baker Scholarship, and the National Institutes of Health Grants P50-CA062924. CVA is supported by a Clinical and Translational Science Institute grant from the National Center of Advancing Translational Sciences (UL1TR001881–01). EJK is supported by the US National Institutes of Health (U54CA210181–01, 1R01CA218004–01A1, 1U01CA200468–01, U01CA196403–01). EC, LO, CH, and WG are funded by Cancer Research UK grants (C7690/A26881, C52547/A28210). EC is funded by North West Cancer Research, Pancreatic Cancer Action, and Pancreatic Cancer Research Fund. CH is supported by National Institute for Health Research (Research for patient benefit), Pancreatic Cancer UK, and the Royal College of Surgeons. The content is solely the responsibility of the authors and does not necessarily represent the official views of funders.
Declaration of interests
CH reports grants from Cancer Research UK, The National Institute for Health Research (Research for patient benefit), Pancreatic Cancer UK, and the Royal College of Surgeons. AML reports grants from the US National Institutes of Health (P50-CA062924), grants from Lustgarten Foundation for Pancreatic Cancer Research, a scholarship from The Benjamin Baker Scholarship, and general support from The Sol Goldman Center for Pancreatic Cancer Research, during the conduct of the study. In addition, AML is named on a pending patent for CancerSEEK, a blood test that screens for eight cancers including pancreatic cancer, the terms of which are managed by Johns Hopkins University in accordance with its conflict of interest policies. EJK reports grants from Philips Healthcare, grants from Project Purple, grants from National Institutes of Health, during the conduct of the study; royalities from Taylor and Francis, a limited liability company, outside the submitted work. SJP reports grants from the US National Institutes of Health, during the conduct of the study. LO, CH, WG, and EC are named on a patent, owned by the University of Liverpool, for two early pancreatic cancer detection biomarkers that are not discussed in the review.
Footnotes
SPP, MGK, PAH, CVA, AN, CYJ, and DL declare no competing interests.
Contributor Information
Stephen P Pereira, Institute for Liver and Digestive Health, University College London, London, UK.
Lucy Oldfield, Department of Molecular and Clinical Cancer Medicine, Institute of Translational Medicine, University of Liverpool, UK.
Alexander Ney, Institute for Liver and Digestive Health, University College London, London, UK.
Phil A Hart, Division of Gastroenterology, Hepatology, and Nutrition, Ohio State University Wexner Medical Center, Columbus, OH, USA.
Margaret G Keane, Division of Gastroenterology and Hepatology, Johns Hopkins University, Baltimore, MD, USA.
Stephen J Pandol, Department of Medicine, Division of Digestive and Liver Diseases, Cedars-Sinai Medical Center, Los Angeles, CA, USA.
Debiao Li, Cedars-Sinai Medical Center, Los Angeles, CA, USA.
William Greenhalf, Department of Molecular and Clinical Cancer Medicine, Institute of Translational Medicine, University of Liverpool, UK.
Christie Y Jeon, Department of Medicine, Division of Digestive and Liver Diseases, Cedars-Sinai Medical Center, Los Angeles, CA, USA.
Eugene J Koay, University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Christopher V Almario, Department of Medicine, Division of Digestive and Liver Diseases, Cedars-Sinai Medical Center, Los Angeles, CA, USA.
Christopher Halloran, Department of Molecular and Clinical Cancer Medicine, Institute of Translational Medicine, University of Liverpool, UK.
Anne Marie Lennon, Division of Gastroenterology and Hepatology, Johns Hopkins University, Baltimore, MD, USA.
Eithne Costello, Department of Molecular and Clinical Cancer Medicine, Institute of Translational Medicine, University of Liverpool, UK.
References
- 1.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014; 74: 2913–21. [DOI] [PubMed] [Google Scholar]
- 2.Allemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018; 391: 1023–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68: 394–424. [DOI] [PubMed] [Google Scholar]
- 4.Arnold M, Rutherford MJ, Bardot A, et al. Progress in cancer survival, mortality, and incidence in seven high-income countries 1995–2014 (ICBP SURVMARK-2): a population-based study. Lancet Oncol 2019; 20: 1493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kamisawa T, Wood LD, Itoi T, Takaori K. Pancreatic cancer. Lancet 2016; 388: 73–85. [DOI] [PubMed] [Google Scholar]
- 6.Canto MI, Harinck F, Hruban RH, et al. International Cancer of the Pancreas Screening (CAPS) Consortium summit on the management of patients with increased risk for familial pancreatic cancer. Gut 2013; 62: 339–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO. Cancer prevention and control in the context of an integrated approach: report by the Secretariat. 2016. https://apps.who.int/gb/ebwha/pdf_files/EB140/B140_31-en.pdf (accessed Dec 5, 2019).
- 8.Poruk KE, Firpo MA, Adler DG, Mulvihill SJ. Screening for pancreatic cancer: why, how, and who? Ann Surg 2013; 257: 17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neoptolemos JP, Kleeff J, Michl P, Costello E, Greenhalf W, Palmer DH. Therapeutic developments in pancreatic cancer: current and future perspectives. Nat Rev Gastroenterol Hepatol 2018; 15: 333–48. [DOI] [PubMed] [Google Scholar]
- 10.Keane MG, Horsfall L, Rait G, Pereira SP. A case-control study comparing the incidence of early symptoms in pancreatic and biliary tract cancer. BMJ Open 2014; 4: e005720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou Y, Abel GA, Hamilton W, et al. Diagnosis of cancer as an emergency: a critical review of current evidence. Nat Rev Clin Oncol 2017; 14: 45–56. [DOI] [PubMed] [Google Scholar]
- 12.Hippisley-Cox J, Coupland C. Identifying patients with suspected pancreatic cancer in primary care: derivation and validation of an algorithm. Br J Gen Pract 2012; 62: e38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stapley S, Peters TJ, Neal RD, Rose PW, Walter FM, Hamilton W. The risk of pancreatic cancer in symptomatic patients in primary care: a large case-control study using electronic records. Br J Cancer 2012; 106: 1940–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strobel O, Neoptolemos J, Jager D, Buchler MW. Optimizing the outcomes of pancreatic cancer surgery. Nat Rev Clin Oncol 2019; 16: 11–26. [DOI] [PubMed] [Google Scholar]
- 15.Ghaneh P, Kleeff J, Halloran CM, et al. The impact of positive resection margins on survival and recurrence following resection and adjuvant chemotherapy for pancreatic ductal adenocarcinoma. Ann Surg 2019; 269: 520–29. [DOI] [PubMed] [Google Scholar]
- 16.Hart PA, Chari ST. Is screening for pancreatic cancer in high-risk individuals one step closer or a fool’s errand? Clin Gastroenterol Hepatol 2019; 17: 36–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petersen GM. Familial pancreatic adenocarcinoma. Hematol Oncol Clin North Am 2015; 29: 641–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Canto MI, Almario JA, Schulick RD, et al. Risk of neoplastic progression in individuals at high risk for pancreatic cancer undergoing long-term surveillance. Gastroenterology 2018; 155: 740–51.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kimura W, Moriya T, Hirai I, et al. Multicenter study of serous cystic neoplasm of the Japan pancreas society. Pancreas 2012; 41: 380–87. [DOI] [PubMed] [Google Scholar]
- 20.Del Chiaro M, Verbeke C, Salvia R, et al. European experts consensus statement on cystic tumours of the pancreas. Dig Liver Dis 2013; 45: 703–11. [DOI] [PubMed] [Google Scholar]
- 21.Vege SS, Ziring B, Jain R, Moayyedi P, Clinical Guidelines Committee, American Gastroenterology Association. American gastroenterological association institute guideline on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology 2015; 148: 819–22; quize12–3. [DOI] [PubMed] [Google Scholar]
- 22.Andersen DK, Korc M, Petersen GM, et al. Diabetes, pancreatogenic diabetes, and pancreatic cancer. Diabetes 2017; 66: 1103–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharma A, Smyrk TC, Levy MJ, Topazian MA, Chari ST. Fasting blood glucose levels provide estimate of duration and progression of pancreatic cancer before diagnosis. Gastroenterology 2018; 155: 490–500.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fernandez E, La Vecchia C, D’Avanzo B, Negri E, Franceschi S. Family history and the risk of liver, gallbladder, and pancreatic cancer. Cancer Epidemiol Biomarkers Prev 1994; 3: 209–12. [PubMed] [Google Scholar]
- 25.Hamada T, Yuan C, Yurgelun MB, et al. Family history of cancer, Ashkenazi Jewish ancestry, and pancreatic cancer risk. Br J Cancer 2019; 120: 848–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hahn SA, Greenhalf B, Ellis I, et al. BRCA2 germline mutations in familial pancreatic carcinoma. J Natl Cancer Inst 2003; 95: 214–21. [DOI] [PubMed] [Google Scholar]
- 27.Slater EP, Langer P, Niemczyk E, et al. PALB2 mutations in European familial pancreatic cancer families. Clin Genet 2010; 78: 490–94. [DOI] [PubMed] [Google Scholar]
- 28.Mukherjee B, Delancey JO, Raskin L, et al. Risk of non-melanoma cancers in first-degree relatives of CDKN2A mutation carriers. J Natl Cancer Inst 2012; 104: 953–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu C, Hart SN, Polley EC, et al. Association between inherited germline mutations in cancer predisposition genes and risk of pancreatic cancer. JAMA 2018; 319: 2401–09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grützmann R, McFaul C, Bartsch DK, et al. No evidence for germline mutations of the LKB1/STK11 gene in familial pancreatic carcinoma. Cancer Lett 2004; 214: 63–68. [DOI] [PubMed] [Google Scholar]
- 31.Greenhalf W, Malats N, Nilsson M, Bartsch D, Neoptolemos J. International registries of families at high risk of pancreatic cancer. Pancreatology 2008; 8: 558–65. [DOI] [PubMed] [Google Scholar]
- 32.Bujanda L, Herreros-Villanueva M. Pancreatic cancer in Lynch syndrome patients. J Cancer 2017; 8: 3667–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iqbal J, Ragone A, Lubinski J, et al. The incidence of pancreatic cancer in BRCA1 and BRCA2 mutation carriers. Br J Cancer 2012; 107: 2005–09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rebbeck TR, Friebel TM, Friedman E, et al. Mutational spectrum in a worldwide study of 29 700 families with BRCA1 or BRCA2 mutations. Hum Mutat 2018; 39: 593–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wendt C, Lindblom A, Arver B, von Wachenfeldt A, Margolin S. Tumour spectrum in non-BRCA hereditary breast cancer families in Sweden. Hered Cancer Clin Pract 2015; 13: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grocock CJ, Vitone LJ, Harcus MJ, Neoptolemos JP, Raraty MG, Greenhalf W. Familial pancreatic cancer: a review and latest advances. Adv Med Sci 2007; 52: 37–49. [PubMed] [Google Scholar]
- 37.Sheel ARG, Harrison S, Sarantitis I, et al. Identification of cystic lesions by secondary screening of familial pancreatic cancer (FPC) kindreds is not associated with the stratified risk of cancer. Am J Gastroenterol 2019; 114: 155–64. [DOI] [PubMed] [Google Scholar]
- 38.Lee KS, Sekhar A, Rofsky NM, Pedrosa I. Prevalence of incidental pancreatic cysts in the adult population on MR imaging. Am J Gastroenterol 2010; 105: 2079–84. [DOI] [PubMed] [Google Scholar]
- 39.Valsangkar NP, Morales-Oyarvide V, Thayer SP, et al. 851 resected cystic tumors of the pancreas: a 33-year experience at the Massachusetts General Hospital. Surgery 2012; 152 (suppl 1): S4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sahora K, Mino-Kenudson M, Brugge W, et al. Branch duct intraductal papillary mucinous neoplasms: does cyst size change the tip of the scale? A critical analysis of the revised international consensus guidelines in a large single-institutional series. Ann Surg 2013; 258: 466–75. [DOI] [PubMed] [Google Scholar]
- 41.Springer S, Masica DL, Dal Molin M, et al. A multimodality test to guide the management of patients with a pancreatic cyst. Sci Transl Med 2019; 11: eaav4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fischer CG, Beleva Guthrie V, Braxton AM, et al. Intraductal papillary mucinous neoplasms arise from multiple independent clones, each with distinct mutations. Gastroenterology 2019; 157: 1123–37.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Singhi AD, McGrath K, Brand RE, et al. Preoperative next-generation sequencing of pancreatic cyst fluid is highly accurate in cyst classification and detection of advanced neoplasia. Gut 2018; 67: 2131–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krishna SG, Hart PA, Malli A, et al. Endoscopic ultrasound-guided confocal laser endomicroscopy increases accuracy of differentiation of pancreatic cystic lesions. Clin Gastroenterol Hepatol 2019; published online Jun 18. DOI: 10.1186/S1542-3565(19)30648-2. [DOI] [PubMed] [Google Scholar]
- 45.Napoleon B, Palazzo M, Lemaistre AI, et al. Needle-based confocal laser endomicroscopy of pancreatic cystic lesions: a prospective multicenter validation study in patients with definite diagnosis. Endoscopy 2019; 51: 825–35. [DOI] [PubMed] [Google Scholar]
- 46.Keane MG, Wehnert N, Perez-Machado M, et al. A prospective trial of CONfocal endomicroscopy in CYSTic lesions of the pancreas: CONCYST-01. Endosc Int Open 2019; 7: E1117–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ben Q, Xu M, Ning X, et al. Diabetes mellitus and risk of pancreatic cancer: a meta-analysis of cohort studies. Eur J Cancer 2011; 47: 1928–37. [DOI] [PubMed] [Google Scholar]
- 48.Hart PA, Bellin MD, Andersen DK, et al. Type 3c (pancreatogenic) diabetes mellitus secondary to chronic pancreatitis and pancreatic cancer. Lancet Gastroenterol Hepatol 2016; 1: 226–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Permert J, Ihse I, Jorfeldt L, von Schenck H, Arnqvist HJ, Larsson J. Pancreatic cancer is associated with impaired glucose metabolism. Eur J Surg 1993; 159: 101–07. [PubMed] [Google Scholar]
- 50.Pannala R, Leirness JB, Bamlet WR, Basu A, Petersen GM, Chari ST. Prevalence and clinical profile of pancreatic cancer-associated diabetes mellitus. Gastroenterology 2008; 134: 981–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aggarwal G, Kamada P, Chari ST. Prevalence of diabetes mellitus in pancreatic cancer compared to common cancers. Pancreas 2013; 42: 198–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Singhi AD, Koay EJ, Chari ST, Maitra A. Early detection of pancreatic cancer: opportunities and challenges. Gastroenterology 2019; 156: 2024–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Owens DK, Davidson KW, Krist AH, et al. Screening for pancreatic cancer: US Preventive Services Task Force Reaffirmation Recommendation Statement. JAMA 2019; 322: 438–44. [DOI] [PubMed] [Google Scholar]
- 54.Kenner BJ, Chari ST, Cleeter DF, Go VL. Early detection of sporadic pancreatic cancer: strategic map for innovation—a white paper. Pancreas 2015; 44: 686–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Henrikson NB, Aiello Bowles EJ, Blasi PR, et al. Screening for pancreatic cancer: updated evidence report and systematic review for the US preventive services task force. JAMA 2019; 322: 445–54. [DOI] [PubMed] [Google Scholar]
- 56.Gullo L, Tomassetti P, Migliori M, Casadei R, Marrano D. Do early symptoms of pancreatic cancer exist that can allow an earlier diagnosis? Pancreas 2001; 22: 210–13. [DOI] [PubMed] [Google Scholar]
- 57.Hruban RH, Petersen GM, Goggins M, et al. Familial pancreatic cancer. Ann Oncol 1999; 10 (suppl 4): 69–73. [PubMed] [Google Scholar]
- 58.Schneider R, Slater EP, Sina M, et al. German national case collection for familial pancreatic cancer (FaPaCa): ten years experience. Fam Cancer 2011; 10: 323–30. [DOI] [PubMed] [Google Scholar]
- 59.Le H, Ziogas A, Rhee JM, Lee JG, Lipkin SM, Zell JA. A population-based, descriptive analysis of malignant intraductal papillary mucinous neoplasms of the pancreas. Cancer Epidemiol Biomarkers Prev 2008; 17: 2737–41. [DOI] [PubMed] [Google Scholar]
- 60.European Study Group on Cystic Tumours of the Pancreas. European evidence-based guidelines on pancreatic cystic neoplasms. Gut 2018; 67: 789–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Maitra A, Sharma A, Brand RE, et al. A prospective study to establish a new-onset diabetes cohort: from the consortium for the study of chronic pancreatitis, diabetes, and pancreatic cancer. Pancreas 2018; 47: 1244–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.UK Early Detection Initiative, Liverpool Clinical Trials Centre, Cancer Research UK. UK early detection initiative for pancreatic cancer. www.uk-edi.co.uk (accessed Sept 17, 2019).
- 63.Lyratzopoulos G, Neal RD, Barbiere JM, Rubin GP, Abel GA. Variation in number of general practitioner consultations before hospital referral for cancer: findings from the 2010 National Cancer Patient Experience Survey in England. Lancet Oncol 2012; 13: 353–65. [DOI] [PubMed] [Google Scholar]
- 64.Pancreatic Cancer UK. Study for survival. 2011. https://www.pancreaticcancer.org.uk/media/86664/study-for-surivial-report-final.pdf (accessed Dec 5, 2019). [Google Scholar]
- 65.Cancer Research UK. Accelerate, Coordinate, Evaluate (ACE) programme. 2019. https://www.cancerresearchuk.org/health-professional/diagnosis/accelerate-coordinate-evaluate-ace-programme/multidisciplinary-diagnostic-centres-mdcs (accessed Sept 2, 2019). [Google Scholar]
- 66.Campbell PJ, Yachida S, Mudie LJ, et al. The patterns and dynamics of genomic instability in metastatic pancreatic cancer. Nature 2010; 467: 1109–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yachida S, Jones S, Bozic I, et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature 2010; 467: 1114–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jenkinson C, Earl J, Ghaneh P, et al. Biomarkers for early diagnosis of pancreatic cancer. Expert Rev Gastroenterol Hepatol 2015; 9: 305–15. [DOI] [PubMed] [Google Scholar]
- 69.Yan L, Tonack S, Smith R, et al. Confounding effect of obstructive jaundice in the interpretation of proteomic plasma profiling data for pancreatic cancer. J Proteome Res 2009; 8: 142–48. [DOI] [PubMed] [Google Scholar]
- 70.Tonack S, Jenkinson C, Cox T, et al. iTRAQ reveals candidate pancreatic cancer serum biomarkers: influence of obstructive jaundice on their performance. Br J Cancer 2013; 108: 1846–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nie S, Lo A, Wu J, et al. Glycoprotein biomarker panel for pancreatic cancer discovered by quantitative proteomics analysis. J Proteome Res 2014; 13: 1873–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Menon U, Gentry-Maharaj A, Ryan A, et al. Recruitment to multicentre trials—lessons from UKCTOCS: descriptive study. BMJ 2008; 337: a2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Riboli E, Hunt KJ, Slimani N, et al. European Prospective Investigation into Cancer and Nutrition (EPIC): study populations and data collection. Public Health Nutr 2002; 5: 1113–24. [DOI] [PubMed] [Google Scholar]
- 74.Ghatnekar O, Andersson R, Svensson M, et al. Modelling the benefits of early diagnosis of pancreatic cancer using a biomarker signature. Int J Cancer 2013; 133: 2392–97. [DOI] [PubMed] [Google Scholar]
- 75.Springer S, Wang Y, Dal Molin M, et al. A combination of molecular markers and clinical features improve the classification of pancreatic cysts. Gastroenterology 2015; 149: 1501–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.O’Brien DP, Sandanayake NS, Jenkinson C, et al. Serum CA19–9 is significantly upregulated up to 2 years before diagnosis with pancreatic cancer: implications for early disease detection. Clin Cancer Res 2015; 21: 622–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Goggins M Molecular markers of early pancreatic cancer. J Clin Oncol 2005; 23: 4524–31. [DOI] [PubMed] [Google Scholar]
- 78.Lee HS, Jang CY, Kim SA, et al. Combined use of CEMIP and CA 19–9 enhances diagnostic accuracy for pancreatic cancer. Sci Rep 2018; 8: 3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kim J, Bamlet WR, Oberg AL, et al. Detection of early pancreatic ductal adenocarcinoma with thrombospondin-2 and CA19–9 blood markers. Sci Transl Med 2017; 9: eaah5583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jenkinson C, Elliott VL, Evans A, et al. Decreased serum thrombospondin-1 levels in pancreatic cancer patients up to 24 months prior to clinical diagnosis: association with diabetes mellitus. Clin Cancer Res 2016; 22: 1734–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tanaka M, Heckler M, Liu B, Heger U, Hackert T, Michalski CW. Cytologic analysis of pancreatic juice increases specificity of detection of malignant IPMN—a systematic review. Clin Gastroenterol Hepatol 2019; 17: 2199–2211.e21. [DOI] [PubMed] [Google Scholar]
- 82.Radon TP, Massat NJ, Jones R, et al. Identification of a three-biomarker panel in urine for early detection of pancreatic adenocarcinoma. Clin Cancer Res 2015; 21: 3512–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Johansen JS, Calatayud D, Albieri V, et al. The potential diagnostic value of serum microRNA signature in patients with pancreatic cancer. Int J Cancer 2016; 139: 2312–24. [DOI] [PubMed] [Google Scholar]
- 84.Kang M, Qin W, Buya M, et al. VNN1, a potential biomarker for pancreatic cancer-associated new-onset diabetes, aggravates paraneoplastic islet dysfunction by increasing oxidative stress. Cancer Lett 2016; 373: 241–50. [DOI] [PubMed] [Google Scholar]
- 85.Gemenetzis G, Bagante F, Griffin JF, et al. Neutrophil-to-lymphocyte ratio is a predictive marker for invasive malignancy in intraductal papillary mucinous neoplasms of the pancreas. Ann Surg 2017; 266: 339–45. [DOI] [PubMed] [Google Scholar]
- 86.Lee HS, Jang CY, Kim SA, et al. Combined use of CEMIP and CA 19–9 enhances diagnostic accuracy for pancreatic cancer. Sci Rep 2018; 8: 3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mellby LD, Nyberg AP, Johansen JS, et al. Serum biomarker signature-based liquid biopsy for diagnosis of early-stage pancreatic cancer. J Clin Oncol 2018; 36: 2887–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cohen JD, Li L, Wang Y, et al. Detection and localization of surgically resectable cancers with a multi-analyte blood test. Science 2018; 359: 926–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kwon EJ, Dudani JS, Bhatia SN. Ultrasensitive tumour-penetrating nanosensors of protease activity. Nat Biomed Eng 2017; 1: 0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zheng X, Mao H, Huo D, Wu W, Liu B, Jiang X. Successively activatable ultrasensitive probe for imaging tumor acidity and hypoxia. Nat Biomed Eng 2017; 1: 0057. [Google Scholar]
- 91.Whitley MJ, Cardona DM, Lazarides AL, et al. A mouse-human phase 1 co-clinical trial of a protease-activated fluorescent probe for imaging cancer. Sci Transl Med 2016; 8: 320ra4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Serrano J, Andersen DK, Forsmark CE, et al. Consortium for the study of chronic pancreatitis, diabetes, and pancreatic cancer: from concept to reality. Pancreas 2018; 47: 1208–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pancreatic Cancer UK. The Pancreatic Cancer UK Early Diagnosis Research Alliance https://www.pancreaticcancer.org.uk/research/about-our-research/our-research-projects/early-diagnosis-projects/the-pancreatic-cancer-uk-early-diagnosis-research-alliance/ (accessed Sept 20, 2019).
- 94.Hart PA, Baichoo E, Bi Y, Hinton A, Kudva YC, Chari ST. Pancreatic polypeptide response to a mixed meal is blunted in pancreatic head cancer associated with diabetes mellitus. Pancreatology 2015; 15: 162–66. [DOI] [PubMed] [Google Scholar]
- 95.Hart PA, Andersen DK, Mather KJ, et al. Evaluation of a mixed meal test for diagnosis and characterization of PancrEaTogEniC DiabeTes secondary to pancreatic cancer and chronic pancreatitis: rationale and methodology for the DETECT study from the consortium for the study of chronic pancreatitis, diabetes, and pancreatic cancer. Pancreas 2018; 47: 1239–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Greenhalf W, Grocock C, Harcus M, Neoptolemos J. Screening of high-risk families for pancreatic cancer. Pancreatology 2009; 9: 215–22. [DOI] [PubMed] [Google Scholar]
- 97.Grocock CJ, Rebours V, Delhaye MN, et al. The variable phenotype of the p.A16V mutation of cationic trypsinogen (PRSS1) in pancreatitis families. Gut 2010; 59: 357–63. [DOI] [PubMed] [Google Scholar]
- 98.Howes N, Greenhalf W, Rutherford S, et al. A new polymorphism for the RI22H mutation in hereditary pancreatitis. Gut 2001; 48: 247–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.McFaul CD, Greenhalf W, Earl J, et al. Anticipation in familial pancreatic cancer. Gut 2006; 55: 252–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kolamunnage-Dona R, Vitone L, Greenhalf W, Henderson R, Williamson PR. A multistate modelling approach for pancreatic cancer development in genetically high-risk families. J R Stat Soc C-Appl 2013; 62: 201–12. [Google Scholar]
- 101.Kemp S Digital in 2017: global overview. January 24, 2017. https://wearesocial.com/special-reports/digital-in-2017-global-overview (accessed Sept 2, 2019). [Google Scholar]
- 102.Bowler GM. Netnography: a method specifically designed to study cultures and communities online. Qual Rep 2010; 15: 1270–75. [Google Scholar]
- 103.Sharma A, Kandlakunta H, Nagpal SJS, et al. Model to determine risk of pancreatic cancer in patients with new-onset diabetes. Gastroenterology 2018; 155: 730–739.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Boursi B, Finkelman B, Giantonio BJ, et al. A clinical prediction model to assess risk for pancreatic cancer among patients with pre-diabetes. Jour Clin Onc 2019; 36: e16226. [Google Scholar]
- 105.Boursi B, Finkelman B, Giantonio BJ, et al. A clinical prediction model to assess risk for pancreatic cancer among patients with new-onset diabetes. Gastroenterology 2017; 152: 840–50.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Baecker A, Kim S, Risch HA, et al. Do changes in health reveal the possibility of undiagnosed pancreatic cancer? Development of a risk-prediction model based on healthcare claims data. PLoS One 2019; 14: e0218580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hsieh MH, Sun LM, Lin CL, Hsieh MJ, Hsu CY, Kao CH. Development of a prediction model for pancreatic cancer in patients with type 2 diabetes using logistic regression and artificial neural network models. Cancer Manag Res 2018; 10: 6317–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yu A, Woo SM, Joo J, et al. Development and validation of a prediction model to estimate individual risk of pancreatic cancer. PLoS One 2016; 11: e0146473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Muhammad W, Hart GR, Nartowt B, et al. Pancreatic cancer prediction through an artificial neural network. Front Artif Intell 2019; 2: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Klein AP, Lindström S, Mendelsohn JB, et al. An absolute risk model to identify individuals at elevated risk for pancreatic cancer in the general population. PLoS One 2013; 8: e72311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Risch HA, Yu H, Lu L, Kidd MS. Detectable symptomatology preceding the diagnosis of pancreatic cancer and absolute risk of pancreatic cancer diagnosis. Am J Epidemiol 2015; 182: 26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chu LC, Park S, Kawamoto S, et al. Utility of CT radiomics features in differentiation of pancreatic ductal adenocarcinoma from normal pancreatic tissue. AJR Am J Roentgenol 2019; 213: 349–57. [DOI] [PubMed] [Google Scholar]
- 113.Amer AM, Zaid M, Chaudhury B, et al. Imaging-based biomarkers: changes in the tumor interface of pancreatic ductal adenocarcinoma on computed tomography scans indicate response to cytotoxic therapy. Cancer 2018; 124: 1701–09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Koay EJ, Lee Y, Cristini V, et al. A visually apparent and quantifiable CT imaging feature identifies biophysical subtypes of pancreatic ductal adenocarcinoma. Clin Cancer Res 2018; 24: 5883–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Koay EJ, Truty MJ, Cristini V, et al. Transport properties of pancreatic cancer describe gemcitabine delivery and response. J Clin Invest 2014; 124: 1525–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Danai LV, Babic A, Rosenthal MH, et al. Altered exocrine function can drive adipose wasting in early pancreatic cancer. Nature 2018; 558: 600–04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ozola Zalite I, Zykus R, Francisco Gonzalez M, et al. Influence of cachexia and sarcopenia on survival in pancreatic ductal adenocarcinoma: a systematic review. Pancreatology 2015; 15: 19–24. [DOI] [PubMed] [Google Scholar]
- 118.Peng P, Hyder O, Firoozmand A, et al. Impact of sarcopenia on outcomes following resection of pancreatic adenocarcinoma. J Gastrointest Surg 2012; 16: 1478–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bridge CP, Rosenthal M, Wright B, et al. Fully-automated analysis of body composition from CT in cancer patients using convolutional neural networks. International workshop on skin image analysis; October 2, 2018. [Google Scholar]
- 120.Pelaez-Luna M, Takahashi N, Fletcher JG, Chari ST. Resectability of presymptomatic pancreatic cancer and its relationship to onset of diabetes: a retrospective review of CT scans and fasting glucose values prior to diagnosis. Am J Gastroenterol 2007; 102: 2157–63. [DOI] [PubMed] [Google Scholar]
- 121.Gangi S, Fletcher JG, Nathan MA, et al. Time interval between abnormalities seen on CT and the clinical diagnosis of pancreatic cancer: retrospective review of CT scans obtained before diagnosis. AJR Am J Roentgenol 2004; 182: 897–903. [DOI] [PubMed] [Google Scholar]
- 122.Canto MI, Goggins M, Yeo CJ, et al. Screening for pancreatic neoplasia in high-risk individuals: an EUS-based approach. Clin Gastroenterol Hepatol 2004; 2: 606–21. [DOI] [PubMed] [Google Scholar]
- 123.Canto MI, Goggins M, Hruban RH, et al. Screening for early pancreatic neoplasia in high-risk individuals: a prospective controlled study. Clin Gastroenterol Hepatol 2006; 4: 766–81, quiz 665. [DOI] [PubMed] [Google Scholar]
- 124.Poley JW, Kluijt I, Gouma DJ, et al. The yield of first-time endoscopic ultrasonography in screening individuals at a high risk of developing pancreatic cancer. Am J Gastroenterol 2009; 104: 2175–81. [DOI] [PubMed] [Google Scholar]
- 125.Verna EC, Hwang C, Stevens PD, et al. Pancreatic cancer screening in a prospective cohort of high-risk patients: a comprehensive strategy of imaging and genetics. Clin Cancer Res 2010; 16: 5028–37. [DOI] [PubMed] [Google Scholar]
- 126.Rosenblatt F The perceptron: a probabilistic model for information storage and organization in the brain. Psychol Rev 1958; 65: 386–408. [DOI] [PubMed] [Google Scholar]
- 127.Roffman D, Hart G, Girardi M, Ko CJ, Deng J. Predicting non-melanoma skin cancer via a multi-parameterized artificial neural network. Sci Rep 2018; 8: 1701. [DOI] [PMC free article] [PubMed] [Google Scholar]