Abstract
Objective
Intraindividual cognitive variability (IIV), a measure of within-person variability across cognitive measures at a single time point, is associated with mild cognitive impairment (MCI) and Alzheimer’s disease (AD). Little is known regarding brain changes underlying IIV, or the relationship between IIV and functional ability. Therefore, we investigated the association between IIV and cerebral atrophy in AD-vulnerable regions and everyday functioning in nondemented older adults.
Method
736 Alzheimer’s Disease Neuroimaging Initiative (ADNI) participants (285 cognitively normal [CN]; 451 MCI) underwent neuropsychological testing and serial MRI over 2 years. Linear mixed effects models examined the association between baseline IIV and change in entorhinal cortex thickness, hippocampal volume, and everyday functioning.
Results
Adjusting for age, sex, apolipoprotein E genotype, amyloid-β positivity, and mean-level of cognitive performance, higher baseline IIV predicted faster rates of entorhinal and hippocampal atrophy, as well as functional decline. Higher IIV was associated with both entorhinal and hippocampal atrophy among MCI participants but selective vulnerability of the entorhinal cortex among cognitively normal (CN) individuals.
Conclusions
IIV was associated with more widespread medial temporal lobe (MTL) atrophy in individuals with MCI relative to CN, suggesting that IIV may be tracking advancing MTL pathologic changes across the continuum of aging, MCI, and dementia. Findings suggest that cognitive dispersion may be a sensitive marker of neurodegeneration and functional decline in nondemented older adults.
Keywords: mild cognitive impairment, neuropsychological assessment, variability, everyday functioning, neurodegeneration
INTRODUCTION
The identification of early cognitive changes in preclinical Alzheimer’s disease (AD) is of critical importance in order to target individuals at risk for decline prior to irreversible neuronal damage and clinically significant cognitive and functional impairments. Cognitive function, as measured by comprehensive neuropsychological assessment, is often reduced to mean level of performance within domains such as memory, language, and executive function. The conventional approach to establishing cognitive decline is to compare mean-level performance across groups of individuals. However, in the search for sensitive and reliable markers of preclinical changes in Alzheimer’s disease, there has been a growing interest in the utility of assessing within-person variability in performance across cognitive measures (Gleason et al., 2018; Koscik et al., 2016; Malek-Ahmadi et al., 2017).
Intraindividual variability (IIV) has traditionally been assessed as an individual’s distribution of reaction times or errors across trials on a given task (referred to as inconsistency) or by examining variability in performance across multiple measures within a single testing session (referred to as dispersion) (Stuss, Murphy, Binns, & Alexander, 2003). Aging is associated with increased dispersion (Christensen et al., 1999), and although some degree of variability across tests or domains is commonly seen in normal cognitive profiles (Schretlen, Munro, Anthony, & Pearlson, 2003), increased variability is thought to reflect decreased neurological integrity (Lovden et al., 2013; Murtha, Cismaru, Waechter, & Chertkow, 2002). Variability across neuropsychological scores may reflect subtle breakdowns in cognitive ability often observed in preclinical AD and therefore provide a more sensitive measure of early decline relative to mean performance. IIV may also reflect subtle changes in cognition that can be detected before conventional neuropsychological thresholds for cognitive impairment are met.
Increased IIV is associated with greater risk of conversion to mild cognitive impairment (MCI) and dementia (Holtzer, Verghese, Wang, Hall, & Lipton, 2008; Koscik et al., 2016), as well as increasing dementia severity (Reckess, Varvaris, Gordon, & Schretlen, 2014), and there associations remain even after adjusting for mean level of cognitive performance. Interestingly, a recent study found that IIV predicted incident MCI and AD to an extent comparable to established cerebrospinal fluid (CSF) AD biomarkers. Unlike CSF measures, IIV is non-invasive and easily implemented (Gleason et al., 2018), and this index has been shown to relate to everyday functioning in nondemented older adults beyond mean-level neuropsychological performance (Fellows & Schmitter-Edgecombe, 2015; Rapp, Schnaider-Beeri, Sano, Silverman, & Haroutunian, 2005). However, to date, no longitudinal studies have examined whether dispersion is associated with changes in everyday functioning over time in older adults at risk for Alzheimer’s disease.
Despite growing evidence linking higher IIV to progression to MCI and AD, little is known regarding the mechanisms underlying IIV in dementia risk. In the only existing study linking IIV to AD neuropathology, higher dispersion was associated with neurofibrillary tangle (NFT) pathology independent of amyloid burden (Malek-Ahmadi et al., 2017), suggesting the existence of an association between dispersion and neurodegeneration. Even less is known about how underlying AD-related brain changes associated with IIV evolve over time. We therefore sought out to build upon prior work by using a well-characterized sample of nondemented older adults from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) in order to investigate the longitudinal association between cognitive IIV and regional neurodegeneration and daily functioning in both normal aging and MCI. We hypothesized that increased baseline IIV would be associated with greater cerebral atrophy in medial temporal lobe (MTL) regions, even after adjusting for mean level of performance and important AD risk factors (e.g., cortical amyloid burden and apolipoprotein E [APOE] e4 genotype), due to selective vulnerability of these brain regions during early stages of NFT pathology. We also expected that greater IIV would be related to functional decline over time, particularly among the MCI participants.
METHOD
The ADNI dataset
Data used in the preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). The ADNI was launched in 2003 as a public-private partnership, led by Principal Investigator Michael W. Weiner, MD. The primary goal of ADNI has been to test whether serial magnetic resonance imaging (MRI), positron emission tomography (PET), other biological markers, and clinical and neuropsychological assessment can be combined to measure the progression of mild cognitive impairment (MCI) and early Alzheimer’s disease (AD). For up-to-date information, see www.adni-info.org.
Participants
All participants included in ADNI were between the ages of 55 and 90 years old, had completed at least 6 years of education, were Spanish or English speakers, had Geriatric Depression Scale scores < 6 (possible score range is 0–15)(Sheikh & Yesavage, 1986), had modified Hachinski Ischemic Scale scores ≤4, and were free of any significant neurological disease or systemic illness. Only participants who were nondemented at baseline and who underwent neuropsychological testing, florbetapir positron emission tomography (PET) amyloid imaging, assessment of everyday functioning with the Functional Activities Questionnaire (FAQ), and T1-weighted anatomical scans with processed data available for download as of November 1, 2017 were included in the present analyses (n = 736). This study was approved by the Institutional Review Boards of all participating institutions. Informed written consent was obtained from all participants at each site.
Participants were diagnosed as MCI (n=451) or classified as cognitively normal (n=285) at their initial screening evaluation based on ADNI diagnostic criteria (Petersen et al., 2010). Diagnostic criteria for MCI were as follows: (1) subjective memory complaint reported by participant or study partner; (2) Mini-Mental State Examination (MMSE) scores between 24–30; (3) global Clinical Dementia Rating Scale (CDR) score of 0.5; (4) abnormal memory function documented by scoring below education-adjusted cutoffs for delayed free recall on Story A of the Wechsler Memory Scale-Revised (WMS-R) Logical Memory II subtest (i.e., out of a maximum score of 25 points, cut-offs were as follows: (a) ≤ 8 for 16 or more years of education; (b) ≤ 4 for 8–15 years of education and (c) ≤ 2 for 0–7 years of education), and (5) general cognitive and functional abilities sufficiently preserved to an extent that they could not qualify for a diagnosis of dementia. Normal baseline cognition was established by ADNI based on cut-scores on the MMSE; CDR; and delayed recall of Story A from the Logical Memory II subscale of the WMS-R.
Participants received follow-up MRI exams and underwent everyday functioning assessment (i.e., FAQ) at 12 and 24 months after baseline. Of the 736 participants in the sample for the present study, 525 participants had complete data at the 24-month follow-up visit. A smaller subset of the sample had MRI and FAQ follow-up at 36 and 48 months. Given the significant reduction in available data after 24 months of follow-up, our primary analyses focused on follow-up to 24 months.
Intra-individual cognitive variability
The primary variable of interest for our study was an IIV index, depicting variability across cognitive measures at a single time point. We calculated the index of dispersion, or IIV, using a procedure similar to that used in previously published reports examining IIV (Gleason et al., 2018; Hilborn, Strauss, Hultsch, & Hunter, 2009; Lindenberger & Baltes, 1997; Morgan, Woods, Delano-Wood, Bondi, & Grant, 2011). As in these previous studies, standard summary measures from tests designed to assess multiple different cognitive abilities were selected for inclusion in the IIV index. Six neuropsychological measures from ADNI were selected because of their routine use in assessing early cognitive changes in AD, administration across all three ADNI grant periods (ADNI-1, -GO, and −2), and coverage of three different domains of cognition (i.e., language, processing speed/executive function; and episodic memory). These six measures were: (1) Animal Fluency, total score; (2) 30-item Boston Naming Test (BNT) total score; (3) Trail Making Test (TMT), Part A; time to completion, (4) TMT, Part B; time to completion, (5) Rey Auditory Verbal Learning Test (AVLT) 30-minute delayed free recall; number of words recalled, and (6) AVLT recognition; number of words correctly recognized. Notably, none of these cognitive measures were employed in ADNI’s diagnostic classification.
Before calculating the baseline IIV index, individual raw scores for each measure were converted into age-, education-, and sex-adjusted z-scores with a mean of 0 and standard deviation of 1 using regression coefficients derived from robust cognitively normal individuals (n=385) who had at least one year of follow-up and did not progress to MCI at any point during their participation in the study (Edmonds et al., 2015). The TMT z-scores were multiplied by −1 so higher z-scores reflected better performance for all scores. The intraindividual standard deviation across the 6 baseline z-scores was computed to create the IIV index. A high score on the IIV index indicated greater variability across cognitive measures whereas a low score on the IIV index reflected more consistency across measures (regardless of scores on the individual neuropsychological measures included in the IIV index).
Assessment of Everyday Functioning
Everyday functioning was quantified using the Functional Assessment Questionnaire (FAQ), a standardized assessment of instrumental activities of daily living (IADL). The FAQ was completed by each participants’ study partner at baseline, 6-month follow-up, and then annually. The study partner rated each participant’s performance over the preceding 4 weeks on 10 separate categories of daily activities including: (1) writing checks, paying bills, or balancing a checkbook; (2) assembling tax records, business affairs, or other papers; (3) shopping alone for clothes, household necessities, or groceries; (4) playing a game of skill such as bridge or chess or working on a hobby; (5) making coffee or tea; (6) preparing a balanced meal; (7) keeping track of current events; (8) paying attention to and understanding a TV program, book, or magazine; (9) remembering appointments, family occasions, holidays, medications; and (10) traveling out of the neighborhood. Each item was rated on a 4-point scale, with higher scores indicating greater dependence (dependent = 3; requires assistance = 2; has difficulty but does by self = 1; normal = 0). The FAQ total score was derived as the sum of the 10 individual activity scores and ranges from 0 to 30.
T1-weighted anatomical MR imaging data acquisition and processing
A detailed description of ADNI MR imaging data acquisition and processing can be found online (www.loni.usc.edu). Briefly, structural scans collected at baseline and follow-up visits were motion corrected, skull-stripped, segmented, and parcellated using FreeSurfer version 5.1 (surfer.nmr.mgh.harvard.edu) (Fischl et al., 2002; Fischl et al., 2004). FreeSurfer derived entorhinal cortical thickness and hippocampal volume served as dependent variables in models. Hippocampal volume was normalized by dividing absolute hippocampal volume by FreeSurfer-derived estimated total intracranial volume and then multiplying the resulting value by 100. Normalized hippocampal volume was examined in all analyses.
Florbetapir PET data acquisition and processing
Amyloid burden was quantified using PET scanning with an 18F-florbetapir tracer. A detailed description of ADNI florbetapir PET imaging data acquisition and processing can be found online (www.loni.usc.edu). Briefly, florbetapir scans were co-registered, averaged, reoriented into a standard 160×160×96 voxel image grid with 1.5 mm cubic voxels, and smoothed to a uniform isotropic resolution of 8 mm full width at half maximum. As described above, structural MR images were skull-stripped, segmented, parcellated using FreeSurfer. This structural image was co-registered to each participant’s first florbetapir image.
A cortical summary standardized uptake value ratio (SUVR) was calculated by dividing the mean florbetapir uptake across four main cortical regions (i.e., frontal, anterior/posterior cingulate, lateral parietal, and lateral temporal cortices) by whole cerebellar (white and gray matter) florbetapir uptake. Increased retention of florbetapir is thought to reflect greater cortical Aβ load. Aβ positivity was determined using the recommended threshold for cross-sectional florbetapir analyses of 1.11 using the whole cerebellum as the reference region.(Clark et al., 2012; Joshi et al., 2012; Landau et al., 2013; Landau et al., 2014)
Statistical analyses
Baseline demographic and clinical characteristics by cognitive status (i.e., MCI versus normal cognition) were examined using analysis of variance (ANOVA) and chi-square tests. Multiple linear regression, adjusting for covariates, was used to examine the cross-sectional relationships between the IIV index, entorhinal cortical thickness, normalized hippocampal volume, and FAQ score at baseline. All linear regression models adjusted for age, sex, APOE ε4 status (carrier versus non-carrier), amyloid-β (Aβ) status (positive versus negative), and mean baseline neuropsychological z-score across the 6 cognitive measures included in the IIV index. The model with FAQ as the dependent variable additionally adjusted for education given the known influence education has on cognitive performance and everyday functioning.
Linear mixed effects models analyzed longitudinal rate of change in entorhinal cortex, normalized hippocampal volume, and FAQ score as a function of baseline IIV over the 2-year interval. The covariates listed above, as well as time (i.e., a visit variable consisting of 3 time points including baseline, month 12, and month 24), the mean baseline neuropsychological z-score x time interaction term, and the IIV x time interaction term, were included as fixed effects and modeled as continuous parameters. The random effect of subject intercept was included. Full information maximum likelihood estimation was used to allow for all available data to be included (Singer & Willett, 2003; Woodard, 2017), which has been demonstrated to be less biased than list-wise deletion (Schafer & Graham, 2002). Of note, there was no missing data at baseline; at 12 months follow up, 590 participants had complete data whereas 146 individuals were missing data; and at 24 months follow up, 525 participants had complete data whereas 211 individuals were missing data. Parameter estimate effect sizes, as indexed by r-values, were interpreted as small (0.10), medium (0.30), and large (0.50) (Cohen, 1988). In addition, model assumptions were met (e.g., there was no multicollinearity of the independent variables, residuals were normally distributed).(Singer & Willett, 2003)
To assess potential selective attrition, ANOVA and chi-square tests were performed to examine whether demographic or clinical characteristics differed between participants in the overall analytic sample who completed the month 24 visit (n=525) and those who were missing data at month 24 (n=211). In addition, as some participants had follow-up beyond 24 months, we ran sensitivity analyses including those participants with follow-up to 48 months (n=117) to confirm if findings from the primary analyses with 24-month follow-up analysis were consistent during this longer follow-up period. All analyses were performed using Statistical Package for the Social Sciences (SPSS) version 24 (SPSS IBM, New York, USA).
3. RESULTS
3.1. Participant characteristics
Baseline demographic and clinical characteristics are shown in Table 1. In comparison to the cognitively normal group, the MCI group was significantly younger at baseline; attained fewer years of education; and had a greater proportion of men, Aβ+ individuals, and APOE ε4 carriers (all p-values ≤ .03). As expected, the MCI group had significantly higher FAQ and IIV scores; lower mean neuropsychological performance as well as lower performance on each of the six individual measures included in the IIV index; and lower entorhinal cortex thickness and smaller hippocampal volume compared to the cognitively normal group (all p-values ≤ .001).
Table 1.
Baseline demographics and clinical characteristics for the overall sample and by cognitive status
| Overall Sample N = 736 |
Normal Cognition n = 285 |
MCI n = 451 |
||||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | F or χ2 | p | |
| Age, years | 72.23 | 6.94 | 73.00 | 6.08 | 71.74 | 7.39 | 5.77 | 0.017 |
| Education, years | 16.37 | 2.62 | 16.63 | 2.53 | 16.20 | 2.66 | 4.71 | 0.030 |
| Gender (% female) | 48.5% | — | 54.4% | — | 44.8% | — | 6.44 | 0.011 |
| APOE ε4 carrier (%)* | 40.8% | — | 28.8% | — | 48.3% | — | 27.69 | <0.001 |
| Aβ + (%)** | 46.3% | — | 31.9% | — | 55.4% | — | 38.80 | <0.001 |
| FAQ Score | 1.71 | 3.15 | 0.33 | 1.16 | 2.59 | 3.65 | 102.19 | <0.001 |
| IIV | 0.91 | 0.46 | 0.80 | 0.40 | 0.98 | 0.48 | 29.22 | <0.001 |
| Mean NP Score*** | −0.44 | 0.79 | −0.06 | 0.60 | −0.69 | 0.81 | 127.34 | <0.001 |
| Animal Fluency | −0.37 | 0.99 | −0.05 | 0.99 | −0.58 | 0.94 | 52.70 | <0.001 |
| Boston Naming Test | −0.42 | 1.38 | 0.00 | 1.02 | −0.68 | 1.51 | 43.94 | <0.001 |
| Trails A | −0.30 | 1.27 | 0.00 | 0.96 | −0.49 | 1.40 | 26.98 | <0.001 |
| Trails B | −0.44 | 1.27 | −0.06 | 1.02 | −0.69 | 1.36 | 45.11 | <0.001 |
| AVLT Delayed Recall | −0.56 | 1.10 | −0.12 | 1.01 | −0.85 | 1.06 | 85.95 | <0.001 |
| AVLT Recognition | −0.56 | 1.25 | −0.13 | 1.05 | −0.84 | 1.30 | 59.81 | <0.001 |
| Entorhinal thickness, mm | 3.44 | 0.41 | 3.55 | 0.28 | 3.38 | 0.46 | 34.29 | <0.001 |
| Normalized hippocampal volume**** | 0.48 | 0.08 | 0.51 | 0.06 | 0.47 | 0.08 | 49.99 | <0.001 |
Results from analysis of variance (ANOVAs) for continuous variables and chi-square tests for dichotomous variables. Data are summarized as mean (standard deviation), unless otherwise indicated. Significant group differences (p < 0.05) appear in bold font.
Abbreviations: MCI = mild cognitive impairment; SD = standard deviation; APOE = apolipoprotein E; amyloid-β (Aβ); FAQ = Functional Assessment Questionnaire; IIV = intraindividual cognitive variability; NP = neuropsychological; AVLT = Rey Auditory Verbal Learning Test; mm = millimeter
APOE ε4+ = at least one APOE ε4 allele
Amyloid-β negativity versus positivity was based on the recommended threshold for cross-sectional florbetapir analyses of 1.11 using the whole cerebellum as the reference region (reference).
Mean NP score is the mean of the six baseline neuropsychological age-, sex-, and education-adjusted z-scores. The six scores were 1) Animal Fluency, total score; (2) 30-item Boston Naming Test (BNT) total score; (3) Trail Making Test (TMT), Part A; time to completion, (4) TMT, Part B; time to completion, (5) Rey Auditory Verbal Learning Test (AVLT) 30-minute delayed free recall; number of words recalled, and (6) AVLT recognition; number of words correctly recognized.
Hippocampal volume was normalized by dividing absolute hippocampal volume by total intracranial volume and then multiplying the resulting value by 100.
3.2. Attrition
To assess potential selective attrition, between-subjects ANOVA and chi-square tests were performed to examine whether demographic or clinical characteristics differed between participants in the analytic sample who completed the month 24 visit (n=525) and those who were missing data at month 24 (n=211). Participants were compared in terms of mean age, sex, APOE ε4 status, Aβ positivity, and cognitive status (MCI versus cognitively normal). Attrition from baseline to 24 months did not differ for any of these variables (all P > 0.05).
3.3. Cross-sectional Associations of Baseline IIV, Entorhinal Cortical Thickness, and Hippocampal Volume
Multiple linear regression models, adjusting for age, sex, Aβ positivity, APOE ε4 genotype, and baseline mean level of cognitive performance, examined the cross-sectional associations between baseline IIV and baseline entorhinal cortical thickness and hippocampal volume across the entire sample. Baseline entorhinal cortical thickness and hippocampal volume were examined as the dependent variable in separate models. There was a trend toward higher levels of IIV being associated with lower entorhinal cortical thickness (Overall model: R2 = .216, F (6,729) = 33.48 p < .001; IIV: Beta = −0.07, p = 0.088). IIV was not a unique predictor of baseline hippocampal volume (Overall model: R2 = .288, F (6,729) = 49.14 p < .001); IIV: Beta = −0.02, p = 0.568).
Analyses were then performed separately for the cognitively normal and MCI groups. Among the MCI group, adjusting for age, sex, Aβ positivity, APOE ε4 genotype, and baseline mean level of cognitive performance, higher levels of IIV were significantly associated with lower entorhinal cortical thickness (Overall model: R2 = .251, F (6,444) = 24.78 p < .001; IIV: Beta = −0.11, p = 0.039). After adjusting for the aforementioned covariates, IIV was not significantly associated with baseline hippocampal volume in the MCI group (Overall model: R2 = .298, F (6,444) = 31.36 p < .001); IIV: Beta = −0.05, p = 0.351). In addition, among cognitively normal older adults, adjusting for age, sex, Aβ positivity, APOE ε4 genotype, and baseline mean level of cognitive performance, there was no significant association between baseline IIV and baseline entorhinal cortical thickness (Overall model: R2 = .073, F (6,278) = 3.65 p = .002; IIV: Beta = 0.1, p = 0.847) or hippocampal volume (Overall model: R2 = .218, F (6,278) = 12.88 p < .001; IIV: Beta < 0.01, p = 0.999).
3.4. Cross-sectional Associations of Baseline IIV and Daily Functioning
Multiple linear regression models, adjusting for age, education, sex, Aβ status, APOE ε4 genotype, and baseline mean level of cognitive performance, examined the cross-sectional relationships between baseline IIV and FAQ score across the entire sample. Higher level of IIV was significantly associated with higher FAQ scores at baseline (Overall model: R2 = .146, F (7,728) = 17.79 p < .001; IIV: Beta = 0.11, p = 0.014).
Analyses were then run for the cognitively normal and MCI groups separately. Among the MCI group, adjusting for age, education, sex, Aβ positivity, APOE ε4 genotype, and baseline mean level of cognitive performance, higher levels of IIV were significantly associated with higher FAQ scores at baseline (Overall model: R2 = .116, F (7,443) = 8.28 p < .001; IIV: Beta = 0.15, p = 0.015). In contrast, after adjusting for the above covariates, among the cognitively normal group, IIV was not significantly associated with FAQ (Overall model: R2 = .141, F (7,277) = 0.799 p = .589); IIV: Beta = 0.06, p = 0.437).
3.5. Longitudinal Prediction of Entorhinal Cortical Thickness and Hippocampal Volume by Baseline IIV
Multilevel modeling, adjusting for baseline age, sex, Aβ status, APOE ε4 genotype, and baseline mean level of cognitive performance, examined whether baseline IIV predicted longitudinal change in entorhinal cortical thickness and hippocampal volume across the 24-month follow-up period. Tables 2 and 3 include the multilevel model parameter estimates for entorhinal cortical thickness and hippocampal volume, respectively. There was a significant interaction between baseline IIV and time, such that higher IIV was associated with decreasing entorhinal cortical thickness (i.e., more atrophy) across time [F(1, 720.04) = 36.72, p < 0.001]. In addition, higher baseline IIV was associated with decreasing hippocampal volume (more atrophy) across time [F(1, 763.23) = 6.98, p = 0.008].
Table 2.
Estimates and effect sizes for the full longitudinal model of the association of IIV and entorhinal cortical thickness
| Estimate | S.E. | df | F | t | p | r | |
|---|---|---|---|---|---|---|---|
| Intercept | 4.760 | 0.150 | 731.91 | 1001.20 | 31.64 | <0.001 | 0.760 |
| Age | −0.016 | 0.002 | 730.67 | 60.10 | −7.75 | <0.001 | 0.276 |
| Gender | −0.029 | 0.027 | 731.14 | 1.13 | −1.06 | 0.288 | 0.039 |
| APOE ε4 status | 0.009 | 0.031 | 730.63 | 0.09 | .31 | 0.760 | 0.011 |
| Aβ status | −0.065 | 0.031 | 731.50 | 4.40 | −2.10 | 0.036 | 0.077 |
| Mean NP Score* | 0.163 | 0.022 | 730.99 | 53.31 | 7.30 | <0.001 | 0.261 |
| Visit | −0.0005 | 0.0008 | 701.81 | 0.38 | −0.61 | 0.539 | 0.023 |
| Baseline IIV | −0.051 | 0.038 | 749.07 | 1.76 | −1.33 | 0.185 | 0.049 |
| IIV × Visit | −0.005 | 0.0008 | 720.04 | 36.72 | −6.06 | <0.001 | 0.220 |
APOE ε4 status = presence or absence of at least one ε4 allele; Aβ = Amyloid-β; IIV = intraindividual cognitive variability; S.E. = standard error of the estimate; df = degrees of freedom.
Mean NP score is the mean of the six baseline neuropsychological age-, sex-, and education-adjusted z-scores. The six scores were Animal Fluency and Boston Naming Test (language); Trail Making Test Parts A and B (processing speed/executive); and Auditory Verbal Learning Test Delayed Recall and Recognition (memory).
Significant effects (p < 0.05) appear in bold font.
Effect size (r values) interpretation: small=0.10, medium=0.30, large=0.50 (Cohen, 1992).
Table 3.
Estimates and effect sizes for the full longitudinal model of the association of IIV and normalized hippocampal volume
| Estimate | S.E. | df | F | t | p | r | |
|---|---|---|---|---|---|---|---|
| Intercept | 0.785 | 0.027 | 735.70 | 872.69 | 29.54 | <0.001 | 0.737 |
| Age | −.004 | 0.0004 | 734.55 | 120.92 | −11.00 | <0.001 | 0.376 |
| Gender | 0.023 | 0.005 | 734.85 | 23.27 | 4.82 | <0.001 | 0.175 |
| APOE ε4 status | 0.0004 | 0.005 | 734.26 | 0.005 | 0.07 | 0.945 | 0.003 |
| Aβ status | −0.020 | 0.005 | 735.04 | 13.16 | −3.63 | <0.001 | 0.133 |
| Mean NP Score* | 0.026 | 0.004 | 734.60 | 43.71 | 6.61 | <0.001 | 0.237 |
| Visit | −.0004 | 0.0001 | 748.25 | 15.30 | −3.91 | <0.001 | 0.142 |
| Baseline IIV | −0.001 | 0.007 | 753.30 | 0.03 | −0.17 | 0.863 | 0.006 |
| IIV × Visit | −0.0003 | 0.0001 | 763.23 | 6.98 | −2.64 | 0.008 | 0.095 |
APOE ε4 status = presence or absence of at least one ε4 allele; Aβ = Amyloid-β; IIV = intraindividual cognitive variability; S.E. = standard error of the estimate; df = degrees of freedom.
Mean NP score is the mean of the six baseline neuropsychological age-, sex-, and education-adjusted z-scores. The six scores were Animal Fluency and Boston Naming Test (language); Trail Making Test Parts A and B (processing speed/executive); and Auditory Verbal Learning Test Delayed Recall and Recognition (memory).
Significant effects (p < 0.05) appear in bold font.
Effect size (r values) interpretation: small=0.10, medium=0.30, large=0.50 (Cohen, 1992).
Analyses were then run separately for normal cognition and MCI groups. Among cognitively normal older adults, higher baseline IIV was significantly associated with greater reduction in entorhinal cortical thickness (more atrophy) across time [F(1, 267.87) = 9.20, p = 0.003]. Baseline IIV was not significantly associated with change in normalized hippocampal volume across time when analyses were restricted to those with normal cognition [F(1, 264.85) = 2.25, p = 0.135]. In contrast, among the MCI group, higher baseline IIV was significantly associated with greater reduction in entorhinal cortical thickness (more atrophy) across time [F(1, 455.64) = 22.40, p < 0.001] and lower normalized hippocampal volume (more atrophy) across time [F(1, 474.90) = 4.81, p = 0.029]. See Supplementary Tables 1a and 1b and Supplementary Figure 1 for models with entorhinal cortical thickness as the dependent variable presented separately for those with MCI and participants with normal cognition. See Supplementary Tables 2a and 2b and Supplementary Figure 2 for models with normalized hippocampal volume as the dependent variable presented separately for those with MCI and participants with normal cognition.
3.6. Longitudinal Prediction of Daily Functioning by Baseline IIV
Multilevel modeling, adjusting for baseline age, sex, education, Aβ status, APOE ε4 genotype, and baseline mean level of cognitive performance, examined whether baseline IIV predicted longitudinal change in FAQ score across the 24-month follow-up period. Higher IIV was associated with higher FAQ scores (greater rate of decline in functional abilities) across time [F(1, 1041.03) = 91.71, p < 0.001]. See Table 4 for multilevel model parameter estimates.
Table 4.
Estimates and effect sizes for the full longitudinal model of the association of IIV and functional abilities
| Estimate | S.E. | df | F | t | p | r | |
|---|---|---|---|---|---|---|---|
| Intercept | 1.081 | 1.541 | 535.12 | 0.492 | 0.70 | 0483 | 0.030 |
| Age | 0.009 | 0.018 | 538.55 | 0.251 | 0.50 | 0.617 | 0.022 |
| Gender | −0.762 | 0.236 | 537.75 | 10.425 | −3.23 | 0.001 | 0.138 |
| Education | −0.059 | 0.045 | 538.20 | 1.712 | −1.31 | 0.191 | 0.056 |
| APOE ε4 status | 0.162 | 0.263 | 538.65 | 0.379 | 0.62 | 0.538 | 0.027 |
| Aβ status | 1.058 | 0.263 | 537.33 | 16.239 | 4.03 | <0.001 | 0.171 |
| Mean NP Score* | −1.211 | 0.189 | 536.24 | 40.942 | −6.40 | <0.001 | 0.266 |
| Visit | −0.048 | 0.012 | 1024.50 | 15.178 | −3.90 | <0.001 | 0.121 |
| Baseline IIV | 0.270 | 0.326 | 471.39 | 0.686 | 0.83 | 0.408 | 0.038 |
| IIV × Visit | 0.117 | 0.012 | 1041.03 | 91.708 | 9.58 | <0.001 | 0.285 |
APOE ε4 status = presence or absence of at least one ε4 allele; Aβ = Amyloid-β; IIV = intraindividual cognitive variability; S.E. = standard error of the estimate; df = degrees of freedom.
Mean NP score is the mean of the six baseline neuropsychological age-, sex-, and education-adjusted z-scores. The six scores were Animal Fluency and Boston Naming Test (language); Trail Making Test Parts A and B (processing speed/executive); and Auditory Verbal Learning Test Delayed Recall and Recognition (memory).
Significant effects (p < 0.05) appear in bold font.
Effect size (r values) interpretation: small=0.10, medium=0.30, large=0.50 (Cohen, 1992).
Analyses were performed again for those with normal cognition and MCI separately. Among cognitively normal older adults, higher baseline IIV was not associated with higher FAQ scores (functional decline) across time [F(1, 354.98) = 0.075, p = 0.784]. In contrast, among the MCI group, higher baseline IIV was significantly associated with higher FAQ scores (functional decline) across time [F(1, 644.12) = 67.85, p < 0.001]. See Supplementary Tables 3a and 3b and Supplementary Figure 3 for models with FAQ score as the dependent variable presented separately for those with MCI and participants with normal cognition.
3.7. Sensitivity Analyses Including Participants with 48-month Follow-up
A subset of the sample had FAQ and MRI follow-up at 36 months (n=92) and 48 months (n=117). Multilevel modeling analyzing longitudinal rate of change in entorhinal cortex, hippocampal volume, and FAQ score as a function of baseline IIV over a 4-year interval, showed that findings remained qualitatively and statistically similar. That is, baseline IIV significantly predicted entorhinal and hippocampal atrophy as well as functional decline (p’s < .001)
DISCUSSION
Our findings showed that higher IIV at baseline predicts faster rates of cerebral atrophy in AD-vulnerable regions and functional decline even after adjusting for mean level of cognitive performance at baseline and AD risk factors including age, sex, APOE ε4 genotype, and Aβ positivity. IIV was associated with more widespread MTL atrophy in individuals with MCI relative to normal cognition, suggesting that IIV may be tracking alongside MTL pathologic changes across the continuum of aging, MCI, and dementia. Findings of this study provide evidence that cognitive IIV may represent an especially sensitive marker of neurodegeneration—even in cognitively normal individuals—that predicts changes in real world, everyday functioning. Our study adds to a growing body of evidence indicating that IIV has utility in predicting outcomes in individuals at risk for AD (Gleason et al., 2018; Holtzer et al., 2008; Koscik et al., 2016) and extends previous work by demonstrating associations with longitudinal changes in MTL integrity and everyday functioning. To our knowledge, this is the first examination of IIV as a longitudinal predictor of MRI markers of neurodegeneration and functional decline in nondemented older adults.
Previous studies have suggested that IIV may serve as a marker for AD pathologic changes, and that increases in IIV may reflect a cortical network disconnection syndrome in AD (Holtzer et al., 2008; Malek-Ahmadi et al., 2017). In the only study to-date linking IIV to AD neuropathology, there was a positive association between IIV and NFTs, whereas IIV was not related to plaque pathology. The authors noted that tangle pathology reflects neuronal degeneration, which may lead to degradation of cortical networks. The present study extends these previous findings linking IIV to neuropathologic makers of neuronal degeneration at autopsy to in vivo MRI markers of neurodegeneration. As noted by Malek-Ahmadi and colleagues (Malek-Ahmadi et al., 2017), the finding of an association between IIV and NFTs raises the possibility that IIV may be a useful behavioral marker in clinical trials of therapies targeting tau (Bakota & Brandt, 2016). Notably, we found that even among cognitively normal individuals who are not impaired on traditional neuropsychological measures, IIV predicted increasing entorhinal cortex atrophy. IIV may relate to neurodegeneration before declines or impairment in mean-level performance is observed. Therefore, considering IIV may identify individuals who might not otherwise be characterized as at risk for neurodegeneration if only mean-level cognitive performance is considered.
In our study, IIV predicted increasing functional difficulty, although it should be noted that the sample was, on average, still functionally independent at the 24-month visit. A cutoff of 6 or higher on the FAQ has been shown to best discriminate between MCI and very mild AD (Teng et al., 2010). While this finding suggests that the change in everyday functioning observed in the present study did not reach dependence in IADLs, increased functional difficulty is a significant risk factor for future functional disability and cognitive impairment (Farias et al., 2017; Nowrangi, Rosenberg, & Leoutsakos, 2016). Our findings therefore provide support for IIV, a noninvasive and easily obtainable marker, as a possible risk factor for decline in daily functioning among nondemented older adults. Notably, IIV predicted functional decline in older adults with MCI who are likely to develop dementia. IIV may be less useful in predicting functional decline in those who are cognitively normal, which may relate to truncated range in the latter group.
That variability in cognitive performance may reflect reduced neurological integrity is an established theory in the field of psychology (Gleason et al., 2018; Hilborn et al., 2009; MacDonald, Hultsch, & Dixon, 2003; Salthouse & Soubelet, 2014). Proposed mechanisms linking higher dispersion and neurological disease include disrupted neural networks, altered functional connectivity, and executive dysfunction or impaired cognitive control (Gleason et al., 2018; Kelly, Uddin, Biswal, Castellanos, & Milham, 2008; West, Murphy, Armilio, Craik, & Stuss, 2002). In contrast to summary scores that reflect function within a single cognitive domain, intraindividual within-session across-neuropsychological test variability can be thought of as a single index of variability across several cognitive domains that are subserved by multiple brain regions and neural networks. As such, this type of intraindividual cognitive variability may reflect declining brain integrity in the early stages of a dementia process (Holtzer et al., 2008). Given that we focused on AD risk, we focused on brain regions affected early in the AD disease process. However, it is likely that other brain regions and networks outside of the MTL relate to dispersion. Future studies examining the associations between dispersion and other brain regions and networks (e.g., frontal regions) as well as the utility of dispersion as a predictor of decline in additional populations (e.g., healthy aging, frontotemporal dementia) are warranted.
IIV can be estimated using multiple approaches. Like previous studies of IIV and AD risk (Gleason et al., 2018; Holtzer et al., 2008; Koscik et al., 2016), we examined IIV as dispersion or within-person variability across separate neuropsychological measures rather than inconsistency across trials of a single task (e.g., variability in reaction time across trials of the same task). Notably, previous studies comparing multiple measures of within-person variability have found that measures of dispersion across tasks and inconsistency within a single task are positively correlated, and both types of indices correlate with increasing age and cognitive decline (Hilborn et al., 2009; Hultsch, MacDonald, & Dixon, 2002).
In selecting the individual measures used to calculate the IIV index, we aimed to maximize our sample size, consider multiple cognitive domains, increase generalizability through including neuropsychological measures that are commonly used in research and clinical settings, and include measures not used in ADNI’s diagnostic classification. Within-person across-neuropsychological measure variability could be calculated using different tests than those used in our study. Future research incorporating additional or different neuropsychological tests may further clarify whether there is an optimal number or combination of measures to include to maximize sensitivity to risk of decline. As described by Holtzer and colleagues (2008), an advantage of the approach we used to compute IIV is that this form of variability can be calculated using standard, widely used neuropsychological assessment methods that are commonly used in both research and clinical settings and are often administered in one testing session. That is, calculation of this type of within-person across-neuropsychological test variability requires no changes to standard neuropsychological assessment procedures commonly used in research studies and clinical settings thereby increasing the potential clinical utility of this IIV index (Holtzer et al., 2008).
Strengths of the present study include a large sample of well-characterized older adults, assessment of neuropsychological functioning and IADLs, analysis of multimodal neuroimaging data, utilization of both neurodegeneration and functional decline as outcomes, and longitudinal design. However, there are also limitations to this study. Twenty four month follow up was a relatively short period of time to see changes in brain structure and everyday functioning particularly among cognitively normal individuals. We would not expect clinically significant functional difficulties in cognitively normal individuals. It is possible that this group experienced subtle functional changes that may not have been captured on the FAQ. In addition, the participants included in this sample were relatively homogeneous and tended to be well educated and Caucasian. ADNI focuses on amnestic forms of MCI and it is possible that findings may have differed in a sample of nonamnestic MCI participants. Future studies replicating the current findings with longer intervals of follow-up and in more diverse samples are warranted. Also, as discussed above, test selection may influence findings and it is possible that the measures selected in this study may not represent the most sensitive IIV index possible.
In summary, our findings show that IIV is a sensitive predictor of neurodegeneration and decline in everyday functioning. IIV predicts these outcomes even after adjusting for mean level of performance and established AD risk factors including APOE status and amyloid positivity. Although normal neuropsychological profiles comprise strengths and weaknesses, our longitudinal design supports the notion that increased IIV reflects neurodegeneration and poorer functioning. Taken together with recent evidence linking IIV to conversion to MCI and dementia due to AD at a level comparable to established CSF biomarkers of AD, as well as evidence linking IIV to NFTs at autopsy, our findings suggest that IIV is a practical and noninvasive alternative to traditional biomarkers in identifying individuals at risk for decline. Moreover, IIV may represent a useful marker in the context of clinical trials targeting neurodegeneration (Gleason et al., 2018; Malek-Ahmadi et al., 2017).
Supplementary Material
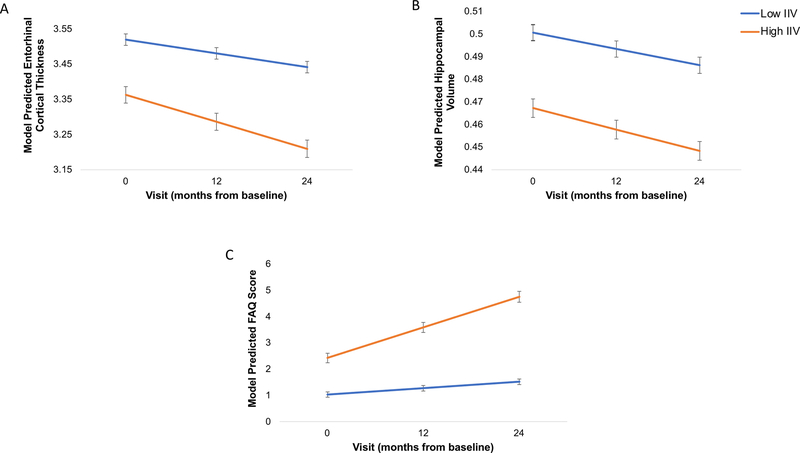
Figure 1.
Baseline IIV Predicts Entorhinal and Hippocampal Atrophy and Functional Decline
Line graphs displaying model predicted values, controlling for age, sex, apolipoprotein E ε4 genotype, PET amyloid-β positivity, and mean level of cognitive performance (and additionally adjusting for education for the model with functional abilities as the depending variable) are shown for (A) entorhinal cortex, (B) normalized hippocampal volume, and (C) Functional Assessment Questionnaire. For visual comparison, the graphs display results for high IIV levels in comparison with low IIV levels which were determined by a median split of the values in the analytic sample (Low = IIV < 0.8195; High = IIV ≥ 0.8195). Lower cortical thickness and hippocampal volume indicate reduced thickness and volume, respectively, (i.e., increasing atrophy). Higher FAQ scores indicate greater functional difficulty. Error bars represent the standard error of the mean.
Public Significance Statement.
Identification of early and reliable cognitive changes in the early stages Alzheimer’s disease (AD) is critical in order to target individuals at risk for significant neuropathologic and functional declines. Our findings suggest that cognitive dispersion may be a sensitive marker of brain changes and functional decline and have added utility above and beyond more conventional AD risk factors including age, genetic risk, and amyloid burden.
ACKNOWLEDGEMENTS
This work was supported by the U.S. Department of Veterans Affairs Clinical Sciences Research and Development Service, NIH, the Alzheimer’s Association, and the Dana Foundation. Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
Footnotes
Data used in preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. A complete listing of ADNI investigators can be found at: http://adni.loni.usc.edu/wp-content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf
REFERENCES
- Bakota L, & Brandt R (2016). Tau Biology and Tau-Directed Therapies for Alzheimer’s Disease. Drugs, 76(3), 301–313. doi: 10.1007/s40265-015-0529-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen H, Mackinnon AJ, Korten AE, Jorm AF, Henderson AS, Jacomb P, & Rodgers B (1999). An analysis of diversity in the cognitive performance of elderly community dwellers: individual differences in change scores as a function of age. Psychology and Aging, 14(3), 365–379. [DOI] [PubMed] [Google Scholar]
- Clark CM, Pontecorvo MJ, Beach TG, Bedell BJ, Coleman RE, Doraiswamy PM, … Skovronsky DM (2012). Cerebral PET with florbetapir compared with neuropathology at autopsy for detection of neuritic amyloid-beta plaques: a prospective cohort study. Lancet Neurol, 11(8), 669–678. doi: 10.1016/s1474-4422(12)70142-4 [DOI] [PubMed] [Google Scholar]
- Cohen J (1988). Statistical Power Analysis for the Behavioral Sciences (2nd ed.). Hillsdale, New Jersey: Lawrence Erlbaum Associates. [Google Scholar]
- Edmonds EC, Delano-Wood L, Clark LR, Jak AJ, Nation DA, McDonald CR, … Bondi MW (2015). Susceptibility of the conventional criteria for mild cognitive impairment to false-positive diagnostic errors. Alzheimers Dement, 11(4), 415–424. doi: 10.1016/j.jalz.2014.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farias ST, Lau K, Harvey D, Denny KG, Barba C, & Mefford AN (2017). Early Functional Limitations in Cognitively Normal Older Adults Predict Diagnostic Conversion to Mild Cognitive Impairment. Journal of the American Geriatrics Society, 65(6), 1152–1158. doi: 10.1111/jgs.14835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellows RP, & Schmitter-Edgecombe M (2015). Between-domain cognitive dispersion and functional abilities in older adults. Journal of Clinical and Experimental Neuropsychology, 37(10), 1013–1023. doi: 10.1080/13803395.2015.1050360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, … Dale AM (2002). Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron, 33(3), 341–355. [DOI] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, … Dale AM (2004). Automatically parcellating the human cerebral cortex. Cerebral Cortex, 14(1), 11–22. [DOI] [PubMed] [Google Scholar]
- Gleason CE, Norton D, Anderson ED, Wahoske M, Washington DT, Umucu E, … Asthana S (2018). Cognitive Variability Predicts Incident Alzheimer’s Disease and Mild Cognitive Impairment Comparable to a Cerebrospinal Fluid Biomarker. J Alzheimers Dis, 61(1), 79–89. doi: 10.3233/jad-170498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilborn JV, Strauss E, Hultsch DF, & Hunter MA (2009). Intraindividual variability across cognitive domains: investigation of dispersion levels and performance profiles in older adults. Journal of Clinical and Experimental Neuropsychology, 31(4), 412–424. doi: 10.1080/13803390802232659 [DOI] [PubMed] [Google Scholar]
- Holtzer R, Verghese J, Wang C, Hall CB, & Lipton RB (2008). Within-person across-neuropsychological test variability and incident dementia. JAMA, 300(7), 823–830. doi: 10.1001/jama.300.7.823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultsch DF, MacDonald SW, & Dixon RA (2002). Variability in reaction time performance of younger and older adults. Journals of Gerontology. Series B, Psychological Sciences and Social Sciences, 57(2), P101–115. [DOI] [PubMed] [Google Scholar]
- Joshi AD, Pontecorvo MJ, Clark CM, Carpenter AP, Jennings DL, Sadowsky CH, … Skovronsky DM (2012). Performance characteristics of amyloid PET with florbetapir F 18 in patients with alzheimer’s disease and cognitively normal subjects. Journal of Nuclear Medicine, 53(3), 378–384. doi: 10.2967/jnumed.111.090340 [DOI] [PubMed] [Google Scholar]
- Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, & Milham MP (2008). Competition between functional brain networks mediates behavioral variability. Neuroimage, 39(1), 527–537. doi: 10.1016/j.neuroimage.2007.08.008 [DOI] [PubMed] [Google Scholar]
- Koscik RL, Berman SE, Clark LR, Mueller KD, Okonkwo OC, Gleason CE, … Johnson SC (2016). Intraindividual Cognitive Variability in Middle Age Predicts Cognitive Impairment 8–10 Years Later: Results from the Wisconsin Registry for Alzheimer’s Prevention. Journal of the International Neuropsychological Society, 22(10), 1016–1025. doi: 10.1017/s135561771600093x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau SM, Breault C, Joshi AD, Pontecorvo M, Mathis CA, Jagust WJ, & Mintun MA (2013). Amyloid-beta imaging with Pittsburgh compound B and florbetapir: comparing radiotracers and quantification methods. Journal of Nuclear Medicine, 54(1), 70–77. doi: 10.2967/jnumed.112.109009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau SM, Thomas BA, Thurfjell L, Schmidt M, Margolin R, Mintun M, … Jagust WJ (2014). Amyloid PET imaging in Alzheimer’s disease: a comparison of three radiotracers. Eur J Nucl Med Mol Imaging, 41(7), 1398–1407. doi: 10.1007/s00259-014-2753-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenberger U, & Baltes PB (1997). Intellectual functioning in old and very old age: cross-sectional results from the Berlin Aging Study. Psychology and Aging, 12(3), 410–432. [DOI] [PubMed] [Google Scholar]
- Lovden M, Schmiedek F, Kennedy KM, Rodrigue KM, Lindenberger U, & Raz N (2013). Does variability in cognitive performance correlate with frontal brain volume? Neuroimage, 64, 209–215. doi: 10.1016/j.neuroimage.2012.09.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald SW, Hultsch DF, & Dixon RA (2003). Performance variability is related to change in cognition: evidence from the Victoria Longitudinal Study. Psychology and Aging, 18(3), 510–523. doi: 10.1037/0882-7974.18.3.510 [DOI] [PubMed] [Google Scholar]
- Malek-Ahmadi M, Lu S, Chan Y, Perez SE, Chen K, & Mufson EJ (2017). Cognitive Domain Dispersion Association with Alzheimer’s Disease Pathology. J Alzheimers Dis, 58(2), 575–583. doi: 10.3233/jad-161233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan EE, Woods SP, Delano-Wood L, Bondi MW, & Grant I (2011). Intraindividual variability in HIV infection: evidence for greater neurocognitive dispersion in older HIV seropositive adults. Neuropsychology, 25(5), 645–654. doi: 10.1037/a0023792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murtha S, Cismaru R, Waechter R, & Chertkow H (2002). Increased variability accompanies frontal lobe damage in dementia. Journal of the International Neuropsychological Society, 8(3), 360–372. [DOI] [PubMed] [Google Scholar]
- Nowrangi MA, Rosenberg PB, & Leoutsakos JS (2016). Subtle changes in daily functioning predict conversion from normal to mild cognitive impairment or dementia: an analysis of the NACC database. International Psychogeriatrics, 28(12), 2009–2018. doi: 10.1017/s1041610216000995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC, Aisen PS, Beckett LA, Donohue MC, Gamst AC, Harvey DJ, … Weiner MW (2010). Alzheimer’s Disease Neuroimaging Initiative (ADNI): clinical characterization. Neurology, 74(3), 201–209. doi: 10.1212/WNL.0b013e3181cb3e25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp MA, Schnaider-Beeri M, Sano M, Silverman JM, & Haroutunian V (2005). Cross-domain variability of cognitive performance in very old nursing home residents and community dwellers: relationship to functional status. Gerontology, 51(3), 206–212. doi: 10.1159/000083995 [DOI] [PubMed] [Google Scholar]
- Reckess GZ, Varvaris M, Gordon B, & Schretlen DJ (2014). Within-person distributions of neuropsychological test scores as a function of dementia severity. Neuropsychology, 28(2), 254–260. doi: 10.1037/neu0000017 [DOI] [PubMed] [Google Scholar]
- Salthouse TA, & Soubelet A (2014). Heterogeneous ability profiles may be a unique indicator of impending cognitive decline. Neuropsychology, 28(5), 812–818. doi: 10.1037/neu0000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schretlen DJ, Munro CA, Anthony JC, & Pearlson GD (2003). Examining the range of normal intraindividual variability in neuropsychological test performance. Journal of the International Neuropsychological Society, 9(6), 864–870. doi: 10.1017/s1355617703960061 [DOI] [PubMed] [Google Scholar]
- Sheikh JI, & Yesavage JA (1986). Geriatric Depression Scale (GDS): Recent evidence and development of a shorter version In Clinical Gerontology: a Guide to Assessment and Intervention (pp. 165–173). New York, NY: The Haworth Press. [Google Scholar]
- Singer J, & Willett J (2003). Applied longitudinal data analysis: Modeling change and event occurrence. New York, NY: Oxford University Press. [Google Scholar]
- Stuss DT, Murphy KJ, Binns MA, & Alexander MP (2003). Staying on the job: the frontal lobes control individual performance variability. Brain, 126(Pt 11), 2363–2380. doi: 10.1093/brain/awg237 [DOI] [PubMed] [Google Scholar]
- Teng E, Becker BW, Woo E, Knopman DS, Cummings JL, & Lu PH (2010). Utility of the functional activities questionnaire for distinguishing mild cognitive impairment from very mild Alzheimer disease. Alzheimer Disease and Associated Disorders, 24(4), 348–353. doi: 10.1097/WAD.0b013e3181e2fc84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West R, Murphy KJ, Armilio ML, Craik FI, & Stuss DT (2002). Lapses of intention and performance variability reveal age-related increases in fluctuations of executive control. Brain and Cognition, 49(3), 402–419. [DOI] [PubMed] [Google Scholar]
- Woodard JL (2017). A quarter century of advances in the statistical analysis of longitudinal neuropsychological data. Neuropsychology, 31(8), 1020–1035. doi: 10.1037/neu0000386 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.