Summary
Background:
We performed a phase II trial of pembrolizumab in patients with NSCLC or melanoma with untreated brain metastases to determine the activity of PD-1 blockade in the CNS. Interim results were previously published, and we now report an updated analysis of the full NSCLC cohort.
Methods:
This was an open-label, single-institution, phase 2 study. Eligible patients were ≥ 18 years of age with advanced NSCLC with ≥1 brain metastasis 5-20mm not previously treated or progressing after prior radiation, no neurologic symptoms or corticosteroid requirement, and performance status <2. Patients were treated with pembrolizumab 10 mg/kg IV every 2 weeks. Cohort 1 was for patients with PD-L1 ≥1% and cohort 2 PD-L1 <1% or unevaluable. The primary endpoint was the proportion of patients achieving a brain metastasis response. All treated patients were analyzed for response and safety endpoints. This study is closed to accrual and is registered with Clinicaltrials.gov, number NCT02085070. Here we report the updated results of the NSCLC cohort.
Findings:
Between March 31, 2014 and May 21, 2018, 42 patients were treated. Median follow-up was 8.3 months (IQR 4.5 to 26.2 months). Eleven of 37 patients in cohort 1 had a brain metastasis response (29.7% [95% CI, 15·9-47·0%]). There were no responses in cohort 2. Grade 3-4 AEs related to treatment included 2 patients with pneumonitis, and 1 each with constitutional symptoms, colitis, adrenal insufficiency, hyperglycemia, and hypokalemia. Treatment-related serious adverse events occurred in 6 (14%) patients and included pneumonitis acute kidney injury, colitis, hypokalemia, and adrenal insufficiency. There were no treatment-related deaths.
Interpretation:
Pembrolizumab has activity in brain metastases from NSCLC with PD-L1 expression ≥1% and is safe in select patients with untreated brain metastases. Further investigation of immunotherapy in patients with CNS disease from NSCLC is warranted.
Funding:
Merck and the Yale Cancer Center
INTRODUCTION
Brain metastases are detected at diagnosis in more than a quarter of patients with stage IV non-small cell lung cancer (NSCLC).1 While radiation therapy continues to be the mainstay of treatment, systemic therapy has shown efficacy for controlling central nervous system (CNS) disease.2 However, this has been mostly limited to the realm of targeted therapies where many drugs have high response rates and good CNS penetration.3,4
Immunotherapy with PD-1 inhibitors is now standard therapy for first-line use in patients with advanced NSCLC, whether as single-agent5 or in combination with chemotherapy.6,7 However, most pivotal trials with immunotherapy excluded patients with untreated brain metastases. There are several possible advantages to using a PD-1 inhibitor to treat brain metastases: local therapy can result in toxicity, particularly with whole brain radiation therapy (WBRT)8; radiation necrosis can occur in more than one-third of patients9; and patients with large-volume systemic disease often cannot tolerate a delay in systemic therapy to undergo local therapy to the brain. Therefore, we embarked on a clinical trial of pembrolizumab in patients with NSCLC and untreated brain metastases to assess the efficacy and safety of a PD-1 inhibitor in this patient population. We previously demonstrated early evidence of activity of pembrolizumab in brain metastases10 and here we present the updated analysis from this study including long-term survival and correlative biomarkers.
METHODS
Study Design and participants
This is a two-arm phase II trial performed at a single institution that allowed patients with stage IV NSCLC or melanoma who had at least one cerebral metastasis measuring at least 5mm but less than 20mm that was not previously treated or was unequivocally progressing following local therapy. Data presented here pertains to the updated results from the NSCLC arm of the trial. A previous report summarizes the interim analysis from this study10 and final results from the melanoma arm have been previously published.11 Any number of prior lines of therapy was allowed, but patients had to be naïve to PD-1 or PD-L1 inhibitors. Additional eligibility requirements included age ≥ 18 years, ECOG performance status < 2, no neurologic symptoms, no corticosteroid requirement, and life expectancy of at least 3 months. Modified RECIST12 (mRECIST) which allowed target lesions ≥ 5mm was used to evaluate CNS disease; systemic disease was not required for participation. Patients were required to have adequate bone marrow, liver and renal function. Patients with active autoimmune disease requiring systemic treatment within the past 3 months, a history of clinically severe autoimmune disease, or a syndrome that required systemic steroids or other immunosuppressive agents were excluded; exceptions were patients with vitiligo, resolved childhood asthma or atopy, hypothyroidism on stable hormone replacement, or Sjorgen’s syndrome. Prior radiation therapy to the CNS was allowed provided it was completed at least 2 weeks before study initiation. Lesions greater than 20mm or located in areas of the brain in which tumor growth would result in symptoms (such as the speech area, motor strip and brain stem) were treated with stereotactic radiosurgery (SRS) prior to initiation of pembrolizumab. Patients with leptomeningeal disease were excluded. Patients with NSCLC were tested centrally for PD-L1 by immunohistochemistry (IHC),13 and those with PD-L1 expression were treated on Cohort 1. Cohort 2 was added during the course of the study as an exploratory cohort to assess patients whose tumors had no PD-L1 expression or those without evaluable tissue; prior platinum-based chemotherapy was required for these patients. Efficacy data for the two cohorts were analyzed separately.
The study was performed after approval by the Yale Human Investigations Committee and all patients provided written informed consent prior to participation. The full protocol is in the appendix pp 9-64.
Procedures
Patients were treated with pembrolizumab 10mg/kg IV every two weeks for up to 24 months or until disease progression (without clinical benefit) or unacceptable toxicity. The dose and schedule were chosen based on the data available at the time of study development. A brain MRI was performed at 4 weeks for safety (i.e. to assess for rapid progression or edema in the CNS); a brain MRI and CT of the chest, abdomen and pelvis were performed every 8 weeks following study initiation for response evaluation. Brain response was evaluated by mRECIST in which the sum of diameters of up to 5 brain metastases were allowed and lesions were considered measurable if they were at least twice the MRI section thickness and ≥5mm in longest diameter. Lesions treated with local therapy immediately prior to study initiation were not assessed for response to pembrolizumab. Systemic response was assessed with RECIST v1.1. Imaging was assessed locally by a neuro-radiologist (A.M.) in conjunction with a neuro-surgeon (V.C.) who knew the patient’s prior local treatment history to adequately choose target lesions that were not previously radiated. No central review of imaging was performed.
Laboratory studies to monitor bone marrow, liver and kidney function were performed every two weeks prior to each treatment, and thyroid function studies were performed every 8 weeks. Adverse events (AEs) were assessed every two weeks using the Common Terminology Criteria for Adverse Events (CTCAE) v4.0 and relationship to study drug was determined by the treating investigator. Dose reductions were not permitted, however dose interruptions were allowed for AEs for up to 12 weeks. Treatment was resumed once AEs improved to grade 0-1 and corticosteroids (if started) were reduced to prednisone ≤ 10mg or equivalent. Treatment discontinuation was required for disease progression without clinical benefit, symptomatic deterioration, imminent risk in the case of progression lesion or new or worsening edema on brain MRI, development of neurologic symptoms or systemic complications following local therapy, unmanageable toxicity attributed to pembrolizumab, development of severe intercurrent illness, patient request, death, or study termination by sponsor. Local therapy to progressing CNS or systemic lesion followed by continuation of study treatment was allowed for patients who were thought to be deriving clinical benefit from therapy despite evidence of disease progression on imaging. Response assessment in the brain and body was continued until removal from study, regardless of whether progression or discontinuation of therapy (such as due to toxicity) occurred. Lesions that were radiated while on trial were considered unevaluable for response thereafter.
For the correlative studies, tumor biopsy samples from brain or extracerebral metastases obtained immediately prior to trial therapy were collected. In cases where tissue from immediately prior to therapy was insufficient for testing, prior archival specimens were used. PD-L1 IHC was assessed using the FDA-approved 22C3 pharmDx kit on the Dako Link 48 platform in a central CLIA-approved laboratory. Semi-quantitative scoring was performed by a trained pathologist using bright field microscopy, and the percentage of tumor and stromal cells expressing PD-L1 was assessed independently. The level of tumor infiltrating lymphocytes (TILs) including major B and T cell populations were measured using multiplexed quantitative immunofluorescence and the AQUA method, as previously described14,15 Targeted transcriptomic analysis of 770 immune-related mRNA transcripts was performed by the nCounter PanCancer Immune profiling panel as previously reported by our group (NanoString Technologies).15 All testing was performed on formalin-fixed paraffin-embedded (FFPE) tissue and analyses include patients from both Cohorts 1 and 2.
Outcomes
The primary endpoint of the trial was the proportion of patients achieving a brain metastasis response (partial response [PR] or complete response [CR]) in the CNS out of all eligible patients. Secondary endpoints included proportion of patients achieving an overall response defined as the percentage of patients who experienced a PR or CR in any metastasis (CNS or systemic) as determined by mRECIST in the brain and RECIST in the systemic disease, progression-free survival defined as the time from start of pembrolizumab to progression (using mRECIST in the brain and RECIST for systemic disease) or death, and overall survival defined as the time from start of pembrolizumab to death, with survival outcomes censored at the date of last follow-up. An additional secondary endpoint was safety and toxicity as measured by the CTCAE v4.0 with all patients who received study drug being considered evaluable. Pre-defined exploratory endpoints included PD-L1 expression and other biomarkers as predictors of clinical efficacy.
Statistical analysis
The primary endpoint of this study was the proportion of patients who achieved a brain metastasis response which we hypothesized would be similar to the systemic response rate of pembrolizumab in PD-L1-positive NSCLC of 25%.16 We aimed to enroll 44 patients to Cohort 1 to provide 80% power to demonstrate that the proportion of patients achieving a brain metastasis response exceeded 10% at an overall one-sided 10% alpha level, if the true proportion was 25%. The minimum criterion for success was that the lower bound of the confidence interval exceeded 10%, which would occur if at least 10 patients had a confirmed brain metastasis response. Post-hoc exploratory analyses included subgroup analyses by baseline demographics, proportion of patients achieving a systemic response, assessment of radiation necrosis, CNS PFS and duration of response.
A sequential monitoring procedure was used to simultaneously evaluate efficacy and futility. Due to shifting treatment paradigms including frontline use of PD-1 inhibitors in combination with chemotherapy, the study was halted early after accrual of 37 patients to Cohort 1. Cohort 2 had a planned accrual of 10 patients and was exploratory in nature. Summary statistics were presented as percentages for categorical variables and as medians with interquartile ranges (IQR) for continuous variables. Continuous variables were compared with the use of Wilcoxon rank sum test, whereas categorical variables were compared with Fisher’s exact test. Survival endpoints were calculated using the Kaplan-Meier method with October 19, 2018 used as the censored date for survival. The effect of baseline variables on survival endpoints was a post-hoc exploratory analysis and was assessed using the Cox model. The proportional hazards assumption was verified with the use of Schoenfeld residuals. Survival curves between different groups were compared using the log-rank test. All patients who received at least one dose of study drug were evaluable for all study endpoints. Patients were considered not assessable for response if they received insufficient imaging after initiating trial therapy. The correlative analyses were exploratory; details of methods are included in appendix p 1. All tests were two-sided with a significance level of 0.05, and were performed using R version 3.6.1. This study is registered with Clinicaltrials.gov, number NCT02085070.
Role of the funding source
The study was funded by Merck and the Yale Cancer Center. Merck contributed to the study design and final manuscript review. The funders of the study had no role in data collection, data analysis, data interpretation, or writing of the report. All authors had full access to all the data in the study and provided approval to submit the manuscript for publication. The corresponding author had final responsibility to submit for publication.
RESULTS
Between March 31, 2014 and May 21, 2018, we screened 71 patients with NSCLC and treated 42 patients: 37 in Cohort 1 and 5 in Cohort 2 (figure 1). Reasons for ineligibility are listed in the appendix p 1. Thirty-six patients (85·7%) had adenocarcinoma histology and 8 patients (20%) had tumors with oncogenic variants previously associated with limited response to checkpoint inhibitor therapy (e.g. EGFR, ALK, or HER2).17 Baseline patient characteristics are shown in table 1; details regarding prior radiation therapy to the brain are in appendix p 1.
Figure 1. Trial Profile.
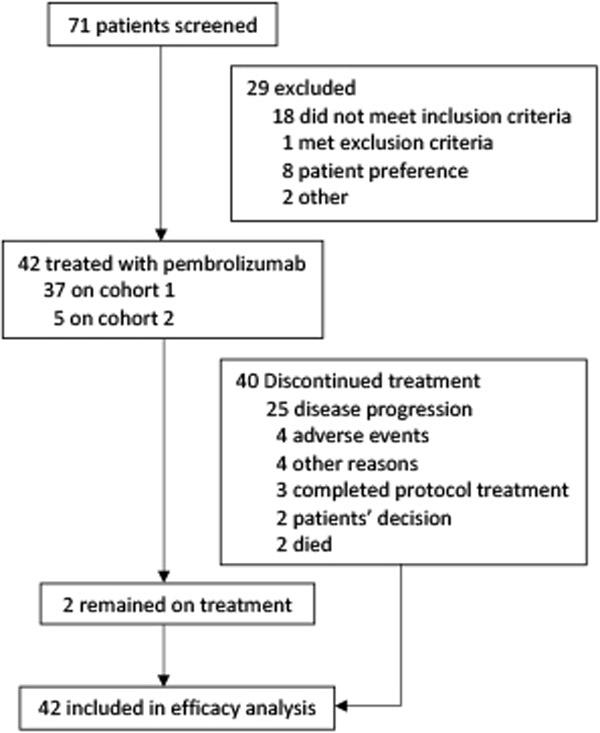
Table 1.
Patient Characteristics
| Characteristic | N=42 |
|---|---|
| Age in years, median (IQR) | 60 (56-71) |
| Female | 28 (67%) |
| Smoking status | |
| Never | 3 (7%) |
| Former | 36 (86%) |
| Current | 3 (7%) |
| ECOG Performance Status | |
| 0 | 4 (10%) |
| 1 | 38 (90%) |
| # lines prior systemic therapy | |
| 0 | 15 (36%) |
| 1 | 14 (33%) |
| 2+ | 13 (31%) |
| Prior local CNS therapy* | |
| None | 21 (50%) |
| Stereotactic radiosurgery | 16 (38%) |
| Whole brain radiation therapy (WBRT) | 8 (19%) |
| Craniotomy/resection | 4 (10%) |
| Histology | |
| Adenocarcinoma | 36 (86%) |
| Squamous cell | 4 (10%) |
| Poorly differentiated carcinoma | 2 (5%) |
| Molecular alteration | |
| KRAS | 14 (33%) |
| EGFR | 6 (14%) |
| ALK | 1 (2%) |
| HER2 | 1 (2%) |
| MET exon 14 | 1 (2%) |
Some patients received more than one treatment modality
IQR, interquartile range
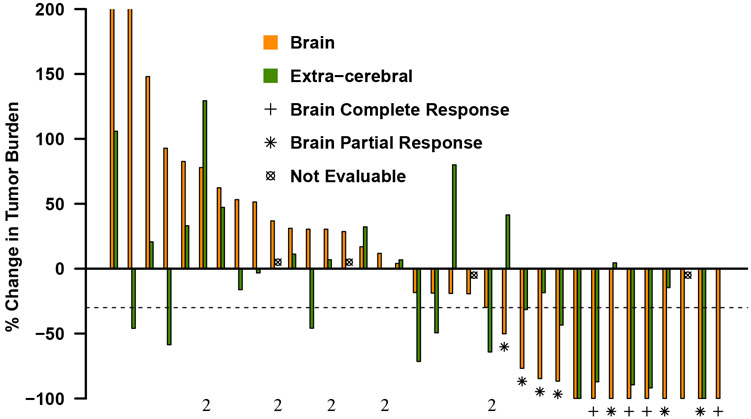
The median number of cycles of pembrolizumab was 5.5 (IQR 4-16.3). Of the 37 PD-L1 positive patients in cohort 1, 11 (29·7% [95% CI, 15·9-47·0%]) had a confirmed brain metastasis response (7 PRs and 4 CRs). This met the prespecified success criteria set for the trial as the lower bound of the confidence interval exceeded 10%. An additional 4 patients had stable disease (SD) in the brain (2 unconfirmed), 16 had progressive disease (PD), and 6 were unevaluable due to rapid systemic progression precluding adequate imaging of the CNS. All brain metastasis responses occurred at either the first or second disease assessment scan, with a median time to response of 1.8 months (IQR 1·7 to 2·4 months). In a post-hoc analysis, duration of response (DOR) in the brain among the 11 CNS responders was 5.7 months (IQR 4·0 to 17·7 months), with only one CNS-responder experiencing progression in the brain by the time of the last MRI on study. Details for patients on Cohort 2 are in the appendix p 1, and figure 2A demonstrates the best brain metastasis and systemic response for each patient in both cohorts. Characteristics of target brain lesions, details regarding patients with oncogenic alterations, information on subsequent CNS therapy, and the association between baseline characteristics and brain metastasis response are in the appendix p 2.
Figure 2. Response characteristics in patients with NSCLC and brain metastases treated with pembrolizumab.
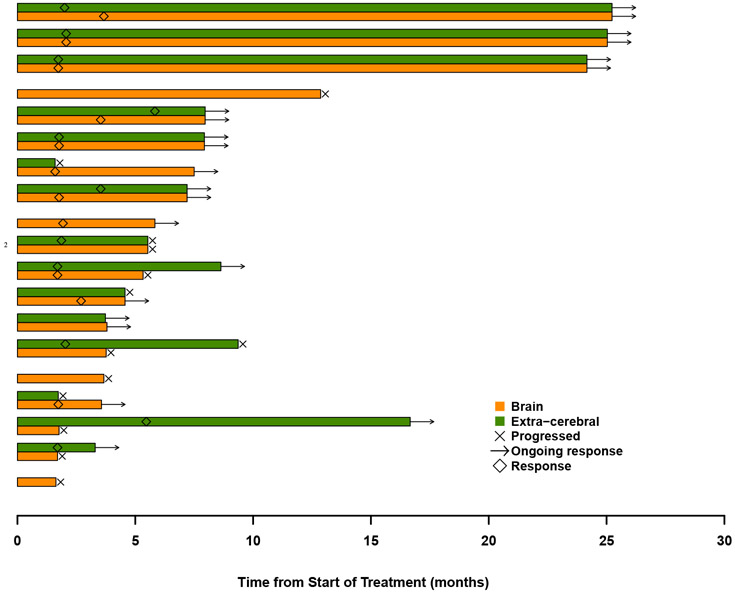
A. Best brain metastasis response by modified RECIST and extra-cerebral response by RECIST version 1.1 in patients evaluable for response in target lesions in the brain (35 of 42 patients). Tumor burden is defined as the sum of diameters of all target lesions (calculated separately for brain and extra-cerebral lesions), and % change in tumor burden indicates the change from baseline to the best response. Each bar represents an individual patient, orange indicating the brain metastasis response and green indicating the extra-cerebral response. The dashed line represents the −30% tumor shrinkage required for a partial response. B. Time to and duration of response in brain (orange bars) and extra-cerebral (green bars) lesions for patients who had a brain metastasis response or remained on trial for at least 4 months (19 of 42 patients). Bars are grouped by the same patient’s brain and extra-cerebral response. Only the orange bar is shown when the extracerebral disease from that patient was unevaluable. C. Time of brain metastasis response, overall progression (brain and/or systemic), and death for the same patient population included in Figure 2B. The arrow at the end of the bar indicates that the patient was last known to be alive at the time of data analysis. Dashed line at 24 months designates the end of the study period. All patients are in Cohort 1 unless otherwise indicated by the number 2.
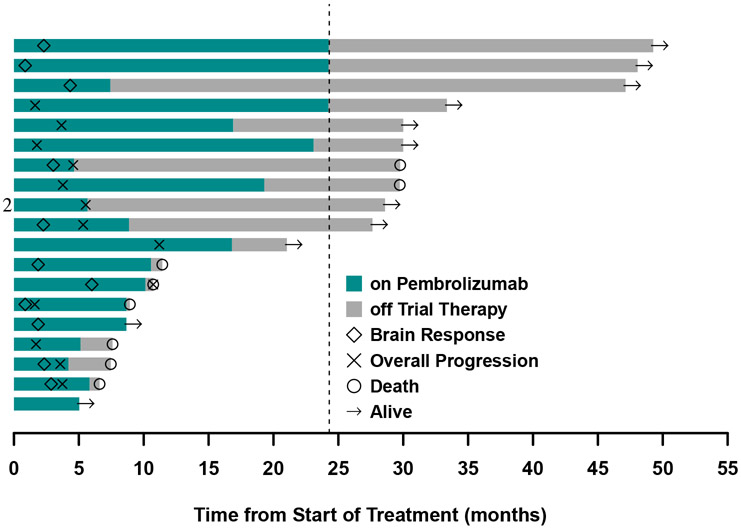
Eleven of the 37 patients (29·7% [95% CI, 15·9-47·0%]) in cohort 1 had a systemic response. Among the 27 patients who were evaluable for both CNS and systemic response, there were 6 patients with discordant outcomes. Of these cases, the brain was the site of progression in 3 patients (11·1%) with response in the body, whereas the other 3 (11·1%) had the reverse scenario. Systemic DOR was 6.9 months (IQR 3·7 to 22·4 months). Figure 2B compares the timing and duration of brain and systemic response and figure 2C demonstrates the patient’s overall course. All patients with discordant responses lived >6 months, with 2 of the 3 patients who had PR in the body but PD in the brain living more than 2 years.
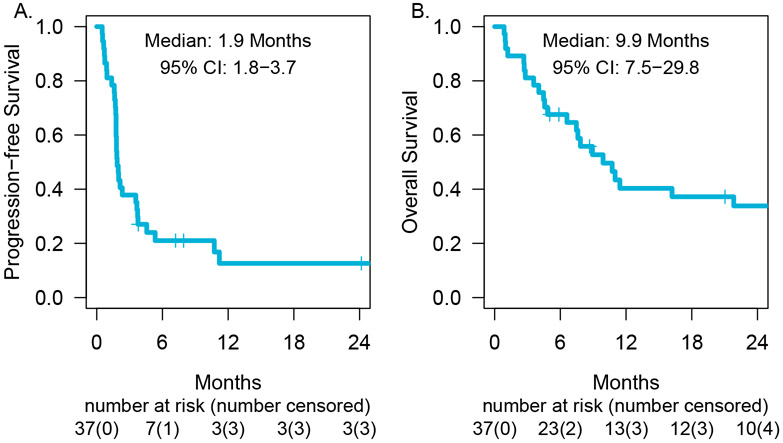
At the time of the data-lock, the median follow-up time was 8·3 months (IQR 4.5 to 26.2 months). Among the 37 patients in cohort 1, 31 patients progressed or died and the median PFS was 1·9 months (95% CI 1·8-3·7 months, figure 3A). Post-hoc analysis demonstrated a CNS PFS of 2·3 months (95% CI 1·9 months-not reached), with 33% of patients progression-free in the CNS at one year (95% CI 19-56%, appendix p 3). Twenty-six of the 37 patients died by the time of data-lack with a median OS of 9·9 months (95% CI, 7·5-29·8 months, figure 3B). The estimates of overall survival at one- and two-years were 40% (95% CI, 30-64%) and 34% (95% CI, 21-54%), respectively. The impact of baseline characteristics on overall survival is summarized in appendix p 3.
Figure 3. Progression-free survival.
(A) and overall survival (B) for patients with NSCLC and brain metastasis treated with pembrolizumab in cohort 1 (PD-L1 expression ≥ 1%).
The toxicity profile of pembrolizumab in this study was consistent with other studies of PD-1 inhibitors in patients with NSCLC (table 2). Neurologic AEs (regardless of attribution) were mostly grade 1-2, with the exception of grade 3 cognitive dysfunction, seizure and stroke in 1 patient each, all considered unrelated to study drug. There were 6 drug-related serious adverse events: 2 patients with pneumonitis and 1 patient each with acute kidney injury, colitis, hypokalemia, and adrenal insufficiency. Four of 42 patients (9·5%) discontinued treatment due to drug-related toxicity. Twenty-six patients died; specific reason for death was not collected however no deaths were considered related to treatment. Details regarding the development of radiation necrosis is included in the appendix p 3.
Table 2.
Adverse events in all treated patients (n=42). All neurologic events are included regardless of attribution to pembrolizumab or frequency; other adverse events are included if occurring in at least 10% of patients or grade 3-5, and considered at least possibly related to pembrolizumab.
| Adverse Event | Grade 1 or 2 |
Grade 3 |
Grade 4 |
Grade 5 |
|---|---|---|---|---|
| Neurologic adverse events, regardless of attribution | ||||
| Cognitive dysfunction | 7 (17%) | 1 (2%) | 0 | 0 |
| Depressed level of consciousness | 1 (2%) | 0 | 0 | 0 |
| Word finding difficulties | 1 (2%) | 0 | 0 | 0 |
| Dizziness | 10 (24%) | 0 | 0 | 0 |
| Headache | 15 (36%) | 0 | 0 | 0 |
| Seizures | 2 (5%) | 1 (2%) | 0 | 0 |
| Gait imbalance | 1 (2%) | 0 | 0 | 0 |
| Paresthesia | 6 (14%) | 0 | 0 | 0 |
| Peripheral neuropathy | 3 (7%) | 0 | 0 | 0 |
| Focal motor weakness | 1 (2%) | 0 | 0 | 0 |
| Speech difficulty | 1 (2%) | 0 | 0 | 0 |
| Stroke | 1 (2%) | 1 (2%) | 0 | 0 |
| Tremors | 1 (2%) | 0 | 0 | 0 |
| Non-neurologic adverse events, treatment-related | ||||
| Constitutional symptoms | 13 (31%) | 1 (2%) | 0 | 0 |
| Pneumonitis | 1 (2%) | 2 (5%) | 0 | 0 |
| Diarrhea/colitis | 4 (10%) | 1 (2%) | 0 | 0 |
| Rash | 8 (19%) | 0 | 0 | 0 |
| Hypothyroidism | 7 (17%) | 0 | 0 | 0 |
| Acute kidney injury | 1 (2%) | 0 | 0 | 0 |
| Adrenal insufficiency | 1 (2%) | 1 (2%) | 0 | 0 |
| Hypokalemia | 0 | 0 | 1 (2%) | 0 |
| Hyperglycemia | 0 | 1 (2%) | 0 | 0 |
Thirty-four patients had tumor tissue available for PD-L1 and TIL analysis, 32 from biopsies obtained immediately prior to trial therapy, and 2 from archival tissue with intervening therapy. Only 4 samples were from brain metastases, with the remainder from systemic sites of disease. Patients with tumors showing PD-L1 expression ≥ 1% in stromal/immune cells had a longer OS than those with PD-L1 <1% (median OS 11·0 months [95% CI 8·73-NR] versus 2·73 months [95% CI 1-NR], p=0·031; appendix p 4). Median OS was numerically higher in those with tumor-cell PD-L1 expression ≥ 1% although results did not reach statistical significance (median OS 11·43 months [95% CI 8·93-NR] versus 4·83 months [95% CI 2·8-NR], p=0·074; appendix p 4). There was no statistically significant association between PD-L1 expression and response or PFS (appendix p 5). The features of patients who had a response in the CNS are shown in the appendix p 6.
We analyzed the impact of baseline TILs on clinical outcomes using multiplexed quantitative immunofluorescence. Elevated levels of studied TIL subsets were associated longer OS but the results did not reach statistical significance (appendix p 7). No statistically significant association was seen between level of TILs and response or PFS.
We also conducted targeted mRNA immune profiling of 23 tumor biopsies using the Nanostring platform. As shown in appendix p 7, tumors from patients who responded to pembrolizumab showed significantly higher levels of multiple pro-inflammatory genes than non-responders including key effector molecules and chemokines such as Granzyme-B (GZMB), C-X-C Motif Chemochkine 9 (CXCL9), C-X-C Motif Chemokine 10 (CXCL10), and Granulysin (GNLY) using p values adjusted for a false discovery rate of < 0·05. We did not find a prominent correlation between the mRNA targets and PD-L1 expression (appendix p 8).
DISCUSSION
This study found that patients with NSCLC and untreated or progressing brain metastases can benefit from systemic treatment with pembrolizumab. Although the study closed early, we were able to demonstrate that 29·7% of PD-L1-positive NSCLC patients achieved a brain metastasis response, which allowed the study to meet its primary endpoint. Survival in this cohort of patients exceeds the historically documented survival for patients with brain metastasis from NSCLC, which is 2-year survival of 14·3%.1
Randomized trials with PD-1 axis inhibitors have changed the treatment landscape for NSCLC.16,18 The response rates overall in these studies are comparable to that in our study, indicating that brain metastases have a similar likelihood of benefit from anti-PD-1 as extra-cerebral disease. To our knowledge, this is the first study to focus on the population of patients with brain metastases from lung cancer and examine the effects of immunotherapy. Other studies have demonstrated that patients with brain metastases from NSCLC can benefit from immunotherapy both as a single agent5,19,20 and in combination with chemotherapy,21 however these reports were based on subset analyses from larger trials and did not assess the specific characteristics of the CNS disease. Several expanded access programs and observational studies examining patients with NSCLC treated with single-agent PD-1 or PD-L1 inhibitors found that clinical outcomes (including efficacy and safety) were similar in patients with versus without brain metastases, highlighting that both PD-1 and PD-L1 inhibitors can be effective in patients with CNS disease.22-25
A limitation of this study is that we only allowed patients with brain metastases between 5 and 20 mm; this was chosen to avoid neurologic compromise in the event of tumor growth. Additionally, we did not evaluate patients with neurologic symptoms or those that required corticosteroid use. We also do not have treatment data on patients after drug was discontinued, therefore we do not know whether rechallenge with immunotherapy was attempted. Another important limitation of this study is that it is a single-arm trial and therefore lacks a control arm that treats patients with radiation to brain metastases followed by systemic therapy, making it difficult to draw definitive conclusions regarding which strategy is superior.
In our study, restricting CNS disease to smaller, asymptomatic lesions resulted in a safe treatment, despite the majority of patients experiencing progression in the brain. We assessed response in the brain with MRIs every 8 weeks (with a safety MRI after 4 weeks of therapy) and found that responses were seen after a median of 1.8 months, allowing the non-responders to receive salvage therapy as appropriate. Development of a treatment strategy for patients should consider tumor histology, biomarkers (i.e. mutation status, PD-L1 expression), size, location, and symptomatology before determining whether to pursue local or systemic therapy upfront.
We found that PD-L1 expression in both tumor and stromal cells was associated with prolonged OS. Measurement of major TIL subsets using quantitative multiplexed immunofluorescence was not significantly associated with clinical outcomes, possibly due to limited sample size, technical aspects of the assay or other biological factors. Several key pro-inflammatory genes associated with adaptive anti-tumor responses with known effector and chemotactic functions relevant in cancer were found to be prominently higher in tumors from patients who responded to pembrolizumab,26-28 An important limitation to these assays is that the patient population was heterogeneous, with many patients having no prior treatment but others receiving 1 or more prior lines of therapy, which might affect the tumor immune milieu. Additionally, most patients only had tissue available from systemic sites of disease, which have a different microenvironment than brain metastases.29 We also were unable to evaluate tumor mutation burden which has been associated with benefit from immunotherapy and may be higher in brain metastases compared to other sites of disease.30 Further validation of these targets and signatures is required to determine whether they could be used as predictive markers.
In summary, pembrolizumab has activity in brain metastases from NSCLC similar to its systemic activity and can result in prolonged survival in a subset of patients. The potential benefit of immunotherapy alone is that all lesions are treated simultaneously and it might reduce the incidence of toxicity from radiation, including radiation necrosis. Studies of combination therapy such as with other immune therapies, radiation or chemotherapy, are warranted to increase the frequency of responses in NSCLC patients with brain metastases.
Supplementary Material
RESEARCH IN CONTEXT.
Evidence before this study:
Immunotherapy with PD-1 axis inhibitors can result in significant and durable responses in patients with non-small cell lung cancer (NSCLC), however most trials excluded patients with active NSCLC brain metastasis. We searched PubMed from January 1, 2009 through November 26, 2019 with the following terms: NSCLC, brain metastases and PD-1 or PD-L1. We found case reports and small case series demonstrating activity of immunotherapy in untreated NSCLC brain metastases. The only prospective trial data was an interim report from the current study which showed preliminary evidence of activity of the PD-1 inhibitor pembrolizumab in the CNS.
Added value of this study:
Here we present the final results and long-term follow-up of NSCLC patients with active brain metastasis and tumor PD-L1 expression ≥ 1%, treated with pembrolizumab. We demonstrate that pembrolizumab can induce responses in the CNS at rates similar to extra-cranial tumors, with overall survival exceeding the historic rates of patients with NSCLC brain metastases. We also provide safety data indicating that treatment is well-tolerated with no new concerns in this patient population.
Implications of all the available evidence:
Immunotherapy with PD-1 axis inhibitors can have activity in the CNS and is safe in patients with NSCLC brain metastases. Pembrolizumab could be an option for treating patients with brain metastases from NSCLC, specifically those with small, asymptomatic lesions. However additional studies are necessary to confirm whether PD-1 inhibitors alone, in combination with other drugs, or with radiation is the optimal strategy.
Acknowledgments:
The study was funded by Merck and the Yale Cancer Center. Additional support was provided by the Department of Defense through the Lung Cancer Research Program X81XWH-15-1-0203 (S. Goldberg, PI) and W81XWH-16-1-0160 (K. Schalper, PI), NIH grants Yale SPORE in Lung Cancer P50CA196530 (R. Herbst, PI), R01 CA158167 (H. Kluger and G. Desir PIs), K24CA172123 (H. Kluger, PI), Yale SPORE in Skin Cancer P50 CA121974 (M. Bosenberg and H. Kluger, PIs), R01 CA204002 (L. Jilaveanu, PI), grant from the Lung Cancer Research Foundation-LUNGevity and Melanoma Research Alliance, Award#308721 (L. Jilaveanu, PI), and Stand Up To Cancer – American Cancer Society Lung Cancer Dream Team Translational Research Grants SU2C-AACR-DT17-15 (P. Janne, A. Shaw, J. Wolchok, PIs), SU2C-AACR-DT22-17 (L. Diaz, PI), and the J. Aron Charitable Foundation (S. Goldberg). Opinions, interpretations, conclusions and recommendations are those of the authors and are not necessarily endorsed by the Department of Defense.
Footnotes
Declaration of interests:
SBG reports grants from Merck during the conduct of the study and grants from AstraZeneca and Boehringer Ingelheim and personal fees from AstraZeneca, Eli Lilly, Bristol-Myers Squibb, Boehringer Ingelheim, Genentech, Amgen and Spectrum outside the submitted work. KAS reports personal fees from Clinica Alemana Santiago, Celgene, Moderna Therapeutics, Shattuck Labs, Pierre-Fabre, Astrazeneca, Dyanamo Therapeutics, EMD Serono, Abbvie, Agenus, Torque Therapeutics. Research funding from: Navigate Bipharma (Novartis), Vasculox/Tioma, Tesaro, Moderna Therapeutics, Takeda Pharmaceuticals, Surface Oncology, Pierre-Fabre Research Institute, Merck, Bristol-Myers Squibb, AstraZeneca and Eli Lilly outside the submitted work. SNG reports Research Support to Institution (Yale) and consultant to Bristol-Myers Squibb, consultant to NEKTAR, Research funding (to Yale) from Genentech/Roche, Research funding (to Yale) from Iovance outside the submitted work. RSH is a member of the board of directors (non-executive/ independent) for Junshi Pharmaceuticals and reports grants from AstraZeneca, Eli Lilly and Company, Merck and Company and personal fees from Abbvie Pharmaceuticals, ARMO Biosciences, AstraZeneca, Biodesix, Bolt Biotherapeutics, Bristol-Myers Squibb, Eli Lilly and Company, EMD Serrano, Genentech/Roche, Genmab, Halozyme, Heat Biologics, IMAB Biopharma, Immunocore, Infinity Pharmaceuticals, Loxo Oncology, Merck and Company, Midas Health Analytics, Nektar, Neon Therapeutics, NextCure, Novartis, Pfizer, Sanofi, Seattle Genetics, Shire PLC, Spectrum Pharmaceuticals, Symphogen, Takeda, Tesaro, Tocagen outside the submitted work. AC reports grants from Bristol-Myers Squibb, Abbvie, AstraZeneca, and Amgen and personal fees from AstraZeneca, Bristol-Myers Squibb, Abbvie, Boehringer-Ingelheim, and Genentech/Roche outside the submitted work. RL reports personal fees from AstraZeneca outside the submitted work. FHW reports grants from Agios and personal fees from Loxo Oncology outside the submitted work. JY reports personal fees from Augmenix / Boston Scientific and Galera Pharmaceuticals outside the submitted work. YK reports grants from Merck during the conduct of the study and grants from Bristol-Myers Squibb and Apexigen and personal fees from Alexion, Corvus, Nektar, Biodesix, Roche-Genentech, Pfizer, Iovance, Immunocore, Celldex, and Array Biopharma, outside the submitted work. VC reports personal fees from Monteris Medical Inc, MRI Interventions, and Brainlab AG outside the submitted work. HMK reports grants from Merck during the conduct of the study and grants from Bristol-Myers Squibb and Apexigen and personal fees from Alexion, Corvus, Nektar, Biodesix, Roche-Genentech, Pfizer, Iovance, Immunocore, Celldex, and Array Biopharma, outside the submitted work. AM, SBO, LJ, TT, KP, ER, HG, AK, RG, HW, MR, GZ, and WW have no conflicts to report.
Data sharing statement:
Deidentified individual participant data may be made available following publication by request to the corresponding author. A research proposal should be included.
Contributor Information
Sarah B. Goldberg, Department of Medicine (Medical Oncology), Yale School of Medicine, New Haven, CT
Kurt A. Schalper, Department of Pathology, Yale School of Medicine, New Haven, CT
Scott N. Gettinger, Department of Medicine (Medical Oncology), Yale School of Medicine, New Haven, CT.
Amit Mahajan, Department of Radiology, Yale School of Medicine, New Haven, CT.
Roy S. Herbst, Department of Medicine (Medical Oncology), Yale School of Medicine, New Haven, CT.
Anne C. Chiang, Department of Medicine (Medical Oncology), Yale School of Medicine, New Haven, CT
Rogerio Lilenbaum, Department of Medicine (Medical Oncology), Yale School of Medicine, New Haven, CT.
Frederick H. Wilson, Department of Medicine (Medical Oncology), Yale School of Medicine, New Haven, CT
Sacit Bulent Omay, Department of Neurosurgery, Yale School of Medicine, New Haven, CT.
James Yu, Department of Therapeutic Radiology, Yale School of Medicine, New Haven, CT.
Lucia Jilaveanu, Department of Medicine (Medical Oncology), Yale School of Medicine, New Haven, CT.
Thuy Tran, Department of Medicine (Medical Oncology), Yale School of Medicine, New Haven, CT.
Kira Pavlik, Department of Medicine (Medical Oncology), Yale School of Medicine, New Haven, CT.
Elin Rowen, Department of Medicine (Medical Oncology), Yale School of Medicine, New Haven, CT.
Heather Gerrish, Department of Medicine (Medical Oncology), Yale School of Medicine, New Haven, CT.
Annette Komlo, Department of Medicine (Medical Oncology), Yale School of Medicine, New Haven, CT.
Richa Gupta, Department of Medicine (Medical Oncology), Yale School of Medicine, New Haven, CT.
Hailey Wyatt, Department of Medicine (Medical Oncology), Yale School of Medicine, New Haven, CT.
Matthew Ribeiro, Department of Medicine (Medical Oncology), Yale School of Medicine, New Haven, CT.
Yuval Kluger, Department of Medicine (Medical Oncology), Yale School of Medicine, New Haven, CT.
Geyu Zhou, Interdepartmental Program in Computational Biology and Bioinformatics, Yale University, New Haven, CT.
Wei Wei, Department of Biostatistics, Yale School of Public Health, New Haven CT.
Veronica Chiang, Department of Neurosurgery, Yale School of Medicine, New Haven, CT.
Harriet M. Kluger, Department of Pathology, Yale School of Medicine, New Haven, CT.
REFERENCES
- 1.Waqar SN, Samson PP, Robinson CG, et al. Non-small-cell Lung Cancer With Brain Metastasis at Presentation. Clin Lung Cancer 2018; 19(4): e373–e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Valiente M, Ahluwalia MS, Boire A, et al. The Evolving Landscape of Brain Metastasis. Trends Cancer 2018; 4(3): 176–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reungwetwattana T, Nakagawa K, Cho BC, et al. CNS Response to Osimertinib Versus Standard Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors in Patients With Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2018: JCO2018783118. [DOI] [PubMed] [Google Scholar]
- 4.Gadgeel S, Peters S, Mok T, et al. Alectinib versus crizotinib in treatment-naive anaplastic lymphoma kinase-positive (ALK+) non-small-cell lung cancer: CNS efficacy results from the ALEX study. Ann Oncol 2018; 29(11): 2214–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016; 375(19): 1823–33. [DOI] [PubMed] [Google Scholar]
- 6.Gandhi L, Rodriguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med 2018; 378(22): 2078–92. [DOI] [PubMed] [Google Scholar]
- 7.Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. N Engl J Med 2018; 379(21): 2040–51. [DOI] [PubMed] [Google Scholar]
- 8.Brown PD, Jaeckle K, Ballman KV, et al. Effect of Radiosurgery Alone vs Radiosurgery With Whole Brain Radiation Therapy on Cognitive Function in Patients With 1 to 3 Brain Metastases: A Randomized Clinical Trial. JAMA 2016; 316(4): 401–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colaco RJ, Martin P, Kluger HM, Yu JB, Chiang VL. Does immunotherapy increase the rate of radiation necrosis after radiosurgical treatment of brain metastases? J Neurosurg 2016; 125(1): 17–23. [DOI] [PubMed] [Google Scholar]
- 10.Goldberg SB, Gettinger SN, Mahajan A, et al. Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncol 2016; 17(7): 976–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kluger HM, Chiang V, Mahajan A, et al. Long-Term Survival of Patients With Melanoma With Active Brain Metastases Treated With Pembrolizumab on a Phase II Trial. J Clin Oncol 2019; 37(1): 52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45(2): 228–47. [DOI] [PubMed] [Google Scholar]
- 13.Dolled-Filhart M, Locke D, Murphy T, et al. Development of a Prototype Immunohistochemistry Assay to Measure Programmed Death Ligand-1 Expression in Tumor Tissue. Arch Pathol Lab Med 2016; 140(11): 1259–66. [DOI] [PubMed] [Google Scholar]
- 14.Schalper KA, Brown J, Carvajal-Hausdorf D, et al. Objective measurement and clinical significance of TILs in non-small cell lung cancer. J Natl Cancer Inst 2015; 107(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schalper KA, Rodriguez-Ruiz ME, Diez-Valle R, et al. Neoadjuvant nivolumab modifies the tumor immune microenvironment in resectable glioblastoma. Nat Med 2019; 25(3): 470–6. [DOI] [PubMed] [Google Scholar]
- 16.Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016; 387(10027): 1540–50. [DOI] [PubMed] [Google Scholar]
- 17.Mazieres J, Drilon AE, Mhanna L, et al. Efficacy of immune-checkpoint inhibitors (ICI) in non-small cell lung cancer (NSCLC) patients harboring activating molecular alterations (ImmunoTarget). Journal of Clinical Oncology 2018; 36(15_suppl): 9010-. [Google Scholar]
- 18.Lopes G, Wu Y-L, Kudaba I, et al. Pembrolizumab (pembro) versus platinum-based chemotherapy (chemo) as first-line therapy for advanced/metastatic NSCLC with a PD-L1 tumor proportion score (TPS) ≥ 1%: Open-label, phase 3 KEYNOTE-042 study. Journal of Clinical Oncology 2018; 36(18_suppl): LBA4–LBA. [Google Scholar]
- 19.Gadgeel SM, Lukas RV, Goldschmidt J, et al. Atezolizumab in patients with advanced non-small cell lung cancer and history of asymptomatic, treated brain metastases: Exploratory analyses of the phase III OAK study. Lung Cancer 2019; 128: 105–12. [DOI] [PubMed] [Google Scholar]
- 20.Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017; 389(10066): 255–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garassino MC, Gadgeel S, Esteban E, et al. Abstract CT043: Outcomes among patients (pts) with metastatic nonsquamous NSCLC with liver metastases or brain metastases treated with pembrolizumab (pembro) plus pemetrexed-platinum: Results from the KEYNOTE-189 study. Cancer Research 2019; 79(13 Supplement): CT043–CT. [Google Scholar]
- 22.Cortinovis D, Chiari R, Catino A, et al. Italian Cohort of the Nivolumab EAP in Squamous NSCLC: Efficacy and Safety in Patients With CNS Metastases. Anticancer Res 2019; 39(8): 4265–71. [DOI] [PubMed] [Google Scholar]
- 23.Crino L, Bronte G, Bidoli P, et al. Nivolumab and brain metastases in patients with advanced non-squamous non-small cell lung cancer. Lung Cancer 2019; 129: 35–40. [DOI] [PubMed] [Google Scholar]
- 24.Hendriks LEL, Henon C, Auclin E, et al. Outcome of Patients with Non-Small Cell Lung Cancer and Brain Metastases Treated with Checkpoint Inhibitors. J Thorac Oncol 2019; 14(7): 1244–54. [DOI] [PubMed] [Google Scholar]
- 25.Molinier O, Audigier-Valette C, Cadranel J, et al. OA 17.05 IFCT-1502 CLINIVO: Real-Life Experience with Nivolumab in 600 Patients (Pts) with Advanced Non-Small Cell Lung Cancer (NSCLC). Journal of Thoracic Oncology 2017; 12(11): S1793. [Google Scholar]
- 26.Arias M, Martinez-Lostao L, Santiago L, Ferrandez A, Granville DJ, Pardo J. The Untold Story of Granzymes in Oncoimmunology: Novel Opportunities with Old Acquaintances. Trends Cancer 2017; 3(6): 407–22. [DOI] [PubMed] [Google Scholar]
- 27.Hsiao YW, Lai TC, Lin YH, et al. Granulysin expressed in a humanized mouse model induces apoptotic cell death and suppresses tumorigenicity. Oncotarget 2017; 8(48): 83495–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tokunaga R, Zhang W, Naseem M, et al. CXCL9, CXCL10, CXCL11/CXCR3 axis for immune activation - A target for novel cancer therapy. Cancer Treat Rev 2018; 63: 40–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu BY, Gupta R, Ribeiro M, et al. PD-L1 expression and tumor-infiltrating lymphocytes in lung cancer brain metastases. Journal of Clinical Oncology 2018; 36(15_suppl): e24116–e. [Google Scholar]
- 30.Stein MK, Pandey M, Xiu J, et al. Tumor Mutational Burden Is Site Specific in Non–Small-Cell Lung Cancer and Is Highest in Lung Adenocarcinoma Brain Metastases. JCO Precision Oncology 2019; (3): 1–13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.