Abstract
Background
Gaps exist in our understanding of the clinical course of pouch-related disorders.
Methods
We evaluated baseline disease activity and longitudinal treatment patterns among patients with inflammatory conditions of the pouch.
Results
Among 468 patients with an ileal pouch-anal anastomosis (IPAA), 94 (20%) had acute pouchitis, 96 (21%) had chronic pouchitis, and 192 (41%) had Crohn disease of the pouch. Following an IPAA, 38% of patients were treated with a biologic and 11% underwent inflammatory bowel disease- or bowel-related surgery.
Conclusions
Treatment patterns after IPAA indicate that pouch-related disorders have a significant impact on individual patients and the healthcare system.
Keywords: pouchitis, ileal pouch-anal anastomosis, biologic therapy
INTRODUCTION
Although restorative proctocolectomy with ileal pouch-anal anastomosis (IPAA) has become the surgical therapy of choice among patients requiring surgery for medically refractory ulcerative colitis (UC) or UC-related dysplasia, multiple complications can occur after IPAA. Pouchitis is the most common complication after IPAA, affecting 40% of patients in the first year after IPAA1 and up to 80% of patients overall.2 Despite the significant burden associated with pouchitis, gaps exist in our understanding of the natural course after IPAA, particularly in our understanding of risk factors for the development of chronic pouchitis and other pouch-related disorders. Additionally, our understanding of patterns of therapy utilization, including the need to reintroduce immunosuppressive and/or biologic therapies has been limited to select populations.
Despite being presented as a curative surgery for UC in many scenarios,3 the burden of acute pouchitis, chronic pouchitis, and Crohn disease (CD) of the pouch can be significant. Patients with pouchitis and pouch-related symptoms have demonstrated significant differences in several domains of health-related quality of life.2 While patients with acute pouchitis respond to short courses of common antibiotics, patients with chronic antibiotic refractory pouchitis and CD of the pouch in particular often require the reinitiation of biologic therapy for the treatment of chronic inflammation and pouch-related symptoms.4–7 Additionally, up to 15% of patients ultimately require excision of the pouch due to pouch failure.8–10 The burden of chronic inflammatory conditions of the pouch has been demonstrated in recent evaluations of tertiary care referral centers, where up to 30% of patients have developed chronic pouchitis11 prompting the consideration of these inflammatory conditions as a spectrum that may require more aggressive early biologic therapy after IPAA.
Given the significant impact of pouchitis and other inflammatory conditions after IPAA, we sought to better characterize the disease course and treatment patterns after IPAA among patients treated for pouch-related conditions in the Sinai-Helmsley Alliance for Research Excellence (SHARE) cohort. The SHARE cohort is a national collaboration of 7 academic medical centers that collected longitudinal clinical and phenotypic data on patients with inflammatory bowel disease (IBD). This prospective study design allows for both the evaluation of any clinical risk factors for the development of pouch-related conditions and the opportunity to evaluate clinical outcomes, therapy, and the need for further surgical treatment over time.
METHODS
Data Source
As described previously, SHARE is a multicenter prospective cohort study.12–14 Patients were recruited from 7 academic centers in the United States (US): Cedars-Sinai Medical Center, Massachusetts General Hospital, the Mayo Clinic, Mount Sinai Medical Center, University of Chicago, University of North Carolina at Chapel Hill, and Washington University, Saint Louis. Data for this study were collected between January 1, 2012 and October 1, 2018. At the time of enrollment, patients provided demographic and clinical information, including past medical, surgical, and family histories, medication use, and the presence of extraintestinal manifestations. Any patient who was <18 years of age, who did not provide informed consent, or who did not have a confirmed diagnosis of IBD was excluded from the study. Patients were prospectively followed after enrollment and follow-up questionnaires were completed every 12 months via telephone, internet, or during a subsequent clinic visit. At the time of follow-up, patients completed information regarding updated medication use and patient reported outcome assessments, as well as healthcare utilization information such as IBD-related surgeries on their bowel or hospitalizations (for any reason) in the interim since the last follow-up assessment.
Outcomes of Interest
The objectives of this study were to evaluate treatment patterns among patients after an IPAA for UC, and to identify factors associated with the development of inflammatory conditions of the pouch, including acute or chronic pouchitis or CD of the pouch. Diagnoses of acute pouchitis, chronic pouchitis, and CD of the pouch were obtained by patient interview and confirmation on review of the medical record. Acute pouchitis was defined by the response to an initial course of antibiotics for an isolated episode of pouchitis while chronic pouchitis was defined by the need for recurrent antibiotics to control symptoms. A diagnosis of CD of the pouch was per the discretion of the treating physician. We evaluated measures of healthcare utilization at baseline and in longitudinal analyses, including need for further IBD- or bowel-related surgery, and medication utilization patterns among patients after IPAA, with a particular focus on those patients who developed chronic inflammatory conditions of the pouch (chronic pouchitis and CD of the pouch). In all therapy analyses, agents were analyzed by individual medications and by therapy groups [antibiotics, immunomodulators, and therapy with biologics (anti-tumor necrosis factor alpha (anti-TNF), anti-interleukin 12/23, and anti-adhesion molecules)]. Disease activity was assessed using the modified Pouchitis Disease Activity Index,15 which was collected at the time of the enrollment visit.
Covariates
Multiple clinical characteristics were analyzed given their potential influence on the development of pouchitis and other inflammatory conditions after IPAA including the age at colectomy, indication for colectomy, type of anastomosis, smoking history, and presence of extraintestinal manifestations. Additional demographic characteristics were analyzed including sex and race.
Statistical Analysis
Continuous variables were summarized using means and standard deviations and compared using Student t tests. Categorical variables were expressed as proportions and compared using Fisher exact and χ 2 testing as appropriate. Multivariable logistic regression models were also used to evaluate the relationship between demographic and clinical variables and the development of inflammatory conditions after IPAA. All covariates included in the multivariable analyses were included based on a prior or suspected association with the development of pouchitis or CD of the pouch, including age, sex, race, and history of primary sclerosing cholangitis, extraintestinal manifestations of IBD, or colectomy for dysplasia. All statistical analyses were performed using SAS (version 9.4) statistical software (SAS Institute, Cary, NC, USA). The study protocol was approved by the Institutional Review Boards of all participating institutions.
RESULTS
Demographics and Clinical Factors
Among 5,906 total patients with IBD, 468 (7.9%) reported a history of a colectomy with an IPAA as a treatment for UC. At the time of enrollment, 86 (18%) patients had no history of pouchitis, while 94 (20%) had experienced acute pouchitis and 98 (21%) had a diagnosis of chronic pouchitis (Table 1). Despite a preoperative diagnosis of UC, 192 patients (41%) had a diagnosis of CD of the pouch at the time of enrollment. Patients were followed for a median of 796 days (interquartile range 567–1043 days).
Table 1.
Comparison of Clinical and Demographic Characteristics of 468 Patients With an IPAA in a Multicenter Cohort, Evaluated by a History of Pouch-Related Disorder
| No Pouchitis (n = 86) | Acute Pouchitis (n = 94) | Chronic Pouchitis (n = 96) | CD of the Pouch (n = 192) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Median | IQR | Median | IQR | P vs No Pouchitis | Median | IQR | P vs No Pouchitis | Median | IQR | P vs No Pouchitis | |
| Age at baseline visit | 36 | 27–52 | 41 | 32–50 | 0.141 | 46 | 33–57 | 0.024 | 41 | 32–51 | 0.083 |
| Age at colectomy | 31 | 24–44 | 32 | 21–43 | 0.377 | 34 | 26–48 | 0.260 | 28 | 22–41 | 0.024 |
| Duration between colectomy and baseline visit | 2 | 0.4–5 | 7 | 3–12 | <0.001 | 7 | 3–14 | <0.001 | 10 | 4–17 | <0.001 |
| Modified Pouchitis Disease Activity Index at enrollmenta | 1 | 0–2 | 3 | 2–4 | <0.001 | 3 | 1–4 | <0.001 | 3 | 1–5 | <0.001 |
| n | % | n | % | n | % | n | % | ||||
| Female sex | 38 | 45 | 42 | 45 | 0.997 | 45 | 47 | 0.770 | 108 | 58 | 0.046 |
| Race | 0.015 | 0.067 | 0.589 | ||||||||
| White | 74 | 87 | 91 | 97 | 91 | 95 | 167 | 89 | |||
| Non-white | 11 | 13 | 3 | 3 | 5 | 5 | 20 | 11 | |||
| Colonic dysplasia at the time of colectomy | 15 | 19 | 8 | 9 | 0.070 | 10 | 11 | 0.171 | 7 | 4 | <0.001 |
| Technique used in IPAAb | 0.584 | 0.630 | 0.531 | ||||||||
| Hand-sewn | 9 | 19 | 7 | 15 | 12 | 29 | 19 | 23 | |||
| Stapled | 39 | 81 | 41 | 85 | 41 | 77 | 62 | 77 | |||
| Any history of smoking cigarettes | 17 | 21 | 32 | 31 | 1.000 | 31 | 33 | 0.597 | 65 | 35 | 0.459 |
| History of extraintestinal manifestationc | 16 | 19 | 23 | 25 | 0.340 | 28 | 29 | 0.097 | 74 | 39 | 0.001 |
| History of primary sclerosing cholangitis | 8 | 10 | 3 | 3 | 0.076 | 12 | 13 | 0.512 | 7 | 4 | 0.054 |
IQR, interquartile range.
aThree hundred two patients had available data for all components of the modified pouchitis disease activity index at the enrollment visit.
bData regarding type of anastomosis available for 236 patients.
cExtraintestinal manifestation defined as ankylosing spondylitis, arthritis (small or large joints), episcleritis/scleritis, erythema nodosum, oral ulcers, pyoderma gangrenosum, sacroiliitis, or uveitis/iritis.
Patients with CD of the pouch were significantly more likely to demonstrate extraintestinal manifestations than patients with no history of pouchitis (39% vs 19%, P = 0.001, Table 1). There was no difference in extraintestinal manifestations when comparing patients with acute or chronic pouchitis to those with no history of pouchitis. Significantly fewer patients with CD of the pouch had colonic dysplasia at the time of colectomy when compared to patients with no history of pouchitis (4% vs 19%, P < 0.001). When compared to patients with no history of pouchitis, patients with a history of acute pouchitis were more likely to be of white race (97% vs 87%, P = 0.015) and demonstrated a longer duration between colectomy and baseline visit (7 vs 2 years, P < 0.001).
In a multivariable analysis adjusting for age, sex, race, and history of primary sclerosing cholangitis or colonic dysplasia, patients with CD of the pouch were significantly more likely to have extraintestinal manifestations when compared to patients with no history of pouchitis [adjusted odds ratio (aOR) 2.76, 95% confidence interval (CI) 1.39–5.51, Table 2]. In multivariable analyses, patients with chronic pouchitis (aOR 0.26, 95% CI 0.09–0.78) and CD of the pouch (aOR 0.17, 95% CI 0.05–0.52) were less likely to undergo colectomy for colonic dysplasia when compared to patients with no history of pouchitis.
Table 2.
Multivariable Analysis, Factors Associated With Developing an Inflammatory Condition of the Pouch After IPAA for UC Among Patients in a Multicenter Cohort, Compared to No Pouchitis (Reference Group)
| Acute Pouchitis | Chronic Pouchitis | CD of the Pouch | |
|---|---|---|---|
| Odds Ratio, 95% CI | Odds Ratio, 95% CI | Odds Ratio, 95% CI | |
| Female sex | 0.78 (0.39–1.50) | 0.83 (0.42–1.64) | 1.22 (0.68–2.19) |
| Age | |||
| 18–30 | 0.49 (0.20–1.25) | 0.22 (0.09–0.57) | 0.46 (0.21–1.02) |
| 30–50 | 1.46 (0.63–3.36) | 0.58 (0.26–1.32) | 1.02 (0.48–2.17) |
| 50–70 | Reference | Reference | Reference |
| >70 | 1.04 (0.06–16.9) | 3.39 (0.49–23.3) | 0.18 (0.01–3.09) |
| Race | |||
| Non-white | 0.11 (0.02–0.55) | 0.28 (0.09–0.89) | 0.51 (0.21–1.25) |
| White | Reference | Reference | Reference |
| Colonic dysplasia at the time of colectomy | 0.42 (0.15–1.18) | 0.26 (0.09–0.78) | 0.17 (0.05–0.52) |
| History of extraintestinal manifestations | 1.53 (0.69–3.43) | 1.43 (0.65–3.17) | 2.76 (1.39–5.51) |
| History of primary sclerosing cholangitis | 2.86 (0.64–12.8) | 0.40 (0.12–1.29) | 1.41 (0.41–4.82) |
All variables included in multivariable analysis are depicted above.
Therapy Use Patterns
At the time of enrollment, a substantial number of patients with chronic pouchitis and CD of the pouch were utilizing medical therapy for their respective inflammatory conditions of the pouch (Table 3). Patients with chronic pouchitis reported use of multiple antibiotics including ciprofloxacin (38%) and metronidazole (23%). Additionally, 11% of patients with chronic pouchitis reported use of anti-TNF therapy at the time of enrollment. Among patients with CD of the pouch, 50% and 3% were being treated with an anti-TNF therapy and vedolizumab, respectively. Biologic use in patients with chronic pouchitis and CD of the pouch did not differ significantly between study sites (Supplementary Table 1). During the follow-up period, an additional 11% of patients with chronic pouchitis and 17% of patients with CD of the pouch started a new anti-TNF therapy. Patients with CD of the pouch also initiated novel biologic therapies, including ustekinumab (7%) and vedolizumab (9%). Including the follow-up period, 179 patients (38%) reported ever use of a biologic after undergoing IPAA. Among patients with chronic inflammatory conditions of the pouch, 25% of patients with chronic pouchitis and 70% of patients with CD of the pouch were treated with a biologic therapy at some point after IPAA.
Table 3.
Therapy Utilization Patterns at Baseline and in Follow-up Among Patients With Chronic Pouchitis and CD of the Pouch in a Multicenter Cohort
| Therapy Use at Enrollment, Chronic Pouchitis (n = 96) | Therapy Use at Enrollment, CD of the Pouch (n = 192) | New Therapy Initiated in Follow-up, Chronic Pouchitis (n = 96) | New Therapy Initiated in Follow-up, CD of the Pouch (n = 192) | |||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |
| Antibioticsa | ||||||||
| Ciprofloxacin | 36 | 38 | 44 | 23 | 7 | 7 | 11 | 6 |
| Metronidazole | 22 | 23 | 18 | 9 | 4 | 4 | 7 | 4 |
| Other antibiotic | 18 | 19 | 15 | 8 | 9 | 9 | 14 | 7 |
| Probiotics | 21 | 22 | 29 | 15 | 6 | 6 | 9 | 5 |
| Methotrexate | 6 | 6 | 24 | 12 | 2 | 2 | 7 | 4 |
| Thiopurine | 5 | 5 | 47 | 25 | 2 | 2 | 14 | 7 |
| Anti-TNF | 11 | 11 | 97 | 51 | 11 | 11 | 32 | 17 |
| Ustekinumab | 0 | 0 | 1 | 1 | 0 | 0 | 14 | 7 |
| Vedolizumab | 0 | 0 | 5 | 3 | 3 | 3 | 18 | 9 |
aAmong patients taking antibiotics, 40 patients were taking a combination of at least 2 antibiotics at enrollment.
Need for Subsequent Surgery
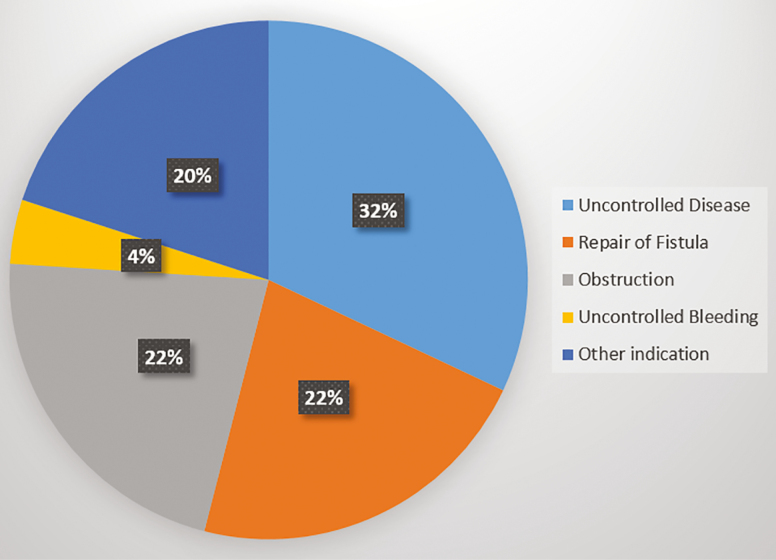
Among all 468 patients with an IPAA in the SHARE population, 54 (11%) underwent an IBD- or bowel-related surgery during the follow-up period, including 6 patients who had confirmed excision of the pouch. Patients with CD of the pouch were significantly more likely than patients with chronic pouchitis to undergo surgery during the follow-up period (21% vs 11%, P = 0.049). Among the 54 patients undergoing surgery, 17 (32%) reported surgery for disease that was uncontrolled, 12 (22%) for repair of a fistula, 12 (22%) for correction of an obstruction, 2 (4%) for uncontrolled bleeding, and 11 (20%) for another indication (Fig. 1). In an evaluation of complications during the follow-up period, 14 of 192 (8%) patients with CD of the pouch developed a new fistula and 6 (3%) patients developed a new abscess. No patients with chronic pouchitis developed either of these complications.
Figure 1.
Indication for IBD-related surgery after IPAA.
DISCUSSION
In a multicenter cohort from 7 academic medical centers, we demonstrated that patients with pouchitis and other inflammatory conditions of the pouch have a high burden of disease. In particular, biologics were utilized in 38% of all patients after an IPAA, and in 70% of patients with CD of the pouch. Coupling the costs of these therapies with the high rate of further surgery noted among patients with chronic pouchitis and CD of the pouch, the burden associated with chronic pouch-related conditions is significant. While a referral bias may exist to some degree given the treating centers, these findings are particularly worrisome when considering all included patients underwent colectomy for a preoperative diagnosis of UC, where colectomy is often viewed as a curative surgery.
In this study we attempted to provide a multicenter view of the burden of pouch-related outcomes, in an effort to better understand the impact of postoperative inflamm atory complications in this population. Patients for the SHARE cohort were largely recruited from clinic visits, where patients with an IPAA would have been more likely to have clinical symptoms, thus the prevalence of disease in our population is reflective of that of the patient population treated in a tertiary referral clinic setting. However, when assessed along with prior reports from IBD Partners2 and long-term cohort studies16,17 it becomes more evident that inflammatory complications after IPAA create a significant burden for patients after colectomy.
Despite the impact of pouchitis and other inflammatory conditions of the pouch demonstrated in the SHARE cohort, there were a limited number of predictors identified in our analyses. Patients with acute pouchitis, chronic pouchitis, and CD of the pouch were more likely to demonstrate a longer duration between colectomy and baseline assessment, a finding that has also been demonstrated in prior cohort studies.18,19 While similar to prior studies, this is unfortunately not an actionable factor after IPAA. In bivariate analysis, patients with CD of the pouch were more likely to demonstrate extraintestinal manifestations when compared to patients without a history of pouchitis. A recent meta-analysis indicated that the presence of extraintestinal manifestations was associated with a significant increase in risk of developing both acute and overall pouchitis,20 however our study demonstrated an increased association with extraintestinal manifestations in the CD of the pouch group alone. Patients with no pouchitis were significantly more likely to demonstrate dysplasia at the time of colectomy when compared to patients with CD of the pouch and chronic pouchitis. These findings may indicate that patients who developed chronic inflammatory conditions of the pouch had increased disease severity at the time of colectomy or a more refractory preoperative disease course leading to colectomy. Similar findings have previously been reported in a single-center study from a tertiary care IBD referral center, leading to a suggestion that surgery may not halt an underlying inflammatory process or phenotype.21 Our ability to further analyze severity of UC in the preoperative period was limited, however.
We demonstrated no significant difference in outcomes among patients with a history of primary sclerosing cholangitis, a predictive factor for pouchitis previously identified in multiple cohort studies.22,23 This may have been due to a relatively small sample size in our analyses. Given the lack of response to normal antibiotic regimens, and a distinct clinical phenotype, some authors have recommended viewing primary sclerosing cholangitis-associated pouchitis as a distinct clinical entity.24 We were unable to assess for multiple serologic or stool biomarkers which have been identified as potential predictors of pouchitis and other pouch-related complications,25 including perinuclear anti-neutrophil cytoplasmic antibodies,26,27 fecal lactoferrin,28 or fecal calprotectin.29 The heterogeneity present in both preoperative variables and postoperative outcomes may require more advanced strategies to better identify those patients at the greatest risk for inflammatory complications after IPAA.
Over 50% of patients with a chronic pouch-related condition (chronic pouchitis or CD of the pouch) in this cohort required treatment with a biologic therapy. While recent retrospective studies have demonstrated the potential utility of ustekinumab5 and vedolizumab4 in treating chronic antibiotic refractory pouchitis and CD of the pouch, the prospects regarding the long-term efficacy of biologic therapies in this population is still relatively concerning. A systematic review and meta-analysis demonstrated a long-term efficacy of only 37% for anti-TNF therapy in the treatment of chronic pouchitis6 and in a more recent evaluation by Kayal et al, biologic use was associated with endoscopic remission in only 40% of patients with CD of the pouch at 12 months after initiation.11
Recent evidence suggests that preoperative anti-TNF use may be an independent predictor of pouchitis.30 Given that preoperative biologic use for UC will likely continue to increase31,32 with the emergence of new mechanisms for the treatment of UC, we may see further increases in pouchitis and particularly chronic pouchitis in the near future. Currently there are no available results from randomized controlled trials evaluating the use of biologics in patients with pouch-related conditions. This absence of prospective data and proven biologic therapy has led to a lack of guidelines and evidence-based treatment algorithms for the management of chronic inflammatory conditions of the pouch. Prospective clinical trials and longitudinal registries are critically needed to improve our understanding of the clinical course of pouch-related conditions.
The rate of CD of the pouch demonstrated in this cohort is among the highest reported. Given a much lower expected incidence,33 and the fact that this was not an inception cohort followed from the time of colectomy, this is likely due to the referral bias inherent in our tertiary care medical centers. Although we would expect that the included medical centers would utilize similar diagnostic criteria in the evaluation of patients with an IPAA, there is existing heterogeneity in the definition and terminology used to describe CD of the pouch.33,34 Standardizing the diagnostic approach to CD of the pouch is an important step in improving our understanding of this disease. Additionally, we should continue to explore the underlying phenotypic differences and drivers of outcomes in this critical population. In recognizing the limitations present in the existing cohort, these data should also provide further motivation for ongoing prospective cohort studies utilizing standardized diagnostic criteria for inflammatory conditions of the pouch and the development of objective outcome assessments at predefined time points in future studies. These findings also indicate the potential importance of longitudinal assessment from the time of IPAA in understanding both the clinical and microbial factors that may influence the development of inflammatory phenotypes after IPAA for UC.
In addition to the costs associated with biologic and other immunosuppressive therapy after IPAA, 11% of all patients underwent an IBD- or pouch-related surgery, including 6 patients who underwent pouch excision. Combined with the cost of continued medical therapy, the need for repeat surgical procedures is further evidence of the significant impact that pouchitis and other inflammatory conditions of the pouch have on the healthcare system. While our cohort likely represents a high-risk population, a prior systematic review has identified the paucity of literature on cost and resource utilization after surgery for UC.35 Future efforts should focus on the financial burden to individual patients and the healthcare system related to pouchitis and other inflammatory conditions after IPAA.
This cohort represents one of the largest multicenter assessments of the impact of pouchitis and other inflammatory conditions of the pouch. Despite the sample size and the structured data collection methods utilized within the SHARE cohort, this study does have limitations. This was not an inception cohort, and thus much of the perioperative history and IBD history prior to colectomy is self-reported. As such, potentially important perioperative events including preoperative therapies, the number of stages required during the proctocolectomy with IPAA, and postoperative complications were not captured for analysis in this particular cohort. Given the referral centers in our cohort, the outcomes represented in this population are not illustrative of the natural history among all patients undergoing restorative proctocolectomy with IPAA for UC. While patients were followed longitudinally and assessed for the development of outcomes, including pouchitis and CD of the pouch, no standardized criteria was used to establish these diagnoses. All diagnoses were based on decisions of the treating physicians and corroborated by patient reported data. Diagnostic criteria have been suggested for both chronic pouchitis36,37 and CD of the pouch,33 and thus a lack of standard criteria may have led to misclassification. Additionally, there were limited supporting data in the form of pouchoscopy, pathology, or other objective data to assess the effectiveness of therapies or changes in disease activity over time.
In conclusion, in a multicenter cohort of 7 academic medical centers, we demonstrated a significant burden associated with inflammatory conditions of the pouch, including acute pouchitis, chronic pouchitis, and CD of the pouch. The impact of these pouch-related conditions on individual patients and the healthcare system should not be underestimated. Future efforts should focus on the earlier evaluation and identification of those individuals at highest risk to develop the worst of these outcomes after colectomy with IPAA, in an effort to develop potential early interventions in these high-risk populations.
Supplementary Material
Funding: This study was funded in part by the Leona M. and Harry B. Helmsley Charitable Trust, the Crohn’s and Colitis Foundation [grant number 567497 (ELB)] and the National Institutes of Health [grant number P30 DK034987 (RSS)].
Conflicts of interest: Edward L. Barnes has served as a consultant for AbbVie, Takeda, and Target Pharmasolutions. Millie D. Long has served as a consultant for AbbVie, UCB, Takeda, Janssen, Pfizer, Salix, Valeant, Target Pharmasolutions and has received research support from Pfizer and Takeda. Benjamin Cohen has served as a consultant for Abbvie, Allergan, Janssen, Target PharmaSolutions, and Sublimity Therapeutics. Joel Pekow has participated in advisory boards for Takeda, Pfizer, and Janssen and has served as a consultant for Verastem and CVS. He has received research funding from Takeda and Abbvie. Hans H. Herfarth has served as a consultant for Allakos, Alivio, AMAG, Finch, Gilead, Merck, Pfizer, Celltrion, Lycera, and Boehringer-Ingelheim and has received research support from Allakos, Artizan, and Pfizer. Laura Raffals, Gaurav Syal, Maia Kayal, Ashwin Ananthakrishnan, Deepak Parakkal, Jean-Fred Colombel, and Robert S. Sandler report no relevant disclosures or conflicts of interest.
DATA AVAILABILITY
The raw data utilized in this study are available from the SHARE Data Management Center. For further information, please contact Dr. Robert Sandler, Director, Center for Gastrointestinal Biology and Disease; robert_sandler@med.unc.edu; CB#7555, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599-7555.
References
- 1. Shen B. Acute and chronic pouchitis—pathogenesis, diagnosis and treatment. Nat Rev Gastroenterol Hepatol. 2012;9:323–333. [DOI] [PubMed] [Google Scholar]
- 2. Barnes EL, Herfarth HH, Sandler RS, et al. Pouch-related symptoms and quality of life in patients with ileal pouch-anal anastomosis. Inflamm Bowel Dis. 2017;23:1218–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Devaraj B, Kaiser AM. Surgical management of ulcerative colitis in the era of biologicals. Inflamm Bowel Dis. 2015;21:208–220. [DOI] [PubMed] [Google Scholar]
- 4. Gregory M, Weaver KN, Hoversten P, et al. Efficacy of vedolizumab for refractory pouchitis of the ileo-anal pouch: results from a multicenter us cohort. Inflamm Bowel Dis. 2019;25:1569–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Weaver KN, Gregory M, Syal G, et al. Ustekinumab is effective for the treatment of Crohn’s disease of the pouch in a multicenter cohort. Inflamm Bowel Dis. 2019;25:767–774. [DOI] [PubMed] [Google Scholar]
- 6. Huguet M, Pereira B, Goutte M, et al. Systematic review with meta-analysis: anti-TNF therapy in refractory pouchitis and Crohn’s disease-like complications of the pouch after ileal pouch-anal anastomosis following colectomy for ulcerative colitis. Inflamm Bowel Dis. 2018;24:261–268. [DOI] [PubMed] [Google Scholar]
- 7. Herfarth HH, Long MD, Isaacs KL. Use of biologics in pouchitis: a systematic review. J Clin Gastroenterol. 2015;49:647–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lightner AL, Shogan BD, Mathis KL, et al. Revisional and reconstructive surgery for failing IPAA is associated with good function and pouch salvage in highly selected patients. Dis Colon Rectum. 2018;61:920–930. [DOI] [PubMed] [Google Scholar]
- 9. Farouk R, Pemberton JH, Wolff BG, et al. Functional outcomes after ileal pouch-anal anastomosis for chronic ulcerative colitis. Ann Surg. 2000;231:919–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tulchinsky H, Hawley PR, Nicholls J. Long-term failure after restorative proctocolectomy for ulcerative colitis. Ann Surg. 2003;238:229–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kayal M, Plietz M, Rizvi A, et al. Inflammatory pouch conditions are common after ileal pouch anal anastomosis in ulcerative colitis patients. Inflamm Bowel Dis. 2019. doi: 10.1093/ibd/izz227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ananthakrishnan AN, Kwon J, Raffals L, et al. Variation in treatment of patients with inflammatory bowel diseases at major referral centers in the United States. Clin Gastroenterol Hepatol. 2015;13:1197–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ananthakrishnan AN, Sakuraba A, Barnes EL, et al. The benefit of combination therapy depends on disease phenotype and duration in Crohn’s disease. Aliment Pharmacol Ther. 2017;46:162–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Barnes EL, Kochar B, Long MD, et al. Lack of difference in treatment patterns and clinical outcomes between black and white patients with inflammatory bowel disease. Inflamm Bowel Dis. 2018;24:2634–2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shen B, Achkar JP, Connor JT, et al. Modified pouchitis disease activity index: a simplified approach to the diagnosis of pouchitis. Dis Colon Rectum. 2003;46:748–753. [DOI] [PubMed] [Google Scholar]
- 16. Lightner AL, Mathis KL, Dozois EJ, et al. Results at up to 30 years after ileal pouch-anal anastomosis for chronic ulcerative colitis. Inflamm Bowel Dis. 2017;23:781–790. [DOI] [PubMed] [Google Scholar]
- 17. Lavryk OA, Stocchi L, Hull TL, et al. Factors associated with long-term quality of life after restorative proctocolectomy with ileal pouch anal anastomosis. J Gastrointest Surg. 2019;23:571–579. [DOI] [PubMed] [Google Scholar]
- 18. Suzuki H, Ogawa H, Shibata C, et al. The long-term clinical course of pouchitis after total proctocolectomy and IPAA for ulcerative colitis. Dis Colon Rectum. 2012;55:330–336. [DOI] [PubMed] [Google Scholar]
- 19. Hashavia E, Dotan I, Rabau M, et al. Risk factors for chronic pouchitis after ileal pouch-anal anastomosis: a prospective cohort study. Colorectal Dis. 2012;14:1365–1371. [DOI] [PubMed] [Google Scholar]
- 20. Hata K, Okada S, Shinagawa T, et al. Meta-analysis of the association of extraintestinal manifestations with the development of pouchitis in patients with ulcerative colitis. BJS Open. 2019;3:436–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yanai H, Ben-Shachar S, Mlynarsky L, et al. The outcome of ulcerative colitis patients undergoing pouch surgery is determined by pre-surgical factors. Aliment Pharmacol Ther. 2017;46:508–515. [DOI] [PubMed] [Google Scholar]
- 22. Hoda KM, Collins JF, Knigge KL, et al. Predictors of pouchitis after ileal pouch-anal anastomosis: a retrospective review. Dis Colon Rectum. 2008;51:554–560. [DOI] [PubMed] [Google Scholar]
- 23. Pavlides M, Cleland J, Rahman M, et al. Outcomes after ileal pouch anal anastomosis in patients with primary sclerosing cholangitis. J Crohns Colitis. 2014;8:662–670. [DOI] [PubMed] [Google Scholar]
- 24. Quinn KP, Lightner AL, Faubion WA, et al. A comprehensive approach to pouch disorders. Inflamm Bowel Dis. 2019;25:460–471. [DOI] [PubMed] [Google Scholar]
- 25. Singh S, Sharma PK, Loftus EV Jr, et al. Meta-analysis: serological markers and the risk of acute and chronic pouchitis. Aliment Pharmacol Ther. 2013;37:867–875. [DOI] [PubMed] [Google Scholar]
- 26. Fleshner P, Ippoliti A, Dubinsky M, et al. Both preoperative perinuclear antineutrophil cytoplasmic antibody and anti-CBir1 expression in ulcerative colitis patients influence pouchitis development after ileal pouch-anal anastomosis. Clin Gastroenterol Hepatol. 2008;6:561–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fleshner PR, Vasiliauskas EA, Kam LY, et al. High level perinuclear antineutrophil cytoplasmic antibody (pANCA) in ulcerative colitis patients before colectomy predicts the development of chronic pouchitis after ileal pouch-anal anastomosis. Gut. 2001;49:671–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gonsalves S, Lim M, Finan P, et al. Fecal lactoferrin: a noninvasive fecal biomarker for the diagnosis and surveillance of pouchitis. Dis Colon Rectum. 2013;56:733–737. [DOI] [PubMed] [Google Scholar]
- 29. Yamamoto T, Shimoyama T, Umegae S, et al. Serial monitoring of faecal calprotectin for the assessment of endoscopic recurrence in asymptomatic patients after ileocolonic resection for Crohn’s disease: a long-term prospective study. Ther Adv Gastroenterol. 2016;9:664–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bertucci Zoccali M, Hyman NH, Skowron KB, et al. Exposure to anti-tumor necrosis factor medications increases the incidence of pouchitis after restorative proctocolectomy in patients with ulcerative colitis. Dis Colon Rectum. 2019;62:1344–1351. [DOI] [PubMed] [Google Scholar]
- 31. Barnes EL, Jiang Y, Kappelman MD, et al. Decreasing colectomy rate for ulcerative colitis in the united states between 2007 and 2016: a time trend analysis. Inflamm Bowel Dis. 2019. doi: 10.1093/ibd/izz247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yu H, MacIsaac D, Wong JJ, et al. Market share and costs of biologic therapies for inflammatory bowel disease in the USA. Aliment Pharmacol Ther. 2018;47:364–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Barnes EL, Kochar B, Jessup HR, et al. The incidence and definition of Crohn’s disease of the pouch: a systematic review and meta-analysis. Inflamm Bowel Dis. 2019;25:1474–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lightner AL, Fletcher JG, Pemberton JH, et al. Crohn’s disease of the pouch: a true diagnosis or an oversubscribed diagnosis of exclusion? Dis Colon Rectum. 2017;60:1201–1208. [DOI] [PubMed] [Google Scholar]
- 35. Lindsay JO, Bergman A, Patel AS, et al. Systematic review: the financial burden of surgical complications in patients with ulcerative colitis. Aliment Pharmacol Ther. 2015;41:1066–1078. [DOI] [PubMed] [Google Scholar]
- 36. Segal JP, Ding NS, Worley G, et al. Systematic review with meta-analysis: the management of chronic refractory pouchitis with an evidence-based treatment algorithm. Aliment Pharmacol Ther. 2017;45:581–592. [DOI] [PubMed] [Google Scholar]
- 37. Barnes EL, Lightner AL, Regueiro M. Perioperative and postoperative management of patients with Crohn’s disease and ulcerative colitis. Clin Gastroenterol Hepatol. 2020;18:1356–1366. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data utilized in this study are available from the SHARE Data Management Center. For further information, please contact Dr. Robert Sandler, Director, Center for Gastrointestinal Biology and Disease; robert_sandler@med.unc.edu; CB#7555, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599-7555.