Abstract
Heat-shock protein 90 (HSP90) is an essential molecular chaperone in eukaryotes. It is important for chaperoning proteins that are important determinants of multistep carcinogenesis. HSP90’s ATPase activity is associated with its chaperone function. Co-chaperones as well as posttranslational modifications (phosphorylation, acetylation, and S-nitrosylation) are important for regulating its ATPase activity. Yeast can be used to express and purify HSP90 and also detect its phosphorylation by pan-phosphoserine or phosphothreonine antibodies.
Keywords: HSP90, Molecular chaperones, Posttranslational modification, Phosphorylation
1. Introduction
Heat-shock protein 90 (HSP90) is an essential molecular chaperone in eukaryotes (1–3). Its cellular functions have been most clearly identified in mammalian cells, Drosophila and baker’s yeast Saccharomyces cerevisiae (4–7). HSP90 creates and maintains the functional conformation of a subset of proteins (8). These targets (or “clients”) are key components of signal transduction pathways and numerous transcription factors. HSP90 and a discrete set of co-chaperone proteins “hold” these clients in a state from which they can respond to activating signals.
HSP90 chaperone activity depends on ATP binding and hydrolysis (9–11) which is coupled to a conformational cycle involving the opening and closing of a dimeric “molecular clamp” via transient association of HSP90’s N-terminal domain (12, 13), which also binds the antitumor antibiotics geldanamycin and radicicol (14–17).
ATPase activity of HSP90is also regulated by co-chaperones. For example, HopSti1 (18–21), pSOCdc37 (22–25), and p23Sbal (26–28) have an inhibitory effect on the ATPase cycle of HSP90 while Ahal (29–31) and Cpr6 (32, 33) have an activating effect.
HSP90 is a phosphoprotein (34–42). Cells treated with the serine/threonine phosphatase inhibitor, okadaic acid, demonstrate HSP90 hyperphosphorylation and decreased association of the chaperone with pp60v-Src, suggesting a link between HSP90 phosphorylation and chaperoning ofits target proteins (35, 43). Recent work has shown that c-Src directly phosphorylates Tyr-300 of HSP90 under basal conditions, reducing its ability to chaperone client proteins (41). Therefore, HSP90 phosphorylation provides an additional means of fine-tuning its chaperone activity. Lack of phospho-specific antibodies has made it difficult to study HSP90 phosphorylation in mammalian cells. Also HSP90 gene knockouts are lethal in mammalian systems, so any mutant HSP90 must be investigated in a background of highly expressed native mammalian HSP90 proteins.
Simple baker’s yeast, S. cerevisiae, is a well-established and valuable tool for studying various aspects of conserved protein chaperone machinery. The yeast system has provided us with a powerful tool to study HSP90 phosphorylation, since it readily allows plasmid exchange whereby any introduced HSP90 gene - provided it is partially functional - can provide 100% of the HSP90 of the cell (Fig. 1). Such genetic modifications are simply not achievable in cultured mammalian cells. This plasmid exchange (Fig. 1) was used to isolate temperature-sensitive (ts) HSP90 mutants.
Fig. 1.

With plasmid shuffling, a mutant HSP90gene can be made to provide all the HSP90 of the yeast cell (yHSP90= Hsp82 and yHsc90=Hsc82). This involves introducing the mutation into yHSP90 on Leu2 plasmid and then introducing it into haploid yeast cells (yHSP90Δ, yHSP90Δ). Growth of these cells on 5-fluoroorotic acid (5-F0A) “cures” the yeast cells of the wild-type yHsc90, therefore creating HSP90 mutant.
This chapter describes the isolation and identification of yeast HSP90 phosphorylation using immunoblotting procedures. Using the yeast system, it is possible to show that HSP90 is constitutively phosphorylated on serine and threonine residues. However, HSP90 threonine phosphorylation is lost upon either heat-shock stress or treatment with the HSP90 inhibitor GA (Fig. 2).
Fig. 2.
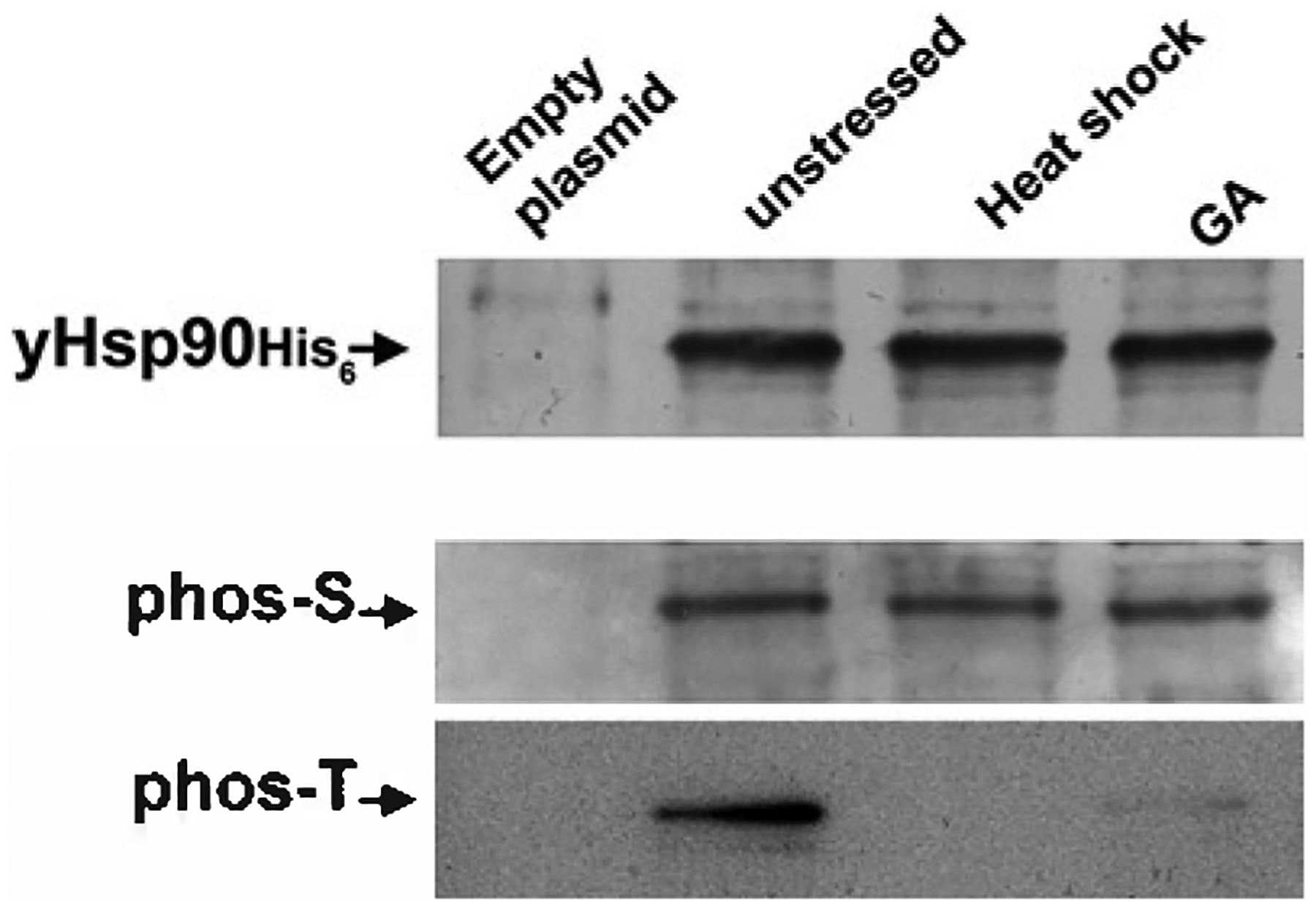
Yeast HSP90 phosphorylation on serine (phos-S) and threonine (phos-T) residues. yHSP90-His6 was purified from yeast cells that were heat shocked at 39° C for 40 min or treated with 100 μM GA for 1 h. Wild-type cells containing the empty plasmid were used as negative control.
2. Materials
YPD (2% (w/v) Bacto peptone, 1% (w/v) yeast extract, 2% (w/v) glucose, 20 mg/1 adenine).
Yeast protein extraction buffer (EB): 50 mM Tris-HCI, pH 6.8, 100 mM NaCl, 50 mM MgCl2. One tablet of complete EDTA-free protease inhibitor cocktail (Roche) and one tablet of PhosphoSTOP (Roche) are added to 50 ml EB.
Bio-Rad Protein Assay solution (Bio-Rad).
425–600 μm glass beads (acid washed), (Sigma).
SDS-PAGE sample buffer (2×): 125 mM Tris-HCI, pH 6.8, 20% glycerol, 2% SDS, 10% 2-mercaptoethanol, 0.01% bromophenol blue, stable at −20°C. Aliquot and avoid freezethaw cycles.
Ponceau S solution (Sigma).
Tris-buffered saline (TBS): 150 mM NaCl, 25 mM Tris base. Adjust pH to 7.4 using HCI. Sterile filter and incubate at 4°C.
Albumin, bovine serum (minimum purity 98%).
Dried skimmed milk.
Protran BASS, 0.45 μm Nitrocellulose membrane (Whatman).
ECL plus Western Blotting Detection System (GE Healthcare).
Ni-NTA agarose (Qiagen).
Imidazole (Sigma).
Phospho-serine QS antibody (Qiagen).
Phospho-threonine Q7 antibody (Qiagen).
Tetra-His antibody (Qiagen).
Anti-secondary mouse antibody; ECL™ antimouse IgG, Horseradish Persoxidase linked whole antibody (GE Healthcare).
X-ray film, X-ray cassette, and X-ray film-developing machine.
3. Methods
The extraction of total yeast protein:
Grow PP30 cells (9) expressing His6 linked at the N-domain of Hsp82 (yHSP90) on 150 ml YPD overnight at 28° C.
Harvest and wash cells two to three times in ice-cold deionized water (dH2O).
Transfer the cell pellet into a screw cap 2-ml tube.
Pellet the cells and remove the supernatant (see Note 1).
Add equal volume of cell pellets, ice-cold glass beads.
Add half the volume of pellet/glass beads, EB.
Bead beat the cells using the mini-beadbeater (BioSpec Products, Inc.) for 30 s.
Incubate the cells on ice for 30 s.
Repeat (steps 7 and 8) 10–12 times.
Centrifuge the tubes at (10,000 ×g) for 10 min at 4° C (see Note 2).
Transfer the supernatants into fresh 1.5-ml microcentrifuge tubes.
Centrifuge the tubes at (10,000 ×g) for 10 min at 4° C (see Note 2).
Transfer the supernatants (soluble protein) into fresh 1.5-ml microcentrifuge tubes.
Protein concentrations were determined using Bio-Rad Protein Assay solution (Bio-Rad).
Transfer 40 μl of Ni-NTA agarose slurry into a 1.5-ml microcentrifuge tube. (see Note 3).
Add 1.0 ml of EB to the Ni-NTA agarose and spin at 10,000 ×g for 1 min at 4°C.
Remove supernatant and add 1.0 ml of EB to the Ni-NTA agarose (see Note 4).
Repeat step (steps 16 and 17) four times.
Resuspend the Ni-NTA agarose in 30 μM imidazole in EB and incubate at 4°C for 30 min (see Note 5).
Repeat steps 16 and 17 twice and remove supernatant.
Add 1 mg of total protein to the Ni-NTA agarose in a total volume of 600 μl.
Incubate the total proteins/Ni-NTA agarose at 4°C for 2 h (see Note 6).
Centrifuge the tubes at (1,000 ×g) for 1 min at 4°C.
Gently remove the supernatant (see Note 7).
Add 1 ml of EB to the Ni-NTA agarose.
Repeat (steps 23–25) five times.
Wash the Ni-NTA agarose with 30-μl EB.
Wash the Ni-NTA agarose with EB once.
Centrifuge the microcentrifuge tube at 15,000 ×g for 1 min at 4°C.
Remove as much supernatant as possible.
Add 40 μl of the protein sample buffer.
Boil the samples for 3–5 min.
Centrifuge the samples at l,000 ×g and load the supernatant on to a 7.5% SDS-PAGE Tris-HCI gel (see Note 8).
Transfer the proteins from SDS-PAGE gel on to ProtranBA85, 0.45 μm nitrocellulose membrane (Whatman).
Examine the quality and efficiency of the transfer by staining the membrane with Ponceau S solution (Sigma) for 2 min (see Note 9).
Wash the membrane with dH2O.
Incubate the membrane in 5% milk-TBS-T for 15–20 min at room temperature.
Wash the membrane with 1× TBS-T for 5 min at room temperature.
Repeat (step 38) three times.
Incubate the membrane with 1:500–1,000 dilution of either phospho-serine (Q5) or phospho-threonine (Q7) antibodies, (Qiagen), in 2% BSA-TBS-T overnight at 4°C (see Note 10).
Wash the membrane three times with 1× TBS-T for 5 min at room temperature.
Incubate the membrane with 1:2,000 dilution of anti-secondary mouse antibody in 5% milk-TBS-T for 1 h (see Note 11) at room temperature.
Wash the membrane three times with 1× TBS-T for 5 min at room temperature.
Remove 1× TBS-T and then apply ECL plus (GE Healthcare) to nitrocellulose membrane for 2–3 min.
Drain nitrocellulose membrane of excess developing solution (do not let dry).
Wrap the blot in saran wrap.
Place the blot in the X-ray film cassette (see Note 12).
Expose the blots to X-ray films by placing X-ray film directly against the western blot for different lengths of time.
4. Notes
The cell pellet must be kept on ice.
At this stage, Bio-Rad Protein Assay solution (Bio-Rad) should be prepared.
Ni-NTA agarose is precharged with Ni2+ ions and appears blue in color. It is provided as a 50% slurry in 30% ethanol.
Do not disturb the Ni-NTA agarose pellet.
Imidazole at low concentrations is commonly used in the binding and wash buffer to minimize binding of unwanted host cell proteins.
Use EppendorfThermomixer R to gently mix total proteins/Ni-NTA agarose solution.
Avoid disturbing the Ni-NTA agarose.
Criterion precast gels from Bio-Rad are suitable for this purpose.
Prepare 5% dry milk (LabScientific Inc.) in 1× TBS-T (0.1% Tween-20, Sigma) buffer before examining the membrane.
Phospho-antibodies stock concentration is 0.1 μg/μl.
1:2,000 dilution of anti-secondary mouse antibody; ECL™ antimouse IgG horseradish peroxidase-inked whole antibody (GE Healthcare).
This procedure must be performed in the dark.
References
- 1.Pearl LH, and Prodromou C (2006) Structure and mechanism of the Hsp90 molecular chaperone machinery, Annu Rev Biochem 75, 271–294. [DOI] [PubMed] [Google Scholar]
- 2.Rutherford S, Knapp JR, and Csermely P (2007) Hsp90 and developmental networks, Adv Exp Med Biol 594, 190–197. [DOI] [PubMed] [Google Scholar]
- 3.Wandinger SK, Richter K, and Buchner J (2008) The Hsp90 chaperone machinery, J Biol Chem 283, 18473–18477. [DOI] [PubMed] [Google Scholar]
- 4.Neckers L (2007) Heat shock protein 90: the cancer chaperone, J Biosci 32, 517–530. [DOI] [PubMed] [Google Scholar]
- 5.Rutherford S, Hirate Y, and Swalla BJ (2007) The Hsp90 capacitor, developmental remodeling, and evolution: the robustness of gene networks and the curious evolvability of metamorphosis, Grit Rev Biochem Mot Biol 42, 355–372. [DOI] [PubMed] [Google Scholar]
- 6.Picard D (2006) Chaperoning steroid hormone action, Trends Endocrinol Metab 17, 229–235. [DOI] [PubMed] [Google Scholar]
- 7.Truman AW, Millson SH, Nuttall JM, King V, Mollapour M, Prodromou C, Pearl LH, and Piper PW (2006) Expressed in the yeast Saccharomyces cerevisiae, human ERKS is a client of the Hsp90 chaperone that complements loss of the Slt2p (Mpklp) cell integrity stress-activated protein kinase, Eukaryot Cell 5, 1914–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prodromou C, and Pearl LH (2003) Structure and functional relationships of Hsp90, Curr Cancer Drug Targets 3, 301–323. [DOI] [PubMed] [Google Scholar]
- 9.Panaretou B, Prodromou C, Roe SM, O’Brien R, Ladbury JE, Piper PW, and Pearl LH (1998) ATP binding and hydrolysis are essential to the function of the Hsp90 molecular chaperone in vivo, EMBO J 17, 4829–4836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Obermann WM, Sondermann H, Russo AA, Pavletich NP, and Hartl FU (1998) In vivo function of Hsp90 is dependent on ATP binding and ATP hydrolysis, J Cell Biol 143, 901–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grenert JP, Johnson BD, and Toft DO (1999) The importance of ATP binding and hydrolysis by hsp90 in formation and function of protein heterocomplexes, J Biol Chem 274, 17525–17533. [DOI] [PubMed] [Google Scholar]
- 12.Prodromou C, Roe SM, O’Brien R, Ladbury JE, Piper PW, and Pearl LH (1997) Identification and structural characterization of the ATP/ ADP-binding site in the Hsp90 molecular chaperone, Cell 90, 65–75. [DOI] [PubMed] [Google Scholar]
- 13.Grenert JP, Sullivan WP, Fadden P, Haystead TA, Clark J, Mimnaugh E, Krutzsch H, Ochel HJ, Schulte TW, Sausville E, Neckers LM, and Toft DO (1997) The amino-terminal domain of heat shock protein 90 (hsp90) that binds geldanamycin is an ATP/ADP switch domain that regulates hsp90 conformation, J Biol Chem 272,23843–23850. [DOI] [PubMed] [Google Scholar]
- 14.Stebbins CE, Russo AA, Schneider C, Rosen N, Hartl FU, and Pavletich NP (1997) Crystal structure of an Hsp90-geldanamycin complex: targeting of a protein chaperone by an antitumor agent, Cell 89, 239–250. [DOI] [PubMed] [Google Scholar]
- 15.Roe SM, Prodromou C, O’Brien R, Ladbury JE, Piper PW, and Pearl LH (1999) Structural basis for inhibition of the Hsp90 molecular chaperone by the antitumor antibiotics radicicol and geldanamycin, J Med Chem 42, 260–266. [DOI] [PubMed] [Google Scholar]
- 16.Workman P, Burrows F, Neckers L, and Rosen N (2007) Drugging the cancer chaperone HSP90: combinatorial therapeutic exploitation of oncogene addiction and tumor stress, Ann NY Acad Sci 1113, 202–216. [DOI] [PubMed] [Google Scholar]
- 17.Neckers L (2006) Chaperoning oncogenes: Hsp90 as a target of geldanamycin, Handb Exp Pharmacol, 259–277. [DOI] [PubMed] [Google Scholar]
- 18.Abbas-Terki T, Briand PA, Donze O, and Picard D (2002) The Hsp90 co-chaperones Cdc37 and Stil interact physically and genetically, Biol Chem 383, 1335–1342. [DOI] [PubMed] [Google Scholar]
- 19.Chang HC, Nathan DF, and Lindquist S (1997) In vivo analysis of the Hsp90 cochaperone Stil (p60), Mot Cell Biol 17, 318–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Richter K, Muschler P, Hainzl O, Reinstein J, and Buchner J (2003) Stil is a non-competitive inhibitor of the Hsp90 ATPase. Binding prevents the N-terminal dimerization reaction during the atpase cycle, J Biol Chem 278, 10328–10333. [DOI] [PubMed] [Google Scholar]
- 21.Song Y, and Masison DC (2005) Independent regulation of Hsp70 and Hsp90 chaperones by Hsp70/Hsp90-organizing protein Stil (Hopl), J Biol Chem 280, 34178–34185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee P, Shabbir A, Cardozo C, and Caplan AJ (2004) Stil and Cdc37 can stabilize Hsp90 in chaperone complexes with a protein kinase, Mol Biol Cell 15, 1785–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.MacLean M, and Picard D (2003) Cdc37 goes beyond Hsp90 and kinases, Cell Stress Chaperones 8, 114–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Siligardi G, Panaretou B, Meyer P, Singh S, Woolfson DN, Piper PW, Pearl LH, and Prodromou C (2002) Regulation of Hsp90 ATPase activity by the co-chaperone Cdc37p/p50cdc37, J Biol Chem 277, 20151–20159. [DOI] [PubMed] [Google Scholar]
- 25.Vaughan CK, Mollapour M, Smith JR, Truman A, Hu B, Good VM, Panaretou B, Neckers L, Clarke PA, Workman P, Piper PW, Prodromou C, and Pearl LH (2008) Hsp90-dependent activation of protein kinases is regulated by chaperone-targeted dephosphorylation ofCdc37, Mot Cell 31, 886–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McLaughlin SH, Sobott F, Yao ZP, Zhang W, Nielsen PR, Grossmann JG, Laue ED, Robinson CV, and Jackson SE (2006) The co-chaperone p23 arrests the Hsp90 ATPase cycle to trap client proteins, J Mol Biol 356, 746–758. [DOI] [PubMed] [Google Scholar]
- 27.Picard D (2006) Intracellular dynamics of the Hsp90 co-chaperone p23 is dictated by Hsp90, Exp Cell Res 312, 198–204. [DOI] [PubMed] [Google Scholar]
- 28.Sullivan WP, Owen BA, and Toft DO (2002) The influence ofA TP and p23 on the conformation of hsp90, J Biol Chem 277, 45942–45948. [DOI] [PubMed] [Google Scholar]
- 29.Lotz GP, Lin H, Harst A, and Obermann WM (2003) Ahal binds to the middle domain of Hsp90, contributes to client protein activation, and stimulates the ATPase activity of the molecular chaperone, J Biol Chem 278, 17228–17235. [DOI] [PubMed] [Google Scholar]
- 30.Meyer P, Prodromou C, Liao C, Hu B, Mark Roe S, Vaughan CK, Vlasic I, Panaretou B, Piper PW, and Pearl LH (2004) Structural basis for recruitment of the ATPase activator Ahal to the Hsp90 chaperone machinery, EMBO J 23, 511–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Panaretou B, Siligardi G, Meyer P, Maloney A, Sullivan JK, Singh S, Millson SH, Clarke PA, Naaby-Hansen S, Stein R, Cramer R, Mollapour M, Workman P, Piper PW, Pearl LH, and Prodromou C (2002) Activation of the ATPase activity of hsp90 by the stress-regulated cochaperone ahal, Mot Cell 10, 1307–1318. [DOI] [PubMed] [Google Scholar]
- 32.Johnson JL, Halas A, and Flom G (2007) Nucleotide-dependent interaction of Saccharomyces cerevisiae Hsp90 with the cochaperone proteins Stil, Cpr6, and Sbal, Mot Cell Biol 27, 768–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mayr C, Richter K, Lilie H, and Buchner J (2000) Cpr6 and Cpr7, two closely related Hsp90-associated immunophilins from Saccharomyces cerevisiae, differ in their functional properties, J Biol Chem 275, 34140–34146. [DOI] [PubMed] [Google Scholar]
- 34.Scroggins BT, and Neckers L (2007) Post-translational modification of heat shock protein 90: impact on chaperone function, Expert Opin. Drug Discop 2, 1403–1414. [DOI] [PubMed] [Google Scholar]
- 35.Mimnaugh EG, Worland PJ, Whitesell L, and Neckers LM (1995) Possible role for serine/threonine phosphorylation in the regulation of the heteroprotein complex between the hsp90 stress protein and the pp60v-src tyrosine kinase, J Biol Chem 270, 28654–28659. [DOI] [PubMed] [Google Scholar]
- 36.Garnier C, Lafitte D, Jorgensen TJ, Jensen ON, Briand C, and Peyrot V (2001) Phosphorylation and oligomerization states of native pig brain HSP90 studied by mass spectrometry, Eur J Biochem 268, 2402–2407. [DOI] [PubMed] [Google Scholar]
- 37.Zhao YG, Gilmore R, Leone G, Coffey MC, Weber B, and Lee PW (2001) Hsp90 phosphorylation is linked to its chaperoning function. Assembly of the reovirus cell attachment protein, J Biol Chem 276, 32822–32827. [DOI] [PubMed] [Google Scholar]
- 38.Fontana J, Fulton D, Chen Y, Fairchild TA, McCabe TJ, Fujita N, Tsuruo T, and Sessa WC (2002) Domain mapping studies reveal that the M domain of hsp90 serves as a molecular scaffold to regulate Akt-dependent phosphorylation of endothelial nitric oxide synthase and NO release, Gire Res 90, 866–873. [DOI] [PubMed] [Google Scholar]
- 39.Adinolfi E, Kim M, Young MT, Di Virgilio F, and Surprenant A (2003) Tyrosine phosphorylation of HSP90 within the P2X7 receptor complex negatively regulates P2X7 receptors, J Biol Chem 278, 37344–37351. [DOI] [PubMed] [Google Scholar]
- 40.Wang C, and Chen J (2003) Phosphorylation and hsp90 binding mediate heat shock stabilization ofp53, J Biol Chem 278, 2066–2071. [DOI] [PubMed] [Google Scholar]
- 41.Duval M, Le Boeuf F, Huot J, and Gratton JP (2007) Src-mediated phosphorylation of Hsp90 in response to vascular endothelial growth factor (VEGF) is required for VEGF receptor-2 signaling to endothelial NO synthase, Mol Biol Cell 18, 4659–4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miyata Y, and Yahara I (1992) The 90-kDa heat shock protein, HSP90, binds and protects casein kinase II from self-aggregation and enhances its kinase activity, J Biol Chem 267, 7042–7047. [PubMed] [Google Scholar]
- 43.Ogiso H, Kagi N, Matsumoto E, Nishimoto M, Arai R, Shirouzu M, Mimura J, FujiiKuriyama Y, and Yokoyama S (2004) Phosphorylation analysis of 90 kDa heat shock protein within the cytosolic arylhydrocarbonreceptor complex, Biochemistry 43, 15510–15519. [DOI] [PubMed] [Google Scholar]


