SUMMARY
Background:
The Seveso accident (Italy) in 1976 caused the contamination of a large population by 2,3,7,8-tetrachlorodibenzo-para-dioxin (2,3,7,8-TCDD). The contaminated territory was divided into three zones: A (very high contamination), B (high contamination), and R (low contamination). We report here the plasma concentrations of seven polychlorinated dibenzo-para-dioxins (PCDDs), 10 polychlorinated dibenzofurans (PCDFs), four non-ortho-polychlorinated biphenyls PCBs (nPCBs), and Toxic Equivalencies (TEQs) in a sample of residents in the most polluted zones A and B and in a reference non-contaminated zone.
Methods:
From December 1992 to March 1994, 62 individuals were randomly selected from the population living in zone A (No. =7) and B (No. =55). A sample of 59 subjects living in a surrounding non-contaminated area (non-ABR), frequency-matched by gender, age, and smoking history, was used as reference. All subjects were administered a questionnaire surveying demographic, lifestyle, medical history, and accident-related factors. We assayed plasma PCDD, PCDF, and nPCB concentrations by high-resolution gas chromatography/high resolution mass spectrometric (HRGC/HRMS) analysis, with results reported as pg/g of lipid, or parts per trillion (ppt). We calculated TEQs using the WHO 2005 Toxic Equivalency Factors (TEFs).
Results:
We found elevated median levels of 2,3,7,8-TCDD in plasma samples of subjects living in zone A (73.3 ppt) and zone B (12.4 ppt), compared with residents in the reference zone (5.5 ppt). In analyses adjusted for gender, age, smoking, and body mass index (BMI), none of the other congeners showed levels higher than reference in the contaminated zones. Compared with men, women showed higher levels (113%) of 2,3,7,8-TCDD and a slight elevation (17%) of TEQ for the other congeners. Age was strongly positively associated with most congener levels; TEQs for PCDDs, PCDFs, and nPCBs showed respectively 12%, 24%, and 41% increases for every 10 years of age. Current smokers had lower (from −37% to −67%) TEQ levels than subjects who had never smoked. BMI was negatively associated with levels of a few congeners, but with no impact on TEQ values.
Conclusions:
The Seveso accident caused a severe exposure of the population to 2,3,7,8-TCDD only. None of the other congeners analyzed showed variation across zones. Age showed a strong positive association with TEQs for all classes of compounds (PCDDs, PCDFs, and nPCBs).
Keywords: Environmental pollution, dioxin, PCDD, PCDF, PCB, TEQ
Abstract
«Livelli plasmatici di diossine, furani, non-orto-PCB e TEQ nella popolazione di Seveso 17 anni dopo l’incidente».
Introduzione:
L’incidente Seveso del 1976 causò la contaminazione di una vasta popolazione dell’area con 2,3,7,8-tetraclorodibenzo-para-diossina (2,3,7,8-TCDD). Il territorio inquinato fu suddiviso in tre zone: A (contaminazione molto alta), B (alta contaminazione), R (bassa contaminazione). In questo articolo riportiamo le concentrazioni plasmatiche di 7 dibenzo-para-diossine policlorurate (PCDD), 10 dibenzofurani policlorurati (PCDF), 4 non-orto bifenili policlorurati (nPCB) ed Equivalenze Tossiche (TEQ) in un campione di residenti nelle zone più inquinate A e B e in una zona non inquinata di riferimento.
Metodi:
Fra il dicembre 1992 e il marzo 1994, 62 soggetti furono selezionati casualmente dalla popolazione residente in zona A (N. 7) e B (N. 55). Come riferimento fu usato un campione di 59 soggetti residenti in una zona circostante non inquinata (non-ABR), appaiato per genere, età e abitudini di fumo. Ai soggetti fu somministrato un questionario su caratteristiche demografiche, abitudini di vita, storia medica e fattori legati all’incidente. Tramite high-resolution gas chromatography/high resolution mass spectrometric (HRGC/HRMS) furono misurate le concentrazioni plasmatiche di PCDD, PCDF e nPCB, espresso in pg/g di lipidi, o parti per trilione (ppt). Le TEQ sono state calcolate utilizzando i fattori di equivalenza tossica (TEF) stabiliti dall’OMS nel 2005.
Risultati:
Abbiamo riscontrato elevate livelli mediani di 2,3,7,8-TCDD nei campioni plasmatici dei soggetti di zona A (73,3 ppt) e zona B (12,4 ppt), in confronto ai residenti nella zona di riferimento (5,5 ppt). In analisi aggiustate per genere, età, fumo e indice di massa corporea (BMI), per nessuno degli altri congeneri le zone contaminate mostravano livelli più elevati del riferimento. In confronto agli uomini le donne mostravano livelli più elevati (113%) di 2,3,7,8-TCDD e una lieve aumento (17%) della TEQ per gli altri congeneri. L’età era fortemente positivamente associata coi livelli della maggior parte dei congeneri; le TEQ per PCDD, PCDF, and nPCB mostravano aumenti rispettivamente del 12%, 24% e 41% per ogni decade di età. Gli attuali fumatori avevano livelli più bassi (da −37% a −67%) di TEQ rispetto ai mai fumatori. Il BMI era negativamente associato coi livelli di pochi congeneri, ma senza impatto sui valori di TEQ.
Conclusioni:
L’incidente di Seveso causò una severa esposizione della popolazione alla sola 2,3,7,8-TCDD. Nessuno degli altri congeneri ha mostrato variazioni tra zone. Per l’età si è evidenziata una forte associazione positiva con le TEQ di tutte le classi di composti (PCDD, PCDF, nPCB).
Keywords: Inquinamento ambientale, diossina, PCDD, PCDF, PCB, TEQ
INTRODUCTION
The term “dioxin(s)” indicates a group of substances which include polychlorinated dibenzo-para-dioxins (PCDDs), polychlorinated dibenzofurans (PCDFs), and a subgroup of polychlorinated biphenyls with dioxin-like properties (dioxin-like PCBs) (11, 26, 27). While PCBs have dielectric properties and were produced for commercial purposes, the other dioxins are unwanted by-products of combustion processes of chlorine containing components. The most toxic compound (congener) of the family is 2,3,7,8-tetrachloro-diben zo-para-dioxin (2,3,7,8-TCDD), which has a wide range of effects and has been classified as carcinogen to humans in 1997 (11). Recently, two other congeners (2,3,4,7,8-PeCDF and PCB 126) have been classified as carcinogens for humans (http://monographs.iarc.fr/ENG/Classification/ClassificationsGroupOrder.pdf). Many of the effects of 2,3,7,8-TCDD are modulated by the Ah receptor (AhR). Several related compounds share the capacity of binding to AhR (hence the term “dioxin-like”) and may therefore have similar effects. Currently, they include seven 2,3,7,8-substituted PCDDs, ten 2,3,7,8-substituted PCDFs, four non-ortho-PCBs (nPCBs), and eight mono-ortho-PCBs (mPCBs). The affinity of a specific compound with the Ah receptor determines its toxic potential, measured by the Toxic Equivalency Factor (TEF), which expresses the order of magnitude of toxicity relative to 2,3,7,8-TCDD (TEF=1). They are established by an international committee of experts and periodically re-evaluated based on scientific evidence. The most used TEF schemes are reported in table 1 (1, 11, 30, 31). To express the global exposure to dioxin-like compounds a TEF-weighted sum of lipid-adjusted concentrations (in pg/g of lipid) is calculated to obtain the Toxic Equivalency (TEQ).
Table 1 -.
| Compound | I-TEF* (NATO-CCMS) | WHO 1998 | WHO 2005 |
|---|---|---|---|
| Polychlorinated-dibenzo-para-dioxins (PCDDs) | |||
| 2,3,7,8-TCDD | 1 | 1 | 1 |
| 1,2,3,7,8-PeCDD | 0.5 | 1 | 1 |
| 1,2,3,4,7,8-HxCDD | 0.1 | 0.1 | 0.1 |
| 1,2,3,6,7,8-HxCDD | 0.1 | 0.1 | 0.1 |
| 1,2,3,7,8,9-HxCDD | 0.1 | 0.1 | 0.1 |
| 1,2,3,4,6,7,8-HpCDD | 0.01 | 0.01 | 0.01 |
| OCDD | 0.001 | 0.0001 | 0.0003 |
| Polychlorinated dibenzofurans (PCDFs) | |||
| 2,3,7,8-TCDF | 0.1 | 0.1 | 0.1 |
| 1,2,3,7,8-PeCDF | 0.05 | 0.05 | 0.03 |
| 2,3,4,7,8-PeCDF | 0.5 | 0.5 | 0.3 |
| 1,2,3,4,7,8-HxCDF | 0.1 | 0.1 | 0.1 |
| 1,2,3,6,7,8-HxCDF | 0.1 | 0.1 | 0.1 |
| 1,2,3,7,8,9-HxCDF | 0.1 | 0.1 | 0.1 |
| 2,3,4,6,7,8-HxCDF | 0.1 | 0.1 | 0.1 |
| 1,2,3,4,6,7,8-HpCDF | 0.01 | 0.01 | 0.01 |
| 1,2,3,4,7,8,9-HpCDF | 0.01 | 0.01 | 0.01 |
| OCDF | 0.001 | 0.0001 | 0.0003 |
| Non-ortho-polychlorinated biphenyls (nPCBs) | |||
| 3,3’,4,4’-TCB (PCB 77) | 0.0005 | 0.0001 | 0.0001 |
| 3,4,4’,5-TCB (PCB 81) | - | 0.0001 | 0.0003 |
| 3,3’,4,4’,5-PeCB (PCB 126) | 0.1 | 0.1 | 0.1 |
| 3,3’,4,4’,5,5’-HxCB (PCB 169) | 0.01 | 0.01 | 0.03 |
| Mono-ortho-polychlorinated biphenyls (mPCBs) | |||
| 2,3,3’,4,4’-PeCB (PCB 105) | 0.0001 | 0.0001 | 0.00003 |
| 2,3,4,4’,5-PeCB (PCB 114) | 0.0005 | 0.0005 | 0.00003 |
| 2,3’,4,4’,5-PeCB (PCB 118) | 0.0001 | 0.0001 | 0.00003 |
| 2’,3,4,4’,5-PeCB (PCB 123) | 0.0001 | 0.0001 | 0.00003 |
| 2,3,3’,4,4’,5-HxCB (PCB 156) | 0.0005 | 0.0005 | 0.00003 |
| 2,3,3’,4,4’,5’-HxCB (PCB 157) | 0.0005 | 0.0005 | 0.00003 |
| 2,3’,4,4’,5,5’-HxCB (PCB 167) | 0.00001 | 0.00001 | 0.00003 |
| 2,3,3’,4,4’,5,5’-HpCB (PCB 189) | 0.0001 | 0.0001 | 0.00003 |
| Di-ortho-polychlorinated biphenyls (dPCBs) | |||
| 2,2’,3,3’,4,4’,5-HpCB (PCB 170) | 0.0001 | - | - |
| 2,2’,3,4,4’,5,5’-HpCB (PCB 180) | 0.00001 | - | - |
Values in bold print indicate changes with respect to previous revision
TEFs for PCBs from Ahlborg 1994 (1)
The Seveso, Italy, dioxin episode caused severe 2,3,7,8-TCDD exposure to a population comprising people of both genders and all ages, The accident took place on July 10, 1976, in the trichlorophenol production department of a chemical plant located near the town of Seveso, 25 km north of Milan. A chemical cloud containing several kilograms of 2,3,7,8-TCDD was released into the environment and contaminated a vast and densely populated area (23, 27). The contaminated area was divided into three zones named A (very high contamination, with displacement of the population), B (high), and R (low) (figure 1). To investigate the long-term health effects of the accident, mortality and cancer incidence studies are on-going. The surveyed population included 4000 subjects in the contaminated zones and 180000 subjects living in the surrounding territory (termed non-ABR), used as reference (6, 24).
Figure 1 -.
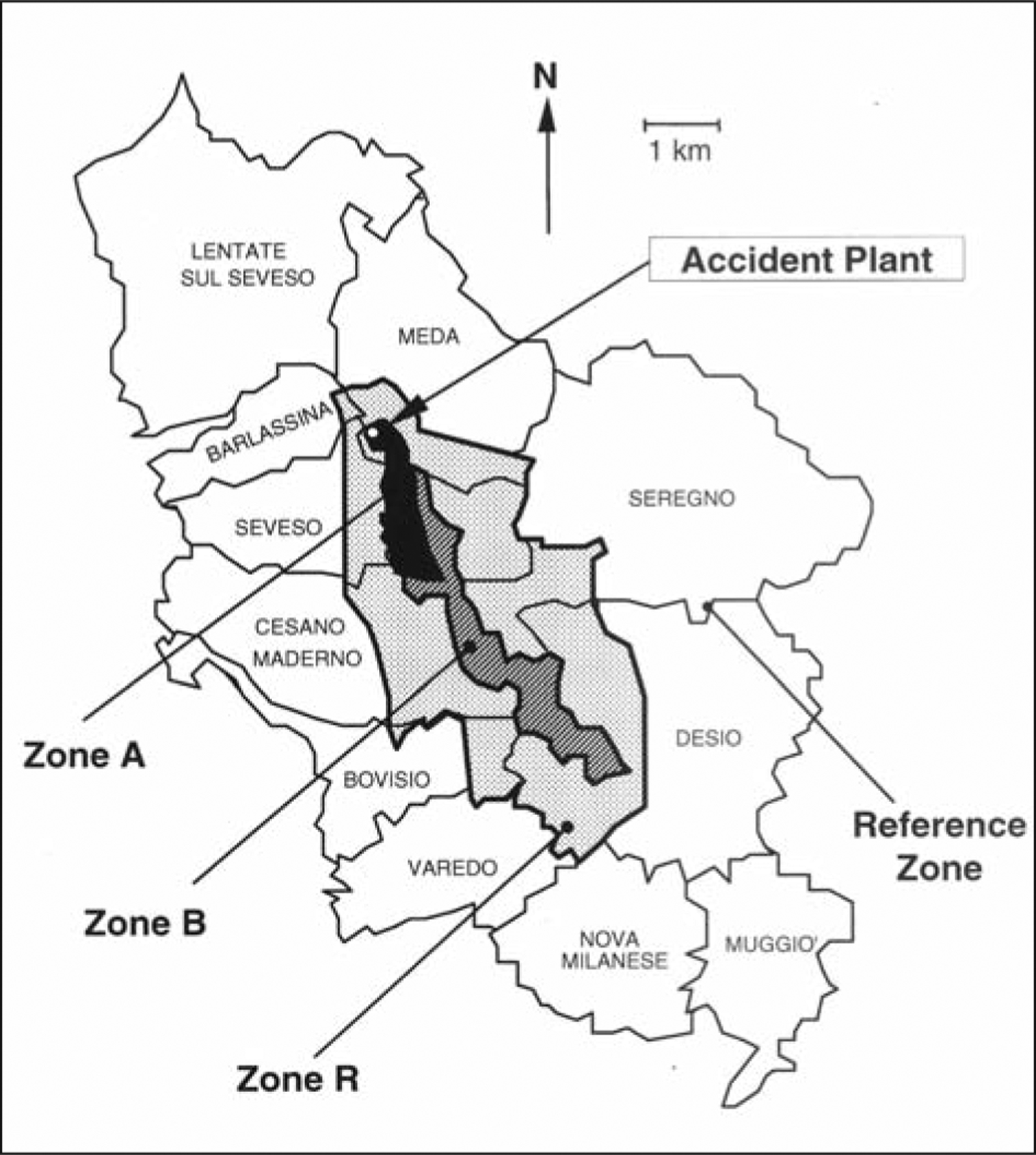
The Seveso, Italy, area, including the territory of 11 towns. The map indicates the three dioxin-contaminated zones with decreasing mean soil levels (A, B, and R) and the surrounding non-contaminated zone (non-ABR) adopted as the reference
Several measurements of plasma concentrations of 2,3,7,8-TCDD were performed in the weeks or months after the accident (18) and many years later on subgroups of the involved population (14, 15), confirming a strong gradient across contamination zones. However, although it had been verified that levels of other congeners were not associated with zone of residence (14), a detailed report of the whole congener profile has never been published. In this paper we report the plasma concentrations of PCDDs, PCDFs, and nPCBs in a sample of the population living in the accident area. In addition, we calculated partial and total TEQs using the WHO 2005 scheme and investigated the relationships with zone of residence, gender, and age.
METHODS
Detailed information on methods has been previously published (14, 15). Briefly, from December 1992 to March 1994, 62 individuals were enrolled from the population living in the most polluted zones A (No. 7) and B (No. 55). A sample of 59 subjects living in a surrounding non-contaminated area (non-ABR), frequency-matched by gender, age, and smoking history, was used as reference. After signing a written informed consent, all subjects were administered a questionnaire surveying demographics, lifestyle, medical history, and accident-related factors. Blood samples were obtained and refrigerated samples were shipped to the Centers for Disease Control and Prevention (CDC), Atlanta, USA for plasma assay using a high-resolution gas chromatography/high resolution mass spectrometric (HRGC/HRMS) analysis (20). Concentrations were reported in pg/g of lipid, or parts per trillion (ppt). Levels below the detection limits (DL) were assigned a value equal to DL/√2 (3). We calculated partial TEQs for PCDDs, PCDFs, nPCBs, and total TEQ (including or not the 2,3,7,8-TCDD contribution) using the WHO 2005 TEFs (31).
Congener and TEQ plasma levels in polluted zones A and B were compared with those found in the reference non-ABR zone using the Mann-Whitney test (2). We then fitted multiple regression models containing the covariates zone of residence, gender (female versus male), age (years/10), smoking status (never, former, current), and body mass index (BMI, in kg/m2). Since congener and TEQ distributions are right-skewed, they were (natural) log-transformed. We then calculated percent changes (%) relative to the intercept with the formula [exp(coefficient )-1]×100.
RESULTS
Gender, age, and smoking status distributions were similar across zones as a result of matching, while subjects in zone A had a higher BMI average (table 2). In crude analyses, only the congener 2,3,7,8-TCDD showed a strong gradient across zones (table 3). Of the other compounds, OCDD and PCB 169 levels were lower in zone B compared with the reference. PCB 126 was somewhat higher in Zone A. The values of TEQs were heavily influenced by the 2,3,7,8-TCDD levels. The high PCB 126 in zone A caused a higher level of TEQ of nPCBs.
Table 2 -.
Characteristics of subjects in the Seveso area, by zone of residence, 1993–1995
| Zone A | Zone B | Zone non-ABR (reference) | |||
|---|---|---|---|---|---|
| N (%) | p* | N (%) | p* | N (%) | |
| Men | 5 (71.4%) | 0.20 | 27 (49.1%) | 0.72 | 27 (45.8%) |
| Women | 2 (28.6%) | 28 (50.9%) | 32 (54.2%) | ||
| Age (years) mean (SD) | 55.2 (16.4) | 0.14 | 47.5 (16.8) | 0.58 | 46.1 (16.7) |
| BMI (kg/m2) mean (SD) | 28.2 (2.3) | 0.005 | 24.5 (3.9) | 0.31 | 23.9 (4.1) |
| Smoking | |||||
| Current | 4 (57.1) | 0.15 | 30 (54.5) | 0.47 | 31 (52.5) |
| Former | 3 (42.9) | 14 (25.4) | 11 (18.6) | ||
| Never smokers | 0 (0.0) | 11 (20.0) | 17 (28.8) | ||
Abbreviations: BMI, body mass index; N, number of subjects; SD, standard deviation
p-value from Mann-Whitney test, compared with the reference zone
Table 3 -.
Plasma concentrations (pg/g lipid) of polychlorinated-dibenzo-para-dioxins (PCDDs), polychlorinated dibenzofurans (PCDFs), non-ortho-polychlorinated biphenyls (PCBs), and toxic equivalencies (TEQs) in the Seveso area, by residence zone, Dec 1992 - Mar 1994
| Zone A | Zone B | Zone non-ABR (reference) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Median | p25–p75 | p* | N | Median | p25–p75 | p* | N | Median | p25–p75 | |
| PCDDs | |||||||||||
| 2,3,7,8-TCDD | 7 | 73.3 | 45.3–80.7 | <0.0001 | 51 | 12.4 | 6.3–24.6 | <0.0001 | 52 | 5.5 | 3.2–7.9 |
| 1,2,3,7,8-PeCDD | 7 | 7.6 | 7.0–9.1 | 0.62 | 51 | 6.8 | 4.9–9.9 | 0.17 | 50 | 7.8 | 5.8–10.0 |
| 1,2,3,4,7,8-HxCDD | 1 | 2.1 | 2.1–2.1 | 0.52 | 25 | 2.5 | 2.2–3.5 | 0.65 | 23 | 3.0 | 2.0–4.2 |
| 1,2,3,6,7,8-HxCDD | ** | ||||||||||
| 1,2,3,7,8,9-HxCDD | 7 | 3.9 | 2.6–8.3 | 0.83 | 52 | 4.0 | 2.8–6.2 | 0.56 | 50 | 4.1 | 2.9–6.8 |
| 1,2,3,4,6,7,8-HpCDD | 7 | 60.1 | 44.6–89.8 | 0.42 | 52 | 51.4 | 33.6–69.6 | 0.81 | 52 | 49.9 | 36.1–80.7 |
| OCDD | 7 | 389 | 228–686 | 0.96 | 50 | 268 | 215–430 | 0.01 | 52 | 412 | 276–585 |
| PCDFs | |||||||||||
| 2,3,7,8-TCDF | 7 | 1.6 | 1.4–2.2 | 0.69 | 50 | 1.6 | 1.3–2.0 | 0.57 | 52 | 1.7 | 1.4–2.0 |
| 1,2,3,7,8-PeCDF | 7 | 1.6 | 1.3–1.8 | 0.63 | 53 | 1.7 | 1.3–2.1 | 0.90 | 55 | 1.8 | 1.3–2.1 |
| 2,3,4,7,8-PeCDF | 7 | 25.5 | 18.5–31.4 | 0.98 | 52 | 21.5 | 15.5–29.4 | 0.29 | 52 | 24.3 | 18.6–30.3 |
| 1,2,3,4,7,8-HxCDF | 7 | 7.7 | 6.7–9.3 | 0.75 | 51 | 6.9 | 5.1–10.6 | 0.70 | 51 | 7.2 | 5.4–10.4 |
| 1,2,3,6,7,8-HxCDF | 7 | 7.7 | 6.1–9.7 | 0.94 | 50 | 7.5 | 5.3–9.8 | 0.76 | 52 | 7.5 | 5.3–10.9 |
| 1,2,3,7,8,9-HxCDF | 7 | 2.4 | 1.6–3.0 | 0.60 | 52 | 2.1 | 1.7–2.8 | 0.87 | 57 | 2.4 | 1.6–2.8 |
| 2,3,4,6,7,8-HxCDF | 7 | 3.3 | 2.1–5.6 | 0.35 | 51 | 2.6 | 1.8–3.7 | 0.47 | 52 | 2.5 | 1.7–3.5 |
| 1,2,3,4,6,7,8-HpCDF | 7 | 10.3 | 6.7–15.7 | 0.94 | 50 | 9.6 | 8.3–12.4 | 0.94 | 52 | 10.2 | 7.5–13.3 |
| 1,2,3,4,7,8,9-HpCDF | 7 | 2.0 | 1.5–4.7 | 0.60 | 53 | 1.8 | 1.6–2.3 | 0.61 | 57 | 1.9 | 1.6–2.5 |
| OCDF | 5 | 2.4 | 1.9–7.8 | 0.03 | 51 | 9.3 | 4.0–12.4 | 0.78 | 52 | 9.8 | 3.0–13.0 |
| Non—ortho PCBs | |||||||||||
| 33’44’-TCB (77) | 4 | 97.6 | 69.6–317 | 0.48 | 52 | 78.1 | 63.5–94.0 | 0.38 | 53 | 82.0 | 62.2–105 |
| 344’5-TCB (81) | 6 | 8.9 | 5.9–12.4 | 0.59 | 52 | 7.5 | 5.7–9.0 | 0.54 | 53 | 7.8 | 5.7–11.7 |
| 33’44’5-PeCB (126) | 7 | 143 | 68.0–137 | 0.10 | 51 | 93.2 | 55.1–159 | 0.56 | 51 | 81.3 | 64.5–121 |
| 33’44’55’-HxCB (169) | 6 | 89.0 | 70.5–125 | 0.88 | 51 | 82.0 | 54.6–106 | 0.05 | 51 | 101.0 | 72.8–122 |
| TEQs | |||||||||||
| PCDDs | 7 | 78.0 | 53.7–90.2 | 0.0001 | 52 | 21.5 | 12.9–25.2 | 0.003 | 52 | 15.1 | 10.7–18.8 |
| PCDDs/no 2378-TCDD | 7 | 9.2 | 8.4–10.1 | 1.00 | 51 | 7.9 | 5.7–11.2 | 0.27 | 52 | 9.3 | 6.5–11.3 |
| PCDFs | 7 | 15.6 | 11.4–18.9 | 0.57 | 54 | 12.5 | 9.4–17.4 | 0.60 | 57 | 14.3 | 10.6–17.9 |
| Non-ortho PCBs | 7 | 15.0 | 6.8–20.8 | 0.08 | 54 | 9.4 | 5.7–15.8 | 0.47 | 57 | 8.9 | 6.5–12.4 |
| Total | 7 | 94.0 | 84.3–118 | <0.0001 | 52 | 43.7 | 32.5–67.4 | 0.11 | 52 | 38.8 | 30.7–50.8 |
| Total/no 2378-TCDD | 7 | 39.0 | 26.7–50.8 | 0.47 | 51 | 31.8 | 20.6–43.7 | 0.55 | 52 | 32.3 | 25.9–44.0 |
Abbreviations: p25-p75: 25th and 75th percentiles.
p-value from Mann-Whitney test, compared with the reference zone.
Undetectable in all plasma samples.
Multiple regression analyses (table 4) confirmed the strong gradient across zones for 2,3,7,8-TCDD (1068% and 130% increase in zones A and B compared with the reference, respectively) and for TEQs. Conversely, the suggestively elevated levels of PCB 126 and nPCBs in Zone A were no longer apparent after adjustment for gender and age. Only 1,2,3,4,7,8,9,-HpCDF was moderately higher (35%) in zone A, while Zone B showed slightly lower levels of OCDD and PCB 169. For some congeners women showed higher levels than men, with a 17% increased TEQ for congeners other than 2,3,7,8-TCDD. For several compounds (five PCDDs, three PCDFs, and two nPCBs) a positive association was found with age; as a result, the TEQs for PCDDs, PCDFs, and nPCBs showed respectively 12%, 24%, and 41% increases for every 10 years of age. Smoking had a negative effect on several congeners. TEQs in former smokers were similar to those in subjects who had never smoked, while current smokers had lower (from −37% to −67%) TEQ levels. BMI was negatively associated with some furans and PCB 169, and positively associated with 1,2,3,4,6,7,8-HpCDD and PCB 126, but no impact on TEQ values was found.
Table 4 -.
Results of multiple regression models* on plasma concentrations (pg/g lipid) of polychlorinated-dibenzo-para-dioxins (PCDDs), polychlorinated dibenzofurans (PCDFs), non-ortho-polychlorinated biphenyls (PCBs), and toxic equivalencies (TEQs) in the Seveso area, by residence zone, Dec 1992 - Mar 1994. Indicated are the relative changes (%) and their 95% confidence intervals (95% CIs)
| Congener | N | Zone A | Zone B | Women | Age (10 yrs) | Former smokers | Current smokers | BMI (kg/m3) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | ||
| PCDDs | |||||||||||||||
| 2,3,7,8-TCDD | 110 | 1068 | 524, 2087 | 130 | 71, 210 | 113 | 52, 198 | 13 | 3, 24 | 11 | −24, 63 | 6 | −28, 58 | 46 | −49, 316 |
| 1,2,3,7,8-PeCDD | 108 | −14 | −43, 28 | −8 | −24, 11 | 24 | 0, 54 | 16 | 10, 23 | −18 | −36, 5 | −5 | −27, 3 | −1 | −3, 2 |
| 1,2,3,4,7,8-HxCDD | 49 | −26 | −79, 159 | −15 | −39, 20 | 6 | −28, 55 | 14 | 0, 30 | 11 | −27, 70 | 23 | −20, 89 | −3 | −8, 2 |
| 1,2,3,6,7,8-HxCDD | ** | ||||||||||||||
| 1,2,3,7,8,9-HxCDD | 109 | −2 | −39, 57 | −1 | −21, 23 | 16 | −10, 50 | 8 | 0, 15 | −32 | −49, −9 | −8 | −32, 24 | 1 | −2, 4 |
| 1,2,3,4,6,7,8-HpCDD | 111 | −10 | −38, 33 | −9 | −24, 9 | 20 | −2, 47 | 3 | −3, 9 | −28 | −43, −9 | −56 | −65, −44 | 3 | 0, 5 |
| OCDD | 109 | −9 | −42, 42 | −26 | −40, −8 | 22 | −4, 56 | 7 | 0, 15 | −27 | −45, −4 | −41 | −56, −22 | 0 | −2, 3 |
| PCDFs | |||||||||||||||
| 2,3,7,8-TCDF | 109 | 23 | −13, 76 | 1 | −15, 19 | −7 | −23, 13 | −4 | −9, 1 | 2 | −18, 27 | 6 | −15, 31 | −2 | −4, 1 |
| 1,2,3,7,8-PeCDF | 115 | 8 | −25, 55 | 5 | −11, 25 | −14 | −29, 4 | −4 | −9, 1 | −6 | −25, 17 | 7 | −13, 33 | −1 | −3, 2 |
| 2,3,4,7,8-PeCDF | 111 | −6 | −28, 22 | −8 | −19, 4 | 12 | −3, 29 | 16 | 12, 21 | −10 | −24, 5 | −23 | −34, −9 | −1 | −3, 0 |
| 1,2,3,4,7,8-HxCDF | 109 | −6 | −31, 29 | −5 | −18, 10 | 14 | −4, 35 | 13 | 8, 18 | −12 | −27, 7 | −25 | −38, −8 | 0 | −2, 2 |
| 1,2,3,6,7,8-HxCDF | 109 | −2 | −30, 37 | −2 | −16, 14 | 20 | 0, 43 | 11 | 6, 17 | −5 | −23, 16 | −29 | −42, −12 | −1 | −3, 1 |
| 1,2,3,7,8,9-HxCDF | 116 | 26 | −8, 73 | 4 | −10, 21 | −2 | −17, 15 | 0 | −5, 4 | 2 | −16, 24 | 4 | −14, 25 | −2 | −4, 0 |
| 2,3,4,6,7,8-HxCDF | 110 | 35 | −13, 110 | 10 | −11, 36 | 19 | −7, 51 | 1 | −5, 8 | −21 | −40, 4 | −25 | −43, −2 | 0 | −3, 2 |
| 1,2,3,4,6,7,8-HpCDF | 109 | −7 | −38, 40 | −8 | −24, 12 | −12 | −29, 10 | −4 | −10, 1 | 5 | −19, 35 | −17 | −36, 8 | −1 | −3, 2 |
| 1,2,3,4,7,8,9-HpCDF | 117 | 35 | 0, 82 | 0 | −12, 15 | −4 | −18, 12 | −2 | −6, 2 | −3 | −19, 16 | 9 | −9, 29 | −2 | −4, 0 |
| OCDF | 108 | −43 | −72, 18 | −4 | −28, 29 | −10 | −36, 25 | −12 | −20, −4 | 9 | −25, 61 | −3 | −34, 42 | 0 | −4, 4 |
| Non-ortho PCBs | |||||||||||||||
| 33’44’-TCB (77) | 109 | 63 | −6, 183 | −15 | −30, 4 | 0 | −20, 26 | −5 | −11, 1 | 5 | −19, 38 | 7 | −18, 39 | −1 | −4, 2 |
| 344’5-TCB (81) | 111 | 25 | −27, 115 | −11 | −30, 13 | 0 | −24, 31 | −7 | −14, 0 | −2 | −28, 35 | 28 | −6, 74 | −2 | −5, 1 |
| 33’44’5-PeCB (126) | 109 | 10 | −23, 58 | 4 | −12, 23 | 18 | −2, 43 | 17 | 11, 23 | −20 | −36, −1 | −41 | −53, −26 | 2 | 0, 4 |
| 33’44’55’-HxCB (169) | 108 | −11 | −33, 19 | −15 | −25, −4 | −15 | −26, −1 | 21 | 16, 26 | −12 | −26, 3 | −13 | −26, 3 | −4 | −5, −2 |
| TEQs | |||||||||||||||
| PCDDs | 111 | 398 | 214, 687 | 54 | 24, 91 | 59 | 24, 103 | 12 | 5, 20 | −1 | −25, 31 | −13 | −35, 16 | 0 | −3, 3 |
| PCDDs/no 2378-TCDD | 110 | −7 | −44, 52 | −4 | −24, 21 | 21 | −7, 58 | 12 | 4, 20 | −18 | −39, 11 | −37 | −54, −14 | −2 | −5, 1 |
| PCDFs | 118 | 6 | −46, 109 | 8 | −20, 48 | 13 | −20, 61 | 24 | 13, 37 | 2 | −32, 53 | −36 | −57, −6 | 0 | −5, 4 |
| Non-ortho PCBs | 118 | 66 | −62, 622 | 30 | −34, 154 | 22 | −43, 163 | 41 | 14, 73 | −11 | −63, 115 | −67 | −86, −21 | −1 | −10, 8 |
| Total | 111 | 149 | 84, 237 | 20 | 4, 38 | 34 | 14, 58 | 14 | 9, 19 | −7 | −22, 12 | −20 | −34, −4 | −1 | −3, 1 |
| Total/no 2378-TCDD | 110 | 0 | −25, 33 | −4 | −16, 10 | 17 | 0, 36 | 15 | 10, 20 | −16 | −29, 1 | −31 | −42, −17 | −1 | −3, 1 |
Abbreviations: BMI, body mass index; 95% CI, 95% confidence interval.
Dependent variables were loge-transformed. Relative change (%) was calculated with the formula: [exp(coefficient)−1]×100. Models contained the following covariates: zone of residence (reference: non-ABR); gender (reference: male); age (years/10); smoking (reference: never smokers); BMI (kg/m2). Each variable is adjusted for the others.
Undetectable in all plasma samples.
DISCUSSION
We confirmed that the population living in the area of the 1976 Seveso accident was heavily exposed to 2,3,7,8-TCDD only. None of the other congeners showed important variation across zones. In interpreting 2,3,7,8-TCDD levels, we note that blood samples were obtained 15–16 years after the accident (corresponding to about two half-lives of the compound). The concentrations extrapolated back to the time of the accident were about four times higher (14).These results are in agreement with findings from other surveys which documented heavy soil contamination (27) and population exposure (18).
One of the most important recognized determinants of congener and TEQ levels is age, reflecting continuous accumulation in the body not balanced by a corresponding elimination (7, 16, 19, 21, 22, 28). In our study age was strongly related with TEQs for all classes of compounds (PCDDs, PCDFs, and nPCBs). It was also positively associated with several single compounds, including those (2,3,7,8-TCDD, 2,3,4,7,8-PeCDF, and PCB 126) classified as human carcinogens by IARC.
Except for 2,3,7,8-TCDD (a 113% increase), women in our study showed a modest increase (17%) of TEQ for congeners other than 2,3,7,8-TCDD. The effect of gender is usually considered to be low. In particular, using the 1998 or 2005 TEFs, males and females in a survey of a large sample of the USA population (2001–2002) had nearly the same distribution of TEQ sub-fractions (21). Coherently, the main TEQ reference ranges for the USA population in the following survey (2003–2004) were presented for both genders combined (22).
Among current smokers we found lower levels of several congeners excluding 2,3,7,8-TCDD and of TEQs which did not include 2,3,7,8-TCDD contribution. Tobacco smoke may be a source of PCDDs (17). On the other hand, it has been shown that smoking increases PCDD/PCDF elimination (10). Our results are in agreement with other studies showing somewhat lower PCDD and/or PCDF blood levels in smokers (4, 8). A similar association has been suggested in a large representative sample of the US population (2001–2002), but only limited to certain age classes (9).
In our study BMI had little or no impact on congeners and was not associated with TEQ values. Percent body fat has been found to be associated with increasing half-life for most of the PCDD/PCDF congeners (10). The role of BMI has been investigated in several studies, but with controversial results (5, 12, 13, 25, 29, 32).
In conclusion, the population living in the Seveso area was highly exposed to 2,3,7,8-TCDD only. This exposure pattern might be relevant to the interpretation of at least some of the discrepancies noted between this population, which exhibited an increased incidence of and mortality from cancers of the lymphatic and haematopoietic tissues (6, 24), and other, mainly occupational, cohorts exposed to a variety of dioxins and dioxin-like compounds, in which the main finding was a moderate increase in all-cancer mortality (11, 27). Age showed a strong positive association with all the classes of compounds (PCDDs, PCDFs, and nPCBs).
ACKNOWLEDGMENTS:
This work was partially funded by the Lombardy Region (Environmental Epidemiology Program).
Footnotes
NO POTENTIAL CONFLICT OF INTEREST RELEVANT TO THIS ARTICLE WAS REPORTED
REFERENCES
- 1.Ahlborg UG, Becking GC, Birnbaum LS, et al. : Toxic Equivalency Factors for dioxin-like PCBs. Report on a WHO-ECEH and IPCS consultation, December 1993. Chemosphere 1994; 28: 1049–1067 [Google Scholar]
- 2.Armitage P, Berry G, Matthews JNS: Statistical methods in medical research. Malden (MA): Blackwell Science, 2001 [Google Scholar]
- 3.Baccarelli A, Pfeiffer R, Consonni D, et al. : Handling of dioxin measurement data in the presence of non-detectable values: overview of available methods and their application in the Seveso chloracne study. Chemosphere 2005; 60: 898–906 [DOI] [PubMed] [Google Scholar]
- 4.Chen HL, Liao PC, Su HJ, et al. : Profile of PCDD/F levels in serum of general Taiwanese between different gender, age and smoking status. Sci Total Environ 2005; 337: 31–43 [DOI] [PubMed] [Google Scholar]
- 5.Collins JJ, Bodner K, Burns CJ, et al. : Body mass index and serum chlorinated dibenzo-p-dioxin and dibenzofuran levels. Chemosphere 2007; 66: 1079–1085 [DOI] [PubMed] [Google Scholar]
- 6.Consonni D, Pesatori AC, Zocchetti C, et al. : Mortality in a population exposed to dioxin after the Seveso, Italy, accident in 1976: 25 years of follow-up. Am J Epidemiol 2008; 167: 847–858 [DOI] [PubMed] [Google Scholar]
- 7.Consonni D, Sindaco R, Bertazzi PA: Blood levels of dioxins, furans, dioxin-like PCBs, and TEQs in general populations: A review, 1989–2010. Environ Int 2012; 44: 151–162 [DOI] [PubMed] [Google Scholar]
- 8.Deml E, Mangelsdorf I, Greim H: Chlorinated dibenzodioxins and dibenzofurans (PCDD/F) in blood and human milk of non-occupationally exposed persons living in the vicinity of a municipal waste incinerator. Chemosphere 1996; 33: 1941–1950 [DOI] [PubMed] [Google Scholar]
- 9.Ferriby LL, Knutsen JS, Harris M, et al. : Evaluation of PCDD/F and dioxin-like PCB serum concentration data from the 2001–2002 National Health and Nutrition Examination Survey of the United States population. J Expo Sci Environ Epidemiol 2007; 17: 358–371 [DOI] [PubMed] [Google Scholar]
- 10.Flesch-Janys D, Becher H, Gurn P, et al. : Elimination of polychlorinated dibenzo-p-dioxins and dibenzofurans in occupationally exposed persons. Journal of toxicology and environmental health 1996; 47: 363–378 [DOI] [PubMed] [Google Scholar]
- 11.International Agency for Research on Cancer: Polychlorinated dibenzo-para-dioxins and polychlorinated dibenzofurans. Lyon: IARC, 1997. (IARC Monographs on the Evaluation of the carcinogenic risk of chemicals to humans No. 69) [Google Scholar]
- 12.Kumagai S, Koda S, Miyakita T, Ueno M: Polychlorinated dibenzo-p-dioxin and dibenzofuran concentrations in serum samples of workers at intermittently burning municipal waste incinerators in Japan. Occup Environ Med 2002; 59: 362–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumagai S, Koda S, Miyakita T, et al. : Polychlorinated dibenzo-p-dioxin and dibenzofuran concentrations in the serum samples of workers at continuously burning municipal waste incinerators in Japan. Occup Environ Med 2000; 57: 204–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Landi MT, Consonni D, Patterson DG Jr, et al. : 2,3,7,8-Tetrachlorodibenzo-p-dioxin plasma levels in Seveso 20 years after the accident. Environ Health Perspect 1998; 106: 273–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Landi MT, Needham LL, Lucier G, et al. : Concentrations of dioxin 20 years after Seveso. Lancet 1997; 349: 1811. [DOI] [PubMed] [Google Scholar]
- 16.Lorber M: A pharmacokinetic model for estimating exposure of Americans to dioxin-like compounds in the past, present, and future. Sci Total Environ 2002; 288: 81–95 [DOI] [PubMed] [Google Scholar]
- 17.Muto H, Takizawa Y: Dioxins in cigarette smoke. Arch Environ Health 1989; 44: 171–174 [DOI] [PubMed] [Google Scholar]
- 18.Needham LL, Gerthoux PM, Patterson DG Jr., et al. : Serum dioxin levels in Seveso, Italy, population in 1976. Teratogenesis, carcinogenesis, and mutagenesis 1997; 17: 225–240 [PubMed] [Google Scholar]
- 19.Patterson DG Jr, Patterson D, Canady R, et al. : Age specific dioxin TEQ reference range. Organohalogen Compounds 2004; 66: 2878–2883 [Google Scholar]
- 20.Patterson DG Jr, Hampton L, Lapeza CR Jr, et al. : High-resolution gas chromatographic/high-resolution mass spectrometric analysis of human serum on a whole-weight and lipid basis for 2,3,7,8-tetrachlorodibenzo-p-dioxin. Analytical chemistry 1987; 59: 2000–2005 [DOI] [PubMed] [Google Scholar]
- 21.Patterson DG Jr., Turner WE, Caudill SP, Needham LL: Total TEQ reference range (PCDDs, PCDFs, cPCBs, mono-PCBs) for the US population 2001–2002. Chemosphere 2008; 73: S261–277 [DOI] [PubMed] [Google Scholar]
- 22.Patterson DG, Wong LY, Turner WE, et al. : Levels in the U.S. population of those persistent organic pollutants (2003–2004) included in the Stockholm Convention or in other long range transboundary air pollution agreements. Environ Sci Technol 2009; 43: 1211–1218 [DOI] [PubMed] [Google Scholar]
- 23.Pesatori AC: Dioxin contamination in Seveso: the social tragedy and the scientific challenge. Med Lav 1995; 86: 111–124 [PubMed] [Google Scholar]
- 24.Pesatori AC, Consonni D, Rubagotti M, et al. : Cancer incidence in the population exposed to dioxin after the “Seveso accident”: twenty years of follow-up. Environ Health 2009; 8: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rylander L, Hagmar L, Wallin E, et al. : Intra-individual variations and temporal trends in dioxin levels in human blood 1987–2002. Chemosphere 2009; 76: 1557–1562 [DOI] [PubMed] [Google Scholar]
- 26.Schecter A, Birnbaum L, Ryan JJ, Constable JD: Dioxins: An overview. Environ Res 2006; 101: 419–428 [DOI] [PubMed] [Google Scholar]
- 27.Schecter A, Gasiewicz TA: Dioxins and Health. New York (NY): Wiley-Interscience, 2003 [Google Scholar]
- 28.Schrey P, Wittsiepe J, Ewers U, et al. : Age-related increase of PCDD/F-levels in human blood - a study with 95 unexposed persons from Germany. Organohalogen Compounds 1992; 9: 261–267 [Google Scholar]
- 29.Tsukino H, Hanaoka T, Sasaki H, et al. : Fish intake and serum levels of organochlorines among Japanese women. Sci Total Environ 2006; 359: 90–100 [DOI] [PubMed] [Google Scholar]
- 30.Van Den Berg M, Birnbaum L, Bosveld ATC, et al. : Toxic equivalency factors (TEFs) for PCBs, PCDDs, PCDFs for humans and wildlife. Environ Health Perspect 1998; 106: 775–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Den Berg M, Birnbaum LS, Denison M, et al. : The 2005 World Health Organization reevaluation of human and mammalian toxic equivalency factors for dioxins and dioxin-like compounds. Toxicol Sci 2006; 93: 223–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wittsiepe J, Schrey P, Lemm F, et al. : Polychlorinated dibenzo-p-dioxins/polychlorinated dibenzofurans (PCDD/Fs), polychlorinated biphenyls (PCBs), and organochlorine pesticides in human blood of pregnant women from Germany. J Toxicol Environ Health A 2008; 71: 703–709 [DOI] [PubMed] [Google Scholar]


