Abstract
BACKGROUND
Induction of reactive oxygen species (ROS) represents a viable strategy to enhance the activity of radiotherapy. We hypothesized that napabucasin would increase ROS via its ability to inhibit NAD(P)H:quinone oxidoreductase 1 (NQO1) and potentiate the response to chemoradiotherapy in rectal cancer (RC) via distinct mechanisms.
METHOD
Proliferation studies, colony formation assays and ROS levels were measured in HCT116 and HT29 cell lines treated with napabucasin, chemoradiation or their combination. DNA damage (p-γH2AX), activation of STAT and downstream angiogenesis was evaluated in both untreated and treated cell lines. Finally, the effects of napabucasin, chemoradiotherapy and the combination were assessed in vivo using subcutaneous mice xenograft models.
RESULTS
Napabucasin significantly potentiated the growth inhibition of chemoradiation in both cell lines. Napabucasin increased ROS generation. Inhibition of ROS by NAC decreased the growth inhibitory effect of napabucasin alone and in combination with chemoradiotherapy. Napabucasin significantly increased p-γH2AX in comparison to chemoradiotherapy alone. Napabucasin reduced the levels of pSTAT-3, VEGF and inhibited angiogenesis through ROS mediated effect. Napabucasin significantly potentiated inhibition of growth and blood vessel formation of chemoradiotherapy in mouse xenografts.
CONCLUSION
Napabucasin is a radiosensitizer with a novel mechanism of action: increasing ROS production and inhibition of angiogenesis. Clinical trials testing the addition of napabucasin to chemoradiotherapy in rectal cancer are needed.
Keywords: colorectal cancer, angiogenesis, DNA damage, ROS, STAT-3, BBI 608
INTRODUCTION
Colorectal cancer (CRC) is among the most deadly cancers worldwide, with a mortality rate of approximately 600,000 per year.1 In the United States alone, CRC is the third leading cause of cancer-associated death.1 Conventional standard-of-care treatments for rectal cancer are suboptimal. These typically comprise neoadjuvant chemoradiotherapy, surgery and adjuvant chemotherapy.2 Unfortunately, resistance to chemoradiotherapy and local disease recurrence remain a major challenge in locally advanced disease.3, 4 To address this issue, we have initiated a series of preclinical studies to enhance the activity of chemoradiotherapy in the setting of rectal cancer by targeting oxidative pathways.
Oxidative stress is a prominent feature of CRC, regardless of tumor genotype. This process is driven in part by the upregulation of quinone oxidoreductase (NQO1) in neoplastic colon tissue.5 NQO1 is a cytosolic flavoprotein antioxidant enzyme that utilizes NADH/NADPH to catalyze quinone reduction, thus stopping the production of ROS and guarding cells from oxidative stress.6, 7 Despite the pathologic role of ROS in inflammation, induction of ROS is a key mechanism by which treatment with ionizing radiation renders tumors susceptible to DNA damage, activates inflammatory pathways (i.e. STAT3) and ultimately promotes cell death.8, 9 ROS production may be inhibited by aberrant angiogenesis, leading to a hypoxic tumor microenvironment.10 These data propose that NQO1 enzymatic activity may be a key mechanism of resistance to conventional therapy in CRC.
We postulated that inhibition of NQO1 may have a unique ability to sensitize rectal cancer to chemoradiotherapy. Although not yet explored in the setting of rectal cancer, treatment with NQO1 inhibitors alone has been revealed to decrease tumor growth in pre-clinical models of lung and hepatocellular cancer. In support of our premise are other pre-clinical investigations showing that NQO1-targeting agents increase sensitivity to ionizing radiation in head and neck cancer.11 Since rectal cancer treatment often involves radiation, targeting NQO1 may be a rational approach to increase cell death and enhance the therapeutic effect of radiotherapy.12
In the present study, we utilized napabucasin (BBI 608), a small molecule that suppresses NQO1 as a novel means to sensitize CRC cells to chemoradiotherapy approaches.13 We hypothesized that napabucasin would increase ROS via its ability to inhibit NQO1 and potentiate the response to radiotherapy via distinct mechanisms. The results of the study showed enhanced DNA damage and growth inhibitory effects of napabucasin combined with chemoradiotherapy on CRC cells in vitro and in mice bearing human CRC xenografts. Importantly, the mechanism of this treatment combination involved ROS production, altered STAT3 signaling and inhibition of VEGF-mediated angiogenesis.
MATERIALS AND METHODS
CRC human cell lines (HCT 116 and HT-29) were procured from the ATCC, USA. Napabucasin was obtained from Boston Biomedical, Inc., USA. 5-Fluorouacil (5-FU), N-acetylcysteine (NAC) and interleukin (IL-6) were bought from Sigma-Aldrich. The Br-dU assay kit was purchased from Roche, USA. Antibodies against pATM (Ser1981; D6H9; no. 5883), ATM (no. sc-28901), pATR (Ser428; no. 2853), Rad51 (no. sc-8349), pγ-H2AX (pS139; no. ab26350), MDM2 (no. sc-965), Chk2 (no. sc-5278), p53 (no. sc-9282), NQO1 (no. sc-32793), STAT-3 (no. sc-8019), pSTAT-3(Tyr705; no. 9138), VEGF (no. sc-7269), and β-Actin (no. sc-8432) were obtained from Cell Signaling, Santa Cruz and Abcam. The VEGF and IL-6 quantification kits were obtained from R&D systems (Cat # DY293B). Eggs were purchased from Charles River (North Franklin, CT, USA).
Cell Line Culture
All human CRC cell lines were grown in McCoy’s 5A media and maintained according to ATCC guidelines and to our previously published protocol.14
Cell Proliferation
HCT 116 and HT-29 cell lines were cultured in 96 well plates then treated with or without napabucasin in a concentration-dependent fashion ranging from 0.3μM to 2.4μM. After 36 hours, CRC cell proliferation was evaluated using the Br-dU assay kit following the manufacturer’s instructions (#11647 229 001, Roche, Indianapolis, IN, USA).15 A microplate reader was used to evaluate absorbance at 450 nm. These experiments were done in triplicate.
Clonogenic Assay
Equal numbers of both CRC cells (100 ± 10) were plated in 6 well plates containing culture media and maintained overnight at 37°C as per published protocol.16 The CRC cell lines were then treated with napabucasin (1μM) alone or in combination with 5-FU (4μM) and subjected to different fractions of radiation at either 0, 2, 4 or 6 Gy. The media containing the treatment was discarded after 36 hours and fresh media was replaced once every 4 days. On day 12 following radiation exposure, the colonies were marked with crystal violet solution for 10 minutes and washed with water. Number of stained colonies were counted with a microscope (DP20 Olympus camera at a magnification of X1.5). For the purposes of this quantification, a clone of 50+ cells was considered as a colony. Survival fraction was calculated according to our previously published protocol.16
Western Blot
Treated or untreated CRC cell lines were attained and lysed using RIPA buffer comprising a phosphatase and protease inhibitors (Sigma-Aldrich, USA). Protein levels for each sample were then estimated using a BCA quantification assay. Equal amounts of protein (100μg) were resolved in SDS page, then transferred to PDVF membranes. The membranes were then blocked using 2.5% BSA or 5% milk, depending on the antibody type. Membranes were next probed using selected primary Abs (antibodies) for 4h at RT. The membranes were then washed three times using PBST buffer for 10-minute intervals each and probed using specific HRP-conjugated secondary Abs for 45 min at RT. The blots were washed again three times using PBST for 10-minute intervals and developed using ECL reagent. The signal was developed using X-ray films.
ROS Measurement
CRC cells were plated in 6 or 96 well plates and treated as indicated in previous sections for 24 hours, following which cells were pretreated with the ROS inhibitors NAC (5mM) for 2 hours prior to staining. After two hours, the CRC cells were stained with CellRox deep red (Thermo fisher Scientific, USA) for one hour at 37°C. DAPI was then added for 10 minutes to estimate cell viability. ROS levels were measured using Becton-Dickinson FACS caliber flow cytometry analysis (BD Bioscience, Germany). Results were analyzed by the FlowJo software (Treestar, Inc., San Carlos, CA). The microplate reader evaluated (96 well plate) absorbance at 640 nm and 665 nm. These experiments were done in triplicate.
Immunofluorescence
Immunofluorescence was performed per our previously published protocol.17 CRC cell lines were incubated in an 8-well chamber slide overnight, after which the cell lines were treated as indicated in the preceding section. After 24h, the media was removed, and fixed with 4% paraformaldehyde for 20 min. Then the cell lines were blocked with 2.5% BSA with 0.02% Triton-X100 for one hour. Subsequently, the blocking buffer was replaced with 2.5% BSA containing antibody against pγ-H2AX for two hours in RT. After this step, buffer containing antibody was removed and cells were washed with PBS. Then 2.5% BSA containing goat-anti-mouse Alexa Fluor–conjugated secondary Ab was supplemented and the cells were incubated for 45 minutes. Cells were later washed with PBS to remove the secondary Ab, allowing for the cell lines to be stained and covered with a slip with DAPI mounting media. Photographed were taken under a fluorescent microscope.
ELISA for Quantification of VEGF Production
CRC cells were treated with or without napabucasin, 5FU for 36 hours and single fraction of 4 Gy radiation at 24 hours. The treatment containing media was removed and substituted with fresh medium. This conditioned media was collected after 16 hours for VEGF quantification and Egg Cam assay. The secretion of VEGF was measured using an ELISA per the manufacturer’s instructions (R&D systems).
Egg Cam Assay
Fertilized eggs were kept in the incubator in a humidified environment at 37°C for 5 days. The eggs were then perforated to form small holes at their tops and injected with conditioned media (as per previous section) or serum free media (control). The eggs were then maintained for 10 days in a humidified environment at 37°C. On day 16, the eggs were opened and CAMs were pictured using microscope (DP20 Olympus camera at a magnification of X1.5) to estimate vascularization using the Angioquant software.18
Animal Studies
Nude mice were purchased from Envigo (Indianapolis, IN, USA) and maintained according to IACUC protocols at Emory University. HCT 116 cells (1 × 106) mixed with 15% matrigel and were subcutaneously inserted into mice. The animals were randomized into 4 groups when the tumor reached 100 mm3 in size. Group 1 received water orally and served as a control group. Group 2 received napabucasin orally (100mg per kg) for 11 days daily. Group 3 received 5-FU (30mg/kg IV) + a single fraction of radiation at 4Gy once a week for two weeks. Finally, group 4 received napabucasin + 5FU + radiation. The tumor volumes were measured once every 4 days using veneal caliber scales. Mice were euthanized via CO2 asphyxiation followed by dissected to peel the skin covering tumors over the inserted matrigel. This was then was photographed under visible light to evaluate angiogenesis.
Statistical Analysis
Statistical significance between the untreated and treated cell lines or animals were assessed by one-way ANOVA tailed by Student’s paired t-test using the Instat software (USA) for Windows. Results are showed as mean ± SD. Variances between means were reflected significant if p< 0.05.
RESULTS
Napabucasin Enhances the Effect of Chemoradiotherapy on Growth and Survival of Human CRC Cell Lines
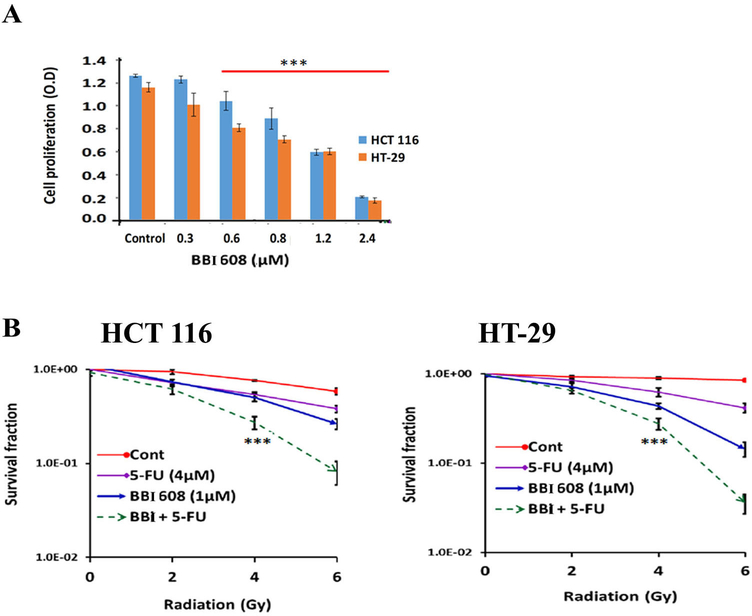
NQO1 activity is linked with control of cell growth via regulation of downstream pathways.19 To evaluate the direct effect of napabucasin on the growth of CRC cell lines, a Br-dU proliferation assay was performed. Napabucasin significantly decreased CRC cell growth in a concentration-dependent manner (0.3μM to 2.4μM; p<0.001) compared to untreated controls. This drug was potent, as the average IC50 for napabucasin in both HCT 116 and HT29 cell lines is 2uM (Fig. 1A). This clinically-relevant concentration was used for all subsequent experiments to assess its impact when combined with conventional therapies.20
Figure 1.
Effect of napabucasin (BBI 608) on proliferation, and survival (A). The Br-dU assay shows that the napabucasin (BBI 608) treatment significantly decreases the CRC cell line proliferation in a dose dependent pattern. (B). Clonogenic evaluations indicate that the napabucasin or 5-FU combination with radiation (IR) treatment significantly decrease CRC cell line survival in a dose dependent (0GY to 6GY) pattern. (Results are showed as mean ± SD (***p<0.0001; NS, non-significant).
We postulated that the ability of napabucasin to modulate NQO1 enzymatic activity might sensitize CRC cell lines to concurrent treatment with 5-FU + IR. While exposure to 5-FU or napabucasin alone had a clear impact on colony formation, clonogenic assays revealed a significantly lesser colony formation in cells treated napabucasin and chemoradiotherapy combined as compared to either single agent alone (Fig. 1B).
Napabucasin Alone or in Combination with Chemoradiotherapy Induces ROS in CRC Cell Lines
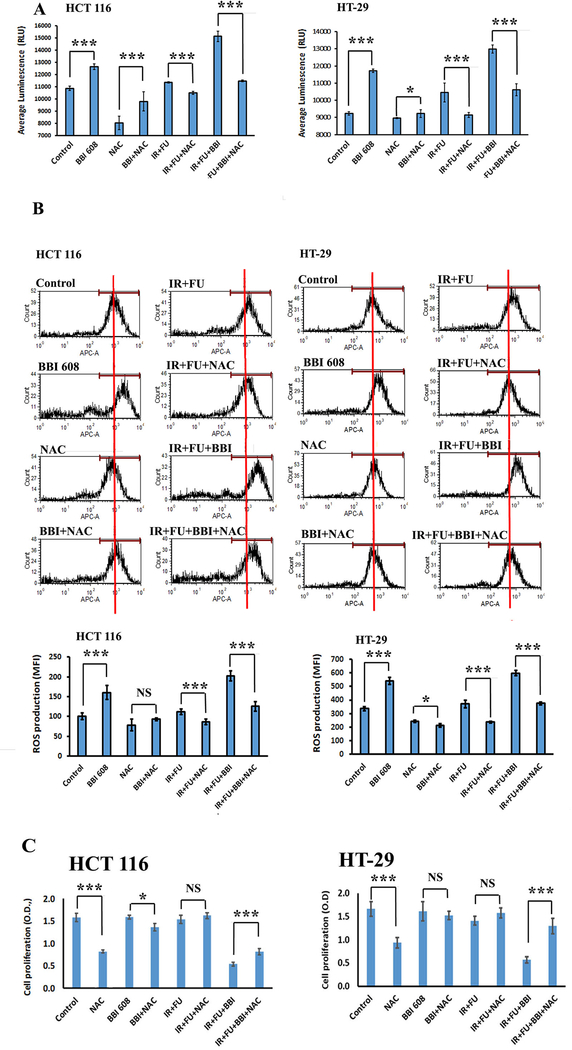
Despite recent data in pancreatic ductal adenocarcinoma (PDAC), it is important to validate the ability of napabucasin to inhibit NQO1 and induce ROS in CRC cells. To confirm induction of ROS was occurring in concert with the reduced cell growth from napabucasin, the levels of intracellular ROS were measured with or without the ROS inhibitor NAC (5mM) in CRC cell lines. Napabucasin alone or in combination with chemoradiotherapy increases ROS production in CRC cells compared to control or chemoradiation alone (Fig. 2A, and 2B). Additionally, inclusion of the ROS inhibitor NAC into the assay antagonizes napabucasin action (alone or in combination with chemoradiotherapy) on ROS (Fig. 2A, and 2B). Importantly, consistent data were obtained when considering the antagonistic effect of NAC on the anti-proliferative effect of napabucasin (alone or in combination with chemoradiotherapy) (Fig. 2C). These observations confirm that the observed sensitization to chemoradiotherapy by napabucasin is mediated through ROS production.
Figure 2.
Effect of napabucasin (BBI 608) alone or in combination with chemoradiotherapy on ROS generation in CRC cell lines (A). Calorimetric analysis shows that the napabucasin (BBI 608) alone or in combination with chemotherapy increases ROS generation while NAC antagonizes napabucasin action in CRC cell lines. (B). Flow cytometry analysis supports these findings. (C). The addition of ROS inhibitor NAC antagonizes napabucasin effects (alone or in combination with chemoradiotherapy) on proliferation (Br-dU assay). Results are showed as mean ± SD (NS, non signofocant; ***p<0.0001; *p<0.01).
Napabucasin Combined with Chemoradiotherapy Alters the DNA Damage Response and STAT3 Signal Transduction in Human CRC Cell Lines
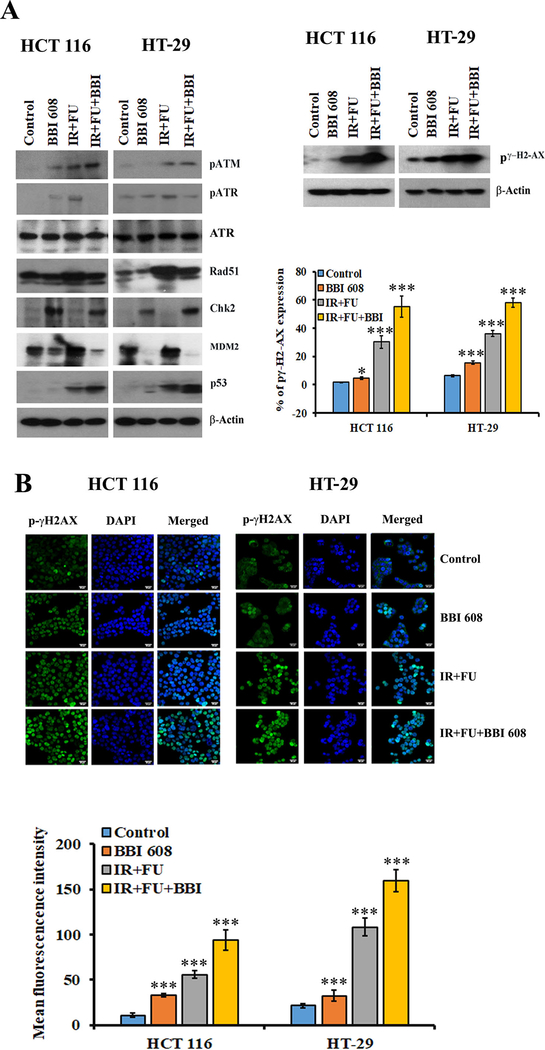
In order to define the mechanism of radio-sensitization by napabucasin we first turned our attention to expression of various DNA repair and damage molecules. Consistent with previously observed ROS induction, the combination of napabucasin and chemoradiotherapy increased the expression of pγH2AX, a marker of DNA damage (Fig 3A). Immunofluorescence analysis further confirmed that napabucasin with chemoradiotherapy increased nuclear pγ-H2AX expression as compared to control or chemoradiotherapy alone treated cell lines (Fig. 3B). Aligned with a DNA damage signature, activation of stress response pathways was also apparent as evidenced by increased expression of pATM, Rad51, Chk2 and p53 (Fig. 3A). This treatment combination also reduced the levels MDM2 and increased p53, which is in both HCT 116 and HT-29 cell lines, could contribute to the observed radiosensitization effects (Fig. 3A).
Figure 3.
Effect of napabucasin (BBI 608) alone or in combination with chemoradiotherapy on DNA damage and repair molecules in CRC cell lines. (A). Western blot analysis shows that the napabucasin (BBI 608) alone or in combination with chemoradiotherapy increased the expression of pATM, pγH2AX, Rad51, Chk2 and p53. In contrast, napabucasin with +5FU+IR reduces the levels of pATR and MDM2. β-Actin acts as a loading control. (B). Immunofluorescence analysis shows that the napabucasin alone or in combination with chemoradiotherapy increased the intensity of pγH2AX in nucleus of both CRC cell lines. Results are showed as mean ± SD (***p<0.0001)
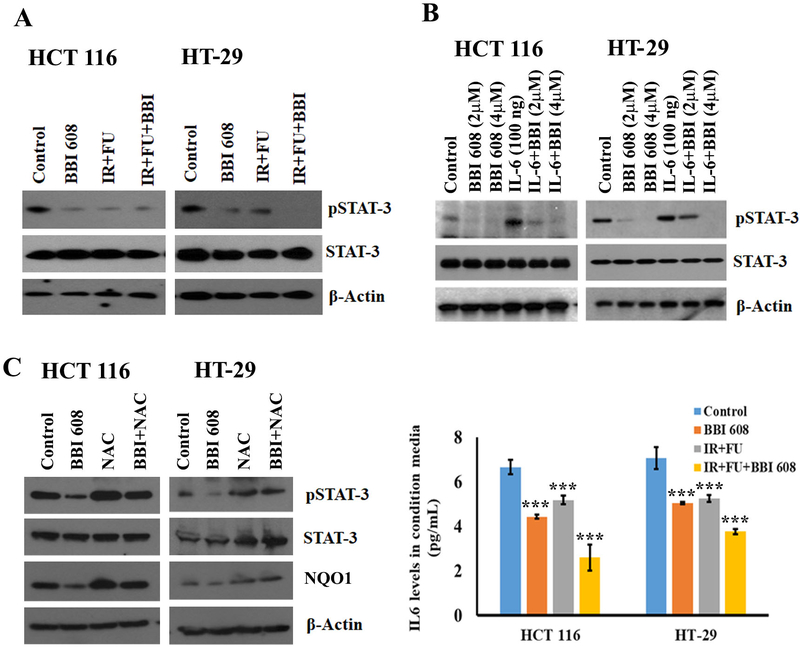
Aside from ROS production and subsequent DNA damage, NQO1 enzymatic activity can also promote proliferation and metastasis via interactions with the STAT-3 transcription factor.20–22 Of note, prior studies in PDAC cell lines indicate a role for STAT3 as an indirect target of napabucasin20, 23, although this has not been validated in human CRC cells. Western Blot analysis showed a clear decrease in pSTAT-3 by napabucasin that was further pronounced in the CRC cell line by concurrent exposure to chemoradiotherapy (Fig. 4A). To confirm the effect of napabucasin on pSTAT-3 in CRC cell lines, and better approximate an inflammatory CRC tumor microenvironment, cells were IL-6 (100ng) was also added to cultures to further increase phosphorylation of STAT-3 (Fig 4B). Even in the presence of elevated levels of this CRC-associated cytokine, napabucasin significantly reduced pSTAT-3 expression in a concentration-dependent manner compared to control treatments in both cell lines (Fig. 4B). To confirm that napabucasin-induced effects on pSTAT-3 are mediated through a mechanism involving ROS-induction, cell lines were treated with napabucasin with or with without NAC and western blot analysis for pSTAT-3 was performed. While napabucasin alone inhibited pSTAT-3 and NQO1 expression in both CRC cell lines and NAC alone increases these proteins (Fig. 4C), the addition of napabucasin to NAC treatment did not downregulate pSTAT-3 and NQO1 confirming the effects of napabucasin are mediated through ROS production (Fig. 4C). Together these data uncover a mechanism by which increased DNA damage occurs and is perpetuated upon exposure to napabucasin and chemoradiotherapy.
Figure 4.
Effect of napabucasin (BBI 608) on phosphorylation, specificity of STAT-3 and IL-6 levels in CRC cell lines. (A). napabucasin combination with 5-FU+IR attenuates pSTAT-3 in both CRC cell lines. (B). napabucasin alone attenuates pSTAT-3 expression in a dose-dependent manner. IL-6 treatment induces pSTAT-3 expression in CRC cell lines, the combination of napabucasin and IL-6 decreases pSTAT-3. (C). Western blot analysis shows that napabucasin attenuates the expression of pSTAT-3. NAC reverts napabucasin action in CRC cell lines. β-Actin acts as a loading control. Results are showed as mean ± SD (***p<0.0001)
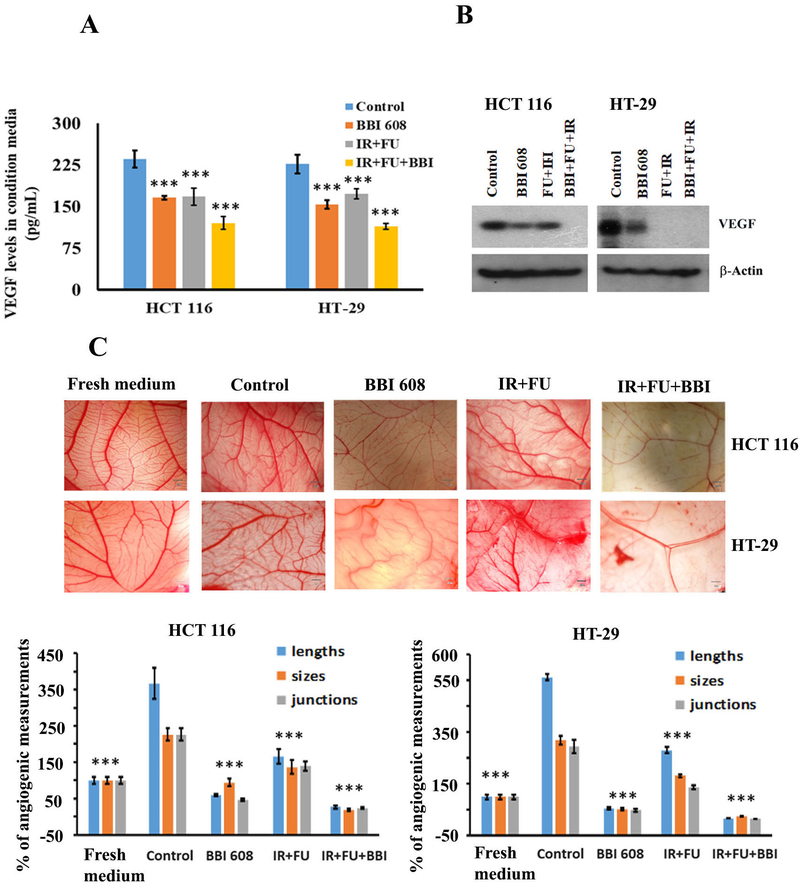
The Combination of Napabucasin and Chemoradiotherapy Reduces VEGF and Impacts Angiogenic Processes
Given the established link between STAT-3 and regulation of angiogenesis, we next considered whether napabucasin might influence this important hallmark of CRC biology. We observed a significantly lower concentration of VEGF in both cell culture supernatants and cell lysates from both HCT 116 and HT-29 human CRC cell lines in response to combined treatment with napabucasin and chemoradiation as compared to any agent alone or to controls (Fig. 5A and 5B). Consistent with these data were other observations indicating that combined treatment with napabucasin and 5-FU + IR significantly decreased blood vessel formation in the well-controlled Egg Cam assay system (Fig. 5C). These results further support a mechanism by which napabucasin may concurrently inhibit VEGF and the overall angiogenic features of tumors.
Figure 5.
Effect of napabucasin (BBI 608) alone or in combination with chemoradiotherapy on angiogenesis and migratory molecules. (A). ELISA analysis indicates that VEGF secretion is significantly lower in individual treatment and in the combination treatment group compared to control CRC cell lines. (B). Western blot analysis shows that the napabucasin alone or in combination with chemoradiotherapy decreased the expression of VEGF in CRC cell lines. β-Actin acts as a loading control. (C). Conditioned media from napabucasin or IR+FU or IR+FU+BBI treated cells inhibit angiogenesis as compared to conditioned media from control or fresh media in Egg CAM assays. Results are showed as mean ± SD (***p<0.0001)
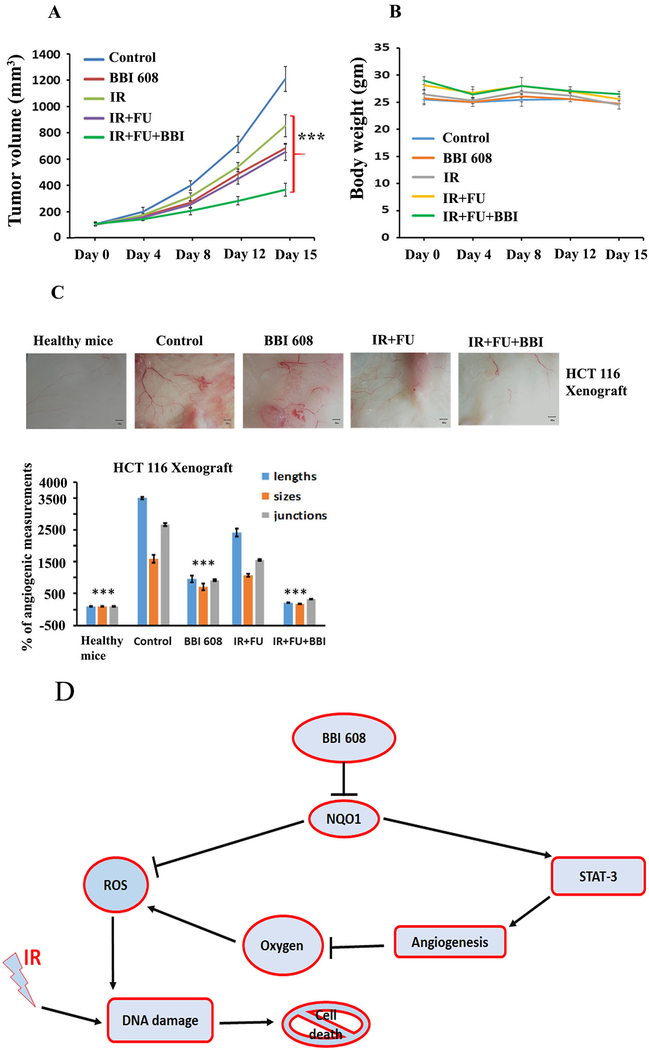
Napabucasin Elicits In Vivo Anti-tumor Activity When Administered in Combination with Chemoradiotherapy in Murine Xenograft Models
To validate our in vitro results, HCT 116-bearing nude mice were treated with vehicle, napabucasin, chemoradiotherapy or the combination of napabucasin and chemoradiotherapy. We observed a significant difference in tumor volume (p<0.0001) was observed in napabucasin or 5-FU + IR treated as compared to the untreated animals (Fig. 6A). Combined therapy with napabucasin and 5-FU + IR led to significant inhibition of tumor growth as compared to chemoradiotherapy alone (p<0.0001) (Fig. 6A). No apparent signs of systemic toxicity were evident in these animals, based on a consistent body weight throughout the study across all treatment groups (Fig. 6B), indicating the combination was well-tolerated. Finally, consistent with in vitro data, we observed significantly less angiogenesis as detected by matrigel plug implantation method in mice treated with the combination when compared to controls or each agent alone (Fig. 6C).
Figure 6.
Effect of napabucasin (BBI 608) alone or in combination with chemoradiotherapy on tumor growth, body weight, and steaminess of HCT 116 xenografts. (A). Significant differences in tumor volumes (p<0.0001) was observed in treated (napabucasin or IR or IR+FU or napabucasin and IR+FU) as compared to the untreated HCT 116 xenograft models. (B). HCT 116 xenograft models show no loss of body weight among the treatment groups. (C). Combined therapy with napabucasin and FU+IR led to significant inhibition of blood vessel formation as compared to chemoradiotherapy or napabucasin alone or control. Results are showed as mean ± SD (***p<0.0001). (D). Mechanism of BBI 608 action on DNA damage in CRC.
DISCUSSION
Our paper has identified napabucasin as a radiosensitizer with a novel mechanism of action increasing ROS in rectal cancer. Our observations suggest that napabucasin could exert its effect by increasing ROS production through NQO1 modulation, causing increased oxidative stress that decreases their survival, as evidenced by reduced CRC tumor volumes in animal models. Napabucasin in combination with chemoradiotherapy also increased DNA damage pathways and reduced aberrant vascularization, further inhibiting growth and survival of CRC cell lines (Fig. 6D).
NQO1 has a key role in tumor development, progression and resistance to therapy. Constitutive upregulation of NQO1 has been observed in colon, breast, pancreatic, prostate, and hepatic cancers.6 Through its role as antioxidant, NQO1 contributes to resistance to chemotherapy and radiation; hence, treatment strategies focusing on modulating or inhibiting NQO1 in cancer have been recently tested in preclinical models. In hepatocellular carcinoma, the knockdown of NQO1 simultaneously inhibited MAPK/ERK and PI3K/AKT signaling cascades; further decreased glutaminolysis, glycolysis and metabolic adaptation, thus reducing the proliferation and tumor growth in liver cancer.24 Similarly, the knockdown of NQO1 using siRNA hindered colony formation and migration of cholangiocarcinoma (CCA), decreased cyclin D1 expression and the ratio of MMP9/TIMP1, indicating that NQO1 could be a potential target for treating CCA.25 These results are further corroborated by another study, which showed that coumarins, a class of natural NQO1 inhibitors, can exert potent anti-migratory activity in CCA.26 NQO1 inhibition in non-small cell lung malignancy reduced xenograft volume by enhancing susceptibility to oxidative stress.27 In preclinical models, dicoumarol, an inhibitor of NQO1, has been revealed to eliminate pancreatic tumor cells by increasing the levels of ROS.28–30 NQO1 has been identified as an important driver for tamoxifen-resistance in breast malignant cells responsive to tamoxifen, indicating that NQO1 could serve as a potential treatment target in the management of estrogen receptor positive breast malignance.31 As these various studies illustrate, NQO1 is an important therapeutic target in malignant cells.
Napabucasin modulates NQO1, leading to increased ROS and cell death, however no preclinical or clinical testing of napabucasin as a radiosensitizer has been previously done.13 Thus, its application to enhance the response of patients with rectal cancer to conventional therapy may be warranted. Prior clinical trials have demonstrated the safety and feasibility of administering napabucasin alone or in combination with cytotoxic chemotherapeutic agents. Also, preliminary data for napabucasin in combination with chemotherapy in colorectal cancer has been encouraging.32 ROS are radiation therapy’s effector molecules, acting through induction of DNA damage.33 Therapeutic strategies aimed at enhancing the production of ROS have been developed to mitigate resistance to radiation therapy. The ROS modulator coroglaucigenin has been shown to enhance ROS generation by antioxidant enzymes and molecules, thus increasing the radiosensitivity of lung cancer cells.34 The bioactivatable NQO1 compound β-lapachone increased ROS production and DNA damage, resulting in enhanced radio-sensitization and lethality in NQO1 overexpressing head and neck cancer cells.11 Similarly, auranofin, improves the susceptibility of breast cancer cells by inhibiting thioredoxin, which elicits hydroxyl radical bursts.35 In nasopharyngeal carcinoma, salinomycin was found to re-sensitize tumor cells to radiotherapy by inactivating Nrf2 and increasing ROS production.36 These various findings provide rationale for evaluating napabucasin as a radiosensitizer with a novel mechanism of action mediated through increased ROS. Our results indicate that napabucasin is a potent radiosensitizer in CRC cell lines as observed through multiple in vitro and in vivo experiments. Furthermore, napabucasin as predicted can increase the levels of ROS in CRC cell lines. The radiosensitization observed with napabucasin is reversed when cells are treated with NAC confirming the effects of napabucasin are mediated through ROS.
ROS exerts its effect on cell lines by increasing irradiation-stimulated DNA double strand breaks and activating the DNA damage response (DDR). Initial DSB sensing is commonly mediated by ATM and downstream sensor proteins.37 Increased levels of ROS such as H2O2 result in the auto-phosphorylation of ATM to produce pATM, leading to the activation of the DDR.38 Increased expression of γH2AX is an additional indicator of oxidative stress and the initiation of the DNA damage pathway.39 The addition of napabucasin to chemoradiotherapy resulted in further increase in the protein levels of pATM, γH2AX, Rad51 and Chk2, suggesting that the increase in ROS production through inhibition of NOQ-1 augmented the DNA damage caused by radiation therapy and led to increase in downstream activation of p53 pathway. Our findings are in agreement with a prior investigation showing that ROS generation by the natural ROS modulator icaritin elicited ROS production in cervical cancer cells, leading to an increase in γH2AX and the activation of DNA damage responses. The cytotoxic effects of icaritin were antagonized by the antioxidant NAC,40 in agreement with our results. A similar synergistic effect between the NQO1 modulator β-lapachone and ionizing radiation was observed in human colon adenocarcinoma cells as well as in pancreatic cancer cells.41–43 These various confirm our observation of the link between NQO1 modulation through small molecules, ROS generation, DNA damage and the resensitization of tumor cells to ionizing radiation.
In addition to directly promoting ROS generation through NQO1 modulation, napabucasin also inhibited pSTAT-3 levels and was also associated with reduced levels of VEGF and vascularization both in CRC cells and tumor models. STAT-3 is a constitutively activated oncogene expressed in many solid tumors, including CRC.44 It stimulates the growth, metastasis of malignant cells by upregulating the STAT-3/JAK pathway in response to EGF or IL-6.45 Our group has previously shown that the knockdown of STAT-3 inhibited angiogenesis in CRC cell lines by decreasing VEGF expression and production.46 The hypoxic tumor microenvironment promotes the production of VEGF. In turn, VEGF promotes development of immature tumor angiogenesis leading to increased vascular leakiness, increased oncotic pressure and decreased perfusion and propagation of tumor hypoxia. VEGF inhibition results in vascular normalization through pruning of immature tumor blood vessels, which enhances the perfusion and oxygenation of tumor tissue.47 Since oxygenation is key in production of ROS and radiosensitization, the combinations of antiangiogenic strategies with chemoradiation have been studied in preclinical and clinical settings.48, 49 Based on these findings, we propose that the anti-angiogenic effects exerted by napabucasin can normalize vascularization in CRC tumor models, which leads to improved oxygenation, thus further improving ROS production and ionizing radiation (Fig. 6C).
In summary, our findings indicate that the addition of napabucasin to currently used chemoradiotherapy regimens in rectal cancer may be a rational approach to develop local disease control and increase organ preservation strategies. Clinical trials testing this combination are warranted given lack of any significant advancement with chemoradiotherapy strategies in locally advanced rectal cancer.
ACKNOWLEDGEMENT
The authors would like to thank Dr. April R. Reedy for helping florescence images and analysis. Florescence images was supported in part by the ICI Microscope Core of the Emory University and NIH/NCI under award number, 2P30CA138292–04. The content is merely the responsibility of the authors and does not necessarily reflect the official views of the NIH.
FUNDING SUPPORT
None
Footnotes
CONFLICT OF INTEREST DISCLOSURES
None to disclosures
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. Ca-Cancer J Clin. 2020;70:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Coyle Y Adjuvant and neoadjuvant therapy for colorectal cancer: molecular-based therapy Shackelford’s surgery of the alimentary Tract. Vol 2: Elsevier; 2019:2126–2136. [Google Scholar]
- 3.Huerta S Rectal cancer and importance of chemoradiation in the treatment Diseases of DNA Repair: Springer; 2010:124–133. [DOI] [PubMed] [Google Scholar]
- 4.Geng L, Wang J. Molecular effectors of radiation resistance in colorectal cancer. Precis Radiat Oncol. 2017;1:27–33. [Google Scholar]
- 5.Traver RD, Horikoshi T, Danenberg KD, et al. NAD (P) H: quinone oxidoreductase gene expression in human colon carcinoma cells: characterization of a mutation which modulates DT-diaphorase activity and mitomycin sensitivity. Cancer Res. 1992;52:797–802. [PubMed] [Google Scholar]
- 6.Oh E-T, Park HJ. Implications of NQO1 in cancer therapy. BMB Rep. 2015;48:609–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan TS, Teng S, Wilson JX, Galati G, Khan S, O’Brien PJ. Coenzyme Q cytoprotective mechanisms for mitochondrial complex I cytopathies involves NAD (P) H: quinone oxidoreductase 1 (NQO1). Free radical research. 2002;36:421–427. [DOI] [PubMed] [Google Scholar]
- 8.Schieber M, Chandel NS. ROS function in redox signaling and oxidative stress. Curr Biol. 2014;24:R453–R462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee SY, Jeong EK, Ju MK, et al. Induction of metastasis, cancer stem cell phenotype, and oncogenic metabolism in cancer cells by ionizing radiation. Mol Cancer. 2017;16:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muz B, de la Puente P, Azab F, Azab AK. The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia. 2015;3:83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li L-S, Reddy S, Lin Z-H, et al. NQO1-mediated tumor-selective lethality and radiosensitization for head and neck cancer. Mol Cancer Ther. 2016;15:1757–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Behr TM, Béhé M, Stabin MG, et al. High-linear energy transfer (LET) α versus low-LET β emitters in radioimmunotherapy of solid tumors: therapeutic efficacy and dose-limiting toxicity of 213Bi-versus 90Y-labeled CO17–1A Fab′ fragments in a human colonic cancer model. Cancer Res. 1999;59:2635–2643. [PubMed] [Google Scholar]
- 13.Froeling F, Chio IIC, Yao M, et al. Bioactivation of napabucasin triggers reactive oxygen species–mediated cancer cell death. Ann Oncol. 2019;30:mdz268. 095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagaraju GP, Long TE, Park W, et al. Heat shock protein 90 promotes epithelial to mesenchymal transition, invasion, and migration in colorectal cancer. Mol Carcinog. 2015;54:1147–1158. [DOI] [PubMed] [Google Scholar]
- 15.Nagaraju GP, Wu C, Merchant N, Chen Z, Lesinski GB, El-Rayes BF. Epigenetic effects of inhibition of heat shock protein 90 (HSP90) in human pancreatic and colon cancer. Cancer Lett. 2017;402:110–116. [DOI] [PubMed] [Google Scholar]
- 16.Rajitha B, Belalcazar A, Nagaraju GP, et al. Inhibition of NF-κB translocation by curcumin analogs induces G0/G1 arrest and downregulates thymidylate synthase in colorectal cancer. Cancer Lett. 2016;373:227–233. [DOI] [PubMed] [Google Scholar]
- 17.Belalcazar A, Shaib WL, Farren MR, et al. Inhibiting heat shock protein 90 and the ubiquitin-proteasome pathway impairs metabolic homeostasis and leads to cell death in human pancreatic cancer cells. Cancer. 2017;123:4924–4933. [DOI] [PubMed] [Google Scholar]
- 18.Niemisto A, Dunmire V, Yli-Harja O, Zhang W, Shmulevich I. Robust quantification of in vitro angiogenesis through image analysis. IEEE Trans Med Imaging. 2005;24:549–553. [DOI] [PubMed] [Google Scholar]
- 19.Oh E-T, Kim J-w, Kim JM, et al. NQO1 inhibits proteasome-mediated degradation of HIF-1α. Nat Commun. 2016;7:13593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Y, Rogoff HA, Keates S, et al. Suppression of cancer relapse and metastasis by inhibiting cancer stemness. Proc Natl Acad Sci U S A. 2015;112:1839–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Löcken H, Clamor C, Müller K. Napabucasin and related heterocycle-fused naphthoquinones as STAT3 inhibitors with antiproliferative activity against cancer cells. J Nat Prod. 2018;81:1636–1644. [DOI] [PubMed] [Google Scholar]
- 22.Pradubyat N, Sakunrangsit N, Mutirangura A, Ketchart W. NADPH: Quinone oxidoreductase 1 (NQO1) mediated anti-cancer effects of plumbagin in endocrine resistant MCF7 breast cancer cells. Phytomedicine 2020;66:153133. [DOI] [PubMed] [Google Scholar]
- 23.Froeling FE, Swamynathan MM, Deschênes A, et al. Bioactivation of napabucasin triggers reactive oxygen species–mediated cancer cell death. Clin Cancer Res. 2019;25:7162–7174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dimri M, Humphries A, Laknaur A, et al. NAD (P) H quinone dehydrogenase 1 ablation inhibits activation of the phosphoinositide 3-kinase/Akt serine/threonine kinase and mitogen-activated protein kinase/extracellular signal-regulated kinase pathways and blocks metabolic adaptation in hepatocellular carcinoma. Hepatology. 2020;71:549–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Butsri S, Kukongviriyapan V, Senggunprai L, Kongpetch S, Zeekpudsa P, Prawan A. Downregulation of NAD (P) H: quinone oxidoreductase 1 inhibits proliferation, cell cycle and migration of cholangiocarcinoma cells. Oncol Lett. 2017;13:4540–4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khunluck T, Kukongviriyapan V, Senggunprai L, Duangarsong W, Prawan A. The inhibition kinetics and potential anti-migration activity of NQO1 inhibitory coumarins on cholangiocarcinoma cells. Integr Cancer Ther. 2019;18:1534735418820444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Madajewski B, Boatman MA, Chakrabarti G, Boothman DA, Bey EA. Depleting tumor-NQO1 potentiates anoikis and inhibits growth of NSCLC. Mol Cancer Res. 2016;14:14–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cullen JJ, Hinkhouse MM, Grady M, et al. Dicumarol inhibition of NADPH: quinone oxidoreductase induces growth inhibition of pancreatic cancer via a superoxide-mediated mechanism. Cancer Res. 2003;63:5513–5520. [PubMed] [Google Scholar]
- 29.Lewis A, Ough M, Li L, et al. Treatment of pancreatic cancer cells with dicumarol induces cytotoxicity and oxidative stress. Clin Cancer Res. 2004;10:4550–4558. [DOI] [PubMed] [Google Scholar]
- 30.Beaver SK, Mesa-Torres N, Pey AL, Timson DJ. NQO1: A target for the treatment of cancer and neurological diseases, and a model to understand loss of function disease mechanisms. Biochim Biophys Acta, Proteins Proteomics. 2019;1867:663–676. [DOI] [PubMed] [Google Scholar]
- 31.Fiorillo M, Sotgia F, Sisci D, Cappello AR, Lisanti MP. Mitochondrial “power” drives tamoxifen resistance: NQO1 and GCLC are new therapeutic targets in breast cancer. Oncotarget. 2017;8:20309–20327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bendell JC, Hubbard JM, O’Neil BH, et al. Phase 1b/II study of cancer stemness inhibitor napabucasin (BBI-608) in combination with FOLFIRI+/−bevacizumab (bev) in metastatic colorectal cancer (mCRC) patients (pts): J. Clin. Oncol; 2017:Abstract 3529. [Google Scholar]
- 33.Wang H, Jiang H, Van De Gucht M, De Ridder M. Hypoxic radioresistance: can ROS be the key to overcome It? Cancers. 2019;11:E112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun M, Pan D, Chen Y, Li Y, Gao K, Hu B. Coroglaucigenin enhances the radiosensitivity of human lung cancer cells through Nrf2/ROS pathway. Oncotarget. 2017;8:32807–32820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zou Z, Chang H, Li H, Wang S. Induction of reactive oxygen species: an emerging approach for cancer therapy. Apoptosis. 2017;22:1321–1335. [DOI] [PubMed] [Google Scholar]
- 36.Zhang G, Wang W, Yao C, Ren J, Zhang S, Han M. Salinomycin overcomes radioresistance in nasopharyngeal carcinoma cells by inhibiting Nrf2 level and promoting ROS generation. Biomed Pharmacother. 2017;91:147–154. [DOI] [PubMed] [Google Scholar]
- 37.Srinivas US, Tan B, Vellayappan BA, Jeyasekharan AD. ROS and the DNA damage response in cancer. Redox Biol. 2019:101084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kozlov SV, Waardenberg AJ, Engholm-Keller K, Arthur JW, Graham ME, Lavin M. Reactive oxygen species (ROS)-activated ATM-dependent phosphorylation of cytoplasmic substrates identified by large-scale phosphoproteomics screen. Mol Cell Proteomics. 2016;15:1032–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li Z, Yang J, Huang H. Oxidative stress induces H2AX phosphorylation in human spermatozoa. FEBS Lett. 2006;580:6161–6168. [DOI] [PubMed] [Google Scholar]
- 40.Chen X, Song L, Hou Y, Li F. Reactive oxygen species induced by icaritin promote DNA strand breaks and apoptosis in human cervical cancer cells. Oncol Rep. 2019;41:765–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim EJ, Ji I-M, Ahn K-J, et al. Synergistic effect of ionizing radiation and β-lapachone against RKO human colon adenocarcinoma cells. Cancer Res Treat. 2005;37:183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beg MS, Huang X, Silvers MA, et al. Using a novel NQO1 bioactivatable drug, beta-lapachone (ARQ761), to enhance chemotherapeutic effects by metabolic modulation in pancreatic cancer. J Surg Oncol. 2017;116:83–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang X, Dong Y, Bey EA, et al. An NQO1 substrate with potent antitumor activity that selectively kills by PARP1-induced programmed necrosis. Cancer Res. 2012;72:3038–3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spano J-P, Milano G, Rixe C, Fagard R. JAK/STAT signalling pathway in colorectal cancer: a new biological target with therapeutic implications. Eur J Cancer. 2006;42:2668–2670. [DOI] [PubMed] [Google Scholar]
- 45.Bharti R, Dey G, Mandal M. Cancer development, chemoresistance, epithelial to mesenchymal transition and stem cells: A snapshot of IL-6 mediated involvement. Cancer Lett. 2016;375:51–61. [DOI] [PubMed] [Google Scholar]
- 46.Nagaraju GP, Park W, Wen J, et al. Antiangiogenic effects of ganetespib in colorectal cancer mediated through inhibition of HIF-1α and STAT-3. Angiogenesis. 2013;16:903–917. [DOI] [PubMed] [Google Scholar]
- 47.Ramjiawan RR, Griffioen AW, Duda DG. Anti-angiogenesis for cancer revisited: Is there a role for combinations with immunotherapy? Angiogenesis. 2017;20:185–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Batchelor TT, Gerstner ER, Emblem KE, et al. Improved tumor oxygenation and survival in glioblastoma patients who show increased blood perfusion after cediranib and chemoradiation. Proc Natl Acad Sci U S A. 2013;110:19059–19064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hsu H-W, Wall NR, Hsueh C-T, et al. Combination antiangiogenic therapy and radiation in head and neck cancers. Oral Oncol. 2014;50:19–26. [DOI] [PubMed] [Google Scholar]