Nearly 20% of annual diagnoses of HIV in the United States occur among women (Centers for Disease Control and Prevention [CDC], 2017a). Although the overall number of new diagnoses among women has fallen modestly in the past 5 years, the trend has leveled off recently, and marked racial and ethnic disparities persist (CDC, 2017a). In New York City in 2017, of 449 women diagnosed with HIV, heterosexual sex was the predominant risk factor (67%), and the majority of women (88%) were African American or Hispanic (New York City Department of Health and Mental Hygiene [NYC DOHMH], 2017).
Pre-exposure prophylaxis (PrEP), a combination of emtricitabine and tenofovir disoproxil fumarate (FTC/TDF) taken daily, can effectively prevent HIV acquisition (Baeten et al., 2012). New York State Department of Health (NYSDOH) and NYC DOHMH PrEP guidelines for women list traditional HIV risk factors such as transactional sex, injection drug use, multiple/anonymous partners, recent sexually transmitted infection (STI), and serodiscordant relationships, as well as circumstances that may disproportionally affect women, such as a history of intimate partner violence or a desire to conceive with a partner living with HIV (NYC DOHMH, 2018; New York State Department of Health [NYSDOH], 2018).
Although PrEP use has increased among White men who have sex with men, uptake among women and racial and ethnic minorities remains low. Of more than 175,000 US women who were estimated to have an indication for PrEP, only 2.1% received a prescription in 2016 (Huang, Zhu, Smith, Harris, & Hoover, 2018). Reasons for this disparity are multifactorial and driven by individual and system-wide factors. PrEP awareness, for example, remains low; a study of women receiving care at an STI clinic in Rhode Island found that of women with an indication for PrEP, most were unaware of it, and notably, African American and Hispanic women were more likely to be unaware of PrEP than White women (Raifman et al., 2019). Additional barriers to care have included, for example, the stigma women experience when accessing services related to HIV; advertising and outreach that have traditionally not focused on heterosexual women; low provider knowledge; and guidelines that do not adequately address the needs of heterosexually active women (Calabrese et al., 2019; CDC, 2017b; Pinto, Berringer, Melendez, & Mmeje, 2018; Sales et al., 2019).
In recognition of gaps in PrEP access for women, NYC DOHMH (2018) released guidance strongly encouraging providers to discuss PrEP with HIV-uninfected women. However, data regarding PrEP use among women in real-world settings are lacking. To address this gap in the literature, we performed a retrospective review to describe a cohort of cisgender women and transgender men in New York City who presented for sexual health and HIV prevention services in Upper Manhattan, an area of high HIV diagnosis among women.
Methods
A retrospective review was performed of all cisgender women and transgender men who accessed sexual health services including PrEP and post-exposure prophylaxis (PEP) between July 2015 and June 2018 at the HIV Prevention Program at New York-Presbyterian Hospital/ Columbia University Irving Medical Center (NYP/CUIMC), a large urban academic health care center in Upper Manhattan. NYP/CUIMC serves a predominantly Hispanic and low-income population; 72% self-identify as Hispanic, 20% to 30% of families live below the federal poverty level, and annual HIV diagnosis rates range from 30.7 to 86.6 per 100,000 persons (DiNapoli & Bleiwas, 2016; NYC DOHMH, 2017). The institutionwide HIV Prevention Program was initiated in July 2015.
The HIV Prevention Program has received grant support from the NYSDOH and NYC DOHMH and makes FTC/TDF available to all patients regardless of insurance status. Patients access the clinic through self-referral or are linked from NYP/CUIMC primary care clinics, the emergency department, NYC DOHMH sexual health clinics, or affiliated community organizations.
As part of routine care, patients presenting for HIV prevention services complete self-administered questionnaires that evaluate demographic characteristics, social determinants of health, and sexual and substance use histories. These questionnaires were developed by the clinic and/or the NYC DOHMH and have not been validated in research settings. We performed a retrospective review of data collected in the questionnaires. Data were stored in a coded spreadsheet to maintain privacy, anonymity, and confidentiality.
Pre-exposure prophylaxis eligibility was based on NYC DOHMH and NYSDOH guidelines. Given the limitations of self-report in identifying those at risk of HIV acquisition, any patient who requested PrEP was considered to have an indication for FTC/TDF.
Pre-exposure prophylaxis initiation, retention in care, and the factors associated with each were assessed. PrEP initiation was defined as filling a prescription for FTC/TDF (as confirmed by PrEP program providers). The Pearson chi-square test and the t-test were used to compare cisgender women and transgender men who initiated PrEP with those who did not. Bivariate analysis was conducted only on patients for whom complete data were available. As per the study design, all cisgender women and transgender men who accessed sexual health services during the study period were included in the review; power calculations to determine sample size were, therefore, not conducted.
Because patients underwent HIV testing at each visit, HIV tests were used as a proxy for retention in care. Retention in care was assessed at 30, 90, and 180 days, which is consistent with the standard-of-care scheduled clinic follow-up for patients taking PrEP. A model was created to assess whether visits on average fell within defined windows. Retention in care at 30 days was defined as having at least one HIV test after Day 21, and HIV testing had to be performed, on average, at least every 45 days. Retention in care at 90 and 180 days was defined as having at least one HIV test after Day 75 or 165, respectively, and HIV testing had to be performed, on average, at least every 105 days. Patients were followed through December 2018 to assess retention in care.
Our study was approved by the CUIMC Institutional Review Board. Informed consent was waived by the Institutional Review Board, as the study posed minimal risk to patients. IBM SPSS (IBM Corp, Released 2018, IBM SPSS Statistics for Windows, Version 26.0, Armonk, NY: IBM Corp) was used to perform all analyses.
Results
One hundred eight cisgender women and transgender men accessed sexual health services during the study period; of which, 78 were eligible for PrEP and 55 started it. The mean age of PrEP-eligible cisgender women and transgender men was 30.2 years; older patients were more likely to start PrEP (31.8 vs. 26.4 years, p = .03). Many cisgender women and transgender men self-identified as African American (44%) or Hispanic (62%); PrEP initiation did not significantly differ based on race or ethnicity (Table 1). Of those who were PrEP eligible, 75 identified as cisgender women and 3 identified as transgender men.
Table 1.
Demographic and Behavioral Characteristics of Study Population
| PrEP Eligible (n = 78) | Started PrEP (n = 55) | Did Not Start PrEP (n = 23) | p-Value | |
|---|---|---|---|---|
| Age (mean) | 30.2 years | 31.8 years | 26.4 years | .03 |
| Race, n (%) | ||||
| Caucasian | 10 (14) | 7 (14) | 3 (14) | .97 |
| African American | 32 (44) | 21 (42) | 11 (50) | .44 |
| Hispanic ethnicity | 43 (62) | 34 (68) | 9 (47) | .11 |
| Reason for clinic visit, n (%) | ||||
| PrEP | 64 (82) | 51 (93) | 13 (57) | <.002 |
| PEP | 18 (23) | 5 (9) | 13 (57) | <.002 |
| PrEP indication, n (%) | ||||
| Partner living with HIV | 33 (42) | 27 (49) | 6 (26) | .06 |
| Intravenous drug use | 8 (10) | 4 (7) | 4 (17) | .18 |
| Transactional sex | 17 (29) | 14 (31) | 3 (23) | .58 |
| STI history | 22 (37) | 18 (43) | 4 (24) | .16 |
| Regular condomless sex | 18 (27) | 13 (26) | 5 (29) | .78 |
| Previous PrEP awareness, n (%) | 52 (96) | 37 (97) | 15 (94) | .52 |
| Insured, n (%) | 51 (78) | 36 (75) | 15 (88) | .25 |
| Stable housing, n (%) | 50 (83) | 41 (87) | 9 (69) | .12 |
| Ever homeless, n (%) | 17(33) | 11 (29) | 6 (43) | .34 |
| Unemployed, n (%) | 18 (33) | 11 (28) | 7 (47) | .18 |
| PCP visit within 1 year, n (%) | 38 (75) | 30 (79) | 8 (62) | .21 |
| Mental health diagnosis, n (%) | 23 (46) | 17 (44) | 6 (55) | .52 |
Note. PCP = primary care provider; PEP = post-exposure prophylaxis; PrEP = pre-exposure prophylaxis; STI = sexually transmitted infection. The Pearson chi-square test and the t-test were used to compare patients who initiated PrEP with those who did not. Variables with a significant p value (p < 0.05) are shown in boldface.
The majority (78%) had health insurance. Of 53 patients who provided citizenship data, 44 (83%) were US citizens. History of homelessness (33%), unstable housing (17%), and unemployment (33%) were common and likely underreported (respectively, 26,18, and 23 patients declined to provide these data). Of 50 patients with complete data, 23 (46%) had a history of a mental health diagnosis. Data regarding intimate partner violence were available for five patients; of the five, two reported a history of physical or sexual abuse. None of these characteristics were significantly associated with PrEP initiation.
Pre-exposure prophylaxis initiation was more common for cisgender women and transgender men who presented for PrEP; 51 of 55 patients starting PrEP presented for it, compared with 13 of 23 patients not starting PrEP (93 vs. 57%, p < .002). In addition, cisgender women and transgender men presenting for PEP were less likely to transition to PrEP; 5 of 55 patients ultimately starting PrEP had initially presented for PEP, compared with 13 of 23 patients ultimately not starting PrEP (9 vs. 57%, p < .002).
The most common indication for PrEP was having a sexual partner living with HIV (42%); a nonsignificant trend suggested that patients with a partner living with HIV were more likely to start PrEP (49 vs. 26%, p = .06). No other PrEP indication was significantly associated with initiation. Nine patients (12%) were diagnosed with an STI during the study period, including three who elected not to start PrEP. Of 51 cisgender women and transgender men with available data, 38 (75%) reported seeing their primary care provider (PCP) within the past year.
Of the 78 cisgender women and transgender men who were eligible for PrEP, 45(58%) were retained in care at 30 days, and 24 (31%) were retained in care at 180 days (Figure 1). Patients who self-identified as Hispanic and patients who had never been homeless were more likely to be retained in care at 180 days (p = .05 and p = .03, respectively).
Figure 1.
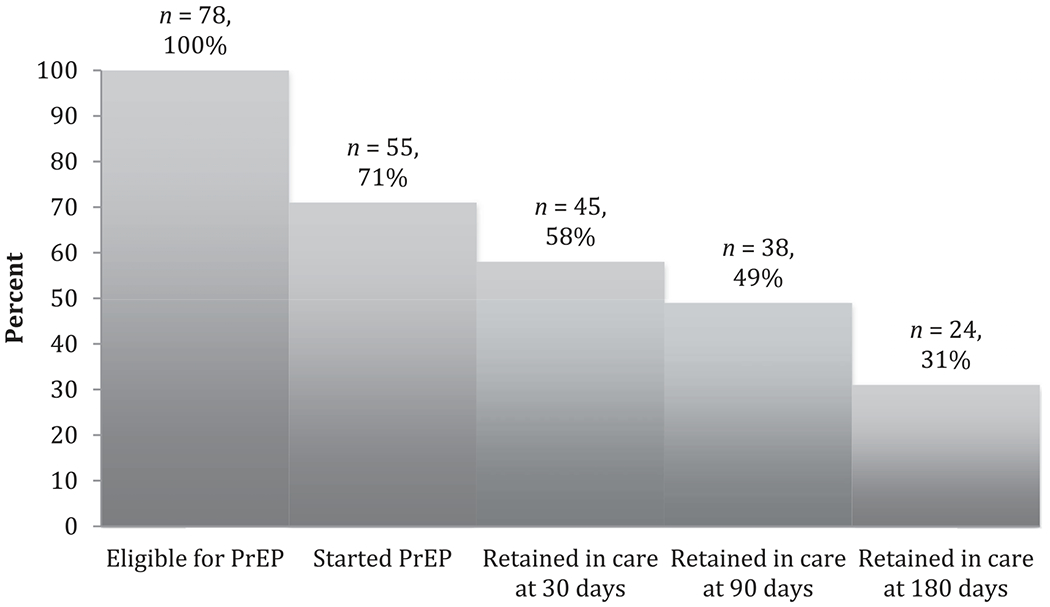
PrEP retention in care over time. Note. PrEP = pre-exposure prophylaxis.
Missing data were frequent and included information on race (n = 6), ethnicity (n = 9), citizenship (n = 25), history of transactional sex (n = 20), STI history (n = 19), frequency of condomless sex (n = 11), previous PrEP awareness (n = 24), insured status (n = 13), current housing (n = 18), history of homelessness (n = 26), unemployment (n = 23), PCP visit history (n = 27), mental health history (n = 28), and intimate partner violence (n = 73). Missing data were due to nonresponse and reflected limitations of self-administered questionnaires; a large proportion of intimate partner violence data were missing because screening questions were added into standard questionnaires only at the end of the study period.
Discussion
Our results were from a cohort of predominantly cisgender women who initiated PrEP in a real-world setting. Most patients eligible for PrEP started it, but retention in preventative care was low and continued to fall over time. Older cisgender women and transgender men and those who presented specifically for PrEP were more likely to start; there was also a trend to suggest that cisgender women and transgender men who had a partner living with HIV were more likely to initiate PrEP. Patients who self-identified as Hispanic and those who had never been homeless were more likely to be retained in care.
A major strength of our study was that our population of predominantly African American and Hispanic women reflected the highest risk female demographic. Our results were also consistent with, and build on, the findings of other studies, which have identified serodiscordant relationships as a common indication for PrEP initiation by women (Blackstock, Patel, Felsen, Park, & Jain, 2017; Seidman et al., 2016). PrEP awareness was higher than that seen in other studies (Patel et al., 2019; Raifman et al., 2019), which was expected, as patients were referred to our program specifically for prevention services including PrEP.
Our study had several limitations. Given its small sample size, generalizability may be limited. In addition, the retrospective nature limited our understanding of the reasons for loss to follow-up. As cisgender women and transgender men self-completed the questionnaires, missing data were substantial. Although we were able to capture the prevalence of traditional risk factors such as condomless sex and intravenous drug use, we had limited data regarding the rate of intimate partner violence, which has been identified as a risk factor for HIV. Finally, our retention in care model provided an approximate, but likely imperfect, measure.
Studies have demonstrated that many women appear unaware of their risk for HIV acquisition (McLellan-Lemal et al., 2013; Sales et al., 2018). With low awareness of HIV risk, women may be less likely to seek HIV prevention care on their own. Thus, PCPs play a critical role in identifying women who would benefit from PrEP and linking them to HIV prevention services. In our study, most cisgender women and transgender men had access to a PCP, and most had seen a provider within a year of their first PrEP program visit. It has been shown that although PCPs recognize the importance of HIV testing and are aware of PrEP, many feel uncomfortable assessing eligibility for PrEP, prescribing it, or linking patients to PrEP care (Blackstock, Moore, et al., 2017; Calabrese, Krakower, & Mayer, 2017; Gold et al., 2018; Zucker et al., 2018). Educating providers on these issues is critical for HIV prevention and women’s health.
Women who are aware of PrEP are interested in it (Sales et al., 2018). Because PrEP can be self-administered and taken discretely, it may be a particularly attractive option for women who face social barriers to condom use. Linking women to preventative care and understanding the barriers to PrEP uptake and retention (including women-specific barriers) are vital to ending the HIV epidemic in women.
Conclusion
Further research is needed to improve PrEP uptake and retention in sexual health care among cisgender women and transgender men. Understanding gender-specific barriers to PrEP care and improving PCP education on PrEP are crucial to ending the HIV epidemic.
Acknowledgments
Disclosures
This work was supported by the New York City Department of Health and Mental Hygiene through a contract with Public Health Solutions. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the funders. Dr. Zucker was supported through the National Institute of Allergy and Infectious Diseases—T32AI007531 “Training in Pediatric Infectious Diseases” (PI: Saiman). Caroline Carnevale and Magdalena E. Sobieszczyk receive funding from Gilead Sciences for investigator-led research.
Contributor Information
Deborah A. Theodore, Instructor in Medicine in the Department of Medicine, Division of Infectious Diseases, Columbia University Irving Medical Center (CUIMC), New York, New York, USA.
Jason Zucker, Instructor in Medicine in the Department of Medicine, Division of Infectious Diseases, CUIMC, New York, New York, USA.
Caroline Carnevale, Principal Investigator, HIV Prevention Program, New York-Presbyterian (NYP) Hospital, New York, NY, USA.
William Grant, medical student, Duke University School of Medicine, Durham, North Carolina, USA.
Matthew Adan, medical student at Columbia University Vagelos College of Physicians and Surgeons, New York, New York, USA.
Alexander Borsa, HIV Treatment and Prevention Coordinator, NYP Hospital, New York, New York, USA.
Paul Richards, Lead Prevention Coordinator, NYP Hospital, New York, New York, USA.
Susan Olender, Assistant Professor of Medicine, Division of Infectious Diseases at CUIMC and the Associate Medical Director of the Comprehensive Health/HIV Program at NYP Hospital, New York, New York, USA.
Alwyn Cohall, Professor of Public Health and Pediatrics, CUIMC and the Mailman School of Public Health, New York, New York, USA.
Peter Gordon, Assistant Professor of Medicine, Division of Infectious Diseases at CUIMC and the Medical Director of the NYP System Select Health, an HIV Special Needs Plan, New York, New York, USA.
Magdalena E. Sobieszczyk, Associate Professor of Medicine and Chief of the Division of Infectious Diseases, CUIMC, New York, New York, USA..
References
- Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J,… Tumwesigye E(2012). Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. The New England Journal of Medicine, 367, 399–410. doi: 10.1056/NEJMoa1108524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackstock OJ, Moore BA, Berkenblit GV, Calabrese SK, Cunningham CO, Fiellin DA, … Edelman EJ (2017). A cross-sectional online survey of HIV pre-exposure prophylaxis adoption among primary care physicians. Journal ofGeneral Internal Medicine, 32(1), 62–70. doi: 10.1007/s11606-016-3903-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackstock OJ, Patel VV, Felsen U, Park C, & Jain S (2017). Pre-exposure prophylaxis prescribing and retention in care among heterosexual women at a community-based comprehensive sexual health clinic. AIDS Care, 29, 866–869. doi: 10.1080/09540121.2017.1286287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese SK, Krakower DS, & Mayer KH (2017). Integrating HIV preexposure prophylaxis (PrEP) into routine preventive health care to avoid exacerbating disparities. American Journal of Public Health, 107(12), 1883–1889. doi: 10.2105/AJPH.2017.304061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese SK, Willie TC, Galvao RW, Tekeste M, Dovidio JF, Safon CB, … Kershaw TS (2019). Current US guidelines for prescribing HIV pre-exposure prophylaxis (PrEP) disqualify many women who are at risk and motivated to use PrEP. Journal of Acquired Immune Deficiency Syndromes, 82(4), 395–405. doi: 10.1097/qai.0000000000002042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (2017a). HIV surveillance report, 2017. Retrieved from https://www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-report-2017-vol-29.pdf
- Centers for Disease Control and Prevention. (2017b). Preexposure prophylaxis for the prevention of HIV infection in the United States—2017 Update. Retrieved from https://www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2017.pdf
- DiNapoli TP, & Bleiwas KB (2016). An economic snapshot of Washington Heights and Inwood. Retrieved from https://www.osc.state.ny.us/osdc/rpt2-2016.pdf
- Gold JA, Zucker J, Theodore DA, Carnevale C, Olender S, Gordon P, & Sobieszczyk ME (2018). Attitudes in assessing patients for pre-exposure prophylaxis among pediatric and internal medicine residents training in New York City. Paper presented at the HIV Research for Prevention Conference, Madrid, Spain, October 23, 2018. [Google Scholar]
- Huang YA, Zhu W, Smith DK, Harris N, & Hoover KW (2018). HIV preexposure prophylaxis, by race and ethnicity—United States, 2014–2016. MMWR Morbidity and Mortality Weekly Report, 67,1147–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan-Lemal E, Toledo L, O’Daniels C, Villar-Loubet O, Simpson C,Adimora AA,& Marks G (2013). “Aman’s gonna do what a man wants to do”: African American and Hispanic women’s perceptions about heterosexual relationships: A qualitative study. BMC Women’s Health, 13, 27. doi: 10.1186/1472-6874-13-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- New York City Department of Health and Mental Hygiene. (2017). HIV surveillance annual report, 2017. Retrieved from https://www1.nyc.gov/assets/doh/downloads/pdf/dires/hiv-surveillance-annualreport-2017.pdf
- New York City Department of Health and Mental Hygiene. (2018). NYC letter to providers about PrEP for women. Retrieved from hhttps://www1.nyc.gov/assets/doh/downloads/pdf/hcp/prepforwomen.pdf
- New York State Department of Health. (2018). PrEP to prevent HIV acquisition. Retrieved from https://www.hivguidelines.org/prep-for-prevention/prep-to-prevent-hiv/#tab_3
- Patel AS, Goparaju L, Sales JM, Mehta CC, Blackstock OJ, Seidman D, … Sheth AN (2019). PrEP eligibility among at-risk women in the southern United States: Associated factors, awareness, and acceptability. Journal of Acquired Immune Deficiency Syndromes, 80(5), 527–532. doi: 10.1097/QAI.0000000000001950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto RM, Berringer KR, Melendez R, & Mmeje O (2018). Improving PrEP implementation through multilevel interventions: A synthesis of the literature. AIDS and Behavior, 22(11), 3681–3691. doi: 10.1007/s10461-018-2184-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raifman JR, Schwartz SR, Sosnowy CD, Montgomery MC, Almonte A, Bazzi AR, … Chan PA (2019). Pre-exposure prophylaxis awareness and use among cisgender women at a sexually transmitted disease clinic. Journal of Acquired Immune Deficiency Syndromes, 80, 36–39. doi: 10.1097/QAI.0000000000001879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sales JM, Cwiak C, Haddad LB, Phillips A, Powell L, Tamler I, & Sheth AN (2019). Impact of PrEP training for family planning providers on HIV prevention counseling and patient interest in PrEP in Atlanta, Georgia. Journal ofAcquired Immune Deficiency Syndromes, 81(4), 414–418. doi: 10.1097/qai.0000000000002057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sales JM, Steiner RJ, Brown JL, Swartzendruber A, Patel AS, & Sheth AN (2018). PrEP eligibility and interest among clinic- and community-recruited young Black women in Atlanta, Georgia, USA. Current HIV Research, 16(3), 250–255. doi: 10.2174/1570162X16666180731143756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman DL, Weber S, Timoney MT, Oza KK, Mullins E, Cohan DL, & Wright RL (2016). Use of HIV pre-exposure prophylaxis during the preconception, antepartum and postpartum periods at two United States medical centers. American Journal of Obstetrics and Gynecology, 215(5), 632. doi: 10.1016/j.ajog.2016.06.020 [DOI] [PubMed] [Google Scholar]
- Zucker J, Carnevale C, Gold JA, Borsa A, Scherer M, Cohall A, … Olender SD (2018). An online survey of HIV testing and pre-exposure prophylaxis attitudes and practice habits among physicians at an academic medical center. Paper presented at the 13th International Conference on HIV Treatment and Prevention Adherence, Miami, FL, USA, June 9, 2018. [Google Scholar]


