The catalase-negative streptococci produce as well as tolerate high levels of H2O2. This work reports the molecular mechanisms of low-H2O2-concentration-induced adaptation to higher H2O2 stress in a Streptococcus species, in which the peroxide-responsive repressor PerR and its redox regulons play the major role. Distinct from the Bacillus subtilis PerR, which is inactivated by H2O2 through histidine oxidation by the Fe2+-triggered Fenton reaction, the streptococcal PerR is inactivated by H2O2 oxidation of the structural Zn2+ binding cysteine residues and thus derepresses the expression of genes defending against oxidative stress. The reversible cysteine oxidation could provide flexibility for PerR regulation in streptococci, and the mechanism might be widely used by lactic acid bacteria, including pathogenic streptococci, containing high levels of cellular manganese, in coping with oxidative stress. The adaptation mechanism could also be applied in oral hygiene by facilitating the fitness and adaptability of the oral commensal streptococci to suppress the pathogens.
KEYWORDS: Streptococcus, cysteine oxidation, hydrogen peroxide, posttranslational regulation, redox signaling, transcriptional regulation
ABSTRACT
Preexposure to a low concentration of H2O2 significantly increases the survivability of catalase-negative streptococci in the presence of a higher concentration of H2O2. However, the mechanisms of this adaptation remain unknown. Here, using a redox proteomics assay, we identified 57 and 35 cysteine-oxidized proteins in Streptococcus oligofermentans bacteria that were anaerobically cultured and then pulsed with 40 μM H2O2 and that were statically grown in a 40-ml culture, respectively. The oxidized proteins included the peroxide-responsive repressor PerR, the manganese uptake repressor MntR, thioredoxin system proteins Trx and Tpx, and most glycolytic proteins. Cysteine oxidations of these proteins were verified through redox Western blotting, immunoprecipitation, and liquid chromatography-tandem mass spectrometry assays. In particular, Zn2+-coordinated Cys139 and Cys142 mutations eliminated the H2O2 oxidation of PerR, and inductively coupled plasma mass spectrometry detected significantly decreased amounts of Zn2+ in H2O2-treated PerR, demonstrating that cysteine oxidation results in Zn2+ loss. An electrophoretic mobility shift assay (EMSA) determined that the DNA binding of Mn2+-bound PerR protein (PerR:Zn,Mn) was abolished by H2O2 treatment but was restored by dithiothreitol reduction, verifying that H2O2 inactivates streptococcal PerR:Zn,Mn through cysteine oxidation, analogous to the findings for MntR. Quantitative PCR and EMSA demonstrated that tpx, mntA, mntR, and dpr belonged to the PerR regulons but that only dpr was directly regulated by PerR; mntA was also controlled by MntR. Deletion of mntR significantly reduced the low-H2O2-concentration-induced adaptation of S. oligofermentans to a higher H2O2 concentration, while the absence of PerR completely abolished the self-protection. Therefore, a low H2O2 concentration resulted in the cysteine-reversible oxidations of PerR and MntR to derepress their regulons, which function in cellular metal and redox homeostasis and which endow streptococci with the antioxidative capability. This work reveals a novel Cys redox-based H2O2 defense strategy employed by catalase-negative streptococci in Mn2+-rich cellular environments.
IMPORTANCE The catalase-negative streptococci produce as well as tolerate high levels of H2O2. This work reports the molecular mechanisms of low-H2O2-concentration-induced adaptation to higher H2O2 stress in a Streptococcus species, in which the peroxide-responsive repressor PerR and its redox regulons play the major role. Distinct from the Bacillus subtilis PerR, which is inactivated by H2O2 through histidine oxidation by the Fe2+-triggered Fenton reaction, the streptococcal PerR is inactivated by H2O2 oxidation of the structural Zn2+ binding cysteine residues and thus derepresses the expression of genes defending against oxidative stress. The reversible cysteine oxidation could provide flexibility for PerR regulation in streptococci, and the mechanism might be widely used by lactic acid bacteria, including pathogenic streptococci, containing high levels of cellular manganese, in coping with oxidative stress. The adaptation mechanism could also be applied in oral hygiene by facilitating the fitness and adaptability of the oral commensal streptococci to suppress the pathogens.
INTRODUCTION
Reactive oxygen species (ROS), such as superoxide anions (O2−), hydrogen peroxide (H2O2), and hydroxyl radicals (HO·), damage almost all biological macromolecules (1–3). Therefore, organisms have evolved diverse mechanisms to cope with ROS (1–4). Facultatively anaerobic streptococci, such as the human opportunistic pathogen Streptococcus pneumoniae and the oral commensal bacterium Streptococcus oligofermentans, do not encode H2O2-scavenging catalase and thus accumulate endogenous H2O2 (5–8). Streptococci are also well-known for surviving in the presence of high concentrations of H2O2 (6, 9, 10). Previously, we determined that statically grown S. oligofermentans cultures have an approximately 200-fold higher survival rate than cells anaerobically cultured in 10 mM H2O2 (11). A similar observation has also been reported for S. pneumoniae (8). This suggests that the low levels of H2O2 that accumulate in statically cultured cells may assist streptococci with resisting the oxidant at higher concentrations. However, the biological basis of this low-H2O2-concentration-induced adaptation remains unknown.
Bacteria usually use cysteine-based redox reactions to sense H2O2 and activate the downstream peroxide detoxification pathways (12–14). Escherichia coli OxyR was the first identified archetype of thiol-based redox regulators in bacteria; it is activated by intramolecular thiol-disulfide formation resulting from H2O2 oxidation and thereby induces expression of the genes involved in defending against oxidative stress (15). Gram-positive bacteria, on the other hand, utilize the peroxide-responsive repressor PerR to sense H2O2 and derepress the H2O2 resistance genes (11, 16, 17). PerR, a member of the Fur family of metal-dependent regulators, possesses two metal-binding sites: a regulatory Fe2+ or Mn2+ binding site consisting of histidine and aspartate residues and a structural Zn2+ binding site comprising four cysteine residues (18, 19). The Bacillus subtilis PerR is inactivated by H2O2 via metal-catalyzed oxidation (MCO) (20). When binding Fe2+, PerR is inactivated by Fenton chemistry-generated HO· from H2O2, which oxidizes the histidine residues. In contrast, the cysteine residues of the B. subtilis PerR that coordinate Zn2+ for structural maintenance are somehow inert to H2O2 (20). Therefore, PerR:Zn,Fe (Fe2+-bound PerR) but not PerR:Zn,Mn responds to H2O2 (17, 19). Makthal et al. (21) also reported that H2O2 inactivates the recombinant Streptococcus pyogenes PerR:Zn,Fe, suggesting that Fe2+-triggered Fenton chemistry could inactivate the streptococcal PerR as well. However, an in vivo study demonstrated that the S. pyogenes PerR:Zn,Mn also displays a weaker response to H2O2 (22). Previously, we found that the S. oligofermentans PerR is inactivated by H2O2 and derepresses the antioxidative non-heme iron-containing ferritin, dpr, and manganese importer mntABC genes (11). However, even if grown in Mn2+-supplemented medium, H2O2 still induces the expression of dpr. This implies that the streptococcal PerR can be inactivated by mechanisms other than Fe2+-triggered Fenton chemistry.
The redox-sensing transcriptional regulators usually respond to H2O2 challenge through cysteine oxidation (12, 13, 23). Recently, this thiol redox switch-based regulatory mechanism was found to be employed by other transcriptional regulators, such as AgrA in the control of the quorum sensing of Staphylococcus aureus (24) and MntR in the regulation of manganese uptake and the oxidative stress resistance of S. oligofermentans (25). Thiol redox proteomics is a powerful approach for the quantification of oxidative thiol modifications and the identification of physiologically important proteins in oxidative stress resistance (26–28). Using this approach, a number of novel redox-regulated proteins that contribute to the protection of E. coli from H2O2 stress (29) have been identified. Recently, proteome-wide quantification and characterization of the oxidation-sensitive cysteine residues have determined complex and multilayered oxidative stress responses in pathogenic bacteria, such as Pseudomonas aeruginosa, S. aureus, and S. pneumoniae (8, 30). Therefore, cysteine-containing proteins not only serve as H2O2-damaged targets but also equip bacteria with the capability to resist H2O2 stress.
To elucidate the mechanisms underlying low-H2O2-concentration-induced resistance to high concentrations of H2O2 in streptococci, we employed physiological, biochemical, genetic, and redox proteomics approaches to investigate the H2O2-sensitive cysteine-containing proteins that may be involved in H2O2 adaptation. We determined that cellular H2O2 levels ranging from 40 to 100 μM protected S. oligofermentans from insult by higher H2O2 concentrations. Redox proteomics identified cysteine oxidation in the H2O2-responsive transcriptional regulators PerR and MntR, which regulate antioxidative stress in response to H2O2, as well as in the thioredoxin system proteins Tpx and Trx, which function in thiol-disulfide homeostasis. Importantly, 40 μM H2O2 oxidized the Zn2+-coordinated cysteine residues and inactivated PerR, thus derepressing its regulons, which function in the thiol redox circuit and metal homeostasis. The high sensitivity of the cysteine residues to H2O2 enables PerR to sense low levels of H2O2 and thus protect the catalase-negative species S. oligofermentans from H2O2 challenge by maneuvering the H2O2 resistance systems. Moreover, the reversible cysteine oxidation resulting from a low H2O2 concentration can also endow the streptococcal PerR with flexibility in H2O2-responsive regulation.
RESULTS
Preexposure to a low H2O2 concentration enables S. oligofermentans to resist higher H2O2 concentrations.
Previously, we found that aerobically cultured S. oligofermentans exhibits significantly higher resistance to H2O2 stress than anaerobic cultures (11), suggesting that the endogenous H2O2 that accumulates in the static culture may protect streptococci from damage in the presence of higher H2O2 concentrations. To validate this presumption, we deleted both the pox and lox genes, which encode pyruvate oxidase and lactate oxidase, respectively, the two major H2O2 producers in S. oligofermentans (5, 6). As expected, when exposed to 20 mM H2O2, only 0.02% survival was found for pox lox mutant cells; in comparison, 30% survival was found for wild-type cells (Table 1).
TABLE 1.
Prepulsing with a low H2O2 concentration increases the survival rates of various S. oligofermentans strains in the presence of a higher H2O2 concentration
| Culture | Survival rate (%)a
|
|||
|---|---|---|---|---|
| Wild-type strain |
Δpox Δlox
mutant |
ΔmntR
mutant |
ΔperR
mutant |
|
| Static culture | 30 ± 3.27* | 0.02 ± 0.01* | ND | ND |
| Anaerobic culture | ||||
| Nonprepulse | 0.21 ± 0.07 | 0.23 ± 0.06 | 7.94 ± 3.18 | 75 ± 11 |
| Prepulse with 40 μM H2O2 | 77 ± 33 | 66 ± 12 | 62 ± 26 | 86 ± 14 |
| Prepulse with 100 μM H2O2 | 46 ± 4 | ND | ND | ND |
Strains were grown anaerobically in a 6-ml BHI culture, and then the survival percentages in 10 mM H2O2 were determined for all strains except for strains labeled with an asterisk, which were statically grown in a 40-ml culture and challenged with 20 mM H2O2. The survival percentage was calculated by dividing the number of CFU in the H2O2-treated culture by that in the untreated culture. The experiments were repeated three times with triplicate batch cultures each time. The results are averages ± SD from three independent experiments. ND, not determined.
To verify if the loss of H2O2 resistance in the pox lox mutant was due to the lack of endogenous H2O2 but not the reduction of acetyl phosphate, which is produced by Pox and which contributes to S. pneumoniae H2O2 resistance (31), we determined whether a preexposure to a low concentration of H2O2 could increase the higher H2O2 resistance of the pox lox mutant. The wild-type and pox lox mutant strains were anaerobically grown until the optical density at 600 nm (OD600) was ∼0.5. One aliquot of the cultures, noted as the prepulse group, was pulsed for 20 min with 40 μM H2O2 prior to a 10-min challenge with 10 mM H2O2. Another aliquot, the nonprepulse group, was directly treated with 10 mM H2O2. Samples not treated with 10 mM H2O2 were included as controls. Table 1 shows that prepulsing with 40 μM H2O2 greatly improved the survival of the pox lox mutant in the presence of 10 mM H2O2 to 66%, whereas that for the nonprepulsed group was 0.23%. Similarly, prepulsing with 40 μM H2O2 increased the survival rate of the wild-type strain to 77%, whereas that for the nonprepulsed group was 0.21%. Moreover, prepulsing with 100 μM H2O2 also elevated the survival rate of the wild-type strain to 46% in the presence of 10 mM H2O2 (Table 1). These results confirm that endogenous H2O2 at low concentrations plays an important role in the protection of Streptococcus from insult by a higher H2O2 concentration.
Estimation of endogenous H2O2 levels for self-protection and oxidative stress by HyPer fluorescent protein.
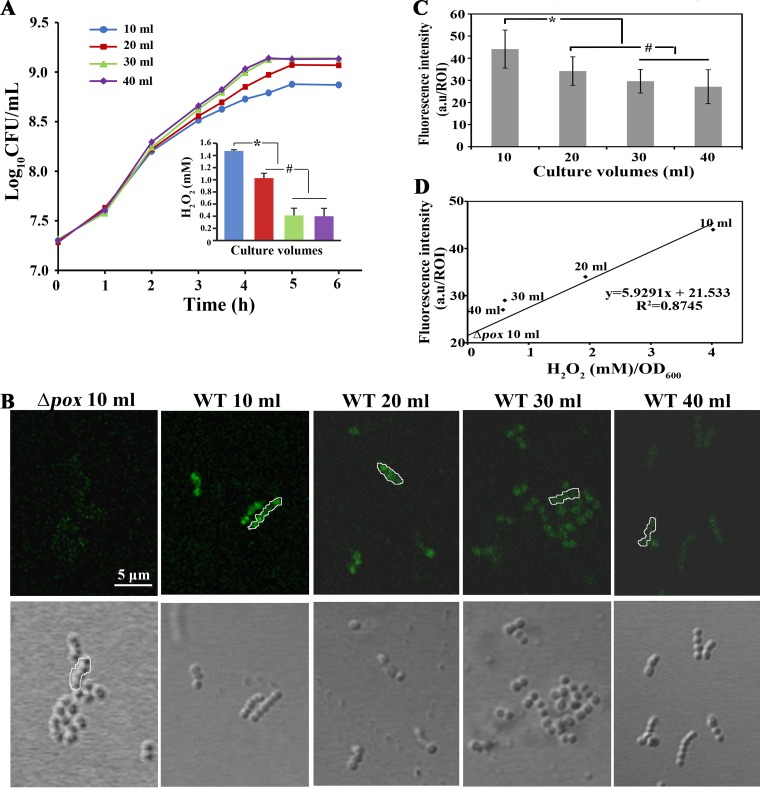
Given that streptococci accumulate endogenous H2O2, we used the HyPer fluorescent protein to estimate intracellular H2O2 levels (32) and compared them with those excreted into the culture. The wild-type (WT) HyPer reporter strain of S. oligofermentans, WT-HyPer (33), was statically grown in 10, 20, 30, and 40 ml of brain heart infusion (BHI) broth in 100-ml flasks, which built an initial O2 supply gradient. The growth profiles and the H2O2 amounts in the cultures were measured. Figure 1A shows that the best growth and the lowest H2O2 concentration (approximately 400 μM) were measured for the 40-ml culture. In contrast, the poorest growth and the highest H2O2 level (approximately 1,400 μM) were detected in the 10-ml culture, indicating that larger amounts of H2O2 are produced by Streptococcus with a rich supply of oxygen and thus suppress its growth.
FIG 1.
Correlation between growth suppression and the cellular H2O2 contents of S. oligofermentans. (A) An overnight culture of the HyPer reporter strain WT-HyPer was diluted 1:30 into 10, 20, 30, or 40 ml of BHI broth in 100-ml Erlenmeyer flasks and statically cultured at 37°C. Growth profiles were monitored by counting the numbers of CFU at the indicated time points. (Inset) H2O2 concentrations in the stationary-phase cultures were determined as described in Materials and Methods and are shown as bar diagrams using the color corresponding to the color of the growth curves for the same culture volumes. Experiments were conducted with three batches of culture and three replicates for each. Averages ± SD from three independent experiments are shown. * and #, the data are significantly different from those determined for 10-ml cultures and those determined for both the 10- and 20-ml cultures, respectively, as verified by one-way analysis of variance followed by Tukey’s post hoc test (P < 0.05). (B) One milliliter of mid-exponential-phase WT-HyPer cells was collected from the cultures for which the results are shown in panel A, washed twice with PBS, and resuspended in 100 μl PBS. After a 30-min air exposure in the dark, HyPer fluorescence was examined using a confocal laser scanning microscope system (Leica model TCS SP8). The Δpox-HyPer cells grown in 10 ml of culture were included as an endogenous H2O2-negative control. Representative fluorescent and corresponding differential interference contrast (DIC) images from three independent experiments are shown. (C) The HyPer fluorescence intensities of the cells shown in panel B were measured using Leica Application Suite (LAS) AF software. At least five images were captured per sample, and 25 regions of interest (ROI; outlines framed in panel B), each containing 5 cells, were measured for calculation of the average fluorescence intensity of each sample. For images with fluorescence that was too weak, the ROI in the corresponding DIC images was framed, and the fluorescence in the corresponding ROI of the fluorescence image was measured. Average fluorescence intensities were calculated and are expressed in arbitrary units (a.u.) per ROI ± standard deviation. *and #, data are significantly different from those obtained from 10-ml cultures and those determined from both 10- and 20-ml cultures, respectively (P < 0.05, one-way analysis of variance followed by Tukey’s post hoc test). (D) A linear regression curve of the HyPer fluorescence intensities in Δpox-HyPer and WT-HyPer cells plotted against the extracellular H2O2 concentrations in the corresponding culture volumes.
Next, the mid-exponential-phase cells of the WT-HyPer strain from each volume of cultures were visualized under a confocal laser scanning microscope (Leica model TCS SP8), and the HyPer fluorescence intensities were measured as described in Materials and Methods. The Δpox-HyPer mutant, the pox deletion mutant carrying the HyPer gene (33), was included as a control from which H2O2 was absent. Figure 1B and C show that the HyPer fluorescence intensities were inversely proportional to the culture volumes but directly proportional to the H2O2 concentrations in the cultures, with a good linear regression (R2 = 0.8745) (Fig. 1D). This indicates that the quantity of H2O2 in a culture indicates an equivalent amount within the cells.
Redox proteomics identifies cysteine-oxidized proteins by the low H2O2 concentration that induces self-protection from oxidative stress.
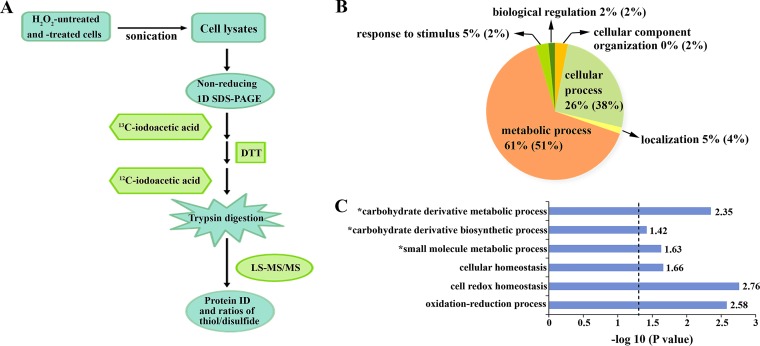
To identify the proteins that are sensitive to a low H2O2 concentration and that might be involved in self-protection from oxidative stress, label-free redox proteomics analysis was performed to identify the cysteine-oxidized proteins in 40 μM H2O2-pulsed anaerobically grown S. oligofermentans. Proteins were extracted from H2O2-treated and -untreated cells, and cysteine thiol group oxidations were analyzed using a combination of differential alkylation and liquid chromatography (LC)-tandem mass spectrometry (MS/MS) (28, 34) (Fig. 2A). The representative MS/MS spectra shown in Fig. S1A and B in the supplemental material demonstrated the reliable identification of the cysteine-modified peptides.
FIG 2.
Redox proteomic identification of proteins whose cysteines were oxidized by endogenous or exogenous H2O2 at levels protecting S. oligofermentans from oxidative stress. (A) Flowchart of redox proteomic analysis, performed using differential alkylation and LC-MS/MS. The free and disulfide-oxidized thiol groups were modified by [13C]- and [12C]iodoacetic acid, respectively. Detailed experimental procedures are described in Materials and Methods. 1D, one dimensional; ID, identifier. (B) Functional classification of the proteins with cysteine residues reversibly or irreversibly oxidized in the wild-type strain statically grown in a 40-ml culture and the 40 μM H2O2-pulsed anaerobically grown wild-type strain (percentages in parentheses), based on Gene Ontology (GO) analysis. (C) Overrepresented biological processes associated with cysteine-oxidized proteins were examined using a statistical overrepresentation test on the Gene Ontology Consortium website. The binomial test was used for statistical significance analysis using a P value of <0.05 as a cutoff, which is indicated by a dashed line. Asterisks indicate the biological processes enriched in both 40 μM H2O2-pulsed cells and cells grown in the 40-ml culture. The remaining items were enriched only in cells grown in the 40-ml culture.
Representative MS/MS spectra for identification of the redox-sensitive proteins and the cysteine-oxidized and -reduced peptide fragment of PerR, Tpx, and Trx of S. oligofermentans. (A and B) Representative MS/MS spectra of the doubly charged fragment ions at m/z 888.9315 (A) and 887.9282 (B), corresponding to peptide fragment AIC235EETGNGHVQLFAK from pyruvate kinase with reduced (13C-carboxymethylated) and oxidized (12C-carboxymethylated) cysteine residues, respectively. (C) The MS/MS spectrum of the doubly charged fragment ion at m/z 1143.6489, corresponding to the peptide fragment SQMVVYGIC139PEC142AQQEQVAS from PerR, in which two cysteine residues were oxidized (12C carboxymethylated). The spectrum was obtained from a preliminary redox proteomic experiment. (D) MS/MS spectrum of a doubly charged fragment ion at m/z 968.5062 for MH22+, corresponding to the Tpx peptide fragment VLSIVPSIDTGVC58STQTR from H2O2-untreated cells, in which the cysteine residue was reduced (13C carboxymethylated). (E and F) MS/MS spectra of a doubly charged fragment ion at m/z 968.5067 and 967.5011 for MH22+, corresponding to the Tpx peptide fragment VLSIVPSIDTGVC58STQTR from H2O2-treated cells, in which the cysteine residue was reduced (13C carboxymethylated) and oxidized (12C carboxymethylated), respectively. (G and H) MS/MS spectra of a triply charged fragment ion at m/z 487.5490 and 486.8803 for MH23+, corresponding to the peptide FWASWC79GPC82KR of Trx from H2O2-untreated cells, in which the Cys82 residue was reduced (13C carboxymethylated) and oxidized (12C carboxymethylated), respectively. (I) MS/MS spectrum of a doubly charged fragment ion at m/z 729.8172 for MH22+, corresponding to the peptide FWASWC79GPC82KR of Trx from H2O2-treated cells, in which the Cys82 residue was oxidized (12C carboxymethylated). (Insets) Fragments of the relevant peptide sequences matching the observed fragmentation ions. Download FIG S1, TIF file, 2.0 MB (2.2MB, tif) .
Copyright © 2020 Tong et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
LC-MS/MS identified 964 proteins in the samples not treated with H2O2 and 1,141 proteins in those pulsed with 40 μM H2O2; 923 were consistently detected in both samples. Among those, 93 cysteine-containing proteins were detected in H2O2-untreated cells and 132 were detected in 40 μM H2O2-treated cells (Data Set S1A to D). Proteins with reversible (S-S or SOH) or irreversible thiol oxidation (SO2H or SO3H) were identified by comparison with those in H2O2-untreated cells. The S-S oxidation ratio (in percent) in each sample was calculated by dividing the intensity of the disulfide-linked peptides by the sum of the peptides and considering a cutoff value of a ≥1.5-fold oxidation ratio in H2O2-treated cells over that in untreated cells to be significant (29). Proteins identified as SOH or SO2H or SO3H oxidations were those found only in H2O2-treated samples or with a ≥1.5-fold elevated peptide intensity compared to that for the control. In summary, 40 μM H2O2 treatment resulted in thiol group oxidation in 57 cysteine-containing proteins (Data Set S1E). Among these, 25 proteins containing 32 cysteine residues were oxidized into disulfide linkages (S-S), with a >50-fold increased disulfide ratio in 21 proteins; 3 proteins were reversibly oxidized as SOH; and the remaining 29 proteins were irreversibly oxidized as SO2H or SO3H. Thus, these 57 proteins are assumed to be involved in self-protection from H2O2 challenge.
As S. oligofermentans cells statically grown in the 40-ml culture survived the 20 mM H2O2 challenge (Table 1), we identified the cysteine-oxidized proteins in this volume of culture. A total of 1,093 proteins were identified by LC-MS/MS analysis, including 108 cysteine-containing proteins (Data Set S2A and B). Calculations indicated that 35 proteins were oxidized, with 26 cysteine residues in 23 proteins being reversibly oxidized into disulfide linkages (S-S) and the remaining 12 proteins being irreversibly oxidized as SO2H or SO3H (Data Set S2E). However, in cells cultured in 10-ml cultures that accumulated larger amounts of H2O2, 66 of the 164 cysteine-containing proteins were oxidized, with 33 being oxidized as S-S and 33 being oxidized as SO2H or SO3H (Data Set S2C to E). Thirty-one proteins that were specifically oxidized in 10- ml cultures belonged to organic acid and organic nitrogen metabolic processes (Data Set S2F), accounting for the growth retardation of S. oligofermentans under oxidative stress.
(A) Redox proteomics-identified proteins in the S. oligofermentans wild-type strain without 40 μM H2O2 treatment. (B) Redox proteomics-identified proteins in the S. oligofermentans wild-type strain pulsed with 40 μM H2O2. (C) Redox proteomics-identified cysteine-containing proteins in the S. oligofermentans wild-type strain without 40 μM H2O2 treatment. (D) Redox proteomics-identified cysteine-containing proteins in the S. oligofermentans wild-type strain pulsed with 40 μM H2O2. (E) Reversible or irreversible oxidized cysteine-containing proteins upon H2O2 treatment in the wild-type strain. +, a peptide with an SOH or SO2H/SO3H modification was identified in the tested sample; a, the thiol/disulfide oxidized ratio was calculated by dividing the intensity of the oxidized disulfide-linked peptide (12C carboxymethylated) over the sum of the disulfide-oxidized (12C-carboxymethylated) and -reduced (13C-carboxymethylated) peptides in the corresponding sample. (F) Overrepresented biological processes of the cysteine-oxidized proteins identified in the S. oligofermentans wild-type strain upon 40 μM H2O2 treatment Download Data Set S1, XLSX file, 0.3 MB (347.8KB, xlsx) .
Copyright © 2020 Tong et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
(A) Redox proteomics-identified proteins in the S. oligofermentans wild-type strain grown in 40-ml cultures. (B) Redox proteomics-identified cysteine-containing proteins in the S. oligofermentans wild-type strain grown in 40-ml cultures. (C) Redox proteomics-identified proteins in the S. oligofermentans wild-type strain grown in 10-ml cultures. (D) Redox proteomics-identified cysteine-containing proteins in the S. oligofermentans wild-type strain grown in 10-ml cultures. (E) Reversible or irreversible oxidized cysteine-containing proteins identified in the S. oligofermentans wild-type strain grown in different volumes of culture medium. *, ND, the peptide fragment was not detected in the tested sample; +, the peptide with the SOH or SO2H/SO3H modification was identified in the tested sample; a, the thiol/disulfide oxidized ratio was calculated by dividing the intensity of oxidized disulfide-linked peptide (12C carboxymethylated) by the sum of the disulfide-oxidized (12C-carboxymethylated) and -reduced (13C-carboxymethylated) peptide in the corresponding sample. (F) 1, overrepresented biological processes of the cysteine-oxidized proteins identified in the S. oligofermentans wild-type strain grown in 40-ml cultures; 2, overrepresented biological processes of the cysteine-oxidized proteins identified in the S. oligofermentans wild-type strain grown in 10-ml cultures. Download Data Set S2, XLSX file, 0.3 MB (345.7KB, xlsx) .
Copyright © 2020 Tong et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
To link the biological functions of the H2O2-sensitive proteins, Gene Ontology (GO) analysis was performed by use of the PANTHER bioinformatics platform (http://www.pantherdb.org/) (35). Figure 2B shows that the proteins oxidized by endogenous H2O2 (in 40-ml aerobic cultures) and exogenously provided H2O2 (for 40 μM H2O2-pulsed anaerobic cells) were categorized into similar biological processes, with approximately 61% and 51% of the proteins, respectively, being involved in metabolic processes and 26% and 38% of the proteins, respectively, being involved in cellular processes. Remarkably, almost all the proteins in the glycolysis and nucleotide salvage pathways were oxidized to form disulfide linkages (Table 2; Fig. 2C; Data Sets S1F and S2F). As expected, the antioxidative thiol-reducing proteins thiol peroxidase (Tpx) and thioredoxin (Trx) were 36.5% to 100% oxidized. It is worth noting that the metalloregulator MntR was markedly oxidized at the thiol group of cysteines (Table 2; Data Sets S1E and S2E). In a preliminary redox proteomic experiment, 40 μM H2O2 treatment also resulted in the oxidations of Cys139 and Cys142 of the peroxide-responsive repressor PerR (Fig. S1C). The consistently identified redox-sensitive proteins in the 40-ml cultures and 40 μM H2O2-pulsed cells (Table 2) either might be involved in self-protection or might simply be hypersensitive to oxidative damage.
TABLE 2.
Redox proteomics identified the cysteine residues and proteins of S. oligofermentans oxidized by both an exogenous 40 μM H2O2 pulse and endogenous H2O2 produced in 40-ml static cultures
| No. | Accession no. in KEGG database |
Protein description | Peptide sequence | Modified cysteine |
Thiol/disulfide oxidized ratio (%)a
|
||
|---|---|---|---|---|---|---|---|
| 40-ml culture |
Nonpulsed culture |
40 μM H2O2-pulsed culture |
|||||
| 1 | I872_01020 | Metal-dependent transcriptional regulator |
CIYEIGTR | C11 | 100 | 0 | 100* |
| 2 | I872_09155 | Glyceraldehyde-3-phosphate dehydrogenase |
TIVFNTNHDVLDGTETVISGASCTTNCLAPMAK | C151, C155 |
C151, 24.7; C155, 100 |
0 | C151, SO3H; C155, 100* |
| 3 | I872_08015 | Fructose-bisphosphate aldolase |
VNVNTECQIAFANATR | C235 | 100 | 0.5 ± 0.5 | 100* |
| 4 | I872_06890 | Pyruvate kinase | AICEETGNGHVQLFAK | C235 | 100 | 0 | 57.4 ± 24.9* |
| 5 | I872_00255 | Aldehyde-alcohol dehydrogenase |
IAEPVGVVCGITPTTNPTSTAIFK | C120 | 100 | 0 | 100* |
| 6 | I872_10355 | 6-Phospho-beta-glucosidase | NVETCLAQPVLLR | C318 | 100 | 0 | 100* |
| 7 | I872_00060 | Hypoxanthine phosphoribosyltransferase |
NLCNLFK | C112 | 100 | 0 | 100* |
| 8 | I872_01620 | Protein translocase subunit SecA |
ELGGLCVIGTER | C507 | 100 | 10 ± 10 | 98 ± 2* |
| 9 | I872_09640 | Probable thiol peroxidase | VLSIVPSIDTGVCSTQTR | C58 | 100 | 0 | 36.5 ± 3.5* |
| 10 | I872_03205 | Thioredoxin family protein | FWASWCGPCKb | C82 | 10 | 80 | 100 |
| 11 | I872_00600 | 50S ribosomal protein L36 | VMVICPANPK | C27 | 100 | 24 ± 6 | 100* |
The thiol/disulfide oxidized ratio was calculated by dividing the intensity of oxidized disulfide-linked peptide by the sum of the intensities of the oxidized and reduced peptides in the corresponding sample. *, a cutoff of a ≥1.5-fold oxidation ratio in H2O2-treated cells over that in untreated cells was considered significant. Experiments were repeated twice, and the results are the averages ± SD from two independent experiments.
This peptide fragment was detected in only one independent experiment.
Redox Western blotting validates the oxidation of cysteine-containing proteins by a low H2O2 concentration, notably, the cysteine oxidation of PerR.
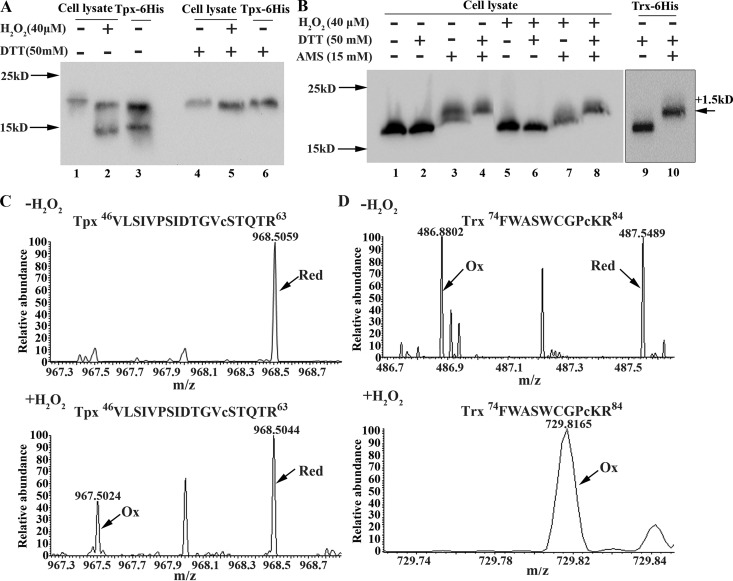
To verify the redox proteomics-identified cysteine residues sensitive to low H2O2 concentrations, Tpx and Trx, the well-known antioxidative proteins, were chosen to examine cysteine oxidation by 40 μM H2O2 in Tpx-6×His and Trx-6×His strains, which carried a 6×His tag fusion at the C terminus of the Tpx and Trx proteins, respectively. Redox Western blotting was performed as described in Materials and Methods. As shown in Fig. 3A, in comparison with the migration of Tpx from H2O2-untreated Tpx-6×His cells (lane 1), a similar faster-migrating band was also detected for both Tpx from H2O2-treated cells (lane 2) and the recombinant Tpx-6×His protein (lane 3), which could be partially oxidized during purification, while upon dithiothreitol (DTT) reduction, the faster-migrating Tpx band from H2O2-treated cells and the Tpx-6×His protein disappeared (lanes 4, 5, and 6). This indicates that H2O2 oxidation results in an intramolecular disulfide linkage in Tpx. Consistently, LC-MS/MS identified the thiol oxidation of Cys58 (Fig. 3C; Fig. S1E and F), and H2O2 treatment caused approximately 36% of the Tpx protein to be oxidized (Fig. 3C; Table 2; Data Set S1E). Although redox proteomics identified Trx Cys82 to be complete oxidized (Fig. 3D; Fig. S1I), redox Western blotting did not detect a differential migration of the Trx protein upon H2O2 oxidation or DTT reduction (Fig. 3B, lanes 1 and 2 versus lanes 5 and 6), while addition of 4-acetamido-4′-maleimidylstilbene-2,2′-disulfonic acid (AMS), a free thiol-reactive reagent, generated different upshifted Trx bands by higher upshifting in DTT-reduced cells than in H2O2-treated cells (Fig. 3B, lanes 4 and 8 versus lanes 3 and 7). By reference to an apparent molecular weight increase of 500 Da per AMS molecule (36) and the migration of three AMS-bound recombinant Trx-6×His proteins (Fig. 3B, lane 10), the differential protein migration in H2O2-treated and DTT-reduced cells suggests that a reversible thiol group oxidation (–SOH) in one cysteine of Trx is formed by H2O2 treatment. Therefore, redox Western blotting confirmed the oxidation of cysteine residues in cells treated with a low concentration of H2O2.
FIG 3.
Verification of the cysteine oxidation of thiol peroxidase (Tpx) and thioredoxin (Trx) in 40 μM H2O2-treated anaerobic cultures. (A) A 6×His tag was fused to the C terminus of the tpx gene (KEGG accession number I872_09640) to construct the S. oligofermentans Tpx-6×His strain. Mid-exponential-phase anaerobically grown Tpx-6×His cells were treated with or without 40 μM H2O2 for 20 min, collected inside an anaerobic glovebox, and then lysed in RIPA buffer containing the free thiol protectant NEM. The cell lysate of each sample was divided into two aliquots; one was left untreated (lanes 1 and 2), and the other was reduced with 50 mM DTT for 1 h (lanes 4 and 5). Redox Western blotting was carried out using an 18% SDS-PAGE gel to detect the Tpx-6×His protein using an anti-His tag antibody. Recombinant Tpx-6×His protein, which was partially oxidized and which formed an intramolecular disulfide linkage during purification, was treated with or without 50 mM DTT (lanes 3 and 6) and used as a reduced and an oxidized molecular control, respectively. (B) Using the same approach described in the legend to panel A, a disulfide linkage upon 40 μM H2O2 oxidation was identified for thioredoxin (KEGG accession number I872_03205) in the Trx-6×His strain (lanes 1 and 2 versus lanes 5 and 6). In addition, 15 mM 4-acetamido-4′-maleimidylstilbene-2,2′-disulfonic acid (AMS), the free thiol-chelating reagent, was used to detect the nondisulfide oxidation of the thiol groups (lanes 3 and 4 and lanes 7 and 8). Cell lysates from the H2O2-untreated strain (lanes 3 and 4) and the H2O2-treated Trx-6×His strain (lanes 7 and 8) were reduced with or without 50 mM DTT. The recombinant Trx-6×His protein was first reduced by 50 mM DTT, and then one aliquot was alkylated with AMS and another was left untreated; these were used as reduced and thiol AMS-bound Trx-6×His protein controls, respectively (lanes 9 and 10). Molecular weight markers are shown at the left, and the increased molecular weight of the protein due to bound AMS molecules (500 Da each) is shown at the right. (C and D) Redox proteomics identified the reduced (Red) and oxidized (Ox) peptide fragments of Tpx VLSIVPSIDTGVC58STQTR (C) and Trx FWASWCGPC82KR (D) in H2O2-untreated (top) and H2O2-treated S. oligofermentans cells (bottom). The relative abundances of the oxidized and reduced peptide fragments are shown.
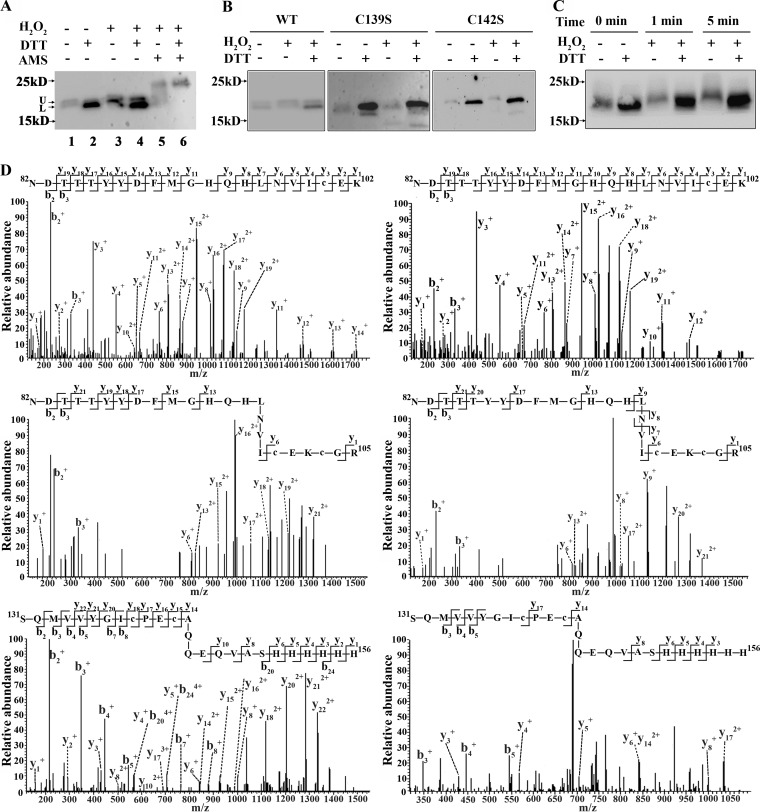
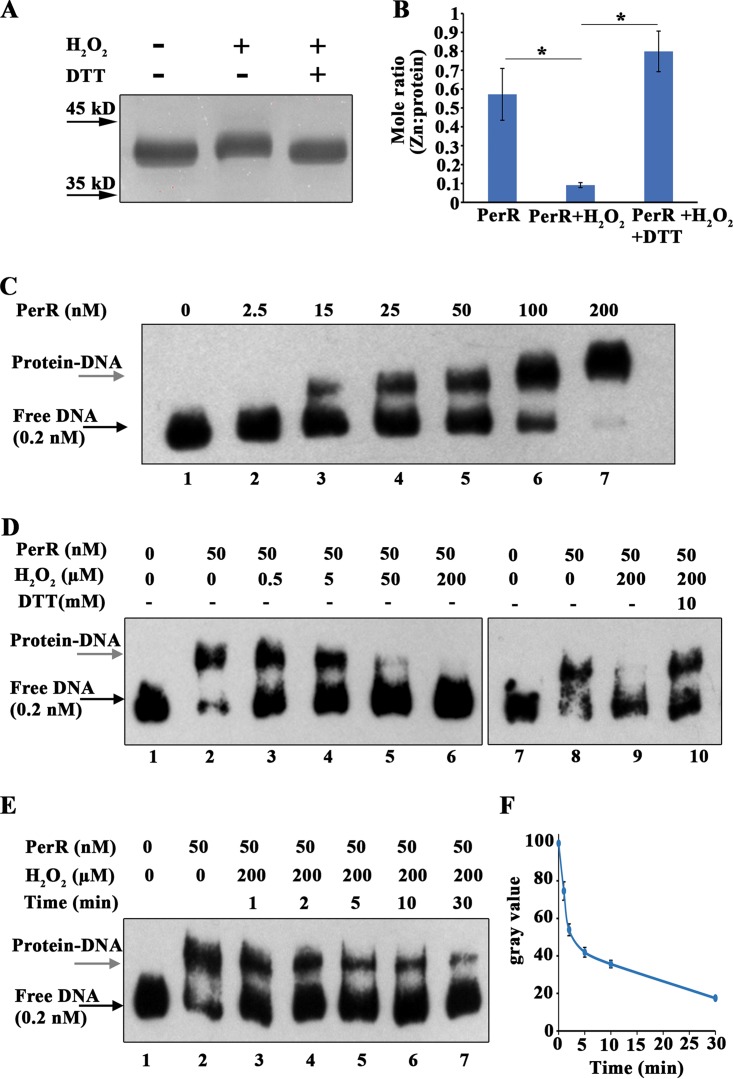
Previously, we demonstrated that the peroxide-responsive repressor PerR and the metalloregulator MntR are involved in the H2O2 resistance of S. oligofermentans (11, 25). Interestingly, redox proteomics detected the cysteine oxidation of the two proteins in 40 μM H2O2-treated cells. Recently, we have validated the increased amount of disulfide-linked MntR oligomer in 40 μM H2O2-pulsed cells (25). Here, we examined H2O2-caused cysteine oxidation in PerR. A PerR-6×His strain, which carries a 6×His tag fusion at the C terminus of PerR, was treated with or without 40 μM H2O2 and then lysed in the presence of the free thiol protectant N-ethylmaleimide (NEM) and 10 mM EDTA, which chelates Fe2+ and so avoids Fenton chemistry-mediated PerR oxidation, as demonstrated in B. subtilis PerR (20). Redox Western blotting detected two bands in the H2O2-untreated PerR-6×His culture, whereas the upper band appeared mainly in the H2O2-treated culture and the lower one appeared exclusively in DTT-treated cell lysates (Fig. 4A, lanes 1 to 4, and Fig. 4B). This is reminiscent of the findings for B. subtilis PerR, which migrated more slowly when the structure maintaining Zn2+ was lost due to the oxidation of cysteine residues (20). Therefore, AMS was employed to examine the cysteine redox status of PerR from H2O2-treated PerR-6×His cells. Figure 4A shows that AMS addition increased the apparent molecular weight of PerR from DTT-reduced cell lysates (upshifted at approximately 0.5 cm, lane 6 versus lane 4) compared to that of PerR from the non-DTT-reduced ones (upshifted at approximately 0.3 cm, lane 5 versus lane 3). This indicates that some but not all of the four Cys residues of the streptococcal PerR are oxidized by pulsing with a low H2O2 concentration.
FIG 4.
Assay of PerR cysteine oxidation in 40 μM H2O2-treated cells. (A) A 6×His tag was fused to the C terminus of perR (KEGG accession number I872_05555) to construct the S. oligofermentans PerR-6×His strain. Using the same approach described in the legends to Fig. 3A and B, redox Western blotting detected cysteine oxidation in the 6×His-tagged PerR protein using the anti-His tag antibody. U and L at the gel left indicate upper and lower protein bands, respectively. (B) A serine substitution of either Cys139 or Cys142 was constructed on a shuttle plasmid (pDL278-perR-6×His), and the plasmid was transformed into the perR deletion strain to construct the perR::pDL278-perRC139S-6×His (C139S) and perR::pDL278-perRC142S-6×His (C142S) strains. The perR deletion mutant harboring pDL278-perR-6×His (WT) was included as a control. The three strains were anaerobically cultured and then treated with 40 μM H2O2. Redox Western blotting, as described in the legend to Fig. 3A, was carried out to detect the oxidation of the PerR mutants. (C) The PerR-6×His strain was anaerobically cultured, and cells were treated with 40 μM H2O2 for 1 min and 5 min. Using the methods described in the legend to Fig. 3A, the cysteine oxidation of PerR was detected by redox Western blotting. (D) The 6×His-tagged PerR protein was immunoprecipitated from the statically grown PerR-6×His strain as described in Materials and Methods and then resolved on an 18% nonreducing SDS-PAGE gel. The protein band was then subjected to differential alkylation and LC-MS/MS analysis. (Top) Representative MS/MS spectra of the triply charged peptide ions at m/z 863.7217 and 862.7243, corresponding to reduced (left) and oxidized (right) NDTTTYYDFMGHQHLNVIC100EK peptide fragments, respectively. (Middle) Representative MS/MS spectra of triply charged peptide ions at m/z 989.1003 and 992.4445, corresponding to both Cys100 and Cys103 reduced (left) and oxidized (right) NDTTTYYDFMGHQHLNVIC100EKC103GR peptide fragments, respectively. (Bottom) The MS/MS spectra represent triple- and quintuple-charged peptide ions at m/z 1038.1281 and 622.0834, respectively, corresponding to both Cys139 and Cys142 reduced (left) and oxidized (right) SQMVVYGIC139PEC142AQQEQVASHHHHHH peptide fragments. The reduced and oxidized cysteine residues were 13C carboxymethylated and 12C carbamidomethylated, respectively.
Redox Western blotting indicated that PerR was also oxidized in statically grown cells (Fig. S2A). To further verify the endogenous cysteine oxidation resulting from H2O2, 6×His-tagged PerR protein immunoprecipitated from statically grown cells was subjected to differential alkylation and LC-MS/MS analysis (Fig. S2B). Figure 4D displays the representative MS/MS spectra of the peptide fragments carrying four cysteine residues. By counting the peptide fragments carrying oxidative and reductive cysteine residues, we calculated the oxidation ratios of the four cysteine residues to be 76% (Cys100), 50% (Cys103), 83% (Cys139), and 82% (Cys142) (Table S1), whereas His40 and His95, whose oxidations inactivate B. subtilis PerR (20), were oxidized approximately 28% and 53%, respectively, in S. oligofermentans PerR (Table S1). Collectively, both MS/MS identification and redox Western blotting determined that the Zn2+-coordinated cysteine residues of S. oligofermentans PerR are hypersensitive to H2O2 oxidation.
Redox Western blotting detects in vivo PerR oxidation (A), and immunoprecipitation detects oxidized PerR (B). (A) Redox Western blotting determined that the PerR proteins were reversibly oxidized in aerobically grown cells. Mid-exponential-phase S. oligofermentans PerR-6×His cells were sonicated in RIPA buffer containing the free thiol protectant NEM and 10 mM EDTA. Cell lysates were divided into two aliquots, in which one was treated with 50 mM DTT for 1 h and one was left untreated. Redox Western blotting was performed to assay the oxidative status of the PerR-6×His protein using anti-6×His antibody, and the molecular weight marker is labeled at the left. (B) Immunoprecipitation of the oxidized cellular PerR. One liter of mid-exponential-phase cells of the statically grown PerR-6×His strain was collected, washed twice with PBS, and resuspended in 10 ml lysis buffer. The 6×His-tagged PerR protein was pulled down using anti-His tag monoclonal antibody-magnetic beads as described in Materials and Methods. Proteins in the elution (IP elution) were separated on an 18% nonreducing SDS-PAGE gel and stained with Coomassie brilliant blue. The molecular mass marker is labeled at the left, and the black arrow indicates the target 6×His-tagged PerR protein with a predicted molecular weight of 17.8 kDa. Download FIG S2, TIF file, 0.2 MB (244KB, tif) .
Copyright © 2020 Tong et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Calculation of the oxidation ratio of cysteine or histidine residues in 6×His-tagged PerR that was immunoprecipitated from statically grown S. oligofermentans. a, the numbers of LC-MS/MS-identified peptide spectral matches (PSMs) of the fragments containing targeting amino acid residues were counted, and the oxidation ratios of the respective residues were calculated by dividing the number of oxidized PSMs by the total number of PSMs. Download Table S1, DOCX file, 0.02 MB (16.4KB, docx) .
Copyright © 2020 Tong et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
H2O2 oxidation of the cysteine residues abolishes PerR binding to DNA due to Zn2+ loss.
To further determine whether H2O2 oxidation occurs at the cysteine residues of the streptococcal PerR in vivo, Cys139 and Cys142 were replaced by serine on the shuttle plasmid pDL278-perR-6×His. Wild-type perR and cysteine-mutated perR were each ectopically expressed in a perR deletion strain, and the resultant complementary strains were treated with or without 40 μM H2O2. Redox Western blotting showed that, different from the findings for wild-type PerR, cysteine-mutated PerR retained the same migration in all samples regardless of 40 μM H2O2 oxidation or DTT reduction (Fig. 4B). This result demonstrates that H2O2 oxidizes Cys139 and Cys142 of the streptococcal PerR.
It is worth noting that even if cells were collected inside an anaerobic glove box and lysed in the presence of NEM, EDTA, and catalase, part of the PerR protein was still oxidized (Fig. 4A, lane 1), suggesting the PerR cysteine residues are hypersensitive to oxidants. This was further confirmed by redox Western blotting, which detected oxidized PerR protein from the cells pulsed by 40 μM H2O2 for only 1 min (Fig. 4C). Noticeably, invariable lower Western blotting signals were detected for PerR from H2O2-treated cells than for PerR from DTT-reduced cells, and this was determined to be because DTT increased the anti-His tag antibody signal (Fig. S3).
DTT reduction increases the hybridization signal of the anti-His tag antibody. Overnight cultures of perR::pDL278-perRC142S-6×His strain were diluted 1:30 into fresh BHI broth and grown statically. The mid-exponential-phase cells were collected, washed twice with PBS, and sonicated in RIPA buffer containing the free thiol protectant NEM and 10 mM EDTA. The cell lysate was divided into seven aliquots; six of these were treated with gradient concentrations of DTT (lanes 2 to 7) for 1 h, and one was not treated with DTT. Redox Western blotting on 18% SDS-PAGE gels was performed to assay for the PerR-C142S protein using the anti-His tag antibody. The black arrow indicates the PerR-C142S hybridization band; the gray arrow shows the molecular weight marker; *, nonspecific hybridization band. Download FIG S3, TIF file, 0.1 MB (137.3KB, tif) .
Copyright © 2020 Tong et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
By reference to the B. subtilis PerR and other Cys4Zn proteins, such as Hsp33 and RsrA (37, 38), oxidation of Zn2+-coordinated cysteine residues would cause Zn2+ loss and, therefore, slower protein migration because of the conformational changes. We subsequently verified whether H2O2 oxidation causes Zn2+ loss from PerR. Overexpressed glutathione S-transferase (GST)-tagged PerR protein (GST-PerR) was treated or not treated with 5 mM H2O2 and subsequently reduced or not reduced with 50 mM DTT. Nonreducing SDS-PAGE analysis did reveal a slower-migrating band for the 5 mM H2O2-treated protein than for the DTT-treated protein (Fig. 5A). Inductively coupled plasma mass spectrometry (ICP-MS) also determined 0.09 mol of Zn2+ per mol of H2O2-treated PerR and 0.79 mol of Zn2+ per mol of DTT-reduced PerR (Fig. 5B), confirming that H2O2 oxidation causes Zn2+ loss from PerR.
FIG 5.
Determination of zinc ion loss and inactivation of the PerR protein caused by H2O2 oxidation. (A and B) The recombinant PerR-GST protein was purified in PBS buffer containing 10 mM EDTA and 3 mM GSH, as described in Materials and Methods. Purified PerR-GST was divided into three aliquots: one was not treated, and the remaining two were treated for 30 min with 5 mM H2O2, with one of these two aliquots subsequently being subjected to 1 h of reduction with 50 mM DTT. Aliquots of the three protein samples were resolved on nonreducing 18% SDS-PAGE gels, and the remaining samples were ultrafiltered and, finally, resuspended in 650 μl PBS buffer. The protein concentrations of PerR-GST were determined using a BCA protein assay kit, and the zinc concentration in the protein was measured using ICP-MS. The molar ratios of zinc ion to the PerR-GST monomer were calculated. The averages ± SD from three independent experiments are shown. *, a result significantly different from that for H2O2-treated PerR-GST protein, as verified by one-way analysis of variance followed by Tukey’s post hoc test (P < 0.05). (C) The PerR-GST protein was digested with 100 U thrombin to remove the GST tag and then eluted into a buffer containing 10 mM EDTA and 1 mM DTT. Next, 1 μM PerR protein was preincubated with 1 mM MnCl2, and then gradient concentrations of the recombinant PerR:Zn,Mn protein (0 to 200 nM) were tested for binding to the 5′-biotin-labeled dpr promoter fragment, as described in Materials and Methods. (D) One micromole of PerR protein was incubated with 1 mM MnCl2 and then treated with increasing concentrations of H2O2 at 30°C for 30 min. After incubation, 150-U/ml catalase was added to decompose the residual H2O2, and 50 nM PerR protein was used for EMSA to test the affinity of binding to the dpr promoter (lanes 3 to 6). To observe whether the H2O2 oxidation-diminished PerR binding could be restored, 10 mM DTT was added to the 200 μM H2O2-treated PerR and the mixture was incubated at 37°C for 1 h (lane 10). E. One micromole of the PerR protein was preincubated with 1 mM MnCl2 and then treated with 200 μM H2O2 at 30°C for different times (lanes 3 to 7). Fifty nM PerR protein was used in the EMSA. Black and gray arrows point to the free DNA probe and protein-DNA complex, respectively. (F) Band densities of the protein-DNA complex in lanes 2 to 7 of panel E were evaluated using ImageJ software, with the density in lane 2 being set as 100%. The density percentages in lanes 3 to 7 were calculated by dividing the band density of the respective lane by that of lane 2. All the experiments were repeated three times, and the averages ± SD from three independent experiments are shown.
Next, we determined whether Zn2+ loss affects DNA binding by PerR. Fifty nanomoles of PerR:Zn,Mn was used for a electrophoretic mobility shift assay (EMSA), based on a calculated Kd (dissociation constant) value of approximate 50 nM for binding (Fig. 5C). Figure 5D shows that 50 μM H2O2 treatment for 30 min diminished PerR’s binding to the dpr promoter (lane 5) and that 200 μM H2O2 treatment completely abolished the binding (lane 6); meanwhile, 10 mM DTT reduction recovered the binding (lane 9 versus lane 10), indicating that PerR is reversibly inactivated by H2O2 oxidation at cysteine residues. Moreover, 5 min of treatment with 200 μM H2O2 inactivated approximately 59% of the PerR protein (Fig. 5E and F). It is worth noting that the EMSA buffer was treated with Chelex 100 to chelate metal ions that might trigger the Fenton reaction. Noticeably, LC-MS/MS did not detect increased histidine residue oxidation in the H2O2-treated PerR:Zn,Mn protein (Table S2), while histidine oxidations that occurred before H2O2 treatment might have been generated during the in vitro purification. In conclusion, H2O2 oxidizes cysteine residues but not histidine residues and inactivates PerR.
Calculation of histidine oxidation in the recombinant PerR:Zn,Mn protein resulting from H2O2. a, the numbers of LC-MS/MS-identified peptide spectral matches (PSMs) of the fragments containing histidine residues were counted, and the oxidation ratios of the histidine residues were calculated by dividing the number of oxidized PSMs by the total number of PSMs. Download Table S2, DOCX file, 0.02 MB (16.8KB, docx) .
Copyright © 2020 Tong et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
PerR and MntR regulate the cellular redox system and metal homeostasis.
Trx and Tpx are involved in cellular redox homeostasis and belong to the S. aureus and Clostridium acetobutylicum PerR regulons (39, 40). Additionally, Dpr, a non-heme iron-containing ferritin, and MntABC, a manganese ABC transporter, are known to play important roles in maintaining cellular metal homeostasis and are under the control of S. oligofermentans PerR and MntR (11, 25). To determine whether the four genes mentioned above plus mntR belong to the PerR or MntR regulons, we performed quantitative PCR (qPCR) to quantify the expression of these genes in 40 μM H2O2-pulsed and nonpulsed anaerobically grown wild-type strain and perR deletion, mntR deletion, and perR mntR double deletion mutants. In comparison with the 40 μM H2O2-induced 3- to 5.8-fold higher levels of expression of tpx, dpr, mntA, and mntR in the wild-type strain, deletion of mntR abolished the H2O2 induction of mntA; however, the H2O2 induction of the four genes almost disappeared in the mutants either with a deletion of perR or with a deletion of both perR and mntR (Table 3). These demonstrate that the H2O2-induced expressions of tpx, dpr, mntA, and mntR are under the control of PerR, while mntA is also controlled by mntR in response to H2O2.
TABLE 3.
Identification of the PerR regulon by qPCR quantification of the gene transcript copies in anaerobically grown wild-type and perR and mntR single and double deletion strains with and without 40 μM H2O2 treatment
| Genea | Transcript copy no./100 16S rRNA copiesb
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Wild-type strain |
ΔperR mutant |
ΔmntR mutant |
ΔperR ΔmntR mutant |
||||||
| Without H2O2 |
With H2O2 | With H2O2 + highc |
Without H2O2 |
With H2O2 | Without H2O2 |
With H2O2 | Without H2O2 |
With H2O2 | |
| mntR | 0.76 ± 0.05 | 2.35 ± 0.21* | 2.16 ± 0.31* | 0.77 ± 0.12 | 0.83 ± 0.02 | ND | ND | ND | ND |
| mntA | 5.52 ± 1.50 | 18.04 ± 3.75* | 20.75 ± 1.20* | 13.81 ± 0.93* | 21.70 ± 3.94* | 18.70 ± 1.59* | 19.70 ± 0.36* | 18.94 ± 2.02* | 22.47 ± 3.53* |
| dpr | 6.26 ± 2.18 | 34.92 ± 6.40* | 29.54 ± 4.74* | 21.40 ± 2.80* | 27.91 ± 0.16* | 7.89 ± 0.76 | 23.62 ± 2.47*# | 25.01 ± 0.26* | 30.77 ± 6.82* |
| tpx | 0.56 ± 0.11 | 3.22 ± 0.17* | 3.10 ± 0.23* | 1.30 ± 0.19* | 1.45 ± 0.24* | 0.66 ± 0.02 | 1.78 ± 0.10*# | 1.60 ± 0.08* | 1.71 ± 0.39* |
| trx | 0.73 ± 0.17 | 1.35 ± 0.35 | 0.75 ± 0.34 | 0.96 ± 0.12 | 1.36 ± 0.18 | 0.82 ± 0.19 | 0.79 ± 0.09 | 0.62 ± 0.02 | 0.80 ± 0.16 |
mntR, Mn-dependent transcriptional regulator (KEGG accession number I872_01020); mntA, manganese transport system substrate-binding protein (KEGG accession number I872_09645); dpr, non-heme iron-containing ferritin (KEGG accession number I872_07415); tpx, thiol peroxidase (KEGG accession number I872_09640); trx, thioredoxin (KEGG accession number I872_03205).
The experiments were repeated three times with triplicate batch cultures each time. The results are the averages ± SD from three independent experiments. The data were significantly different from those obtained for the wild-type strain without 40 μM H2O2 treatment (*) and from those obtained with the same strain not treated with H2O2 (#), as verified by one-way analysis of variance followed by Tukey’s post hoc test (P < 0.05). ND, not determined.
qPCR was implemented with the wild-type strain prepulsed with 40 μM H2O2 and then further challenged by 10 mM H2O2.
Notably, even in the absence of H2O2, 2.5- to 4.0-fold higher levels of expression of tpx, dpr, and mntA were detected in the ΔperR and ΔperR ΔmntR mutants than in the wild-type strain, suggesting that PerR may directly regulate the tpx, dpr, and mntA genes. However, the conserved PerR binding sequence (TTAATTAGAAGCATTATAATTAA) was found only in the dpr promoter region; consistently, EMSA indicated PerR binding to the dpr promoter (Fig. 5C) but not to the promoters of mntABC, tpx, and mntR (Fig. S4). Our previous work found that MntR bound to the mntABC promoter (25), indicating the direct regulation of mntA by MntR. Thus, PerR directly regulates dpr but indirectly regulates mntA, tpx, and mntR via unknown mechanisms. Of note, similar expression levels of the PerR-regulated genes were detected in cells pulsed only with 40 μM H2O2 and cells that were pulsed and then further challenged by 10 mM H2O2 (Table 3), indicating that these genes are under the control of PerR, which is already inactivated by as little as 40 μM H2O2.
EMSA of PerR binding to the mntABC, tpx, and mntR promoters. The PerR-GST protein was digested with 100 U thrombin to remove the GST tag and then eluted into a buffer containing 10 mM EDTA and 1 mM DTT. Next, 1 μM PerR protein was preincubated with 1 mM MnCl2, and then gradient concentrations of the recombinant PerR:Zn,Mn protein (0 to 200 nM) were used for binding to 5′-biotin-labeled mntABC (KEGG accession numbers I872_09645, I872_09650, and I872_09655), tpx (KEGG accession number I872_09640), and mntR (KEGG accession number I872_01020) promoters, as described in Materials and Methods. Black arrows point to the free DNA probes (0.2 nM for each). Download FIG S4, TIF file, 0.6 MB (586.2KB, tif) .
Copyright © 2020 Tong et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
PerR, MntR, and the regulated cellular redox and metal homeostatic proteins are involved in the self-protection of S. oligofermentans from H2O2 stress.
Given that both PerR and MntR contribute to the high H2O2 resistance of S. oligofermentans (11, 25), to determine their roles in self-protection against H2O2 stress, the ΔperR and ΔmntR mutants were prepulsed with or without 40 μM H2O2, and then their survival with 10 mM H2O2 challenge was determined as described above. Table 1 shows that the mntR deletion dramatically reduced the 40 μM H2O2-induced protection from high H2O2 challenge to 8-fold, compared to the 367-fold protection in the wild-type strain, while perR deletion almost completely abolished the low-H2O2-concentration-induced adaptation (Table 1). Together, the two redox regulators MntR and, in particular, PerR play important roles in the low-H2O2-concentration-induced self-protection of S. oligofermentans from a higher-concentration H2O2 stress, most likely by H2O2 inactivating the two transcriptional repressors and thereby derepressing the antioxidative systems. Notably, a 9.4-fold elevated survival rate in the presence of a higher concentration of H2O2 was observed for the ΔperR mutant than for the ΔmntR mutant (Table 1), suggesting that Dpr and redox system proteins might play the major role in protecting S. oligofermentans from challenge with a higher H2O2 concentration.
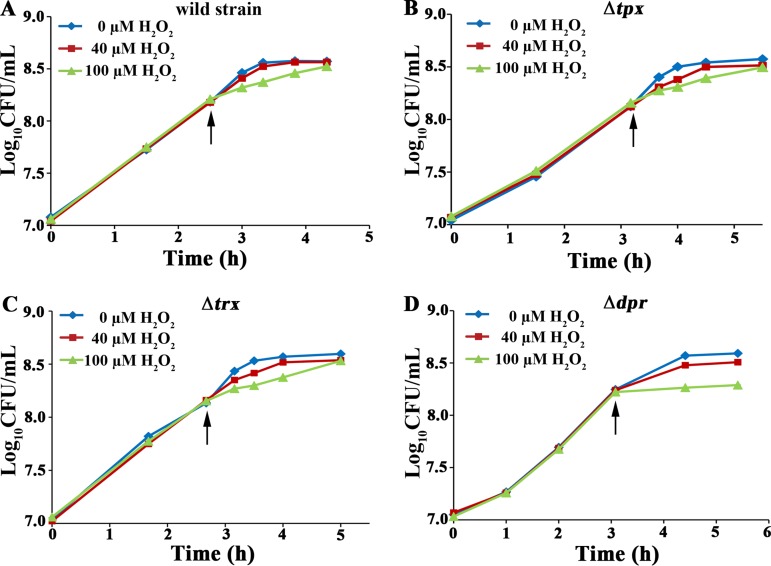
Next, the role of Dpr and redox system proteins in the H2O2 resistance of S. oligofermentans was determined. The tpx, trx, dpr, and mntABC genes were each deleted, and the mutants were compared with the wild-type strain for growth suppression by 40 and 100 μM H2O2. As shown in Fig. 6, 100 μM H2O2 slightly suppressed the growth of the wild-type strain, but 40 μM H2O2 already retarded the growth of the tpx, trx, and dpr deletion mutants, with the Δdpr mutant being the most severely inhibited. The ΔmntABC mutant was reported to exhibit reduced resistance to 10 mM H2O2 (11), but its growth was not significantly inhibited by 100 μM H2O2 (data not shown). Collectively, the results indicate that the redox regulators PerR and MntR and their regulated cellular redox and metal homeostasis proteins are involved in the self-protection of S. oligofermentans from H2O2 stress.
FIG 6.
H2O2 sensitivity assay of mutants with deletions of the genes involved in cellular redox and metal homeostasis. Overnight cultures of the tested strains were diluted 1:30 into fresh BHI broth and anaerobically cultured. Triplicate cultures were used for each strain. When the OD600 reached approximately 0.5, one replicate was left as the H2O2-untreated control and the other two were supplemented with 40 and 100 μM H2O2, respectively. The growth profiles of the tested strains were monitored by counting the number of CFU at the indicated time points. The experiments were repeated three times, with triplicate cultures being used each time. The averages from three independent experiments are shown. Black arrows indicate the time point of H2O2 supplementation.
DISCUSSION
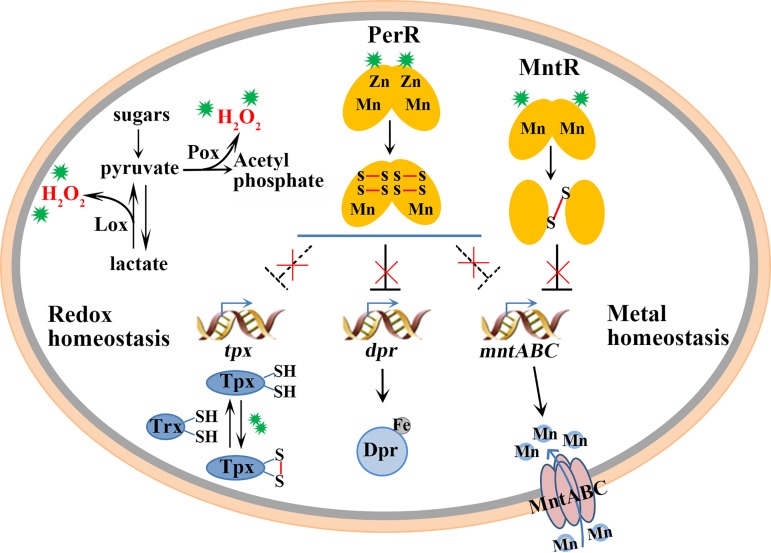
Although a few studies have reported that endogenous H2O2 protects streptococci from challenge with a higher H2O2 concentration (8, 22), the mechanism remains unclear. In the present study, through a combination of physiological, biochemical, genetic, and redox proteomic studies, we elucidated the mechanism underlying the low-H2O2-concentration-induced adaptation of catalase-negative streptococci to a higher H2O2 concentration. Figure 7 depicts that streptococci employ pyruvate oxidase (Pox) and lactate oxidase (Lox) to produce endogenous H2O2. Two H2O2-sensing redox regulators, the peroxide-responsive repressor PerR and the metalloregulator MntR, are inactivated by H2O2 oxidation of the cysteine residues. PerR cysteine oxidation results in Zn2+ loss and the subsequent derepression of dpr, mntABC, tpx, and mntR. H2O2 oxidation of MntR leads to disulfide-linked intermolecular polymers and inactivates the regulator, thus derepressing the manganese uptake regulon mntABC (25). In addition to dpr and mntABC, as indicated in our previous work (11), mntR and the thiol peroxidase-encoding gene tpx were identified to be the PerR regulons. Deletion of these functional genes as well as the redox circuit protein Trx increased the sensitivity of S. oligofermentans to a low H2O2 concentration, and correspondingly, deletion of either mntR or perR resulted in the streptococci becoming constitutively resistant to a higher H2O2 concentration. Thus, this work reveals a redox-regulated anti-H2O2 defense network, in which PerR has evolved to sense H2O2 by a Cys-based redox reaction in the manganese-rich cellular environments of the catalase-negative streptococci.
FIG 7.
Diagram depicting the low-H2O2-concentration-induced adaptive mechanism against higher H2O2 stress in streptococci. The catalase-void streptococci use pyruvate oxidase (Pox) and lactate oxidase (Lox) to generate low levels of endogenous H2O2, which induces an adaptation to avoid attack by a higher H2O2 concentration. Redox proteomics analysis and physiological and genetic experiments identified a hierarchal H2O2-sensing and resistance network consisting of the H2O2-sensitive cysteine-containing proteins. These include redox transcriptional regulators, e.g., the peroxide response repressor PerR and the metalloregulator MntR, a repressor of the Mn2+ uptake regulon mntABC, as well as the redox homeostatic proteins, e.g., thiol peroxidase (Tpx), which catalyzes the reduction of H2O2, and thioredoxin (Trx), which specifically reduces H2O2 oxidation-generated disulfide linkages. Inactivation of PerR by trace H2O2 derepresses tpx and the genes encoding metal ion homeostatic proteins, like mntR, dpr, and mntABC, whereas oxidation inactivation of MntR derepresses mntABC (25). Dpr chelates free ferrous ion to avoid Fenton chemistry, whereas MntABC imports Mn2+ to decompose the cellular H2O2. These functional proteins help S. oligofermentans resist the stress associated with a higher H2O2 concentration. Of note, PerR directly represses the dpr gene and controls the tpx, mntABC, and mntR genes indirectly by unknown mechanisms. H2O2 is identified by the green symbols.
Cysteine residues are the most sensitive to H2O2 oxidation (41), and therefore, reversibly oxidized cysteine thiol modifications, such as SOH and the disulfide bond, usually function in the activation of redox regulatory proteins. Some redox regulators, such as the E. coli chaperone protein Hsp33 (37) and the Streptomyces coelicolor anti-sigma factor RsrA (38) and Fur-like repressor CatR (42), possess a structural Cys4Zn. The Zn2+ at the Cys4Zn site stabilizes the cysteine residues as thiolate, which may increase the reactivity of cysteine toward electrophilic H2O2 (43). The S. oligofermentans PerR possesses the structural Cys4Zn as well. Redox proteomics, redox Western blotting, and LC-MS/MS identification of the immunoprecipitated protein all demonstrated that the cysteine residues of the streptococcal PerR are oxidized by a low H2O2 concentration (Fig. 4). Oxidation of the cysteine residues causes Zn2+ loss and inactivates PerR (Fig. 5), thereby derepressing the antioxidative genes (Table 3). This explains the underlying mechanism of the PerR-mediated H2O2 adaptation of streptococci.
It has been reported that the B. subtilis PerR, an ortholog of the streptococcal PerR (see Fig. S5 in the supplemental material), is inactivated by histidine oxidation, whereas its Zn2+-coordinated cysteine residues are inert to H2O2 oxidation (20). In contrast, the streptococcal PerR is inactivated by H2O2 oxidation at the Zn2+-coordinated cysteine residues (Fig. 4 and 5). Subsequent structural homology modeling of the S. oligofermentans PerR was performed with the SWISS-MODEL server by automatically selecting the S. pyogenes PerR (PDB accession number 4LMY) as a template. Structural comparison with B. subtilis PerR (PDB accession number 2FE3) did show some differences between the two at the C-terminal Cys4Zn site (Fig. S5); specifically, Cys103 and Cys142 of the S. oligofermentans PerR are situated close to the N terminus of the S4 β-strand and at a short H6 helix, respectively, while Cys96 and Cys139 of the B. subtilis PerR are situated at the C terminus of the S3 β-strand and in the center of a long H6 helix, respectively. These differences may render the two PerRs with different H2O2 sensitivities, as a cysteine residue near the N terminus of a helix more likely possesses lower pKa values (43). Cellular metal environments could be another clue to the distinct inactivation mechanisms of the two PerRs. A much higher ratio of Mn/Fe was determined in S. oligofermentans cells (1.02 ± 0.25) than in B. subtilis cells (0.05 ± 0.01), which was paralleled in this study, in accordance with the Mn-centric definition of streptococci (44–46). Especially, when grown in a medium supplemented with 2.5 μM and 100 μM Mn2+, 1.78 ± 0.46 and 8.04 ± 0.42 cellular Mn/Fe ratios were found in S. oligofermentans, respectively (25), indicating an active manganese uptake system in this bacterium. The higher cellular Mn/Fe ratio in streptococci could result in higher percentages of PerR:Zn,Mn than of the PerR:Zn,Fe found in bacilli; thus, cysteine oxidation contributes to H2O2 inactivation of the streptococcal PerR:Zn,Mn proteins (Fig. 4 and 5; Table S1). Nevertheless, the possibility of Fe2+-triggered streptococcal PerR inactivation cannot be excluded, as approximately 28% of His40 residues and 53% of His95 residues in PerR were oxidized when S. oligofermentans was grown in BHI broth containing 0.5 μM Mn2+ and 15 μM Fe2+ (Table S1). Therefore, the dual-H2O2-sensing mechanisms of the redox regulator PerR could provide protection for the catalase-negative streptococci from oxidative stress in environments with different metal ions.
Sequence alignment and structural comparison of Zn2+-coordinated cysteine residues of the PerR proteins from S. oligofermentans and B. subtilis. (A) The amino acid sequences of the S. oligofermentans (UniProt accession number A0A512AEK5) and B. subtilis (UniProt accession number P71086) PerRs were retrieved from the UniProt protein database. Sequence alignment was performed using the DNAMAN program. *, conserved amino acid residues essential for manganese or ferric ion binding in B. subtilis; #, conserved cysteine residues for zinc ion binding. Black shadings indicate 100% homology. (B) Structural homology modeling of the S. oligofermentans PerR, performed using the SWISS-MODEL server by automatically selecting S. pyogenes PerR (PDB accession number 4LMY) as a template (left) and the crystal structure of B. subtilis PerR (PDB accession number 2FE3) (right). Four Zn2+-coordinated cysteine residues are shown as sticks, and the corresponding cysteine residues are labeled. Download FIG S5, TIF file, 2.1 MB (2.2MB, tif) .
Copyright © 2020 Tong et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Metal homeostasis plays a central role in oxidative stress resistance in Gram-positive bacteria (4, 44, 47, 48). In addition to PerR, streptococci also employ MntR, a metalloregulator protein, to control cellular manganese and iron homeostasis (11, 16, 21, 25). Oxidative inactivation of PerR and MntR derepresses the expression of the metal homeostasis-related genes dpr and mntABC (Table 3) (25). Dpr chelates cellular Fe2+ and so prevents the production of highly toxic HO·, and the manganese importer MntABC takes up Mn2+ to decompose cellular H2O2. MntABC and Dpr have been verified to protect S. oligofermentans from challenge by a high H2O2 concentration in our previous studies (11). Here, Dpr was further verified to resist a sublethal H2O2 concentration.
The thioredoxin (Trx) system, which is comprised of NADPH, thioredoxin reductase (TrxR), and thioredoxin, plays a key role in defense against oxidative stress, particularly in the catalase-lacking streptococci (9, 49–51). Oxidation of the cysteine thiol groups of Trx and Tpx has been found in 40 μM H2O2-pretreated S. oligofermentans cells (Fig. 3), and deletion of two genes increases the H2O2 sensitivity of the streptococcus (Fig. 6). This observation indicates that the Trx system is involved in the H2O2 adaptation of S. oligofermentans, which is presumably under the control of PerR.
In conclusion, this work reports a novel H2O2 adaptation mechanism. Trace amounts of cellular H2O2 cause thiol oxidation of the redox-based regulatory and functional proteins and activate antioxidative systems; meanwhile, they reduce the level of glycolysis, which generates ROS precursors. This H2O2 adaptation mechanism could be an important antioxidative defense strategy of the catalase-void anaerobes.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
S. oligofermentans AS 1.3089 (52) and its derivative strains (see Table S3 in the supplemental material) were grown in brain heart infusion (BHI) broth (BD Difco, Franklin Lakes, NJ) statically or anaerobically under 100% N2. Escherichia coli DH5α, used for cloning, was grown in Luria-Bertani (LB) broth at 37°C under shaking. When required, kanamycin (1 mg/ml) and spectinomycin (1 mg/ml) were used for the selection of Streptococcus transformants, while ampicillin (100 μg/ml) and spectinomycin (250 μg/ml) were used to select E. coli transformants.
Strains, plasmids, and primers used in this study. *, Amp, ampicillin; Kan, kanamycin; Sp, spectinomycin; r, resistant; s, sensitive. Italic nucleotide bases indicate restriction enzyme digestion sites. Download Table S3, DOCX file, 0.02 MB (26.2KB, docx) .
Copyright © 2020 Tong et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Construction of genetically altered strains.
All primers used in this study are listed in Table S3. tpx, trx, dpr, and perR deletion strains were constructed using the PCR-ligation method (53). The upstream and downstream DNA fragments of each gene were amplified from the genomic DNA. The purified, BamHI-digested PCR products were ligated with a kanamycin resistance gene fragment from plasmid pALH124 (54) or a spectinomycin resistance gene fragment from pDL278 (55) at compatible sites. For construction of 6×His-tagged strains, the tpx, trx, and perR genes were amplified from the genomic DNA using a pair of primers, with the reverse primer carrying a sequence encoding 6 histidines just before the termination codon. Meanwhile, an ∼600-bp DNA fragment immediately downstream of the termination codon of each gene was amplified. The purified PCR products were digested with BamHI and ligated with the kanamycin resistance gene fragment. The ligation mixtures were transformed into the S. oligofermentans wild-type strain, except that the ligation mixture for perR deletion was transformed into the ΔmntR strain (11) to construct a ΔperR ΔmntR strain, as described previously (6). For construction of the perR::pDL278-perRC139S-6×His and perR::pDL278-perRC142S-6×His strains, the perR-6×His gene fusion was amplified from the genomic DNA of the strain with 6×His-tagged PerR. After digestion with EcoRI and SalI, the purified product was inserted into the compatible sites on the E. coli-streptococci shuttle vector pDL278 (55) to produce pDL278-perR-6×His. Then, Cys139 and Cys142 were mutated into serine using a site-directed gene mutagenesis kit (Beyotime Biotechnology Co., Shanghai, China). The correct pDL278-perR-6×His, pDL278-perRC139S-6×His, and pDL278-perRC142S-6×His plasmids were transformed into the perR deletion strain to produce strains ectopically expressing wild-type and cysteine-mutated perR.
Detection of intracellular hydrogen peroxide by HyPer imaging.
Mid-exponential-phase HyPer reporter cells were pelleted, washed twice with phosphate-buffered saline (PBS), resuspended in 100 μl of PBS, and exposed to air in the dark for 30 min. Forty microliters of cells was placed on a Polysine microscope slide (25 by 75 by 1 mm; Thermo Scientific, Waltham, MA), covered with a Fisher-brand microscope glass coverslip (diameter, 15 mm; thickness, 0.13 to 0.17 mm; Thermo Scientific), and then visualized under a confocal laser scanning microscope (Leica model TCS SP8; Leica Microsystems, Buffalo Grove, IL, USA). Excitation was provided at 488 nm, with emission being collected from a wavelength range of 500 to 530 nm (32, 56). For each sample, at least 5 fluorescent and differential interference contrast (DIC) images were captured. The fluorescence intensities of 25 regions of interest (ROI), with each ROI containing 5 cells, from each sample were measured using Leica Application Suite (LAS) AF software. For images with fluorescence that was too weak, the ROI in the corresponding DIC images was framed, and the fluorescence was measured in the same ROI in the fluorescence image. The average fluorescence intensities of 25 ROIs were calculated and are expressed in arbitrary units (a.u.) per ROI ± standard deviation.
Redox proteomics analysis by differential alkylation and LC-MS/MS.
The differential alkylation method (34) was used to identify H2O2-induced changes in the thiol redox status of the proteins. Briefly, mid-exponential-phase cells in the tested samples were collected by centrifugation and resuspended in radioimmunoprecipitation assay (RIPA) buffer (50 mM Tris [pH 7.4], 150 mM NaCl, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, 2 mM sodium pyrophosphate, 25 mM β-glycerophosphate, 1 mM sodium orthovanadate, sodium fluoride, 1 mM EDTA, 0.5 μg/ml leupeptin) containing 1 mM phenylmethylsulfonyl fluoride (PMSF). To minimize an artificial oxidation during sample preparation, cell breakage by sonication was performed inside an anaerobic chamber (Thermo Scientific); moreover, 10 mM EDTA and 1 kU/ml catalase were included in the lysis buffer to prevent an Fe2+-triggered Fenton reaction and decompose H2O2, respectively. The sonication was implemented on ice in the dark using a UP-400S ultrasonicator (Xinzi Company, Ningbo, China), and cell lysates were centrifuged at 8,000 × g for 15 min, and then the protein concentration in the supernatant was measured using a Pierce bicinchoninic acid (BCA) protein assay kit (Thermo Scientific). The same amounts of protein from all the samples were separated on a nonreducing one-dimensional SDS-PAGE gel, and each gel lane was cut into 6 slices and washed with MS-grade water three times. The proteins in the gel were alkylated for 30 min with 55 mM [13C]iodoacetic acid in 50 mM NH4HCO3 (pH 8.0) in the dark. After removing the iodoacetic acid, 25 mM DTT reduction was performed for 45 min at 55°C, and then the DTT was removed and the proteins were alkylated with 55 mM [12C]iodoacetic acid for 30 min in the dark. Upon in-gel digestion with MS-grade trypsin (Promega, Fitchburg, WI), LC-MS/MS analysis was implemented with an Easy-nLC integrated nano-high-performance liquid chromatography system (Proxeon, Odense, Denmark) and a Q-Extractive mass spectrometer (Thermo Scientific, Waltham, MA), as described previously (28).
MS/MS spectra were searched against the forward and reverse S. oligofermentans protein database, downloaded from UniProt, using the SEQUEST search engine of Proteome Discoverer software (v1.4). The precursor ion mass tolerance was 20 ppm for all mass spectra acquired in an Orbitrap mass analyzer, and the fragment ion mass tolerance was 0.02 Da for all MS/MS spectra. The following search criteria were employed: full tryptic specificity was required; two missed cleavages were allowed; 13C carboxymethylation (free cysteine residue), 12C carboxymethylation (disulfide linkage cysteine residue) and sulfenic, sulfinic, and sulfonic acids were variable modifications for cysteine; oxidation was a variable modification for methionine; and the false discovery rate (FDR) was set to 0.01. All the cysteine-modified MS/MS spectra were manually confirmed. The MaxQuant software package was used to obtain the intensity of the cysteine-modified peptides. Duplicate experiments were performed in parallel.
Protein GO category analysis.
Homologues of the S. oligofermentans redox-sensitive proteins were searched for in S. pneumoniae and put into the PANTHER bioinformatics platform (http://www.pantherdb.org/) for Gene Ontology (GO) analysis. GO enrichment analysis was implemented on the Gene Ontology Consortium website (http://www.geneontology.org), the binomial test was used for analysis of statistical significance, and a P value of <0.05 was used as a cutoff.
Redox Western blotting.
Cells were collected by centrifugation and resuspended in RIPA buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, sodium orthovanadate, sodium fluoride, EDTA, leupeptin) with addition of 40 mM N-ethylmaleimide (NEM), 1 mM PMSF, 10 mM EDTA, and 1 kU/ml catalase. Cells were sonicated on ice in the dark for 45 min and alkylated in the dark for 20 min, and then the supernatant were collected by centrifugation. Reduced samples were prepared by incubating the lysates with 50 mM DTT for 1 h. For the 4-acetamido-4′-maleimidylstilbene-2,2′-disulfonic acid (AMS) alkylating experiment, cells were resuspended in PBS buffer containing 15 mM AMS, 1 mM PMSF, 10 mM EDTA, and 1 kU/ml catalase, sonicated, and then incubated at 4°C for 2 h in the dark. Half of the samples were reduced with 50 mM DTT for 1 h, and then the DTT was removed and the samples were alkylated with 15 mM AMS at 4°C for 2 h in the dark. The protein concentration of the lysate was determined using a BCA protein assay kit. Protein samples were diluted in nonreducing loading buffer (4×; 0.2 M Tris-HCl, pH 6.8, 40% glycerol, 8% SDS, 0.4% bromphenol blue), separated by SDS-PAGE, transferred onto a nitrocellulose membrane, and hybridized with an anti-His tag antibody (Abmart Company, Shanghai, China) at a 4,000-fold dilution. Detection was performed using a chemiluminescent nucleic acid detection module kit (Thermo Scientific).
IP and LC-MS/MS identification of cysteine thiol oxidation of the PerR protein in vivo.
6×His-tagged PerR protein was purified by immunoprecipitation (IP) using anti-His tag monoclonal antibody-magnetic agarose (MBL International Corporation, Woburn, MA) according to the instructions of the manufacturer. Briefly, the 6×His-tagged-PerR-expressing strain PerR-6×His was statically grown in BHI broth. The mid-exponential-phase cells were collected and washed with PBS three times. Then, the cells were resuspended in lysis buffer (50 mM Tris-HCl, 150 mM NaCl, 0.05% NP-40, 1 mM DTT) containing 55 mM [13C]iodoacetic acid, 10 mM EDTA and 1 kU/ml catalase. The cells were sonicated on ice in the dark for 45 min and alkylated in the dark for 20 min, and then the cell lysate was subjected to centrifugation. The obtained supernatant was mixed and incubated with the magnetic beads. After washing 4 times with lysis buffer, the immunoprecipitated 6×His-tagged PerR protein was eluted by boiling in nonreducing SDS sample buffer (4% SDS, 125 mM Tris-HCl, pH 8.0, 20% glycerol) and separated using 18% nonreducing SDS-PAGE. The target PerR protein band with the expected molecular size was cut from the gel, and cysteine residue oxidation was identified by differential alkylation and LC-MS/MS, as described above, except that the reduced and oxidized cysteine residues were alkylated with 55 mM [13C]iodoacetic acid and [12C]iodoacetamide, respectively.
Overexpression of PerR-GST, Tpx-6×His, and Trx-6×His proteins.
A 450-bp DNA fragment containing the entire perR coding gene was PCR amplified. The purified PCR product was digested with EcoRI/XhoI and ligated into the compatible sites on pGEX4T-1 (GE Healthcare, Boston, MA), and the produced pGEX-PerR was transformed into E. coli BL21(DE3) cells (Novagen, Madison, WI). Correct transformants were grown at 37°C to an OD600 of 0.4 to 0.6, and 0.1 mM isopropyl-β-d-thiogalactopyranoside (IPTG; Sigma-Aldrich, St. Louis, MO) was added to induce PerR-GST expression at 22°C overnight. Then, the cells were collected by centrifugation and resuspended in phosphate-buffered saline (PBS; 10 mM Na2HPO4, 1.8 mM KH2PO4, 137 mM NaCl, 2.7 mM KCl, pH 7.4) containing 1 mM DTT and 10 mM EDTA and then lysed by sonication for 30 min. The cell lysate was centrifuged at 8,000 × g for 30 min, and the supernatant was filtered through a 0.22-nm-pore-size polyvinylidene difluoride membrane (Millipore, Billerica, MA) and then applied to a GSTrap HP column (GE Healthcare, Boston, MA). The proteins were eluted with elution buffer (20 mM Tris-HCl buffer containing 1 mM DTT, 10 mM EDTA, and 10 mM reduced glutathione [GSH], pH 8.0), and the elution fractions were analyzed by electrophoresis on a 12% SDS-PAGE gel. The fractions with the desired protein were pooled and dialyzed against PBS buffer containing 3 mM GSH and 10 mM EDTA three times. Then, the purified proteins were stored in aliquots in 10% glycerol at −80°C until use.
For the overexpression of the Tpx-6×His and Trx-6×His proteins, 492- and 552-bp DNA fragments containing the entire tpx and trx coding genes, respectively, were PCR amplified with the primer pairs listed in Table S3. The resultant products were integrated into pET-28a (Novagen, Madison, WI) by Gibson assembly (New England Biolabs, Beverly, MA) to produce pET-28a-Tpx and pET-28a-Trx. The correct constructs were transformed into E. coli BL21(DE3) (Novagen, Madison, WI) cells. Correct transformants were grown at 37°C to an OD600 of 0.6 to 0.8, 0.1 mM IPTG (Sigma-Aldrich, St. Louis, MO) was added, and the cells were incubated at 22°C overnight. Then, the cells were collected by centrifugation, resuspended in binding buffer (20 mM sodium phosphate, 500 mM NaCl, 30 mM imidazole, 1 mM EDTA, 1 mM DTT, pH 7.4), and lysed by sonication for 30 min. The supernatant was filtered and then applied to an Ni2+-charged chelating column (GE Healthcare, Piscataway, NJ) that had previously been equilibrated with binding buffer. Proteins were eluted with elution buffer (20 mM sodium phosphate, 500 mM NaCl, 500 mM imidazole, 1 mM DTT, pH 7.4). The fractions with the desired protein were pooled and dialyzed against buffer containing 20 mM Tris-HCl, 150 mM NaCl, 1 mM DTT, and 1 mM EDTA. The purified Tpx-6×His and Trx-6×His proteins were stored in aliquots in 10% glycerol at −80°C until use.
Nonreducing SDS-PAGE.
Five micrograms of PerR-GST protein was treated or not treated with 5 mM H2O2 for 30 min and with or without a subsequent reduction by 50 mM DTT for 1 h. Before electrophoresis, 40 mM NEM was added, and the mixture was kept in the dark for 30 min. The protein samples were diluted in nonreducing SDS loading buffer (4×; 0.2 M Tris-HCl, pH 6.8, 40% glycerol, 8% SDS, 0.4% bromphenol blue) and then separated on a 12% SDS-PAGE gel.
Determination of zinc content in PerR-GST using ICP-MS.
The PerR-GST protein was treated or not treated with 5 mM H2O2 for 30 min and with or without a subsequent reduction by 50 mM DTT for 1 h and was then transferred into Chelex 100-treated PBS buffer via ultrafiltration. Protein concentrations were measured with a BCA protein assay kit. The protein samples were treated with nitric acid (ultrapure), and then the zinc content was analyzed by inductively coupled plasma mass spectrometry (ICP-MS; DRCII apparatus; PerkinElmer, USA) at Peking University Health Science Center. Beryllium, indium, and uranium standard solutions (NIST certified; PerkinElmer) were used to calibrate the ICP-MS. Experiments were conducted for triplicate samples and repeated at least three times.
Electrophoretic mobility shift assay (EMSA).
The target gene promoter fragments were generated by PCR amplification using the biotin-labeled primer pair listed in Table S3. The PerR-GST protein was first dialyzed into PBS buffer containing 10 mM EDTA and 1 mM DTT and then digested with 100 U thrombin to remove the GST tag. One micromole of the PerR protein was preincubated with 1 mM MnCl2, and then 0.2 nM a biotin-labeled double-stranded DNA probe and increasing amounts of PerR (0 to 200 nM) were mixed in the binding buffer [10 mM Tris-HCl, pH 8.0, 5% glycerol, 50 mM NaCl, 10 μg/ml bovine serum albumin, 2 ng/μl poly(dI·dC), 0.5 mM DTT, 1 mM MnCl2]. The reaction proceeded at 30°C for 30 min. To observe the effect of H2O2 on PerR binding, 1 μM PerR protein was preincubated with 1 mM MnCl2 and then treated with various concentrations of H2O2 (0 to 200 μM) at 30°C for 30 min or with 200 μM H2O2 at 30°C for various times (0 to 30 min), and then catalase was added to a final concentration of 150 U/ml and the mixture was incubated at 37°C for 30 min. To determine whether oxidation was reversible, 10 mM DTT was added to reduce the 200 μM H2O2-treated PerR at 37°C for 1 h. Then, 50 nM H2O2-oxidized or DTT-reduced PerR protein was tested for binding to a 0.2 nM biotin-labeled dpr promoter fragment. The binding mixtures were electrophoresed on a 6% polyacrylamide gel on ice. The DNA-protein complex was transferred onto a nylon membrane and detected with a chemiluminescent nucleic acid detection module kit (Thermo Scientific).
Determination of H2O2 survival rate.
Overnight cultures of the tested strains were diluted 1:30 into fresh BHI broth and incubated strictly anaerobically. When the OD600 reached 0.4 to 0.5, the cells were separated into three aliquots. One aliquot was treated with 10 mM H2O2 for 10 min, and another was prepulsed with 40 μM H2O2 for 20 min before being subjected to 10 mM H2O2 treatment, while an aliquot not treated with 10 mM H2O2 was used as a control. Then, the cells were collected, washed twice with PBS, and resuspended in 200 μl BHI broth. Cell chains were separated by sonication for 30 s with an XC-3200D ultrasonic cleaner (Xinchen Company, Nanjing, China), and then 10-fold serial dilutions were performed. Appropriate dilutions were plated on BHI agar plates, and the numbers of CFU were counted after 24 h of incubation in a candle jar at 37°C. The survival percentage was calculated by dividing the number of CFU of the H2O2-challenged sample by the number of CFU of the corresponding controls. Experiments were executed in triplicate, and each experiment was repeated at least three times independently.
Assay of growth under H2O2 stress.
S. oligofermentans wild-type and gene deletion strains were grown anaerobically in BHI broth until the OD600 reached ∼0.5, with three replicates of each strain being included. Two replicate cultures were supplemented with 40 and 100 μM H2O2, respectively, leaving one replicate as an H2O2-untreated control. The growth profiles were measured by counting the number of CFU at the different time intervals. Triplicates for each sample were measured, and the experiments were repeated at least three times.
Determination of excreted hydrogen peroxide in culture.
The hydrogen peroxide in the culture suspension was quantified as described previously (11). Briefly, 650 μl of culture supernatant was added to 600 μl of a solution containing 2.5 mM 4-amino-antipyrine (4-amino-2,3-dimethyl-1-phenyl-3-pyrazolin-5-one) (Sigma-Aldrich) and 0.17 M phenol. The reaction proceeded for 4 min at room temperature; horseradish peroxidase (Sigma-Aldrich) was then added to a final concentration of 50 mU/ml in 0.2 M potassium phosphate buffer (pH 7.2). After 4 min of incubation at room temperature, the optical density at 510 nm was measured with a Unico 2100 visible spectrophotometer (Unico, Shanghai, China). A standard curve was generated with known concentrations of chemical H2O2.
Quantitative PCR.
Total RNA was extracted from mid-exponential-phase (OD600, ∼0.4 to 0.5) H2O2-treated and -untreated S. oligofermentans cells using the TRIzol reagent (Invitrogen, Carlsbad, CA), as recommended by the supplier. After quality confirmation with a 1% agarose gel, the RNA was treated with RNase-free DNase (Promega, Madison, WI) and analyzed by PCR for possible chromosomal DNA contamination. cDNA was generated from 2 μg total RNA with random primers using Moloney murine leukemia virus reverse transcriptase (Promega, Madison, WI), according to the supplier’s instructions, and was used for quantitative PCR (qPCR) amplification with the corresponding primers (Table S3). Amplifications were performed with a Mastercycler ep realplex2 instrument (Eppendorf, Germany). To estimate the copy numbers of the tested genes, a standard curve for each tested gene was generated by quantitative PCR using a 10-fold serially diluted PCR product as the template. The 16S rRNA gene was used as the biomass reference. The number of copies of the tested gene transcript per 100 16S rRNA copies is shown. All measurements were done for triplicate samples, and the experiments were repeated at least three times.
ACKNOWLEDGMENT
This study was supported by the National Natural Science Foundation of China (grant no. 31370098 and 31970035).
REFERENCES
- 1.Miller RA, Britigan BE. 1997. Role of oxidants in microbial pathophysiology. Clin Microbiol Rev 10:1–18. doi: 10.1128/CMR.10.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Winterbourn CC, Kettle AJ, Hampton MB. 2016. Reactive oxygen species and neutrophil function. Annu Rev Biochem 85:765–792. doi: 10.1146/annurev-biochem-060815-014442. [DOI] [PubMed] [Google Scholar]
- 3.Imlay JA. 2013. The molecular mechanisms and physiological consequences of oxidative stress: lessons from a model bacterium. Nat Rev Microbiol 11:443–454. doi: 10.1038/nrmicro3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Faulkner MJ, Helmann JD. 2011. Peroxide stress elicits adaptive changes in bacterial metal ion homeostasis. Antioxid Redox Signal 15:175–189. doi: 10.1089/ars.2010.3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu L, Tong H, Dong X. 2012. Function of the pyruvate oxidase-lactate oxidase cascade in interspecies competition between Streptococcus oligofermentans and Streptococcus mutans. Appl Environ Microbiol 78:2120–2127. doi: 10.1128/AEM.07539-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tong H, Chen W, Merritt J, Qi F, Shi W, Dong X. 2007. Streptococcus oligofermentans inhibits Streptococcus mutans through conversion of lactic acid into inhibitory H2O2: a possible counteroffensive strategy for interspecies competition. Mol Microbiol 63:872–880. doi: 10.1111/j.1365-2958.2006.05546.x. [DOI] [PubMed] [Google Scholar]
- 7.Tong H, Chen W, Shi W, Qi F, Dong X. 2008. SO-LAAO, a novel l-amino acid oxidase that enables Streptococcus oligofermentans to outcompete Streptococcus mutans by generating H2O2 from peptone. J Bacteriol 190:4716–4721. doi: 10.1128/JB.00363-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lisher JP, Tsui HT, Ramos-Montanez S, Hentchel KL, Martin JE, Trinidad JC, Winkler ME, Giedroc DP. 2017. Biological and chemical adaptation to endogenous hydrogen peroxide production in Streptococcus pneumoniae D39. mSphere 2:e00291-16. doi: 10.1128/mSphere.00291-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henningham A, Dohrmann S, Nizet V, Cole JN. 2015. Mechanisms of group A Streptococcus resistance to reactive oxygen species. FEMS Microbiol Rev 39:488–508. doi: 10.1093/femsre/fuu009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yesilkaya H, Andisi VF, Andrew PW, Bijlsma JJ. 2013. Streptococcus pneumoniae and reactive oxygen species: an unusual approach to living with radicals. Trends Microbiol 21:187–195. doi: 10.1016/j.tim.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 11.Wang X, Tong H, Dong X. 2014. PerR-regulated manganese ion uptake contributes to oxidative stress defense in an oral streptococcus. Appl Environ Microbiol 80:2351–2359. doi: 10.1128/AEM.00064-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hillion M, Antelmann H. 2015. Thiol-based redox switches in prokaryotes. Biol Chem 396:415–444. doi: 10.1515/hsz-2015-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Antelmann H, Helmann JD. 2011. Thiol-based redox switches and gene regulation. Antioxid Redox Signal 14:1049–1063. doi: 10.1089/ars.2010.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marinho HS, Real C, Cyrne L, Soares H, Antunes F. 2014. Hydrogen peroxide sensing, signaling and regulation of transcription factors. Redox Biol 2:535–562. doi: 10.1016/j.redox.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zheng M, Aslund F, Storz G. 1998. Activation of the OxyR transcription factor by reversible disulfide bond formation. Science 279:1718–1721. doi: 10.1126/science.279.5357.1718. [DOI] [PubMed] [Google Scholar]
- 16.Turner AG, Ong CY, Djoko KY, West NP, Davies MR, McEwan AG, Walker MJ. 2017. The PerR-regulated P1B-4-type ATPase (PmtA) acts as a ferrous iron efflux pump in Streptococcus pyogenes. Infect Immun 85:e00140-17. doi: 10.1128/IAI.00140-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fuangthong M, Herbig AF, Bsat N, Helmann JD. 2002. Regulation of the Bacillus subtilis fur and perR genes by PerR: not all members of the PerR regulon are peroxide inducible. J Bacteriol 184:3276–3286. doi: 10.1128/jb.184.12.3276-3286.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacquamet L, Traore DA, Ferrer JL, Proux O, Testemale D, Hazemann JL, Nazarenko E, El Ghazouani A, Caux-Thang C, Duarte V, Latour JM. 2009. Structural characterization of the active form of PerR: insights into the metal-induced activation of PerR and Fur proteins for DNA binding. Mol Microbiol 73:20–31. doi: 10.1111/j.1365-2958.2009.06753.x. [DOI] [PubMed] [Google Scholar]
- 19.Herbig AF, Helmann JD. 2001. Roles of metal ions and hydrogen peroxide in modulating the interaction of the Bacillus subtilis PerR peroxide regulon repressor with operator DNA. Mol Microbiol 41:849–859. doi: 10.1046/j.1365-2958.2001.02543.x. [DOI] [PubMed] [Google Scholar]
- 20.Lee JW, Helmann JD. 2006. The PerR transcription factor senses H2O2 by metal-catalysed histidine oxidation. Nature 440:363–367. doi: 10.1038/nature04537. [DOI] [PubMed] [Google Scholar]
- 21.Makthal N, Rastegari S, Sanson M, Ma Z, Olsen RJ, Helmann JD, Musser JM, Kumaraswami M. 2013. Crystal structure of peroxide stress regulator from Streptococcus pyogenes provides functional insights into the mechanism of oxidative stress sensing. J Biol Chem 288:18311–18324. doi: 10.1074/jbc.M113.456590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grifantini R, Toukoki C, Colaprico A, Gryllos I. 2011. Peroxide stimulon and role of PerR in group A Streptococcus. J Bacteriol 193:6539–6551. doi: 10.1128/JB.05924-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rhee SG. 2006. Cell signaling. H2O2, a necessary evil for cell signaling. Science 312:1882–1883. doi: 10.1126/science.1130481. [DOI] [PubMed] [Google Scholar]
- 24.Sun F, Liang H, Kong X, Xie S, Cho H, Deng X, Ji Q, Zhang H, Alvarez S, Hicks LM, Bae T, Luo C, Jiang H, He C. 2012. Quorum-sensing agr mediates bacterial oxidation response via an intramolecular disulfide redox switch in the response regulator AgrA. Proc Natl Acad Sci U S A 109:9095–9100. doi: 10.1073/pnas.1200603109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen Z, Wang X, Yang F, Hu Q, Tong H, Dong X. 2017. Molecular insights into hydrogen peroxide-sensing mechanism of the metalloregulator MntR in controlling bacterial resistance to oxidative stresses. J Biol Chem 292:5519–5531. doi: 10.1074/jbc.M116.764126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lindahl M, Mata-Cabana A, Kieselbach T. 2011. The disulfide proteome and other reactive cysteine proteomes: analysis and functional significance. Antioxid Redox Signal 14:2581–2642. doi: 10.1089/ars.2010.3551. [DOI] [PubMed] [Google Scholar]
- 27.Leonard SE, Carroll KS. 2011. Chemical ‘omics’ approaches for understanding protein cysteine oxidation in biology. Curr Opin Chem Biol 15:88–102. doi: 10.1016/j.cbpa.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 28.Wang J, Jin L, Li X, Deng H, Chen Y, Lian Q, Ge R, Deng H. 2013. Gossypol induces apoptosis in ovarian cancer cells through oxidative stress. Mol Biosyst 9:1489–1497. doi: 10.1039/c3mb25461e. [DOI] [PubMed] [Google Scholar]
- 29.Leichert LI, Gehrke F, Gudiseva HV, Blackwell T, Ilbert M, Walker AK, Strahler JR, Andrews PC, Jakob U. 2008. Quantifying changes in the thiol redox proteome upon oxidative stress in vivo. Proc Natl Acad Sci U S A 105:8197–8202. doi: 10.1073/pnas.0707723105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deng X, Weerapana E, Ulanovskaya O, Sun F, Liang H, Ji Q, Ye Y, Fu Y, Zhou L, Li J, Zhang H, Wang C, Alvarez S, Hicks LM, Lan L, Wu M, Cravatt BF, He C. 2013. Proteome-wide quantification and characterization of oxidation-sensitive cysteines in pathogenic bacteria. Cell Host Microbe 13:358–370. doi: 10.1016/j.chom.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pericone CD, Park S, Imlay JA, Weiser JN. 2003. Factors contributing to hydrogen peroxide resistance in Streptococcus pneumoniae include pyruvate oxidase (SpxB) and avoidance of the toxic effects of the Fenton reaction. J Bacteriol 185:6815–6825. doi: 10.1128/jb.185.23.6815-6825.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Belousov VV, Fradkov AF, Lukyanov KA, Staroverov DB, Shakhbazov KS, Terskikh AV, Lukyanov S. 2006. Genetically encoded fluorescent indicator for intracellular hydrogen peroxide. Nat Methods 3:281–286. doi: 10.1038/nmeth866. [DOI] [PubMed] [Google Scholar]
- 33.Tong H, Wang X, Dong Y, Hu Q, Zhao Z, Zhu Y, Dong L, Bai F, Dong X. 2019. A Streptococcus aquaporin acts as peroxiporin for efflux of cellular hydrogen peroxide and alleviation of oxidative stress. J Biol Chem 294:4583–4595. doi: 10.1074/jbc.RA118.006877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schilling B, Yoo CB, Collins CJ, Gibson BW. 2004. Determining cysteine oxidation status using differential alkylation. Int J Mass Spectrom 236:117–127. doi: 10.1016/j.ijms.2004.06.004. [DOI] [Google Scholar]
- 35.Mi H, Huang X, Muruganujan A, Tang H, Mills C, Kang D, Thomas PD. 2017. PANTHER version 11: expanded annotation data from Gene Ontology and Reactome pathways, and data analysis tool enhancements. Nucleic Acids Res 45:D183–D189. doi: 10.1093/nar/gkw1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hajaj B, Yesilkaya H, Benisty R, David M, Andrew PW, Porat N. 2012. Thiol peroxidase is an important component of Streptococcus pneumoniae in oxygenated environments. Infect Immun 80:4333–4343. doi: 10.1128/IAI.00126-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jakob U, Eser M, Bardwell JC. 2000. Redox switch of Hsp33 has a novel zinc-binding motif. J Biol Chem 275:38302–38310. doi: 10.1074/jbc.M005957200. [DOI] [PubMed] [Google Scholar]
- 38.Bae JB, Park JH, Hahn MY, Kim MS, Roe JH. 2004. Redox-dependent changes in RsrA, an anti-sigma factor in Streptomyces coelicolor: zinc release and disulfide bond formation. J Mol Biol 335:425–435. doi: 10.1016/j.jmb.2003.10.065. [DOI] [PubMed] [Google Scholar]
- 39.Horsburgh MJ, Clements MO, Crossley H, Ingham E, Foster SJ. 2001. PerR controls oxidative stress resistance and iron storage proteins and is required for virulence in Staphylococcus aureus. Infect Immun 69:3744–3754. doi: 10.1128/IAI.69.6.3744-3754.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hillmann F, Doring C, Riebe O, Ehrenreich A, Fischer RJ, Bahl H. 2009. The role of PerR in O2-affected gene expression of Clostridium acetobutylicum. J Bacteriol 191:6082–6093. doi: 10.1128/JB.00351-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Di Simplicio P, Franconi F, Frosali S, Di Giuseppe D. 2003. Thiolation and nitrosation of cysteines in biological fluids and cells. Amino Acids 25:323–339. doi: 10.1007/s00726-003-0020-1. [DOI] [PubMed] [Google Scholar]
- 42.Hahn JS, Oh SY, Chater KF, Cho YH, Roe JH. 2000. H2O2-sensitive Fur-like repressor CatR regulating the major catalase gene in Streptomyces coelicolor. J Biol Chem 275:38254–38260. doi: 10.1074/jbc.M006079200. [DOI] [PubMed] [Google Scholar]
- 43.Cremers CM, Jakob U. 2013. Oxidant sensing by reversible disulfide bond formation. J Biol Chem 288:26489–26496. doi: 10.1074/jbc.R113.462929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Helmann JD. 2014. Specificity of metal sensing: iron and manganese homeostasis in Bacillus subtilis. J Biol Chem 289:28112–28120. doi: 10.1074/jbc.R114.587071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lisher JP, Giedroc DP. 2013. Manganese acquisition and homeostasis at the host-pathogen interface. Front Cell Infect Microbiol 3:91. doi: 10.3389/fcimb.2013.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Imlay JA. 2019. Where in the world do bacteria experience oxidative stress? Environ Microbiol 21:521–530. doi: 10.1111/1462-2920.14445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aguirre JD, Culotta VC. 2012. Battles with iron: manganese in oxidative stress protection. J Biol Chem 287:13541–13548. doi: 10.1074/jbc.R111.312181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Turner AG, Ong CL, Gillen CM, Davies MR, West NP, McEwan AG, Walker MJ. 2015. Manganese homeostasis in group A Streptococcus is critical for resistance to oxidative stress and virulence. mBio 6:e00278-15. doi: 10.1128/mBio.00278-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rhodes DV, Crump KE, Makhlynets O, Snyder M, Ge X, Xu P, Stubbe J, Kitten T. 2014. Genetic characterization and role in virulence of the ribonucleotide reductases of Streptococcus sanguinis. J Biol Chem 289:6273–6287. doi: 10.1074/jbc.M113.533620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu Y, Itzek A, Kreth J. 2014. Comparison of genes required for H2O2 resistance in Streptococcus gordonii and Streptococcus sanguinis. Microbiology 160:2627–2638. doi: 10.1099/mic.0.082156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marco S, Rullo R, Albino A, Masullo M, De Vendittis E, Amato M. 2013. The thioredoxin system in the dental caries pathogen Streptococcus mutans and the food-industry bacterium Streptococcus thermophilus. Biochimie 95:2145–2156. doi: 10.1016/j.biochi.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 52.Tong H, Gao X, Dong X. 2003. Streptococcus oligofermentans sp. nov., a novel oral isolate from caries-free humans. Int J Syst Evol Microbiol 53:1101–1104. doi: 10.1099/ijs.0.02493-0. [DOI] [PubMed] [Google Scholar]
- 53.Lau PC, Sung CK, Lee JH, Morrison DA, Cvitkovitch DG. 2002. PCR ligation mutagenesis in transformable streptococci: application and efficiency. J Microbiol Methods 49:193–205. doi: 10.1016/s0167-7012(01)00369-4. [DOI] [PubMed] [Google Scholar]
- 54.Liu Y, Zeng L, Burne RA. 2009. AguR is required for induction of the Streptococcus mutans agmatine deiminase system by low pH and agmatine. Appl Environ Microbiol 75:2629–2637. doi: 10.1128/AEM.02145-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.LeBlanc DJ, Lee LN, Abu-Al-Jaibat A. 1992. Molecular, genetic, and functional analysis of the basic replicon of pVA380-1, a plasmid of oral streptococcal origin. Plasmid 28:130–145. doi: 10.1016/0147-619X(92)90044-B. [DOI] [PubMed] [Google Scholar]
- 56.Markvicheva KN, Bogdanova EA, Staroverov DB, Lukyanov S, Belousov VV. 2008. Imaging of intracellular hydrogen peroxide production with HyPer upon stimulation of HeLa cells with epidermal growth factor. Methods Mol Biol 476:79–86. doi: 10.1007/978-1-59745-129-1_6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Representative MS/MS spectra for identification of the redox-sensitive proteins and the cysteine-oxidized and -reduced peptide fragment of PerR, Tpx, and Trx of S. oligofermentans. (A and B) Representative MS/MS spectra of the doubly charged fragment ions at m/z 888.9315 (A) and 887.9282 (B), corresponding to peptide fragment AIC235EETGNGHVQLFAK from pyruvate kinase with reduced (13C-carboxymethylated) and oxidized (12C-carboxymethylated) cysteine residues, respectively. (C) The MS/MS spectrum of the doubly charged fragment ion at m/z 1143.6489, corresponding to the peptide fragment SQMVVYGIC139PEC142AQQEQVAS from PerR, in which two cysteine residues were oxidized (12C carboxymethylated). The spectrum was obtained from a preliminary redox proteomic experiment. (D) MS/MS spectrum of a doubly charged fragment ion at m/z 968.5062 for MH22+, corresponding to the Tpx peptide fragment VLSIVPSIDTGVC58STQTR from H2O2-untreated cells, in which the cysteine residue was reduced (13C carboxymethylated). (E and F) MS/MS spectra of a doubly charged fragment ion at m/z 968.5067 and 967.5011 for MH22+, corresponding to the Tpx peptide fragment VLSIVPSIDTGVC58STQTR from H2O2-treated cells, in which the cysteine residue was reduced (13C carboxymethylated) and oxidized (12C carboxymethylated), respectively. (G and H) MS/MS spectra of a triply charged fragment ion at m/z 487.5490 and 486.8803 for MH23+, corresponding to the peptide FWASWC79GPC82KR of Trx from H2O2-untreated cells, in which the Cys82 residue was reduced (13C carboxymethylated) and oxidized (12C carboxymethylated), respectively. (I) MS/MS spectrum of a doubly charged fragment ion at m/z 729.8172 for MH22+, corresponding to the peptide FWASWC79GPC82KR of Trx from H2O2-treated cells, in which the Cys82 residue was oxidized (12C carboxymethylated). (Insets) Fragments of the relevant peptide sequences matching the observed fragmentation ions. Download FIG S1, TIF file, 2.0 MB (2.2MB, tif) .
Copyright © 2020 Tong et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
(A) Redox proteomics-identified proteins in the S. oligofermentans wild-type strain without 40 μM H2O2 treatment. (B) Redox proteomics-identified proteins in the S. oligofermentans wild-type strain pulsed with 40 μM H2O2. (C) Redox proteomics-identified cysteine-containing proteins in the S. oligofermentans wild-type strain without 40 μM H2O2 treatment. (D) Redox proteomics-identified cysteine-containing proteins in the S. oligofermentans wild-type strain pulsed with 40 μM H2O2. (E) Reversible or irreversible oxidized cysteine-containing proteins upon H2O2 treatment in the wild-type strain. +, a peptide with an SOH or SO2H/SO3H modification was identified in the tested sample; a, the thiol/disulfide oxidized ratio was calculated by dividing the intensity of the oxidized disulfide-linked peptide (12C carboxymethylated) over the sum of the disulfide-oxidized (12C-carboxymethylated) and -reduced (13C-carboxymethylated) peptides in the corresponding sample. (F) Overrepresented biological processes of the cysteine-oxidized proteins identified in the S. oligofermentans wild-type strain upon 40 μM H2O2 treatment Download Data Set S1, XLSX file, 0.3 MB (347.8KB, xlsx) .
Copyright © 2020 Tong et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
(A) Redox proteomics-identified proteins in the S. oligofermentans wild-type strain grown in 40-ml cultures. (B) Redox proteomics-identified cysteine-containing proteins in the S. oligofermentans wild-type strain grown in 40-ml cultures. (C) Redox proteomics-identified proteins in the S. oligofermentans wild-type strain grown in 10-ml cultures. (D) Redox proteomics-identified cysteine-containing proteins in the S. oligofermentans wild-type strain grown in 10-ml cultures. (E) Reversible or irreversible oxidized cysteine-containing proteins identified in the S. oligofermentans wild-type strain grown in different volumes of culture medium. *, ND, the peptide fragment was not detected in the tested sample; +, the peptide with the SOH or SO2H/SO3H modification was identified in the tested sample; a, the thiol/disulfide oxidized ratio was calculated by dividing the intensity of oxidized disulfide-linked peptide (12C carboxymethylated) by the sum of the disulfide-oxidized (12C-carboxymethylated) and -reduced (13C-carboxymethylated) peptide in the corresponding sample. (F) 1, overrepresented biological processes of the cysteine-oxidized proteins identified in the S. oligofermentans wild-type strain grown in 40-ml cultures; 2, overrepresented biological processes of the cysteine-oxidized proteins identified in the S. oligofermentans wild-type strain grown in 10-ml cultures. Download Data Set S2, XLSX file, 0.3 MB (345.7KB, xlsx) .
Copyright © 2020 Tong et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Redox Western blotting detects in vivo PerR oxidation (A), and immunoprecipitation detects oxidized PerR (B). (A) Redox Western blotting determined that the PerR proteins were reversibly oxidized in aerobically grown cells. Mid-exponential-phase S. oligofermentans PerR-6×His cells were sonicated in RIPA buffer containing the free thiol protectant NEM and 10 mM EDTA. Cell lysates were divided into two aliquots, in which one was treated with 50 mM DTT for 1 h and one was left untreated. Redox Western blotting was performed to assay the oxidative status of the PerR-6×His protein using anti-6×His antibody, and the molecular weight marker is labeled at the left. (B) Immunoprecipitation of the oxidized cellular PerR. One liter of mid-exponential-phase cells of the statically grown PerR-6×His strain was collected, washed twice with PBS, and resuspended in 10 ml lysis buffer. The 6×His-tagged PerR protein was pulled down using anti-His tag monoclonal antibody-magnetic beads as described in Materials and Methods. Proteins in the elution (IP elution) were separated on an 18% nonreducing SDS-PAGE gel and stained with Coomassie brilliant blue. The molecular mass marker is labeled at the left, and the black arrow indicates the target 6×His-tagged PerR protein with a predicted molecular weight of 17.8 kDa. Download FIG S2, TIF file, 0.2 MB (244KB, tif) .
Copyright © 2020 Tong et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Calculation of the oxidation ratio of cysteine or histidine residues in 6×His-tagged PerR that was immunoprecipitated from statically grown S. oligofermentans. a, the numbers of LC-MS/MS-identified peptide spectral matches (PSMs) of the fragments containing targeting amino acid residues were counted, and the oxidation ratios of the respective residues were calculated by dividing the number of oxidized PSMs by the total number of PSMs. Download Table S1, DOCX file, 0.02 MB (16.4KB, docx) .
Copyright © 2020 Tong et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
DTT reduction increases the hybridization signal of the anti-His tag antibody. Overnight cultures of perR::pDL278-perRC142S-6×His strain were diluted 1:30 into fresh BHI broth and grown statically. The mid-exponential-phase cells were collected, washed twice with PBS, and sonicated in RIPA buffer containing the free thiol protectant NEM and 10 mM EDTA. The cell lysate was divided into seven aliquots; six of these were treated with gradient concentrations of DTT (lanes 2 to 7) for 1 h, and one was not treated with DTT. Redox Western blotting on 18% SDS-PAGE gels was performed to assay for the PerR-C142S protein using the anti-His tag antibody. The black arrow indicates the PerR-C142S hybridization band; the gray arrow shows the molecular weight marker; *, nonspecific hybridization band. Download FIG S3, TIF file, 0.1 MB (137.3KB, tif) .
Copyright © 2020 Tong et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Calculation of histidine oxidation in the recombinant PerR:Zn,Mn protein resulting from H2O2. a, the numbers of LC-MS/MS-identified peptide spectral matches (PSMs) of the fragments containing histidine residues were counted, and the oxidation ratios of the histidine residues were calculated by dividing the number of oxidized PSMs by the total number of PSMs. Download Table S2, DOCX file, 0.02 MB (16.8KB, docx) .
Copyright © 2020 Tong et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
EMSA of PerR binding to the mntABC, tpx, and mntR promoters. The PerR-GST protein was digested with 100 U thrombin to remove the GST tag and then eluted into a buffer containing 10 mM EDTA and 1 mM DTT. Next, 1 μM PerR protein was preincubated with 1 mM MnCl2, and then gradient concentrations of the recombinant PerR:Zn,Mn protein (0 to 200 nM) were used for binding to 5′-biotin-labeled mntABC (KEGG accession numbers I872_09645, I872_09650, and I872_09655), tpx (KEGG accession number I872_09640), and mntR (KEGG accession number I872_01020) promoters, as described in Materials and Methods. Black arrows point to the free DNA probes (0.2 nM for each). Download FIG S4, TIF file, 0.6 MB (586.2KB, tif) .
Copyright © 2020 Tong et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Sequence alignment and structural comparison of Zn2+-coordinated cysteine residues of the PerR proteins from S. oligofermentans and B. subtilis. (A) The amino acid sequences of the S. oligofermentans (UniProt accession number A0A512AEK5) and B. subtilis (UniProt accession number P71086) PerRs were retrieved from the UniProt protein database. Sequence alignment was performed using the DNAMAN program. *, conserved amino acid residues essential for manganese or ferric ion binding in B. subtilis; #, conserved cysteine residues for zinc ion binding. Black shadings indicate 100% homology. (B) Structural homology modeling of the S. oligofermentans PerR, performed using the SWISS-MODEL server by automatically selecting S. pyogenes PerR (PDB accession number 4LMY) as a template (left) and the crystal structure of B. subtilis PerR (PDB accession number 2FE3) (right). Four Zn2+-coordinated cysteine residues are shown as sticks, and the corresponding cysteine residues are labeled. Download FIG S5, TIF file, 2.1 MB (2.2MB, tif) .
Copyright © 2020 Tong et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Strains, plasmids, and primers used in this study. *, Amp, ampicillin; Kan, kanamycin; Sp, spectinomycin; r, resistant; s, sensitive. Italic nucleotide bases indicate restriction enzyme digestion sites. Download Table S3, DOCX file, 0.02 MB (26.2KB, docx) .
Copyright © 2020 Tong et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.