Abstract
Background:
While almond-specific IgE-mediated food allergies have traditionally been equated with other tree nut allergies, outcomes of oral food challenges to almond and the utility of clinical testing to predict IgE-mediated almond hypersensitivity is not well known.
Objective:
To describe almond oral challenge outcomes and assess the predictive value of clinical testing.
Methods:
603 almond challenges performed for 590 patients, aged 1 to 66 years, were analyzed from Massachusetts General Hospital allergy practices. Reactions were graded using the Niggemann and Beyer allergic reaction grading system and the Sampson 2006 NIAID anaphylaxis definition.
Results:
Almond challenges included 545 passes (92%), 15 (3%) indeterminates, and 30 (5%) failures, in contrast with 31% challenge failures for other foods. Most reactions were mild; 21 (4%) had Grade 2/3 allergic symptoms, and 3 (0.5%) had anaphylaxis. Median almond-specific IgE was 0.89 kU/L (range: <0.35, >100), median skin prick test (SPT) was 4.0 mm (0, 28), and 475 subjects (81%) were sensitized to almond. Failure was associated with higher almond-specific IgE (p<0.001), larger almond SPT (p=0.001), higher peanut IgE (p=0.003), and a history of almond reaction (p<0.029). Almond-specific IgE, almond SPT, and age at challenge combined demonstrated good predictive value for Grade 2/3 allergic reactions by ROC analysis (AUC 0.83).
Conclusions:
The proportion of failed almond challenges (5%) was low in contrast with other allergens, suggesting that some almond challenges may be safely conducted with higher patient-to-staff ratios or potentially introduced at home. Though reactions are usually uncommon and mild, anaphylaxis is possible with high almond sensitization.
Keywords: oral food challenge, almond allergy, safety, food allergy, almond hypersensitivity
Background:
Almond is the most commonly consumed and produced tree nut in the U.S. in 2017.(1) Approximately 0.4%−1.2% of the U.S. population is allergic to tree nuts, and almond allergy is one of the most common tree nut allergens reported at 0.7% in the United States.(2–4) Almond sensitization is common, with 71% of patients with birch allergy demonstrating cross sensitization with almond (compared with 84% to hazelnut and 60% to peanut).(5) The rate of almond sensitization in patients with peanut allergy is high, with 50% sensitized by skin prick testing to almonds.(3, 6) However, studies describing almond challenges show surprisingly low rates of true clinical reactivity, with reaction rates generally ranging from 0–6% (Table 1).(7–9) Only one study by Andorf et al describing the outcome of 21 almond challenges showed a much higher reaction rate of 48%, and this study was distinct in its selection of highly sensitized multi-food allergic individuals.(10) Even in this report, reactions were mostly mild with none requiring epinephrine use. Interpretation of reactions is also confounded by the possibility of oral allergy syndrome to almond due to birch cross-reactivity, resulting in mild symptoms like oral itch and a low risk of systemic reactivity{Ortolani, 1993 #3644}. Due to these factors and limited sample sizes, there is no available information regarding the predictive value of skin testing and serum specific IgE testing with regards to clinically relevant almond hypersensitivity.
Table 1:
Literature Review of Almond Challenge Outcomes
| Study Design | Almond Diagnostics | Almond Challenge Outcomes |
|---|---|---|
| Andorf et al JACI in Practice 2017(10) • n=165 patients with possible allergy to 2+foods receiving 311 challenges |
• median SPT 9.5 mm (range 6–25 mm) • median IgE 5.2 kU/L (range 0.4–100 kU/L) |
• n=21 challenges to almond • 10 reactions = 52% passage rate • 48% with skin reaction, 0% with GI reaction, 10% with respiratory symptoms, 0% with epi |
| Couch et al Ann Allergy Asthma Immunol 2017(9) • n=109 patients with tree nut allergy receiving 156 challenges |
• mean sIgE 1.18 (median 0) • mean SPT 1.27 (median 0) • 42 with sIgE <2 • 6 with sIgE >=2 • 37 with SPT <3 • 11 with SPT >3 |
• 57 challenges • 100% passage rate |
| Elizur et al Allergy 2018(8) • n=83 patients with peanut / tree nut allergy receiving 232 challenges |
• 49 with SPT >3 mm • exact SPT & IgE not specified |
• 49 challenges • 1 clinical reaction = 98% passage rate |
| Rodriguez et al JACI 2000(9) • n=34 with suspected Rosaceae food allergy • performed SPT, IgE and if positive, then OFC to all Rosaceae foods (apricot, almond, plum, strawberry, apple, peach, pear) |
• 4 with reported almond clinical history • 18 with positive almond-specific SPT or IgE • exact SPT & IgE not specified |
• 18 challenges to individuals with sensitization • 1 clinical reaction = 94% passage rate • Only oral symptoms • Rate of clinical reactivity for those with positive SPT (n=15): 7% • Rate of clinical reactivity for those with positive IgE (n=6): 17% |
Our main objectives were to use a large retrospective cohort of pediatric and adult subjects receiving oral food challenges to (1) determine the frequency and severity of reactions to almonds during oral food challenges, and (2) assess the utility of clinical history, skin prick testing and serum specific IgE testing to almond and other allergens in predicting clinically relevant almond allergy.
Methods
Study population
All patients, pediatric and adult, who had been referred for an oral open food challenge (OFC) for suspected almond allergy from 2009 – 2018 were included. The referral base consisted of 11 allergists from 2 main practice locations, Massachusetts General Hospital for Children - Food Allergy Center and Massachusetts General Hospital Allergy Associates. The decision to refer for challenge was determined based on the allergists’ clinical judgment and no guidelines were set regarding the criteria for challenge.
Study Design
We conducted a retrospective cohort analysis of all patients undergoing an almond challenge. Provider documentation, nursing flowsheets, and symptom score sheets were reviewed to evaluate signs and symptoms of clinical hypersensitivity during OFCs. We also collected clinical history regarding reaction to almonds and peanuts, seasonal allergies, atopic dermatitis, and SPT and IgE data on commonly cross-reactive allergens, including peanut, tree nuts (hazelnut, cashew, pistachio, walnut, pecan), and birch. This project was undertaken as a quality improvement initiative at Massachusetts General Hospital (Boston, MA, USA), and as such was not formally supervised by the Institutional Review Board per their policies.
Patient SPT and Immunocap IgE results from the closest date (median 6–7 months) prior to their OFC were recorded. Positive SPTs were defined as mean wheal diameter ≥3 mm above the saline control, and positive almond-specific IgE were defined as >0.35 kU/L. Any IgEs results reported as >100 kU/L were analyzed as 100.1 kU/L and <0.35 kU/L as 0.34. For any SPT or allergen-specific IgE values that were documented as positive without details regarding the numeric value, the data was imputed as 3 mm for SPT. This imputation was done for 2 almond SPT, 15 peanut SPT, and 5 hazelnut SPT values.
Oral Food Challenges
OFCs were supervised by clinical allergists and nurse practitioners. The protocol involved graduated administration of a total of six grams of almond protein (two tablespoons of almond butter or twenty-four almonds divided in 5 doses of 1%, 3%, 10%, 30%, and 56%) every 10–15 minutes over the course of an hour, with two hours of observation following the administration of the final dose. Vitals and symptoms were documented every fifteen minutes using a standardized flow sheet. If the in office challenge was tolerated, patients or their caregivers were instructed to give six grams of almond protein daily for four days to evaluate any subacute allergic reactions or intolerances.
Challenge outcomes were categorized as either pass, fail, or indeterminate based on the clinician’s judgment. Failed challenges were generally defined as the patient displaying signs or symptoms of an IgE-mediated response during the observed challenge. A challenge was labeled as indeterminate if either (1) no evidence of IgE-mediated symptoms were present, but the patient was unable to consume the full amount of almond protein, or (2) the patient developed very mild symptoms that were difficult to confirm as IgE-mediated in origin. At the clinician’s discretion, some patients with indeterminate challenges were offered the opportunity to introduce almond at home at the dose tolerated in clinic. Patients who tolerated this home introduction were recategorized as passing their challenge. For patients who were challenged multiple times, repeat challenges were described separately and only the most recent challenge was included in the main analysis.
We noted variability in clinician interpretation of challenge outcomes with some challenges being reported as failures with only mild subjective symptoms, while other clinicians described similar symptoms in patients and were able to successfully reintroduce almond. For these challenge failures, it was difficult to determine if their reactions represented the early stages of a potentially life-threatening IgE mediated reaction, oral allergy syndrome, non-IgE mediated symptoms, or anxiety triggered symptoms. To identify the subgroup of reactions that were more clearly potentially life-threatening IgE mediated reactions, we retrospectively categorized reactions based on the Niggemann and Beyer classification system for grading allergic reactions.(11) We labeled all reactions that met criteria for Grade 2 or Grade 3 reactions (presence of urticaria, angioedema, generalized flushing, abdominal pain, vomiting, diarrhea, cough, wheeze, laryngeal symptoms, tachycardia, low blood pressure, or mental status changes) as “Grade 2 or 3 Niggemann-Beyer reactions (Grade 2/3 reactions)”. Reactions that were Grade I (isolated redness, local swelling (not angioedema), pruritus) were excluded from these analyses. All reactions that met criteria for anaphylaxis as defined by the 2006 Sampson NIAID guidelines were labeled as such. We reported outcomes for all three categories: clinician-determined challenge outcome, grade 2/3 reactions, and anaphylaxis.
Statistical Analysis
We analyzed clinical and demographic variables, including clinical almond reactivity, serum specific IgE measurements, total IgE level, and SPT results, using Fisher’s exact tests, chi-square tests, t-tests or ANOVA, where appropriate, with an alpha=0.05. We reported unadjusted and adjusted odds ratios with univariable and multivariable logistic regression to assess potential clinical predictors of challenge outcomes and used stepwise elimination based on Akaike information criterion to identify the variables most predictive of challenge outcome. We also conducted a sensitivity analysis including only subjects who were sensitized to assess variables most strongly associated with challenge outcome. Receiver operator curve (ROC) analysis was performed to assess the sensitivity and specificity of almond-specific IgE and almond skin prick testing, and comparison of area under the curve (AUC) was conducted by DeLong’s test (R packages ROCR_1.0–7, pROC_1.13.0). All analyses were conducted using R version 3.5.1.(12)
Results
Study Population and Challenge Outcomes
Of the 3689 challenges, 603 (16%) almond challenges were performed for 590 patients (Figure 1). The population was mostly pediatric, with 90% of subjects <18 years old, and was mostly Caucasian with a slight male predominance (Table 2). Allergic comorbidities were common with 502 (85%) subjects having either atopic dermatitis or seasonal allergies, and high levels of birch sensitization (Table 2). The majority of subjects, 475 (81%) had almond sensitization with either positive almond SPT or almond-specific IgE, and 304 (52%) had both positive SPT and almond-specific IgE. Median SPT was 4.0 mm (range: 0, 28), median IgE was 0.89 kU/L (range: <0.35, >100), and 106 (18%) had high levels of sensitization with either almond-specific IgE ≥15 kU/L or almond SPT ≥ 8 mm (Figure 2). Of the 60 subjects (11%) with self-reported history of prior reaction to almond, 7 were confirmed by prior challenge and 27 (45%) either reported or had challenge proven objective symptoms (usually hives or local flushing). Of the 115 non-sensitized patients, at least 19 (2%) reported a prior history of almond reaction and all but 2 of these passed the almond challenge. Regarding the remainder of challenges performed on non-sensitized subjects without a history of prior reaction, clinicians reported conducting these challenges primarily to reduce parental or patient anxiety about introduction of almond or about future tree nut challenges.
Figure 1. Consort Diagram of Study Participants receiving an Almond Challenge:
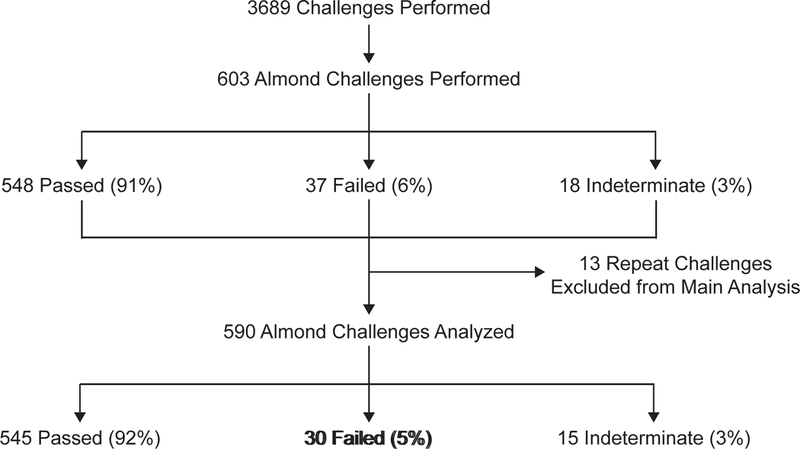
*11 individuals had 13 repeat challenges performed to almond. In all cases, the latest challenge was selected for analysis and all 13 prior challenges were excluded. 10 of the 11 participants eventually went on to pass the final challenge.
Table 2: Demographics and Allergic Clinical Characteristics.
Ratios of specific IgEs to total IgE were not significantly different between groups
| n | Overall n=590 |
Pass n=545 |
Indeterminate n=15 |
Fail n=30 |
p | |
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Female, n (%) | 588 | 249 (42.3) | 231 (42.5) | 6 (40.0) | 12 (40.0) | 0.995 |
| Age at challenge in years, mean (sd) | 590 | 10.2 (8.1) | 10.3 (8.0) | 10.9 (13.9) | 8.1 (6.4) | 0.317 |
| Race, n (%) | 554 | 0.220 | ||||
| White | 461 (83.2) | 428 (83.8) | 13 (86.7) | 20 (71.4) | ||
| Asian | 57 (10.3) | 51 (10.0) | 0 (0.0) | 6 (21.4) | ||
| Black or African American | 7 (1.3) | 7 (1.4) | 0 (0.0) | 0 (0.0) | ||
| Other | 29 (5.2) | 25 (4.9) | 2 (13.3) | 2 (7.1) | ||
| Hispanic/Latino, n (%) | 482 | 26 (5.8) | 24 (6) | 0 (0.0) | 2 (10.0) | 0.486 |
| Allergic History | ||||||
| Atopic Dermatitis, n (%) | 569 | 370 (65.0) | 340 (64.9) | 10 (66.7) | 20 (66.7) | 0.972 |
| Seasonal allergies, n (%) | 570 | 348 (61.1) | 321 (61.0) | 8 (57.1) | 19 (63.3) | 0.925 |
| Positive Birch Testing, n (%) | 259 | 194 (74.9) | 182 (75.8) | 5 (55.6) | 7 (70.0) | 0.362 |
| Total IgE, mean (sd) | 451 | 633.2 (986.1) | 639.1 (1007.5) | 650.3 (824.7) | 521.5 (643.8) | 0.85 |
| Almond History | ||||||
| Almond-specific IgE, mean (sd) | 570 | 3.5 (9.4) | 3.1 (7.7) | 3.4 (5.6) | 11.2 (23.7) | <0.001 |
| Ratio of Almond:Total IgE, mean (sd) | 441 | 0.01 (0.03) | 0.04 (0.12) | 0.01 (0.01) | 0.01 (0.02) | <0.001 |
| Almond SPT in mm, mean (sd) | 570 | 4.0 (3.7) | 3.8 (3.6) | 3.6 (3.8) | 6.4 (5.6) | 0.001 |
| History of Previous Almond Reaction, n (%) | 559 | 60 (10.7) | 51 (9.8) | 2 (14.3) | 7 (25.9) | 0.029 |
| Other Allergic Sensitization | ||||||
| Peanut IgE, mean (sd) | 503 | 27.9 (37.9) | 27.0 (37.3) | 14.9 (17.6) | 52.4 (49.2) | 0.003 |
| Peanut SPT in mm, mean (sd) | 403 | 8.5 (6.8) | 8.4 (6.9) | 10.1 (5.0) | 10.2 (5.6) | 0.462 |
| Peanut Allergy, n (%) | 197 | 69 (35.0) | 64 (35.6) | 1 (20.0) | 4 (33.3) | 0.766 |
| Hazelnut IgE, mean (sd) | 522 | 14.9 (24.9) | 14.6 (24.8) | 12.4 (20.4) | 21.7 (30.4) | 0.369 |
| Hazelnut SPT in mm, mean (sd) | 500 | 4.3 (4.8) | 4.3 (4.9) | 4.2 (4.6) | 3.9 (4.1) | 0.929 |
| Pistachio IgE, mean (sd) | 525 | 10.9 (21.2) | 10.7 (20.9) | 7.1 (13.4) | 15.7 (28.9) | 0.420 |
| Cashew IgE, mean (sd) | 531 | 9.2 (19.5) | 9.1 (19.2) | 8.8 (16.2) | 12.5 (24.8) | 0.677 |
| Pecan IgE, mean (sd) | 532 | 5.3 (13.6) | 5.0 (12.9) | 10.8 (23.5) | 8.6 (19.5) | 0.131 |
| Walnut IgE, mean (sd) | 541 | 10.8 (23.4) | 10.3 (22.1) | 16.9 (35.5) | 17.4 (36.1) | 0.203 |
Figure 2. Almond-specific IgE versus Almond SPT by Challenge Outcome.
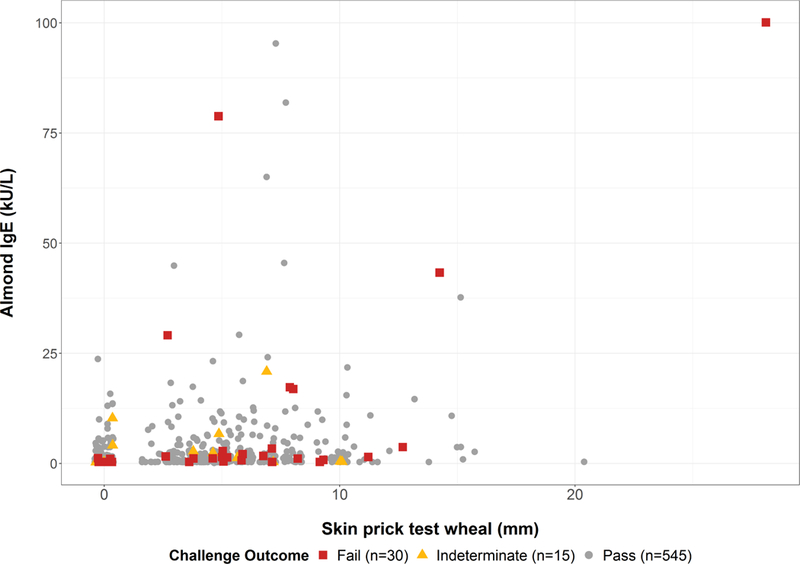
All specific IgE values <0.35 kU/L were plotted as 0.34, and all values >100 kU/L were plotted as 100.1
Most patients tolerated the almond challenges, with 545 passed (92%), 30 failed (5%) and 15 indeterminate challenges (3%). In comparison, the proportion of failed outcomes for 1,352 other challenges to common allergens was 31% (31% for peanut, 27% for tree nut, 34% for milk, 37% for baked milk, 16% for egg, and 47% for baked egg). Among the 30 failed challenges, 21 (4%) met the criteria for Grade 2/3 allergic reactions and 3 (0.5%) were anaphylactic reactions.
Repeat challenges
Eleven subjects had multiple challenges to almond, comprising 24 challenges total (Table E1). Ten patients initially had failed or indeterminate challenges and then eventually went on to pass the final challenge. The median time to re-challenge was 2.7 years (mean 2.3 years). Most subjects had mild symptoms (oral itching, pruritus, nasal symptoms, urticaria). One subject (Subject 5) had diffuse wheezing and then passed a challenge within the same year, and the original reaction was later attributed to uncontrolled asthma. Only 1 subject passed a challenge and then went on to fail a challenge (Subject 9). During home introduction of almond following his initial challenge, this patient experienced oral itching and eliminated almond from the diet. In the two years between the repeat challenges, the subject’s almond SPT increased from 5 to 14 mm and almond-specific IgE from 0.42 to 43.3 kU/L. He had no history of allergic rhinitis symptoms and birch testing was not performed. Upon repeat challenge, the subject failed developing perioral hives, oral itching, and mild abdominal pain.
Predictors of Challenge outcome
Failure to pass the challenge was associated with larger almond-specific SPT, higher almond-specific IgE, higher almond-specific IgE to total IgE ratio, history of previous reaction to almond, and larger peanut specific IgE (Table 2). There was no association between challenge outcome and nut sensitization (nut-specific IgE or SPT for peanut, pistachio, cashew, pecan, hazelnut, walnut), total IgE, atopic dermatitis, seasonal allergies, or birch sensitization. There was also no association between challenge outcome and the season in which the challenge was performed (p=0.12). Despite the association of almond SPT and IgE with challenge failure, the estimated 95% predicted probability of failing a challenge would be at an almond SPT response of 46 mm and almond-specific IgE of 174 kU/L, both well outside the range of our cohort for typically observed SPT IgE values (Figure 3A and 3B). At the upper limits of the range of sensitization seen in our cohort, predicted probability of failing a challenge was 60% for SPT of 28mm and 60% for IgE of 100 kU/L. ROC analysis revealed that almond SPT (AUC 0.66) and almond-specific IgE (AUC 0.61) individually, or combined (AUC 0.67), were poor predictors of failed challenge outcome (Figure 3C), consistent with the wide range of skin and serum sensitization among patients who passed challenges (Figure 2).
Figure 3. Predicted Probabilities for Almond-specific IgE (A) and Almond SPT (B) and ROC Analysis of Almond SPT, Almond specific IgE, and age at challenge in predicting Challenge Outcome (C) and IgE-mediated reactions (D).
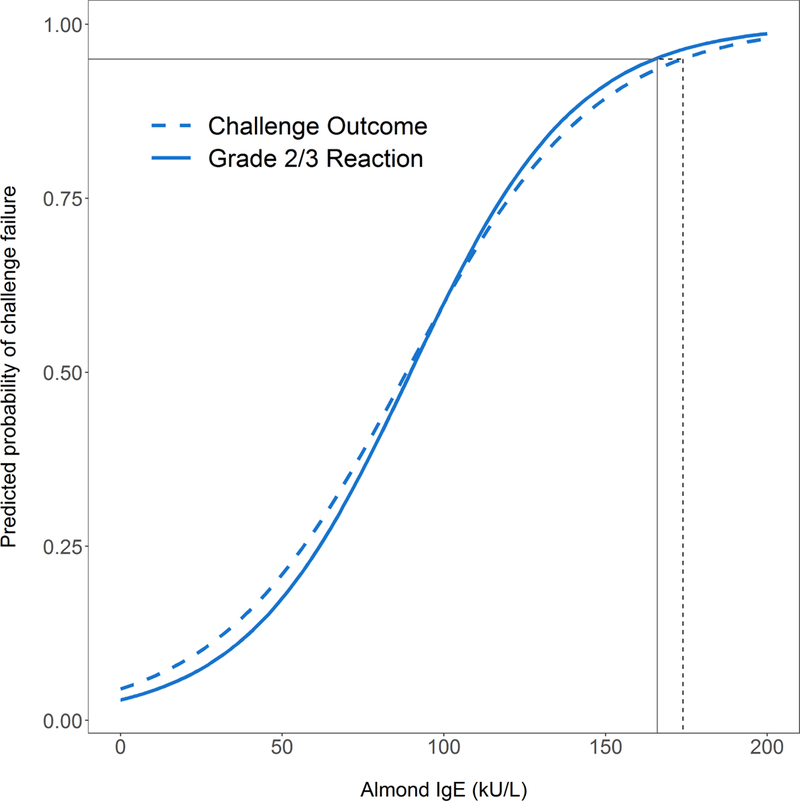
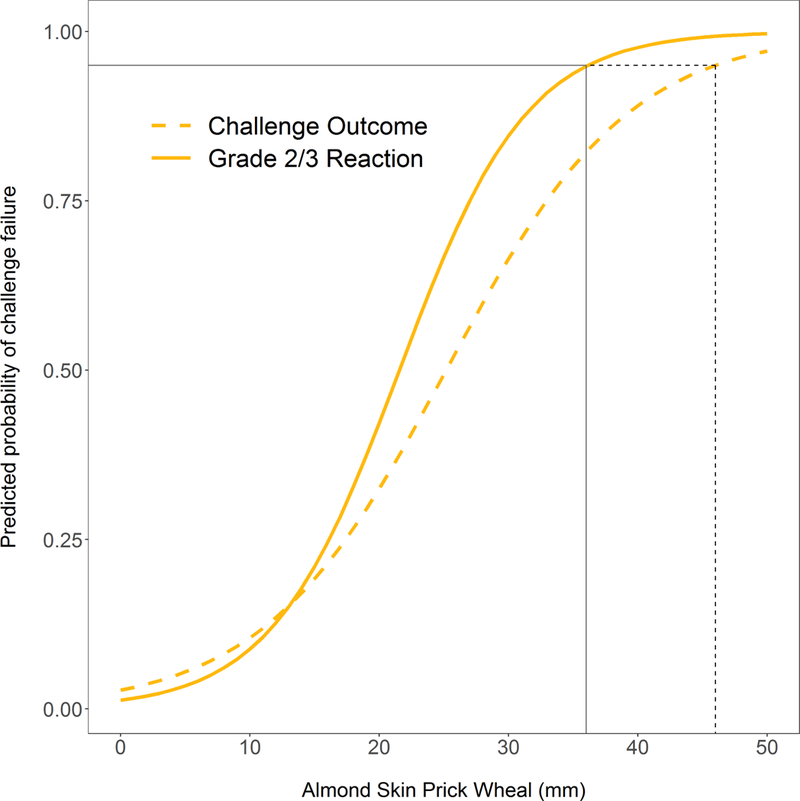
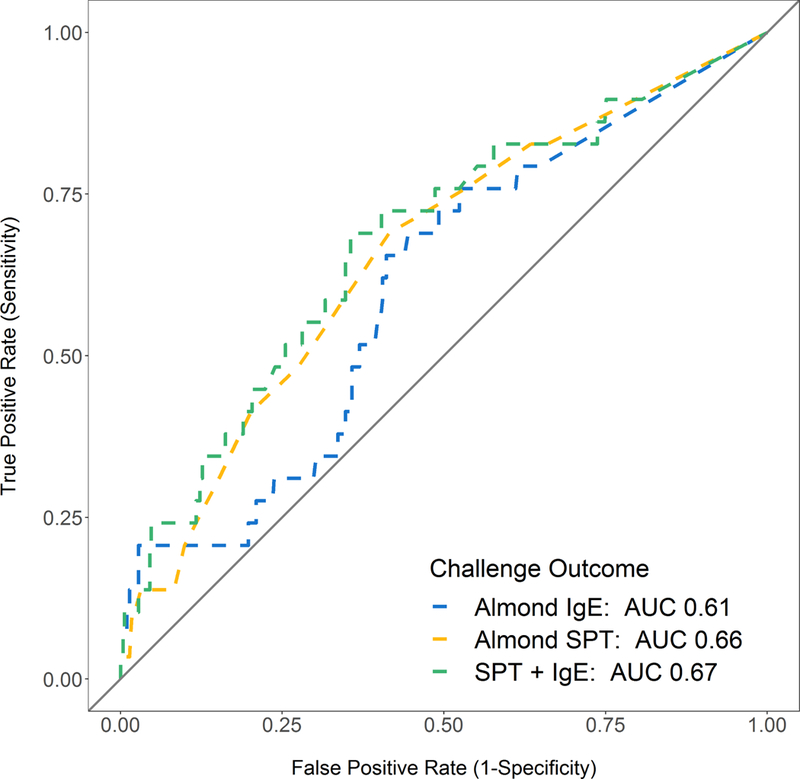
Almond SPT of 46 mm and almond-specific IgE of 174 kU/L were associated with 95% predicted probability of failing a challenge (A & B, dashed black line). Almond SPT of 36 mm and almond-specific IgE of 166 kU/L were associated with 95% predicted probability of having an Grade 2/3 allergic reaction during a challenge (A & B, solid line).
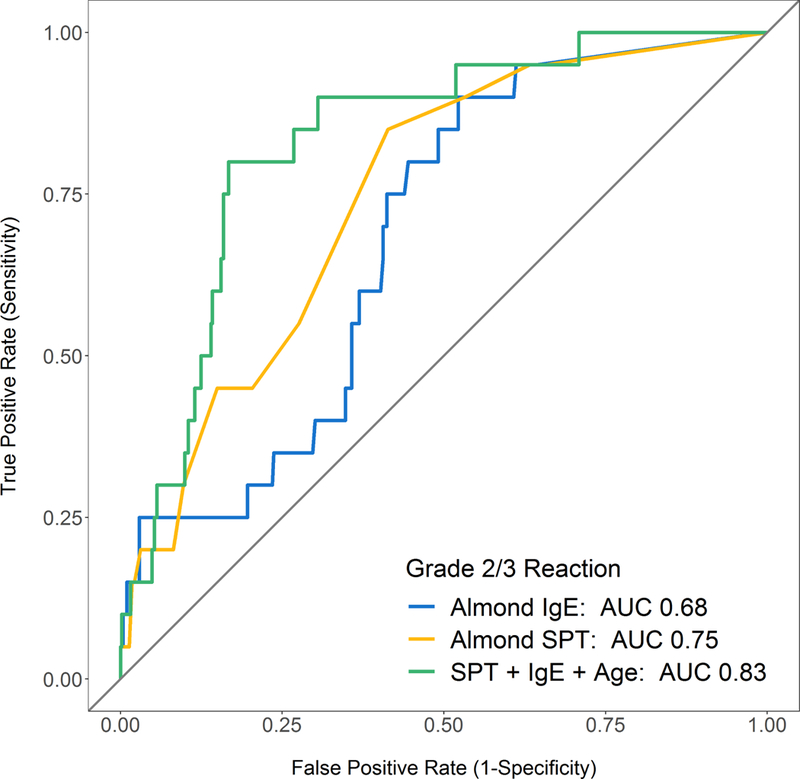
Narrowing the challenge outcome to Grade 2/3 allergic reactions confirmed the association with higher almond sensitization and also revealed further associations with failed challenge outcome, including younger age at challenge and higher specific IgEs to peanut, pecan, walnut, and pistachio (Table 2). Using multivariable logistic regression, after adjusting for the other variables in the model, younger age at challenge, increased almond SPT, and increased almond-specific IgE remained robust predictors of Grade 2/3 allergic reactions (Table 2). Sensitivity analysis removing any non-sensitized patients showed similar findings on univariable logistic regression with associations between Grade 2/3 allergic reactions and age at challenge, almond-specific IgE, almond SPT, and peanut-specific IgE (Table E2). Almond SPT of 36 mm and almond-specific IgE of 166 kU/L were associated with 95% predicted probability of having a Grade 2/3 allergic reaction during a challenge, once again outside of the range seen in our cohort (Figure 3A and 3B). At the upper limits of the range of sensitization seen in our cohort, predicted probability of a Grade 2/3 allergic reaction was 79% for SPT of 28mm and 60% for IgE of 100. Sensitivity and specificity of almond SPT and almond-specific IgE for Grade 2/3 allergic reactions remain poor (Table E3). ROC analysis using Grade 2/3 allergic as an outcome demonstrated improved predictive ability of almond SPT (AUC 0.75), and most of all the combination of almond SPT, almond-specific IgE, and age at challenge (AUC 0.83) which had significantly greater predictive value than SPT alone (p=0.008) and almond-specific IgE alone (p=0.006) (Figure 3D).
Based on SPT, subjects with almond SPT 0–4 mm had a 3% reaction rate (with 0.9% Grade 2/3 reactions and 0% anaphylactic reactions), while the reaction rate among those with >5 mm wheal was 8% (with 7% Grade 2/3 N-B reactions and 1% anaphylactic reactions) (Table 4). For almond-specific IgE <1 kU/L, the reaction rate was 3% (with 1% Grade 2/3 reactions, and 0% anaphylactic reactions), and almond IgE>1 kU/L had a reaction rate of 8% (with 6% Grade 2/3 reactions and 1% anaphylactic reactions).
Table 4:
Challenge Fail Rates by Almond SPT (A), Almond-specific IgE (B), and Age (C)
| A | ||||
|---|---|---|---|---|
| SPT Size | n (%) | Failed challenge | Grade 2/3 Reaction | Anaphylactic Reaction |
| 0–2 mm | 203 (34%) | 3% | 0.5% | 0% |
| 3–4 mm | 122 (21%) | 3% | 2% | 0% |
| 5–6 mm | 124 (21%) | 7% | 6% | 1% |
| 7–8 mm | 60 (10%) | 10% | 5% | 2% |
| >8 mm | 61 (10%) | 10% | 10% | 2% |
| B | ||||
| Specific IgE | n (%) | Failed challenge | Grade 2/3 Reaction | Anaphylactic Reaction |
| <0.35 | 195 (33%) | 3% | 0.5% | 0% |
| 0.35 – 1.00 | 109 (18%) | 3% | 3% | 0% |
| 1.01 – 2.00 | 86 (15%) | 12% | 9% | 1% |
| 2.01 – 10.00 | 136 (23%) | 3% | 2% | 0% |
| > 10.00 | 44 (7%) | 17% | 14% | 5% |
| C | ||||
| Age at Challenge (years) |
n (%) | Failed challenge | Grade 2/3 Reaction | Anaphylactic Reaction |
| 0–4 | 146 (25%) | 7% | 6% | 0% |
| 5–9 | 203 (34%) | 6% | 4% | 1% |
| 10–17 | 180 (31%) | 4% | 2% | 0% |
| 18+ | 61 (10%) | 2% | 0% | 0% |
Failed challenges
The failed reactions (Table 5, Table E4) were mostly characterized by oral symptoms 7 (23%), with 3 (10%) patients experiencing respiratory symptoms, 6 (20%) gastrointestinal symptoms, and 1 (3%) with cardiovascular symptoms. Most challenge failures were treated with antihistamines, and for the anaphylactic reactions, 1(3%) received only albuterol for uncontrolled asthma potentially independent of the oral challenge because they started the challenge with a brief transient wheeze (Table E4), and two (7%) received epinephrine for symptoms described in more detail below.
Table 5: Description of Failed Challenge Reactions.
Symptom categories were derived from PRACTALL guidelines and definitions of reactions.
| Overall (n=30) |
Grade 2/3 Reaction (n=21) |
Anaphylactic Reaction (n=3) |
|
|---|---|---|---|
| Percent Ingested, mean (sd) | 37.0 (38.3) | 39.8 (41.8) | 57.0 (60.8) |
| Reaction Symptoms: Subjective | |||
| Oral Itching | 8 (27%) | 3 (14%) | 0 (0%) |
| Pruritus | 8 (27%) | 6 (29%) | 1 (33%) |
| Nausea | 2 (7%) | 2 (10%) | 0 (0%) |
| Abdominal Pain | 3 (10%) | 3 (14%) | 1 (33%) |
| Irritability | 2 (7%) | 2 (10%) | 0 (0%) |
| Dizziness | 0 (0%) | 0 (0%) | 0 (0%) |
| Reaction Symptoms: Objective | |||
| Hives / Angioedema | 17 (57%) | 17 (81%) *** | 2 (67%) |
| Non-urticarial Rash | 3 (10%) | 1 (5%) | 0 (0%) |
| Flushing (local) | 6 (20%) | 3 (14%) | 1 (33%) |
| Flushing (generalized) | 0 (0%) | 0 (0%) | 0 (0%) |
| Nasal symptoms | 12 (40%) | 10 (48%) | 3 (100%) |
| Wheeze | 2 (7%) | 2 (10%) | 2 (67%)* |
| Laryngeal symptoms† | 1 (3%) | 1 (5%) | 0 (0%) |
| Vomiting | 3 (10%) | 3 (14%) | 1 (33%) |
| Diarrhea | 0 (0%) | 0 (0%) | 0 (0%) |
| Tachycardia | 1 (3%) | 1 (5%) | 1 (33%) |
| Low blood pressure | 0 (0%) | 0 (0%) | 0 (0%) |
| Reaction Treatment | |||
| Treatment given | 21 (70%) | 14 (67%) | 3 (100%) |
| Antihistamines | 21 (70%) | 14 (67%) | 3 (100%) |
| H2 blocker | 3 (10%) | 3 (14%) | 2 (67%)* |
| Steroid | 2 (7%) | 2 (10%) | 2 (67%)* |
| Albuterol | 1 (3%) | 1 (5%) | 1 (33%) |
| Epinephrine | 2 (7%) | 2 (10%) | 2 (67%)* |
In this cohort, the 1 subject experiencing laryngeal symptoms had throat clearing.
p<0.05
p<0.01
p<0.001
Many of the challenges that were categorized as failed challenges presented with only subjective symptoms. Of note, there was a trend of higher oral itching in those without Grade 2/3 allergic symptoms versus those with Grade 2/3 allergic symptoms (57% v. 14%, p=0.058). Among the 475 sensitized patients, the proportion of failed challenges was 6%, whereas among the 115 non-sensitized patients, the proportion of failed challenges was 2.6% (3 subjects). Furthermore, the three subjects who failed challenges in the setting of negative almond SPT and almond-specific IgE developed only oral itch, without any other cutaneous, respiratory, or gastrointestinal symptoms.
Anaphylaxis Cases
The first case of anaphylaxis was a 7-year-old female with atopic dermatitis, allergic rhinitis, asthma requiring controller medication, multiple food allergies (egg, peanut, and tree nut), and a history of food protein-induced allergic proctocolitis. She had never ingested almond. Her laboratory testing five years prior showed total IgE of 40 kU/L and almond-specific IgE of 4.26 kU/L and SPT of 11 mm. Seven months prior, her total IgE was 2104 kU/L, almond-specific IgE was ≥100 kU/L, and SPT was 28 mm. During the challenge, the patient initially complained of transient mouth itch after the 1% dose, but eventually ingested 100% of the dose. One hour and 45 minutes later, she developed pruritus, hives, flushing, tachycardia, objective and subjective gastrointestinal symptoms. She was given intramuscular epinephrine 0.15 mg (0.007 mg/kg) and oral cetirizine. Shortly afterwards, she vomited and had progressive urticaria so was treated with another 0.15 mg of epinephrine (total 0.014 mg/kg), oral steroid and oral H2 blocker. Her condition returned to baseline 2.5 hours after initial administration of epinephrine.
The second case was a 5 year-old female with a history of mild persistent asthma and food allergies to egg and tree nuts. She had a history of hives, left eye and lip swelling at 14 months of age following ingestion of almonds and walnuts together. Her most recent almond SPT was 8 mm, total IgE was 409 kU/L and almond-specific IgE was 17.3 kU/L. During the challenge, she ingested 100% of the dose. Following the last dose, she developed increased congestion and diffuse wheezing. She was treated with one 0.15 mg (0.009 mg/kg) dose of intramuscular epinephrine, antihistamine, oral H2 blocker, and oral steroid. Her wheezing resolved within 5 minutes of epinephrine administration. Common attributes of these two cases with anaphylaxis include ingestion of 100% of the dose, SPT ≥8 millimeters, younger age, female sex, and persistent asthma, even though well controlled at the time of challenge.
Discussion
This paper describes the largest cohort of almond challenges in the literature to date. Almond is a particularly useful nut for re-introduction for several reasons. The prevalence of growth concerns in the food allergic population(13) indicates that for patients with concomitant milk allergy or growth concerns, supplementation of the diet with almond based products, such as almond milk or almond butter, could be a useful alternative for improving nutrition in the food allergic population. Almond based products are also often readily available without cross-contamination concerns. Finally, for families who are anxious about the challenge process, in our experience beginning with the almond challenge has been a useful way to reduce stress.
Most studies of almond challenge outcomes reported passage rates ranging from 94–100%, often with low sensitization levels and with uniformly mild reactions. In their description of 21 challenges to almond, Andorf et al challenged patients with median SPT of 9.5 mm and median almond-specific IgE of 5.2 kU/L and found a 52% passage rate. However, even for this cohort, none of the patients required epinephrine. Our population spans a large range of sensitization, with 19% of patients having no almond sensitization and 18% of patients having either almond-specific IgE ≥15 kU/L or almond SPT ≥ 8 mm. In this diverse population, we demonstrated a similarly high passage rate of 92% to other studies, which contrasted with our pass rate (69%) for other common allergens such as peanuts, non-almond tree nuts, milk, and egg.
Unlike for other tree nuts, almond-specific SPT and IgE serve as poor predictors of challenge outcome. While almond-specific SPT and IgE and history of prior reaction were significantly associated with challenge outcome, the sensitivity and specificity of these tests remain poor. In particular, the almond-specific SPT and IgE values associated with a 95% predicted probability of failing a challenge are unrealistically high and markedly different than the 15kU/L cutoff commonly identified for peanut and other tree nuts,(14–16) further illustrating the unreliability of almond specific testing in screening appropriate patients for challenge.
However, it appears that there are two distinct types of failed almond challenges, the first with more objective symptoms which we termed Grade 2/3 allergic reactions, and the second with mostly oral or very mild symptoms. This is supported by our finding that oral itching was less featured in reactions of subjects with Grade 2/3 allergic symptoms, though oral itch as a symptom should not be used to rule out an IgE-mediated reaction. Unfortunately, due to limited data on birch sensitization, we were unable to statistically determine if subjects without Grade 2/3 allergic symptoms were experiencing symptoms consistent with oral allergy syndrome. However, we suspect that this subgroup may confound many of the prior analyses of the predictive value of almond skin and serum testing. To address the possible confounding nature of oral allergy syndrome reactions, we created the subgroup of “Grade 2/3 allergic reactions”. Here, we not only confirmed the association of failed challenges with increased almond-specific IgE, increased almond SPT, and increased peanut IgE, but also extended these findings to younger age at challenge and increased sensitization to other tree nuts, including hazelnut, walnut, pecan, and pistachio. We suspected that many of these associations were linked to the high correlations between tree nut and peanut IgE’s, and using multivariable logistic regression with stepwise model selection, were able to narrow the predictors that remained associated with challenge failure even after adjusting for each other: almond SPT, almond-specific IgE, and age. We hypothesize that younger age is associated with the Grade 2/3 allergic reactions, because children being challenged at a younger age are less likely to have allergic rhinitis and therefore oral allergy syndrome. Using ROC analysis, almond SPT and almond-specific IgE individually remain limited in their sensitivity and specificity for even IgE-mediated reactions. However, the combination of all three factors shows relative strong ability to predict potentially life-threatening IgE-mediated reactions. These findings, however, need to be replicated in equally large, well-characterized cohorts of almond challenges before being implemented as criteria for determining patients at risk for true IgE-mediated almond allergy.
It should be noted that while almond challenges appear to be safer than those of other tree nuts, anaphylaxis can occur. As this cohort spanned 9 years of clinical practice, our physicians began referring more highly sensitized patients over time, which was reflected in statistically significantly higher proportions of failed almond challenges (data not shown). We began to see more anaphylactic reactions, including the two cases who received epinephrine. With only 2 subjects, we lack the statistical power to assess predictors of epinephrine use, but it should be noted that there appears to be a few commonalities including SPT>8 mm, elevated almond-specific IgE (>17 kU/L) controller medication use for asthma, and larger quantity of almond ingested prior to symptom onset.
Given the access issues for oral food challenges, it has become essential to develop safe practices where we can increase the availability of food challenges for patients truly at risk for allergic reactions and anaphylaxis. Almond challenge pass rates are so high that some authors have discussed the possibility of challenging more aggressively (7). Potential strategies might include the following steps. First, all patients with negative almond specific skin test and IgE, even with a positive history, could have home introductions since 97% passed their challenge and the handful of failures we observed only had oral itch. Second, since the risk of anaphylactic reactions was 0% for those with SPT <5 mm and IgE <1 kU/L, these data may support introducing almond at home for patients with low levels of sensitization, using gradual introduction methods that span 1–2 weeks. Third, the data certainly support challenging patients with higher levels of sensitization in outpatient clinics, as 80–90% of these patients will still pass an almond challenge. Fourth, given a 92% overall pass rate, clinics could increase the patient to provider ratio to challenging 4 subjects at a time and still maintain a 72% probability of all subjects passing (25% probability of only 1 patient reacting or having an indeterminate challenge and 3% chance of more than 1 patient doing so).
All of these suggestions should be tempered by the limitations of this study. Our cohort represents a variety of clinical practitioners with corresponding variability in referral, diagnostic, and management practice. However, this does improve the generalizability of our findings. Regarding the 18% of the cohort with very high levels of sensitization, sample sizes in the higher range of sensitization are lower, so final conclusions about the risk of challenging subjects with high levels of sensitization will require further study with larger sample sizes. Regarding the 19% of subjects who showed no sensitization, while a small proportion (3% of the cohort) were challenged due to history of reaction, our physicians reported that the remainder of challenges were generally performed when parents had high levels of anxiety about introduction of almond at home. This supports the need for better education of families about the low risk of almond introduction. Furthermore, removal of these non-sensitized patients in our sensitivity analyses showed similar findings for predictors of challenge outcomes. Finally, in our efforts to identify the subjects who truly experienced potentially life-threatening IgE mediated reactions, we acknowledge that the definition of the subgroup of “Grade 2/3 allergic reactions” may have missed some of the potentially life-threatening reactions that were simply stopped prior to the development of objective and more severe systemic symptoms. However, we determined that the loss of a few subjects in the subgroup analysis allowed for a more accurate characterization of predictors of true potentially life-threatening IgE mediated reactions.
In conclusion, examining 603 almond challenges, we have observed low rates of true clinical reactivity to almond, despite in some cases very high almond sensitization both by skin and serum testing. Most reactions were mild, though anaphylaxis to almond is possible and the two cases we observed both had high sensitization. While the combination of almond SPT, almond-specific IgE, and age at challenge may prove useful in predicting which patients are truly at risk for developing almond allergy, these findings will need to be validated in other cohorts. We anticipate that by incorporating these findings into clinical practice, we can reduce the number of unnecessary almond challenges and improve access for food allergic patients truly at risk for life-threatening reactions.
Supplementary Material
Table 3: Allergic Clinical Characteristics associated with Grade 2/3 allergic reaction during Almond Challenge.
OR for specific IgE represent the increased odds for every 10 kU/L change in IgE. OR for SPT represent the increased odds for every 5 mm change in wheal. Variables not meeting significance criteria were excluded from the table.
| No Grade 2/3 symptoms (n=569) |
Grade 2/3 N-B reaction (n= 21) |
Unadj. OR [95% CI] |
p | Adj. OR [95% CI] |
p | |
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Age at challenge in years, mean (sd) | 10.4 (8.2) | 6.1 (3.4) | 0.86 [0.77, 0.97] | 0.01 | 0.85 [0.75, 0.97] | 0.01 |
| Almond History | ||||||
| Almond-specific IgE, mean (sd) | 3.1 (7.7) | 14.3 (27.3) | 1.51 [1.22, 1.87] | <0.001 | 1.25 [0.96, 1.64] | 0.10 |
| Almond SPT in mm, mean (sd) | 3.8 (3.6) | 7.8 (5.8) | 2.75 [1.69, 4.47] | <0.001 | 2.57 [1.47, 4.47] | <0.001 |
| Allergic Sensitization | ||||||
| Peanut IgE, mean (sd) | 26.9 (37.1) | 52.8 (49.3) | 1.16 [1.04, 1.29] | 0.006 | - | - |
| Pistachio IgE, mean (sd) | 10.5 (20.6) | 21.2 (32.5) | 1.17 [1.01, 1.36] | 0.044 | - | - |
| Pecan IgE, mean (sd) | 5.1 (13.2) | 12.0 (22.8) | 1.24 [1.01, 1.52] | 0.048 | - | - |
| Walnut IgE, mean (sd) | 10.4 (22.4) | 23.1 (41.0) | 1.16 [1.02, 1.33] | 0.028 | - | - |
Highlights:
What is already known about this topic? Almond-specific IgE-mediated food allergy is often equated with other tree nuts.
What does this article add to our knowledge? True almond reactivity is uncommon and mostly mild.
How does this study impact current management guidelines? Some almond challenges may be safely conducted with higher patient-to-staff ratios or at home.
Funding/Acknowledgments:
Y.V. is supported by the National Institutes of Health (NIH, K23-AI130408) and the Massachusetts General Hospital, Office of Women’s Careers Scholarly Writing Award. We would also like to acknowledge the contributions of Caroline Southwick to this work.
Abbreviations:
- OFC
oral food challenge
- SPT
skin prick test
- IgE
Immunoglobulin E
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: The authors have no conflicts of interest to disclose.
References:
- 1.Perez A. Fruit and Tree Nut Yearbook Tables In: Agriculture USDo, editor. https://www.ers.usda.gov/data-products/fruit-and-tree-nut-data/fruit-and-tree-nut-yearbook-tables/#Tree%20Nuts: Economic Research Service; 2018. [Google Scholar]
- 2.Sicherer SH, Furlong TJ, Munoz-Furlong A, Burks AW, Sampson HA. A voluntary registry for peanut and tree nut allergy: characteristics of the first 5149 registrants. J Allergy Clin Immunol 2001;108(1):128–32. [DOI] [PubMed] [Google Scholar]
- 3.Sicherer SH, Munoz-Furlong A, Sampson HA. Prevalence of peanut and tree nut allergy in the United States determined by means of a random digit dial telephone survey: a 5-year follow-up study. J Allergy Clin Immunol 2003;112(6):1203–7. [DOI] [PubMed] [Google Scholar]
- 4.Gupta RS, Warren CM, Smith BM, Blumenstock JA, Jiang J, Davis MM, et al. The Public Health Impact of Parent-Reported Childhood Food Allergies in the United States. Pediatrics 2018;142(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uotila R, Kukkonen AK, Pelkonen AS, Makela MJ. Cross-sensitization profiles of edible nuts in a birch-endemic area. Allergy 2016;71(4):514–21. [DOI] [PubMed] [Google Scholar]
- 6.Moneret-Vautrin DA, Rance F, Kanny G, Olsewski A, Gueant JL, Dutau G, et al. Food allergy to peanuts in France--evaluation of 142 observations. Clin Exp Allergy 1998;28(9):1113–9. [DOI] [PubMed] [Google Scholar]
- 7.Couch C, Franxman T, Greenhawt M. Characteristics of tree nut challenges in tree nut allergic and tree nut sensitized individuals. Ann Allergy Asthma Immunol 2017;118(5):591–6.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elizur A, Appel MY, Nachshon L, Levy MB, Epstein-Rigbi N, Golobov K, et al. NUT Co Reactivity - ACquiring Knowledge for Elimination Recommendations (NUT CRACKER) study. Allergy 2018;73(3):593–601. [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez J, Crespo JF, Lopez-Rubio A, De La Cruz-Bertolo J, Ferrando-Vivas P, Vives R, et al. Clinical cross-reactivity among foods of the Rosaceae family. J Allergy Clin Immunol 2000;106(1 Pt 1):183–9. [DOI] [PubMed] [Google Scholar]
- 10.Andorf S, Borres MP, Block W, Tupa D, Bollyky JB, Sampath V, et al. Association of Clinical Reactivity with Sensitization to Allergen Components in Multifood-Allergic Children. J Allergy Clin Immunol Pract 2017;5(5):1325–34.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Niggemann B, Beyer K. Time for a new grading system for allergic reactions? Allergy 2016;71(2):135–6. [DOI] [PubMed] [Google Scholar]
- 12.R Core Team. R: A language and environment for statistical computing R Foundation for Statistical Computing [Internet]. 2018. Available from: https://www.R-project.org/.
- 13.Hobbs CB, Skinner AC, Burks AW, Vickery BP. Food allergies affect growth in children. J Allergy Clin Immunol Pract 2015;3(1):133–4 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sampson HA, Ho DG. Relationship between food-specific IgE concentrations and the risk of positive food challenges in children and adolescents. J Allergy Clin Immunol 1997;100(4):444–51. [DOI] [PubMed] [Google Scholar]
- 15.Sampson HA. Utility of food-specific IgE concentrations in predicting symptomatic food allergy. J Allergy Clin Immunol 2001;107(5):891–6. [DOI] [PubMed] [Google Scholar]
- 16.Clark AT, Ewan PW. Interpretation of tests for nut allergy in one thousand patients, in relation to allergy or tolerance. Clin Exp Allergy 2003;33(8):1041–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


