Abstract
Auditory steady-state responses (ASSRs) are states in which the electrical activity of the brain reacts steadily to repeated auditory stimuli. They are known to be useful for testing the functional integrity of neural circuits in the cortex, as well as for their capacity to generate synchronous activity in both human and animal models. Furthermore, abnormal gamma oscillations on ASSR are typically observed in patients with schizophrenia (SZ). Changes in neural synchrony may reflect aberrations in cortical gamma-aminobutyric acid (GABA) neurotransmission. However, GABA’s impact and effects related to ASSR are still unclear. Here, we examined the effect of a GABAa receptor antagonist, (+)-bicuculline, on ASSR in free-moving rats. (+)-Bicuculline (1, 2 and 4 mg/kg, sc) markedly and dose-dependently reduced ASSR signals, consistent with current hypotheses. In particular, (+)-bicuculline significantly reduced event-related spectral perturbations (ERSPs) at 2 and 4 mg/kg between 10 and 30 minutes post-dose. Further, bicuculline (2 and 4 mg/kg) significantly and dose-dependently increased baseline gamma power. Furthermore, the occurrence of convulsions was consistent with the drug’s pharmacokinetics. For example, high doses of (+)-bicuculline such as those greater than 880 ng/g in the brain induced convulsion. Additionally, time-dependent changes in ERSP with (+)-bicuculline were observed in accordance with drug concentration. This study partially unraveled the contribution of GABAa receptor signals to the generation of ASSR.
1 Introduction
Auditory steady-state response (ASSR) measures the intrinsic ability of auditory neuronal ensembles to entrain to rhythmically presented stimuli and can be used to test the functional integrity of neural circuits that support synchronization [1–3] across frequencies in both human and animal models. Human studies have consistently shown a 40-Hz deficit in ASSR, or in evoked gamma power, in patients with schizophrenia (SZ) [4–8] and in family members with increased risk of SZ [9]. Acute treatment with N-methyl-D-aspartate (NMDA) receptor antagonists like phencyclidine and ketamine is known to mimic a brief SZ-like state in healthy individuals [10–12]. Taking advantage of the clinical evidence, preclinical studies often also employ a short-term disruption to NMDA neurotransmission to mimic a psychosis-like state [13–15]. The effects of NMDA antagonists MK-801 and ketamine have been tested with the 40-Hz ASSR, and results have provided understanding of the role of NMDA receptors in 40-Hz ASSR [16–19]. ASSR is a translatable biomarker which is driving research into neuropsychiatric disorders like schizophrenia.
ASSR deficits in SZ could reflect neurophysiological abnormalities [1, 20] and developmental alterations in the neurotransmission of excitatory (glutamate) and inhibitory (gamma-aminobutyric acid: GABA) transmitter systems [21]. Cortical GABAergic interneurons strongly regulate neuronal network oscillations, particularly in the gamma band [22, 23], and dysfunction of these cells is one of the putative pathophysiological mechanisms of SZ [24, 25].
Furthermore, studies have suggested that ASSR also reflects control by GABA-agonist- activated inhibitory interneurons of the timing of pyramidal neuron firing in cortical layers 2–3 [26], with the interaction between pyramidal neurons and inhibitory neurons thought to underlie the occurrence of neural oscillations [27]. The potential role of the GABA neurotransmitter system in the generation and maintenance of synchronous oscillations [28] has led to increased focus on the system’s possible involvement in the pathophysiology of SZ [29–31], with some neurobiological alterations observed in SZ suggested to result from a compensatory response to restore inhibitory synaptic efficacy [26]. It is likely that the GABAa receptor subtype, which propagates network synchronization [32], is associated with the oscillatory abnormalities observed in patients with SZ. Given that the temporal cortex is thought to play an important role in the generation of ASSRs, these findings suggest a potential link between GABAa abnormalities and ASSR disturbances in SZ. Studies have examined the effects of the GABAa agonist muscimol [33] and GABAa antagonists picrotoxin and (-)-bicuculline methiodide [18, 33] on ASSR in rats; however, the interpretation of these studies has been insufficient and unclear (not significantly).
Here, therefore, we assessed the effects of the GABAa receptor antagonist bicuculline in rats ASSR models which we constructed previously with the same sound protocol in a clinical setting [16]. To evaluate the pharmacological effects of GABAa receptor antagonists, we also examined the pharmacokinetics of bicuculline and its effects on convulsion.
2. Experimental procedures
2.1 Animals
Sprague-Dawley (SD) rats (Japan Charles River Laboratories International, Inc., Atsugi-shi, Japan) with electroencephalogram (EEG) electrode implants were housed in groups of three in temperature- and humidity-controlled rooms (23 ± 2°C and 55 ± 10%) under a 12-h light/dark cycle. Food and water were available ad libitum in the home cages. All animal experimental procedures were approved by the Institutional Animal Care and Use Committee of Astellas Pharma Inc. Tsukuba Research Center is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International.
2.2 Drugs
The GABAa antagonist bicuculline (Sigma-Aldrich Co. LLC., St. Louis, MO, USA) was dissolved in 0.1 mol/L HCl (pH 5.0). The drug was subcutaneously (sc) administered at 1 mL/kg to rats.
2.3 Bicuculline concentration in rat brain
Male SD rats aged 11 weeks (Charles River Laboratories Japan, Inc.) that had been freely feeding received a sc dose of (+)-bicuculline (1, 4 mg/kg). The animals were sacrificed under isoflurane anesthesia, and whole blood samples and brain tissue were taken at 10, 30, and 110 min post-dose. Whole blood samples were taken using a syringe containing heparin sodium. After collection of whole blood, the cerebrum was removed from each animal. Whole blood samples were centrifuged to separate plasma. Plasma and cerebrum samples were stored at −20°C until assay.
(+)-Bicuculline concentrations in plasma and 25% brain homogenate samples were determined using a liquid chromatography-tandem mass spectrometry method with a calibration curve range of 3 to 1000 ng/mL. Mean plasma and brain concentrations were calculated using Microsoft Excel ® (Microsoft Corp, Redmond, WA, USA).
2.4 Convulsion testing
A convulsion test was performed during the pharmacokinetics testing of bicuculline, using a minimum dose of 1 mg/kg based on Vohs [33] and a maximum dose of 4 mg/kg based on our preliminary results that this dose was not lethal in rats. Regarding efforts to minimize harm and suffering, Astellas Pharma Inc. has acquired AAALAC accreditation and the convulsion experiments were conducted under veterinary supervision. During the convulsion testing, the experimenter observed and monitored the animals from immediately after compound administration until 110 minutes after the end of the experiment. Evaluation was done using a modification of the method of Racine [34]. Convulsion severity was categorized as Grade 0, No convulsion; Grade I, rhythmic mouth and facial twitching; Grade II, rhythmic nodding or tail flicking; Grade III, single limb twitch; Grade IV, bilateral anterior limb rigidity or twitching with standing; and Grade V, comprehensive tonic-clonic with fall. Grade III or higher was judged to indicate induced convulsion. Animals were euthanized after the experiments.
2.5 Electrode implantation
Male SD rats aged 8 weeks were anesthetized using isoflurane and then placed into a stereotactic apparatus. The apparatus and experimental procedures were similar to those described previously [16]. After applying lidocaine as a local anesthetic, an incision was made to expose the skull. The location of bregma was identified, and stainless-steel screw electrodes with wire leads were implanted epidurally over the temporal cortex (AP, −4.5 mm; ML, −7.5 mm; and DV, −4.0 mm from bregma), cerebellum (ground), and frontal sinus (reference). Lead wires were connected to the pedestal, and the entire assembly was secured to the skull using dental cement. Recordings were conducted for at least 10 days after the rats recovered from surgery.
2.6 EEG recordings
EEG recordings [16] were performed using a programming script with a data acquisition and analysis software package (Spike2®, Cambridge Electronic Design, Milton, Cambridge, UK). Rats were hooked to customized electrode cables up to the pedestal. The electrode cables were connected to a high-impedance differential AC amplifier (sampling rate: 1000 Hz, low cut-off filter: 1 Hz, high cut-off filter: 500 Hz; model #1800; A-M Systems, Carlsborg, WA, USA) and versatile data acquisition unit (Micro1401, Cambridge Electronic Design). Rats were individually placed into a recording chamber in an electrically shielded cage with a speaker attached to the top of the cage and moved freely during the EEG recording. For habituation, the EEG recording was started at least 30 min after placement in the recording chamber. Auditory stimuli consisted of click sounds (80 dB, 1 ms), which were presented as 500-ms trains at 40 Hz, with 20 clicks per train. Click sound trains were repeated 200 times/trial, with an inter-train interval of 600 ms. The ASSR was recorded at −10 (baseline), 10, 30, 70 and 110 min after drug injection.
2.7 Data analysis
The EEG data from Spike2 were converted for analysis in Matlab. By using the MATLAB toolbox EEGLAB® (MathWorks, Natick MA, USA), signal trial epochs between −250 and 750 ms (for ASSR) or -600 and 0 ms (for baseline gamma power analysis) relative to the first click of the train were extracted from continuous data. All outliers in each split file were rejected for movement artifacts based on a criterion of 2 times the root mean square amplitude per mouse. In ASSR analysis, a low-pass filter (100 Hz) was applied to the EEG data to remove artifacts. Averaging stimuli during each click sound train was performed by wavelet transformation (frequency limits: 10 to 100 Hz, wavelet cycles: 0, epoch size: 1.0 sec). As output data, the measurable factors were divided into two categories: event-related spectral perturbations (ERSPs) (pre-stimulations from −250 to 0 ms) and inter-trial coherence (ITC) using the EEGLAB® and were gathered using open source software, KNIME® (KNIME AG, Zurich, Switzerland). Mean ERSP and ITC were calculated by averaging the data from 100 to 450 msec within a trial (0–500 ms) for 40 Hz (38–42 Hz). ERSP is defined as an event-related change in power relative to a pre-stimulus baseline, while ITC is defined as phase consistency across trials, and ranges between 0 (random phase across trials) and 1 (identical phase across trials) [35]. Baseline power analysis, i.e. stimulus-free and non-time locked analysis, was performed using fast Fourier transform (FFT) analysis with EEGLAB®. The value obtained between 30 and 80 Hz was derived from a power spectrum as the baseline gamma power. Both the ASSR and baseline gamma power were expressed relative to pre-dosing values (−10 min). The same animals were used for ASSR and baseline power recordings.
2.8 Statistical analysis
For (+)-bicuculline concentration, all values are expressed as mean ± standard deviation (SD). For EEG data, all values are expressed as mean ± standard error of the mean (SEM). Statistical comparisons were performed using one- or two-way analysis of variance (ANOVA) with Dunnett’s multiple comparisons test to compare differences among multiple groups (GraphPad Prism 7®, GraphPad Software, San Diego CA, USA). For all tests, p<0.05 was considered significant.
3. Results
3.1 (+)-bicuculline concentration in rat brain (Fig 1)
Fig 1. Brain concentrations of (+)-bicuculline in rats.
Rats received a subcutaneous dose of (+)-bicuculline (1, 4 mg/kg) in distilled water with 0.1N HCl and 0.1N NaOH (pH 5.0). Animals were sacrificed and the cerebrum was removed at 10, 30, and 110 min post-dose. Brain concentrations were measured by liquid chromatography-tandem mass spectrometry. Values are mean ± SD.
(+)-bicuculline penetrated the brain in a dose-dependent manner and correspondingly induced convulsion at a bicuculline concentration greater than 880 ng/g in the brain. Time to maximum concentration (Tmax) of (+)-bicuculline concentration in the brain was 10 and 30 min at 1 and 4 mg/kg, respectively.
3.2 ASSR (Figs 2 and 3)
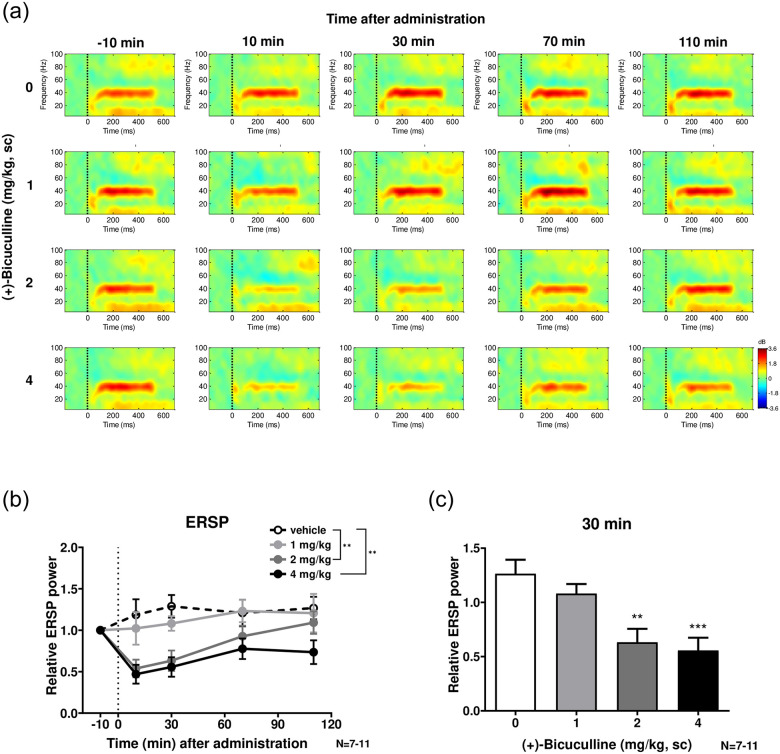
Fig 2. The effect of (+)-bicuculline on the 40 Hz ERSP.
a, Heat map representation of mean time-frequency plots of ASSR at 40-Hz stimulation at −10 (baseline), 10, 30, 70 and 110 min following (+)-bicuculline (0, 1, 2 and 4 mg/kg, sc) treatment. b, Time-course of the 40 Hz ERSP following (+)-bicuculline treatment. c, Effect of bicuculline on the 40 Hz ERSP at 30 min after treatment. Bicuculline significantly (4 mg/kg) and dose-dependently decreased ERSP. Values are mean ± SEM. The number of rats was 12 in each group.
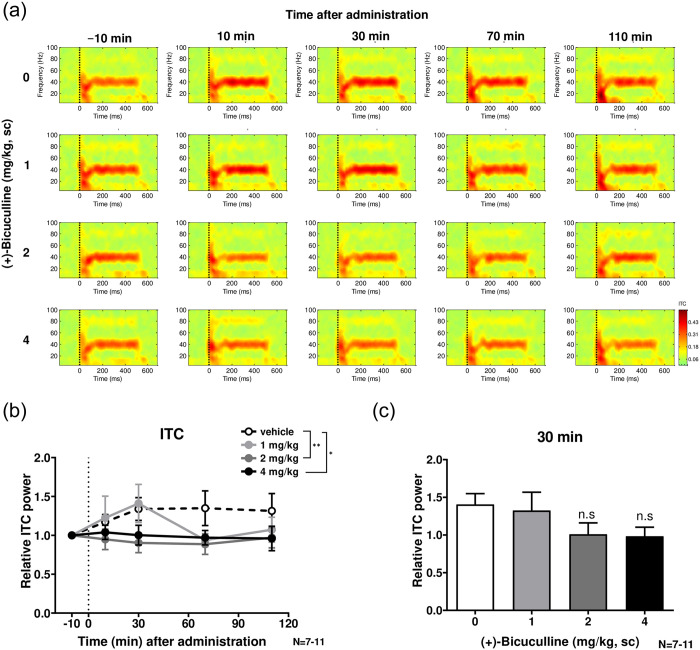
Fig 3. The effect of (+)-bicuculline on the 40 Hz ITC.
a, Heat map representation of mean time-frequency plots of ASSR at 40-Hz stimulation at −10 (baseline), 10, 30, 70 and 110 min following (+)-bicuculline (0, 1, 2 and 4 mg/kg, sc) treatment, as shown for ERSP in Fig 2. b, Time-course of the 40 Hz ITC following (+)-bicuculline treatment. The 40 Hz ITC was significantly reduced in bicuculline (2 and 4 mg/kg)-treated rats than vehicle-treated rats. c, Effect of bicuculline on the 40 Hz ITC at 30 min, the time at which bicuculline achieves Cmax. No statistically significant effect was observed on ITC with bicuculline treatment. Values are mean ± SEM. The number of rats was 12 in each group.
Fig 2 shows the effects of (+)-bicuculline on ASSR according to ERSP. Fig 2a shows a heat map representation of mean time-frequency plots of ASSR at 40-Hz stimulation at −10 (baseline), 10, 30, 70 and 110 min following (+)-bicuculline (0, 1, 2 and 4 mg/kg, sc) treatment. The time-course of ERSP of ASSR at 40 Hz following (+)-bicuculline treatment is summarized in Fig 2b. Two-way ANOVA revealed significant time (F(2.274, 75.04) = 4.440; P<0.05) and treatment (F(3, 33) = 8.580; P<0.01) effects. Post hoc comparisons showed significantly smaller effects in bicuculline (2 and 4 mg/kg)-treated rats than vehicle-treated rats on Dunnett’s multiple comparisons test (P<0.01). Furthermore, bicuculline significantly (4 mg/kg, p < 0.05, Dunnett’s multiple comparisons test after one-way ANOVA) and dose-dependently decreased ERSP at 40 Hz (Fig 2c) at 30 min, the time at which maximum bicuculline concentration is observed (Cmax; maximum drug concentration).
Fig 3 shows the effects of (+)-bicuculline on ASSR according to ITC at 40 Hz. Fig 3a shows a heat map representation of mean time-frequency plots of ASSR at 40-Hz stimulation at −10 (baseline), 10, 30, 70 and 110 min following (+)-bicuculline (0, 1, 2 and 4 mg/kg, sc) treatment, as was shown for ERSP in Fig 2. In Fig 3b, relative ITC due to treatment at 1, 2 and 4 mg/kg across four time points is compared with that following vehicle treatment. Two-way ANOVA revealed no significant time (F(2.170, 71.61) = 0.6592; P = 0.5323>0.05) or treatment (F(3, 33) = 2.118; P = 0.1167>0.05) effects. However, the ITC of ASSR at 40 Hz was significantly smaller in bicuculline (2 and 4 mg/kg)-treated rats than vehicle-treated rats in Dunnett’s multiple comparisons test. At 30 min, the time at which bicuculline achieves Cmax, the ITC at 40 Hz decreased following treatment with bicuculline (2 and 4 mg/kg, sc), as was observed for ERSP, albeit not significantly so (Fig 3c).
3.3. Baseline gamma power (Fig 4)
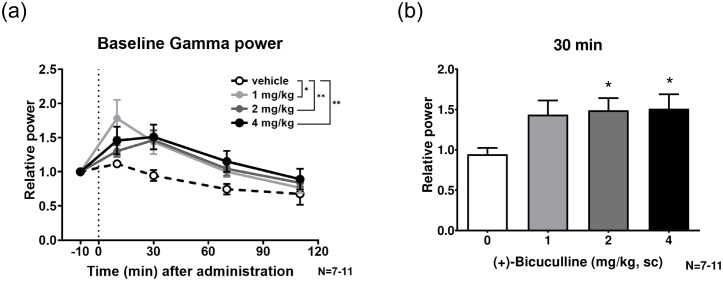
Fig 4. Baseline gamma power (30–80 Hz) following treatment with 1 to 4 mg/kg bicuculline.
a, Time-course of relative baseline gamma of ASSR following (+)-bicuculline treatment. b, Effect of bicuculline on baseline gamma at 30 min. Bicuculline significantly (2 and 4 mg/kg) and dose-dependently increased the baseline gamma power at 30 min.
Fig 4 shows the baseline gamma power (30–80 Hz) following treatment with 1 to 4 mg/kg bicuculline. The time-course of the relative baseline gamma of ASSR following (+)-bicuculline treatment is summarized in Fig 4a. Two-way ANOVA revealed significant time (F(2.297, 75.80) = 21.02; P <0.0001) and treatment (F(3, 33) = 4.200; P<0.05) effects. Post hoc comparisons showed a significant increase in bicuculline (1, 2 and 4 mg/kg)-treated rats compared to vehicle-treated rats in Dunnett’s multiple comparisons test (P<0.05, 0.01 and 0.01, respectively). Furthermore, bicuculline significantly (2 and 4 mg/kg, p < 0.05, Dunnett’s multiple comparisons test after one-way ANOVA) and dose-dependently increased the baseline gamma power at 30 min, the time at which the drug achieves Cmax (Fig 4b).
4. Discussion
In this study, we examined the effect of the GABAa receptor antagonist (+)-bicuculline on ASSR in free-moving rats. Subcutaneous doses of (+)-bicuculline (1, 2 and 4 mg/kg) caused a marked and dose-dependent decrease the 40 Hz ERSP. In particular, (+)-bicuculline significantly reduced ERSP at 2–4 mg/kg between 10 and 30 min post-dose. (+)-Bicuculline also dose-dependently reduced the 40 Hz ITC, but the efficacy was moderate. Further, baseline gamma power was increased with (+)-bicuculline treatment in a dose-dependent manner. Given that bicuculline is a GABAa receptor-specific full antagonist, disinhibition could always have occurred regardless of the presence or absence of stimulation. ITC is defined as phase consistency across trials and could more directly reflect neural synchrony. In contrast, ERSP is defined as an event-related response relative to prestimulus baseline power, meaning that it would be affected by alteration in baseline gamma power. In the present study, (+)-bicuculline exerted its effects preferentially on ERSP over ITC, suggesting that (+)-bicuculline could preferentially increase baseline gamma power and consequently reduce ASSR.
More importantly, these results for (+)-bicuculline paralleled its pharmacokinetics. Our finding that (+)-bicuculline reduced ERSP suggests that the neuronal activity of pyramidal neurons may be disinhibited regardless of synchronicity because (+)-bicuculline inhibits input signals from GABAergic interneurons to pyramidal neurons. Time-dependent changes in ERSP with (+)-bicuculline occurred in accordance with the drug concentration in brain. Furthermore, the pharmacokinetics of (+)-bicuculline were consistent with its convulsion-inducing effects. Sullivan et al. [18] reported that acute and chronic administration of GABAa receptor antagonists had no effect on 40-Hz ASSR despite strong evidence suggesting that GABAergic inhibition is responsible for oscillations and the maintenance of synchronous firing [23, 28, 30]. In that paper, however, the authors used bicuculline methiodide, which may not have penetrated the brain because it has a quaternary amine showing high polarity [36–38]. Drugs that enhance GABAergic signaling have been used to treat seizure disorders since the discovery of phenobarbital in 1912 and the development of benzodiazepines in the 1950s [39]. Soukupová et al. [40] and Eder et al. [41] reported a focal convulsant action using the hydrophilic form of bicuculline, bicuculline methiodide, on parietal neocortex injection in adult and immature rodents. Graham [42] reported that quaternary bicuculline methiodide (‘N-methyl bicuculline’) [43] and methochloride [44] are much more stable than (+)-bicuculline, and are more water soluble and of similar potency to GABA antagonists, but do not appear to cross the blood brain barrier upon systemic administration. Injected intracisternally, bicuculline methiodide is a more potent convulsant than bicuculline [43]. These studies suggest that bicuculline methiodide does not have the potential to penetrate the brain.
In contrast, (+)-bicuculline penetrated the brain in a dose-dependent manner and induced convulsion at a bicuculline concentration greater than 880 ng/g in the brain. The Tmax of (+)-bicuculline concentration in the brain was 10 and 30 min at 1 and 4 mg/kg, respectively. Johnston et al. [44] reported that bicuculline reduces strychnine-insensitive inhibition of pyramidal cells in the cerebral cortex and is a potent convulsant when applied to the cerebral cortex. Taken together, these findings suggest that bicuculline could affect ASSR in the cerebral cortex if sufficient brain concentrations are achieved.
GABA is an important neurotransmitter in the central nervous system. GABAa receptors belong to the family of Cys-loop ligand-gated ion channels [45]. Investigations on the postmortem brains of patients with SZ have revealed abnormalities in GABAergic interneurons, including reduced expression of the GABA-synthesizing enzyme glutamic acid decarboxylase 67 (GAD67) and parvalbumin (PV) in cortical neurons [26, 46, 47]. Furthermore, clinical studies have shown a reduction in GABA in the anterior cingulate cortex as measured using proton magnetic resonance spectroscopy in patients with chronic SZ [48] and first-episode SZ [49]. Nakazawa et al. [50] reported that NMDA receptor hypofunction occurs in PV-positive GABA interneurons in early postnatal development, which leads to impairment of cortical maturation, causing a reduction in intrinsic excitability and impaired GABA release and a subsequent disinhibition of pyramidal neurons.
Human studies have consistently shown a 40-Hz deficit in ASSR in patients with SZ [4–8] and in family members with increased risk of SZ [9]. Furthermore, GABAergic (particularly PV-positive) interneurons are disrupted in SZ [51], and the GABAergic system is altered in SZ and PV-positive interneurons offer a potential target for treatment [52]. GABAa receptor expresses in pyramidal neurons and receive input from interneurons [53], suggesting that GABAa receptor hypofunction, such as via administration of a GABAa receptor antagonist, could be a model of SZ. Whether GABAa receptor hypofunction could give rise to the behavioral domain of SZ, i.e. positive symptoms such as hyperactivity and stereotypy, and negative symptoms such as social deficits, as well as cognitive impairment warrants further investigation.
As we mentioned in the Introduction, preclinical studies often employ short-term disruption of NMDA neurotransmission to mimic a psychosis-like state [13–15]. Ketamine is known to alter both the amplitude and latency of auditory ERPs in clinical and preclinical studies [54, 55]. Several groups have used this approach to understand the role of NMDA receptors in the 40-Hz ASSR [16–19], and have demonstrated that NMDA receptor antagonists showed bi-phasic effects; moderate or lower occupancy of NMDA receptors causes augmentation of ASSR while higher occupancy causes blunted ASSR signals. More basically, ketamine causes a persistent reduction in neuronal firing of GABAergic interneurons in the cortex [56, 57], although this would not completely explain the effect of ketamine on ASSR. Note that ketamine is also known to enhance the function of GABAa receptors in cortical neurons [58], which might counteract the hypofunction of interneurons. Blockade of GABAa receptors might therefore be an effective strategy for disrupting ASSR.
In summary, the present study partially unraveled the mechanism of ASSR, namely that GABAa receptors underpin ASSR signal, as has long been hypothesized. This finding helps elucidate the pathogenesis of SZ and will therefore contribute to drug discovery for this condition.
5. Conclusion
Subcutaneous doses of (+)-bicuculline markedly and dose-dependently reduced ASSR signals. Furthermore, the occurrence of convulsions was consistent with the drug’s pharmacokinetics. Abnormal gamma oscillations on ASSR are known to be typically observed in patients with SZ. Taken together, this study partially unravels the mechanism of ASSR, and thereby contributes to elucidating the pathogenesis of SZ. ASSR may be a candidate electrophysiological index for GABAergic abnormalities in the auditory cortex.
Supporting information
For illustration, a representative ERP image (trial by time) from a subject at baseline recording is shown. An averaged ERP wave form is in the panel at bottom. The onset of click trains is set as time zero. A number of epochs is 182 after outlier rejection (see Experimental procedure).
(PPTX)
Acknowledgments
The authors thank Dr. Mitsuyuki Matsumoto of Astellas Pharma Inc. for his assistance throughout this study and input during the preparation of the manuscript.
Abbreviations
- ANOVA
analysis of variance
- ASSRs
auditory steady-state responses
- EEG
electroencephalogram
- ERSPs
event-related spectral perturbations
- FFT
fourier transform
- GABA
gamma-aminobutyric acid
- GAD67
glutamic acid decarboxylase 67
- ITC
inter-trial coherence
- NMDA
N-methyl-D-aspartate
- PV
parvalbumin
- sc
subcutaneously
- SD
Sprague-Dawley
- SD
standard deviation
- SEM
standard error of the mean
- SZ
schizophrenia
- Tmax
Time to maximum concentration
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The authors declare that, other than income received from our primary employers, no financial support or compensation has been received for this research and that there are no personal conflicts of interest to declare. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The funding organization (Astellas Pharma Inc.) did not play a role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript and only provided financial support in the form of authors’ salaries and/or research materials. The funder provided support in the form of salaries for authors (MY, SH, KT, MI and TM), but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Brenner CA, Krishnan GP, Vohs JL, Ahn WY, Hetrick WP, Morzorati SL, et al. Steady state responses: electrophysiological assessment of sensory function in schizophrenia. Schizophr Bull. 2009;35:1065‒1077. 10.1093/schbul/sbp091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lins OG, Picton TW. Auditory steady-state responses to multiple simultaneous stimuli. Electroencephalography Clinical Neurophysiology. 1995;96:420–432. 10.1016/0168-5597(95)00048-w [DOI] [PubMed] [Google Scholar]
- 3.Regan D. Human Brain Electrophysiology: Evoked Potentials and Evoked Magnetic Fields in Science and Medicine. Amsterdam: Elsevier; 1989. [Google Scholar]
- 4.Kwon JS, O’Donnell BF, Wallenstein GV, Greene RW, Hirayasu Y, Nestor PG, et al. Gamma frequency-range abnormalities to auditory stimulation in schizophrenia. Arch Gen Psychiatry. 1999;56:1001–1005. 10.1001/archpsyc.56.11.1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brenner CA, Sporns O, Lysaker PH, O’Donnell BF. EEG synchronization to modulated auditory tones in schizophrenia, schizoaffective disorder, and schizotypal personality disorder. Am J Psychiatry. 2003;160:2238–2240. 10.1176/appi.ajp.160.12.2238 [DOI] [PubMed] [Google Scholar]
- 6.Light GA, Hsu JL, Hsieh MH, Meyer-Gomes K, Sprock J, Swerdlow NR, et al. Gamma band EEG oscillations reveal neural network cortical coherence dysfunction in schizophrenia patients. Biological Psychiatry. 2006;60:1231–1240. 10.1016/j.biopsych.2006.03.055 [DOI] [PubMed] [Google Scholar]
- 7.Krishnan GP, Hetrick WP, Brenner CA, Shekhar A, Steffen AN, O’Donnell BF. Steady state and induced auditory gamma deficits in schizophrenia. Neuroimage. 2009;47:1711–1719. 10.1016/j.neuroimage.2009.03.085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spencer KM. The functional consequences of cortical circuit abnormalities on gamma oscillations in schizophrenia: insights from computational modeling. Front Hum Neurosci. 2009;3:33 10.3389/neuro.09.033.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hong LE, Summerfelt A, McMahon R, Adami H, Francis G, Elliott A, et al. Evoked gamma band synchronization and the liability for schizophrenia. Schizophr Res. 2004;70: 293–302,. 10.1016/j.schres.2003.12.011 [DOI] [PubMed] [Google Scholar]
- 10.Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry. 1991;148:1301–1308. 10.1176/ajp.148.10.1301 [DOI] [PubMed] [Google Scholar]
- 11.Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, et al. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51:199–214. 10.1001/archpsyc.1994.03950030035004 [DOI] [PubMed] [Google Scholar]
- 12.Luby ED, Gottlieb JS, Cohen BD, Rosenbaum G, Domino EF. Model psychoses and schizophrenia. Am J Psychiatry. 1962;119:61–67. 10.1176/ajp.119.1.61 [DOI] [PubMed] [Google Scholar]
- 13.Adell A, Jimenez-Sanchez L, Lopez-Gil X and Romon T. Is the acute NMDA receptor hypofunction a valid of schizophrenia? Schizophr Bull. 2012;38(1):9–14 10.1093/schbul/sbr133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frohlich J, Van Horn JD. Reviewing the ketamine model for schizophrenia. J Psychopharmacol. 2014;28: 287–302. 10.1177/0269881113512909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gunduz-Bruce H. The acute effects of NMDA antagonism: from the rodent to the human brain. Brain Res Rev. 2009;60:279–286. 10.1016/j.brainresrev.2008.07.006 [DOI] [PubMed] [Google Scholar]
- 16.Kozono N, Honda S, Tada M, Kirihara K, Zhao Z, Jinde S, et al. Auditory Steady State Response; nature and utility as a translational science tool. Scientific Reports. 2019;9:8454–8464. 10.1038/s41598-019-44936-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sivarao DV, Frenkel M, Chen P, Healy FL, Lodge NJ, Zaczek R. MK-801 disrupts and nicotine augments 40 Hz auditory steady state responses in the auditory cortex of the urethane anesthetized rat. Neuropharmacology. 2013;73:1–9. 10.1016/j.neuropharm.2013.05.006 [DOI] [PubMed] [Google Scholar]
- 18.Sullivan EM, Timi P, Hong LE, O’Donnell P. Effects of NMDA and GABA-A receptor antagonism on auditory steady state synchronization in awake behaving rats. Int J Neuropsychopharmacol. 2015;18:pyu118 10.1093/ijnp/pyu118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vohs JL, Chambers RA, O’Donnell BF, Krishnan GP & Morzorati SL. Auditory steady state responses in a schizophrenia rat model probed by excitatory/inhibitory receptor manipulation. Int J Psychophysiol. 2012;86:136–142. 10.1016/j.ijpsycho.2012.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Teale P, Collins D, Maharajh K, Rojas DC, Kronberg E, Reite M. Cortical source estimates of gamma band amplitude and phase are different in schizophrenia. Neuroimage. 2008;42:1481–1489. 10.1016/j.neuroimage.2008.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uhlhaas PJ, Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci. 2010;11:100–113. 10.1038/nrn2774 [DOI] [PubMed] [Google Scholar]
- 22.Cardin JA, Carlen M, Meletis K, Knoblich U, Zhang F, Deisseroth K, et al. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459:663–667. 10.1038/nature08002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459:698–702. 10.1038/nature07991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akbarian S, Kim JJ, Potkin SG, Hagman JO, Tafazzoli A, Bunney WE, et al. Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Arch Gen Psychiatry. 1995;52:258–266. 10.1001/archpsyc.1995.03950160008002 [DOI] [PubMed] [Google Scholar]
- 25.Hashimoto T, Volk DW, Eggan SM, Mirnics K, Pierri JN, Sun Z, et al. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J Neurosci. 2003;23:6315–6326. 10.1523/JNEUROSCI.23-15-06315.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nature Review Neuroscience. 2005;6:312–324. [DOI] [PubMed] [Google Scholar]
- 27.Sohal V.S. Insights into cortical oscillations arising from optogenetic studies. Biol Psychiatry. 2012;71:1039‒1045. 10.1016/j.biopsych.2012.01.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gonzalez BG and Lewis DA. GABA neurons and the mechanisms of network oscillations: implications for understanding cortical dysfunction in schizophrenia. Schizophr Bull. 2008;34:944‒961. 10.1093/schbul/sbn070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benes FM & Berretta S. GABAergic interneurons: implications for understanding schizophrenia and bipolar disorder [Review]. Neuropsychopharmacology. 2001;25:1–27. 10.1016/S0893-133X(01)00225-1 [DOI] [PubMed] [Google Scholar]
- 30.Lewis DA, Moghaddam B. Cognitive dysfunction in schizophrenia: convergence of γ-aminobutyric acid and glutamate alterations. Neurological Review. 2006;63:1372–1376. [DOI] [PubMed] [Google Scholar]
- 31.Wassef A, Baker J, and Kochan LD. GABA and schizophrenia: a review of basic science and clinical studies. Journal of Clinical Psychopharmacology. 2003;23:601–640. 10.1097/01.jcp.0000095349.32154.a5 [DOI] [PubMed] [Google Scholar]
- 32.Lewis DA, Gonzalez-Burgos G. Neuroplasticity of neocortical circuits in schizophrenia. Neuropsychopharmacology. 2008;33:141–165. 10.1038/sj.npp.1301563 [DOI] [PubMed] [Google Scholar]
- 33.Vohs JL, Chambers RA, Krishnan GP, O’Donnell BF, Berg S & Morzorati SL. GABAergic modulation of the 40 Hz auditory steady-state response in a rat model of schizophrenia. Int J Neuropsychopharmacol. 2010;13:487–497. 10.1017/S1461145709990307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Racine. Modification of seizure activity by electrical stimulation II motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32(3):281–294 10.1016/0013-4694(72)90177-0 [DOI] [PubMed] [Google Scholar]
- 35.Tada M., Nagai T., Kirihara K., Koike S., Suga M., Araki T., et al. Differential alterations of auditory gamma oscillatory responses between pre-onset high-risk individuals and first-episode schizophrenia. Cerebral Cortex. 2016;26(3):2027–1035. [DOI] [PubMed] [Google Scholar]
- 36.Levin V.A. Relationship of octanol/water partition coefficient and molecular weight to rat brain capillary permeability. J. Med. Chem., 1980;23:682–684. 10.1021/jm00180a022 [DOI] [PubMed] [Google Scholar]
- 37.Liu X, Tu M, Kelly RS, Chen C and Smith BJ. Development of a computational approach to predict blood-brain barrier permeability. Drug Metab. Dispos., 2004;32:132–139. 10.1124/dmd.32.1.132 [DOI] [PubMed] [Google Scholar]
- 38.Summerfield SG, Read K, Begley DJ, Obradovic T, Hidalgo IJ, Coggon S, et al. Central nervous system drug disposition: the relationship between in situ brain permeability and brain free fraction. J. Pharmacol. Exp. Ther. 2007;322:205–213. 10.1124/jpet.107.121525 [DOI] [PubMed] [Google Scholar]
- 39.Magiorkinis E, Diamantis A, Sidiropouloe K and Panteliadis C. Highights in the history of Epilepsy: The Last 200 Years. Epilepsy Res Treat. 2014;2014:24–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Soukupová S, Mikolasova R, Kubova H, Mares P. New model of cortical epileptic foci in freely moving developing rats. Epilepsy Res. 1993;15:27–33. 10.1016/0920-1211(93)90006-s [DOI] [PubMed] [Google Scholar]
- 41.Eder HG, Jones DB, Fisher RS. Local perfusion of diazepam attenuates interictal and ictal events in the bicuculline model of epilepsy in rats. Epilepsia. 1997;38:516–521. 10.1111/j.1528-1157.1997.tb01134.x [DOI] [PubMed] [Google Scholar]
- 42.Johnston Graham AR. Advantages of an antagonist: bicuculline and other GABA antagonists. British Journal of Pharmacology. 2013;169:328–336. 10.1111/bph.12127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pong SF and Graham LT. N-methyl bicuculline, a convulsant more potent than bicuculline. Brain Res. 1972;42:486–490. 10.1016/0006-8993(72)90547-1 [DOI] [PubMed] [Google Scholar]
- 44.Johnston GAR, Beart PM, Curtis DR, Game CJ, McCulloch RM, Maclachlan RM. Bicuculline methochloride as a GABA antagonist. Nat New Biol. 1972;240:219–220. 10.1038/newbio240219a0 [DOI] [PubMed] [Google Scholar]
- 45.Goetz T, Arslan A, Wisden W, and Wulff P. GABAA receptors. Prog Brain Res. 2007;160:21–41. 10.1016/S0079-6123(06)60003-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Akbarian S. & Huang HS. Molecular and cellular mechanisms of altered GAD1/GAD67 expression in schizophrenia and related disorders. Brain Res. Rev. 2006;52: 293–304. 10.1016/j.brainresrev.2006.04.001 [DOI] [PubMed] [Google Scholar]
- 47.Eyles DW, McGrath JJ and Reynolds GP. Neuronal calcium-binding proteins and schizophrenia. Schizophr. Res. 2002;57:27–34. 10.1016/s0920-9964(01)00299-7 [DOI] [PubMed] [Google Scholar]
- 48.Rowland LM, Krause BW, Wijtenburg SA, McMahon RP, Chiappelli J, Nugent KL, et al. Medial frontal GABA is lower in older schizophrenia: a MEGA-PRESS with macromolecule suppression study. Mol. Psychiatry. 2016;21:198–204. 10.1038/mp.2015.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chiu PW, Lui SSY, Hung KSY, Chan RCK, Chan Q, Sharn PC, et al. In vivo gamma-aminobutyric acid and glutamate levels in people with first-episode schizophrenia: a proton magnetic resonance spectroscopy study. Schizophr. Res. 2018;193:295–303. 10.1016/j.schres.2017.07.021 [DOI] [PubMed] [Google Scholar]
- 50.Nakazawa K, Jeevakumar V & Nakao K. Spatial and temporal boundaries of NMDA receptor hypofunction leading to schizophrenia. NPJ Schizophr. 2017;3:7 10.1038/s41537-016-0003-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nakazawa K, Zsiros V, Jiang Z, Nakao K, Kolata S, Zhang S, et al. GABAergic interneuron origin of schizophrenia pathophysiology. Neuropharmacology. 2012;62(3):1574–1583. 10.1016/j.neuropharm.2011.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kaar SJ, Marques TR and Howes OD. Pre-frontal parvalbumin interneurons in schizophrenia: a meta-analysis of post-mortem studies. Journal of Neural Transmission. 2019;126:1637–1651 10.1007/s00702-019-02080-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lewis DA, Cuely AA, Glausia JR and Volk DW. Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci. 2012;35(1):57–67. 10.1016/j.tins.2011.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Saunders JA, Gandal M J, Roberts T P & Siegel SJ. NMDA antagonist MK801 recreates auditory electrophysiology disruption present in autism and other neurodevelopmental disorders. Behav Brain Res. 2012;234:233–237. 10.1016/j.bbr.2012.06.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Umbricht D, Schmid L, Koller R, Vollenweider FX, Hell D, and Javitt DC. Ketamine-induced deficits in auditory and visual context-dependent processing in healthy volunteers: Implications for models of cognitive deficits in schizophrenia. Archives of General Psychiatry. 2000;57:1139–1147. 10.1001/archpsyc.57.12.1139 [DOI] [PubMed] [Google Scholar]
- 56.Wang M, and Arnsten AFT. Contribution of NMDA receptors to dorsolateral prefrontal cortical networks in primates. Neuroscience Bulletin. 2015;31:191–197. 10.1007/s12264-014-1504-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Roopun AK, Cunningham MO, Racca C., Alter K, Traub RD and Whittington MA. Region-specific changes in gamma and beta2 rhythms in NMDA receptor dysfunction models of schizophrenia. Schizophr Bull. 2008;34:962–973. 10.1093/schbul/sbn059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang DS., Pennma A. and Orser BA. Ketamine increases the function of r-aminobutyric acid type A receptors in hippocampal and cortical neurons. Anesthesiology. 2017;126:666–677. 10.1097/ALN.0000000000001483 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For illustration, a representative ERP image (trial by time) from a subject at baseline recording is shown. An averaged ERP wave form is in the panel at bottom. The onset of click trains is set as time zero. A number of epochs is 182 after outlier rejection (see Experimental procedure).
(PPTX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.