Abstract
Introduction
Surgical resection and systemic chemotherapy with temozolomide remain the mainstay for treatment of glioblastoma. However, many patients are not candidates for surgical resection given inaccessible tumor location or poor health status. Furthermore, despite being first line treatment, temozolomide has only limited efficacy.
Methods
The development of injectable hydrogel-based carrier systems allows for the delivery of a wide range of chemotherapeutics that can achieve high local concentrations, thus potentially avoiding systemic side effects and wide-spread neurotoxicity. To test this modality in a realistic environment, we developed a diblock copolypeptide hydrogel (DCH) capable of carrying and releasing paclitaxel, a compound that we found to be highly potent against primary gliomasphere cells.
Results
The DCH produced minimal tissue reactivity and was well tolerated in the immune-competent mouse brain. Paclitaxel-loaded hydrogel induced less tissue damage, cellular inflammation and reactive astrocytes than cremaphor-taxol (typical taxol-carrier) or hydrogel alone. In a deep subcortical xenograft model of glioblastoma in immunodeficient mice, injection of paclitaxel-loaded hydrogel led to local tumor control and improved survival. However, the tumor cells were highly migratory and were able to eventually escape the area of treatment.
Conclusions
These findings suggest this technology may be ultimately applicable to patients with deep-seated inoperable tumors, but as currently formulated, complete tumor eradication would be highly unlikely. Future studies should focus on targeting the migratory potential of surviving cells.
Introduction
Glioblastoma accounts for 40% of primary brain tumors and results in over 15,000 deaths a year in America alone [1]. Despite maximal therapy including surgical resection, average survival is only 20 months [2]. Some patients are not candidates for surgery due to poor health, deep or bilateral location or proximity to eloquent structures [3, 4]. Without cytoreductive surgery, the average survival is four months [2].
In addition to cytoreductive surgery, current standard of care also includes temozolomide and radiation. Temozolomide treatment has a small but statistically significant improvement in survival [5]. However, it also induces mutations leading to more aggressive recurrent tumors [6]. Further, treatment with temozolomide can result in enhanced resistance by demethylation of the MGMT promoter [7]. There are many promising chemotherapy options that show improved potency over temozolomide without the genotoxic damage. However, often these drugs are limited by their pharmacokinetics, inability to cross the blood-brain barrier or difficulties and side effects with systemic administration [8].
To address these limitations, drug releasing implants are being developed, most often composed of biodegradable polymers, that may provide improved local delivery of promising chemotherapeutics in the CNS [9]. While multiple studies have evaluated the ability of different carriers to deliver chemotherapeutic payloads to glioblastoma cells, the majority of these studies have tested them in sub-optimal conditions using either in vitro [10] or subcutaneous flank environments [11], or testing them on suboptimal, multi-passaged glioma cell lines that often do not recapitulate the migratory capacity of patient tumors [12–14]. Fully understanding the potential of these vehicles requires testing in realistic in vivo environments. To this end, we have constructed diblock copolypeptide hydrogels (DCH) capable of delivering paclitaxel to a patient-derived gliomasphere model of GBM in a mouse brain. We found that paclitaxel-loaded hydrogels led to local control and improved survival in this mouse model, but surviving cells were able to migrate out of the treatment zone.
Methods
Gliomasphere culture and in vitro proliferation assay
Cancerous stem cells can be derived from brain tumors and propagated in vitro as neurosphere-like gliomaspheres [15, 16]. For this study, we utilized the sample HK308, a gliomasphere line derived from a recurrent GBM in a 50-year-old male as previously described [15, 16]. The culture and the original tumor sample have an amplification of the epidermal growth factor receptor gene, and express the EGFRvIII mutation. MGMT was unmethylated in the original tumor sample as reported by the neuropathologist [15, 16]. For cell proliferation experiments used to determine potency of chemotherapeutic agents, gliomaspheres were initially plated in a 6 well plate using 3ml of serum-free media (DMEM F12 +B27+bFGF+EGF+heparin) at a concentration of 100,000 cells/ml. Chemotherapeutic drugs were solubilized in either aqueous or DMSO-based solutions at various concentrations and applied to the proliferating gliomasphere cells for seven days. On the seventh day the total number of viable cells were counted using Trypan blue with the Growth Rate (GR) value for the various drug concentrations determined by estimating the exponential growth kinetics over the seven days and comparing these to an untreated control using methods described previously [15]. Gliomasphere-derived hGBM cells used for in vivo mouse studies were transduced with a 3rd generation, self-inactivating lentiviral construct expressing GFP and firefly luciferase [17] by dissociating 100,000 hGBM cells in 2mL of serum free media and adding 1mL of lentivirus and incubating for 3 days. Infected cells were purified by cell sorting for GFP and expanded in vitro.
Hydrogel synthesis and fabrication
The diblock copolypeptide, K180A40 was the DCH used in this study, and was synthesized using techniques described in detail previously [18]. Briefly, within an inert, dinitrogen filled glovebox environment a solution of Nε-carbobenzyloxy-L-lysine N-carboxyanhydride ((Z-L-lysine NCA) in THF solvent was polymerized upon addition of the transition metal initiator, Co(PMe3)4. The polymerization of the Nε-carbobenzyloxy-L-lysine block was allowed to proceed for approximately 45 minutes and was monitored for completeness by FTIR before the subsequent addition of a solution of L-alanine NCA in THF. The consumption of the second NCA was also allowed to proceed for 45 minutes before the reaction was removed from the glovebox for subsequent deprotection of the Nε-carbobenzyloxy-L-lysine residues by HBr in an acetic acid/TFA solution followed by polymer precipitation in diethyl ether. The precipitated polymer was re-dissolved in nonpyrogenic DI water and then dialyzed within a 2000 Da MWCO dialysis bag exhaustively against NaCl for two days and then nonpyrogenic DI water for two days before being lyophilized. Paclitaxel was loaded into the DCH by dissolving and mixing the DCH and paclitaxel in equal mass in an 80/20 Methanol/water solution and then drying under vacuum overnight to remove the solvent. The dry DCH/paclitaxel powder was resuspended in nonpyrogenic DI water at 3 wt% for subsequent in vitro and in vivo evaluation.
In vitro evaluation of release of paclitaxel from DCH
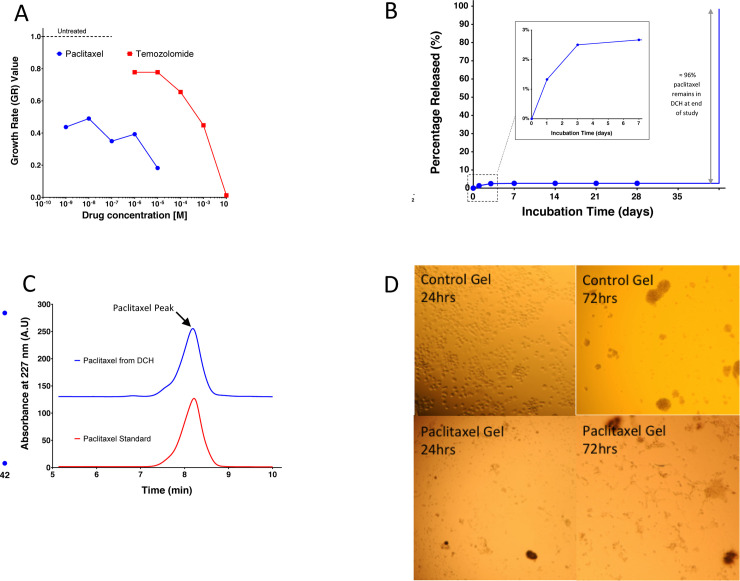
For the in vitro paclitaxel release assay, 100 μL of 3 weight-percent (wt%) DCH loaded with paclitaxel (DCH-paclitaxel) was injected into pre-hydrated 20,000 MWCO Slide-A-Lyzer dialysis cassettes before being placed in a sink of 100 mL of 5% Fetal Bovine Serum (FBS) containing 1xPBS. A cassette contacting 100 μL of paclitaxel in DMSO was used as a control. The complete incubation media was collected and replaced on days 1, 3, 7, 14, 21, 28 and 42. To analyze the amount of released paclitaxel, 50 mL of the incubation media from each time point was extracted with four separate 50 mL ethyl acetate washes. The extracted organic layer was evaporated to dryness under vacuum, resolubilized in acetonitrile and filtered with a 0.2 μm syringe filter prior to analysis by HPLC. HPLC analysis of paclitaxel concentration was performed using methods adapted from a previous report [19]. In this report, which also used paclitaxel, the limit of detection (LOD) was 5ng/ml and the limit of quantification (LOD) was 10ng/ml. Standards were prepared by weighing out a known amount of paclitaxel and dissolving in a known volume of acetonitrile. Ten μL of release assay samples of unknown paclitaxel concentration and paclitaxel standards were run on a C18 150 x 4.6 mm I.D. Agilent column using an isocratic 50/50 Acetonitrile/Water mobile phase and detected by UV absorption detection at a wavelength of 227 nm. On day 1, we detected over 1% recovery from 0.1g sample of gel loaded with 3% paclitaxel (1ug/ml, 200 times the detection limit).
Surgery and hydrogel implantation
Biocompatibility evaluations of DCH and Cremophor vehicle with and without paclitaxel were performed using adult male and female C57Bl6 mice aged 8–14 weeks (purchased from Jackson Labs). For gliomasphere-derived hGBM cell injections, Non-obese Diabetic/ Severe Combined Immunodeficiency/Gamma Null (NSG) mice were used (An NSG colony is maintained by Division of Laboratory Animal Medicine and made available to researchers). Mice were anesthetized using isoflurane and a craniectomy was performed by drilling a rectangular exposure window in the bone with a high-speed dental drill. Following surgery, mice were treated with non-steroidal antiflammatory (NSAID) medication for 48 hours. From that point all mice were monitored daily for signs of neurological comprise including but not limited to: weight loss, decreased mobility, hunching or hemi-paresis. Following the discovery of neurologic compromise mice were removed from the vivarium and euthanized with an overdose of pentobarbital. In total 35 mice were used for this study and 7 mice were found dead before neurological symptoms developed. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol and this specific study was approved by the University of California at Los Angeles Committee on the Ethics of Animal Experiments and the Animal Research committee. All mice were sacrificed with terminal intraperitoneal doses of pentobarbital.
For biocompatibility evaluations, DCH or vehicle were injected stereotactically into the center of the caudate putamen nucleus using the target coordinates of 0.5 mm anterior to Bregma, 2 mm lateral to Bregma and a depth of 3.0 mm below the cortical surface. A 2 μL volume of DCH was injected using a pulled glass micropipettes ground to a beveled tip with 150–250 μm inner diameter attached via specialized connectors and high-pressure tubing to a 10 μL syringe that was mounted to a stereotaxic frame and controlled by an automated microdrive pump. For studies on hGBM cells, a 2μL suspension of 100,000 hGBM cells was injected first into the striatum using the target coordinates of 0.5 mm anterior to Bregma, 2 mm lateral to Bregma and a depth of 3.0 mm below the cortical surface. In the first two cohorts this first injection was followed immediately by a second injection of 2 μL of DCH placed directly above using the target coordinates of 0.5 mm anterior to Bregma, 2 mm lateral to Bregma and a depth of 2.0 mm below the cortical surface. In the third cohort, 100,000 hGBM cells were injected into the striatum and allowed to grow for two weeks. The wound was then re-opened and 2ul of DCH were placed at the same stereotactic location. Postoperative care and analgesia was administered preoperatively as well as for two days following surgery to alleviate pain.
IVIS spectrum in vivo imaging
The gliomasphere cell proliferation in vivo within the brain was monitored at discrete time intervals using non-invasive bioluminescence imaging on an IVIS® Spectrum in vivo imaging system. Mice received intraperitoneal injections of 200 μL of luciferin (30mg/ml) and were scanned 5 minutes later with a 10 second exposure. Results were listed as flux (photons/second) through the area of interest.
Animal perfusions and histology
At specific predetermined times after hydrogel/cell injection, or when tumors reached a terminal size requiring euthanasia, animals were given a lethal dose of pentobarbital and a transcardial perfusion was performed with heparinized saline and 4% paraformaldehyde. Brains were excised, post-fixed in 4% paraformaldehyde for 5 hours and then stored in 30% sucrose. Brains were sectioned along the coronal plane using a cryostat. Tissue sections (40 μM) were stained using standard fluorescent immunohistochemical techniques as previously described [20] to visualize host tissue, injected gliomaspheres cells and hydrogel deposit localization. Primary antibodies were: rabbit anti-GFAP (1:1000; Dako, Carpinteria, CA); rat anti-GFAP (1:1000, Zymed Laboratories); rabbit anti-Iba1 (1:1000, Wako Chemicals, Richmond VA); rat anti-CD68 (1:100; AbD Serotec, Biorad, CA). Fluorescence secondary antibodies were conjugated to: Alexa 488 (green) or Alexa 405 (blue) (Molecular Probes), or Cy3 (550, red) or Cy5 (649, far red) all from (Jackson Immunoresearch Laboratories). Nuclear stain: 4',6'-diamidino-2-phenylindole dihydrochloride (DAPI; 2ng/ml; Molecular Probes). Sections were cover-slipped using ProLong Gold anti-fade reagent (InVitrogen, Grand Island, NY). Sections were examined and photographed using deconvolution fluorescence microscopy and scanning confocal laser microscopy (Zeiss, Oberkochen, Germany).
Results
Paclitaxel is more potent than temozolomide
Temozolomide is available in an oral formulation, is well tolerated, and is able to cross the blood-brain barrier making it a convenient therapeutic option. However, clinically temozolomide has only been shown to increase survival by a few months [5]. An additional local delivery chemotherapy option may be helpful to increase survival. Given space limitations the ideal candidate chemotherapeutic agent would have both high efficacy and high potency. We tested both temozolomide and paclitaxel at various doses against our patient-derived gliomasphere line (HK308). Both of the two candidate drugs were effective at suppressing the proliferation of the gliomaspheres in a concentration dependent manner over the seven-day evaluation period (Fig 1A). By applying growth rate (GR) inhibition calculations [21], we quantified the relative effectiveness of each drug’s capacity to suppress cell growth in the endpoint assays. The GR50 value (representing the drug concentration at which the cell proliferation rate is reduced by half), was less than 1 nM (the lowest dose tested) for paclitaxel, and was approximately 0.5 mM for temozolomide. Based on these results, paclitaxel was at least 500,000 times more potent on our human patient derived gliomasphere cell line than temozolomide and was considered to be the more appropriate candidate for further exploration in subsequent in vivo studies.
Fig 1. In vitro evaluation of DCH-paclitaxel system.
A. On a per weight basis, paclitaxel is 500,000 times more potent than temozolomide at suppressing the growth of human gliomaspheres over a 7-day period in vitro. Growth rate (GR) inhibition calculations27 were used to quantify the relative effectiveness of various concentrations of each drug and normalized to untreated controls. B. Cumulative release curve for paclitaxel loaded into the DCH over a 6 week incubation in vitro. A 1000 fold sink of 5% FBS in 1XPBS was used. An initial burst release of paclitaxel of over the first three days was noted and 96% of drug was recovered at the end of the 6 week incubation period. C. HPLC chromatograms show that paclitaxel recovered from the DCH at the end of the 6 week in vitro incubation was chemically identical to freshly prepared drug. D. 2ul of DCH control and paclitaxel loaded were placed in 3ml of media and 100,000 HK308 cells. Gels dissolved in the media. Photos were taken at 24 and 72 hours.
DCH-paclitaxel maintains a high local concentration over a prolonged period
The ideal chemotherapeautic agent should maintain high local concentrations and not become diluted through diffusion. To determine in vitro drug release kinetics we prepared a 3 wt% K180A40 DCH at a drug concentration of 3% w/v and placed the gel in a dialysis cassette suspended in an incubation buffer with 5% v/v fetal bovine serum (FBS) to simulate the brain environment. The incubation media was replaced at discrete time points and the concentration of paclitaxel was evaluated by HPLC following organic extraction. A solution of paclitaxel in DMSO at the same concentration was also incubated within a separate dialysis cassette over the same time course to serve as a control. The paclitaxel stayed inside the DCH matrix throughout the six-week period indicating that the paclitaxel does not diffuse out of the matrix but is rather only released as the gel is degraded (Fig 1B). The extraction efficiency was nearly 100%. After analyzing the last day (day 42) the content of the dialysis bag was removed and the paclitaxel remaining in the gel was extracted and analyzed. The remaining 97% of the paclitaxel was recovered. This paclitaxel was tested and found to have retained its chemical signature and biological activity (Fig 1C and 1D).
DCH-paclitaxel maintains a high local concentration over a prolonged period
To test the CNS biocompatibility of the DCH vehicle, we injected the K180A40 DCH with and without paclitaxel into the caudate putamen of healthy adult mice in the absence of hGBM cells and evaluated the general foreign body response using standard immunohistochemistry markers. For comparison, we injected an equivalent volume of 50% v/v Cremophor EL (polyoxyethylated castor oil) in ethanol with and without paclitaxel in separate cohorts of mice. Cremophor EL is used as the surfactant vehicle for paclitaxel within the standard Taxol formulation approved clinically for intravenous administration of the drug. The purpose of this experiment is to show that a delivery vehicle for paclitaxel is needed because the current IV formulation is too toxic to be injected into the brain. We do not believe that cremophor has significant BBB penetrance. Animals tolerated all four formulations well after injection and there were no observable adverse health events throughout the duration of the study. At one week post injection, which was previously characterized as the time of maximum foreign body response to DCH alone (without cargo molecules) [22], brain tissue was evaluated at and around the injection site using immunohistochemical markers for macrophages and activated microglia (CD68, IBA-1) and astrocyte reactivity (GFAP) (Fig 2A–2D). To quantify the extent of the foreign body response, we characterized the intensity of immunohistochemical staining across a radial distance of 1 mm originating from the center of the injection (Fig 2E and 2F). To establish a cumulative measure of immunostaining across this circular tissue injection zone, we calculated the area under the curve (AUC) on the intensity plots (Fig 2F).
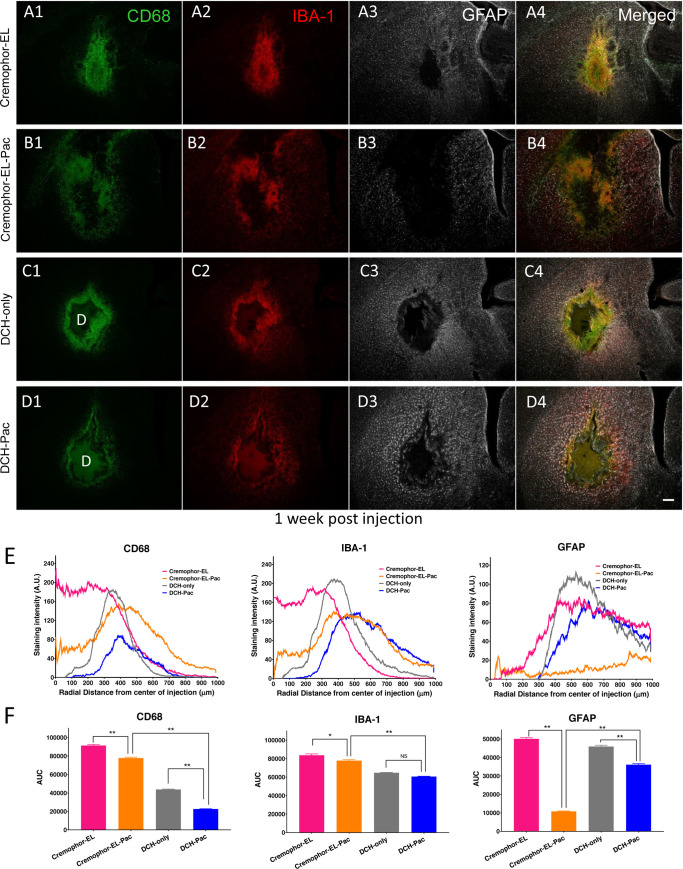
Fig 2. DCH-paclitaxel exhibits good biocompatibility in healthy CNS in contrast to paclitaxel in Cremophor EL vehicle.
A-D. Images of caudate putamen at 1 week after injection into healthy, uninjured tissue of Cremophor EL vehicle (A), Cremophor EL + paclitaxel (B), DCH only (C), or DCH + paclitaxel (D), showing single channel and merged multichannel immunofluorescence for multiple markers of inflammation and gliosis, CD 68, IBA-1 and GFAP. Scale bar, 200 μm for all images, D = DCH depot. E. Quantification of immunofluorescence intensity for each treatment group across a radial area of 1 mm originating from the center of the injection (n = 3 mice per stain per treatment). F. Area Under the Curve (AUC) calculations for the various immunofluorescence intensity traces from E. provide a single measure of cumulative staining within the 1 mm radial field. The DCH-paclitaxel system showed markedly and significantly less staining intensity, indicative of a more favorable foreign body response, compared with paclitaxel administered using the Cremophor EL vehicle * p < 0.01 or ** p < 0.001 (ANOVA with post-hoc Tukey’s multiple comparisons test).
The Cremophor EL vehicle treated group exhibited a small, localized core of densely packed CD68- and IBA-1-positive cells at the injection center, surrounded by a border of compact astrocyte reactivity typical of that around focal CNS lesions (Fig 2A). Compared with other treatment groups, the Cremophor EL vehicle showed the largest total of CD68-positive, IBA-1-positive and GFAP-positive cells within the field of analysis. By comparison, the inclusion of paclitaxel within this Cremophor EL vehicle resulted in a larger and more diffuse volume of tissue disruption and inflammation but significantly fewer total CD 68-positive and IBA-1-positive cells within the analysis field (Fig 2B and 2F). Interestingly, in the Cremophor EL plus paclitaxel group there was the appearance of increased microglia reactivity within the preserved tissue outside the direct lesion/disrupted tissue field suggesting soluble paclitaxel had reached these tissue regions and was mildly stimulating this cell population and/or that these inflammatory cells were suppressed by the drug in the core injection area (Fig 2B). Notably, the injection region in the Cremophor EL plus paclitaxel group exhibited a remarkable and statistically significant absence of GFAP-positive cells, such that there was no compact border of reactive astrocytes in this group (Fig 2B and 2F).
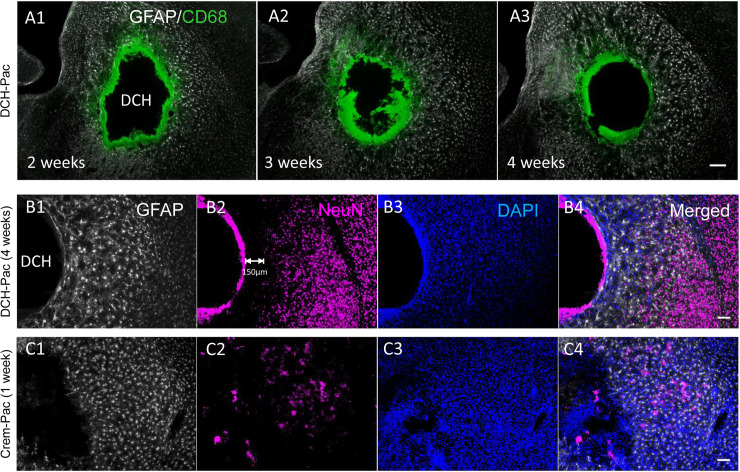
Compared to the Cremophor EL treated groups, the DCH injected groups showed a milder and more focal foreign body response (Fig 2C–2F), and in particular, there was significantly less CD68 immunoreactivity in the DCH and DCH plus paclitaxel groups (Fig 2E and 2F). The DCH-paclitaxel group also exhibited significantly less CD68 staining around the DCH boundary compared to the DCH only control (Fig 2C–2F). Host neuronal viability was well preserved around the deposit margins, with only a limited radial zone of about 150 μm radially becoming depleted of NeuN positive neurons after 4 weeks of paclitaxel exposure (Fig 3B). By contrast paclitaxel delivered using Cremophor-El caused more profuse NeuN positive neuron loss around the injection site (Fig 3C) as well as a considerable depletion of GFAP positive astrocytes (Fig 2B). These findings provide strong evidence that the DCH-paclitaxel system caused less damage to host tissue compared to the standard paclitaxel in Cremophor-El vehicle, while at the same time providing a local depot of high drug concentration for at least four weeks after injection.
Fig 3. Paclitaxel-DCH depots persist locally for up to 4 weeks in vivo and are superior at preserving viable adjacent healthy CNS tissue compared to paclitaxel in Cremophor EL vehicle.
A. CD68 positive cells are present at the DCH-paclitaxel interface for up to 4 weeks post injection but there is minimal diminution of the depot size, minimal material resorption and minimal infiltration of inflammatory cells into the DCH-paclitaxel depot. B. NeuN positive, viable neurons are present in normal density and intermingled with mildly reactive astrocytes in close proximity with the DCH-paclitaxel depot (DCH-Pac). C. In contrast, there is pronounced depletion of NeuN positive neurons and severe proliferative reactive astrogliosis in tissue adjacent to injection of paclitaxel in Cremophor EL vehicle (Crem-Pac). Scale bar, 200 μm for all images. N = 3 mice per stain per treatment.
DCH-paclitaxel depots reduce bioluminescence imaging of hGBM cells, delay tumor expansion, and confer a significant survival advantage in NSG mice
Since the DCH-paclitaxel system showed prolonged local availability of active drug in combination with a favorable foreign body response in healthy mice compared to the standard Cremophor EL vehicle, we evaluated its effects on in vivo xenotransplantation model of glioblastoma. We injected a 2 μL suspension of 100,000 cells from a patient-derived gliomasphere line (GFP-positive, Luciferase-positive) into the striatum, followed immediately, but separately, by a second injection of 2 μL of DCH placed directly above the hGBM cells (Fig 4A). Animals received either DCH-only or DCH-paclitaxel. We used non-invasive, semi-quantitative bioluminescence imaging to estimate hGBM tumor size and follow the approximate progression of tumor growth in live animals.
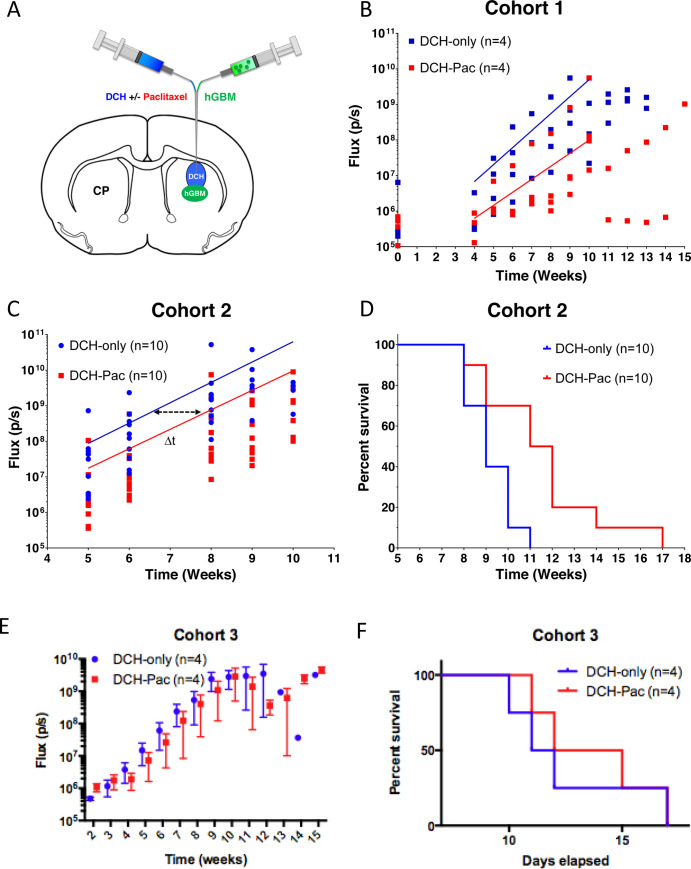
Fig 4. DCH-paclitaxel reduces hGBM growth progression rates monitored by luciferase bioluminescence imaging in vivo and prolongs survival.
A. Schematic of the mouse brain identifying the anatomical location of the gliomasphere and then DCH injections within the caudate putamen (CP) region of the striatum. B and C. Graphs showing Luciferase Total Flux (p/s = photons/second) progression as a function of time as measured by bioluminescence IVIS imaging for each animal receiving either DCH only or DCH-paclitaxel in cohort 1 (n = 4 animals per group) (B) or cohort 2 (n = 10 animals per group) (C). An exponential growth regression was applied to each treatment group in B and C which is represented by a straight line on the semi-log plot. The delta t (Δt) is the time between the same absolute average flux value between the two treatment groups and was calculated to be approximately 1.5 weeks for cohort 2 D. Kaplan Meyer survival curve for cohort 2 animals which demonstrated that DHC-paclitaxel conferred a significant median survival time increase of 2.5 week (or a 23% extension of life) compared to the DCH alone (p = 0.0063). Statistic calculated by log rank test calculated by Prism 6 software. E. In a third cohort, animals were injected with gliomasphere cells followed by DHC two weeks later. The effect was smaller and the survival curve did not meet statistical significance.
We first conducted a small sample pilot study (Cohort 1, n = 4 animals per group) in which bioluminescence imaging was conducted immediately after brain injections and at weekly intervals from 4 weeks onwards (Fig 4B). In both treatment groups, there was no significant increase in the bioluminescence flux signal at 4 weeks compared to several hours after injection. In the hGBM/DCH-only group, there was a measurable increase in the bioluminescence flux signal at 5 weeks, which continued to increase exponentially until 10 weeks when the signal plateaued as the hGBM tumors reached a terminal size and animals required euthanasia (Fig 4B). To evaluate the effect of paclitaxel treatment on hGBM cells, we applied exponential regression analysis on the bioluminescence flux signal values across the interval of 5 to 9 weeks of exponential growth for each animal to determine (i) the extrapolated flux signal at the initial time in which tumor growth kinetics appear to begin exponential growth (Ao); and (ii) the kinetic rate constant of the exponential growth (k). Paclitaxel treatment resulted in (i) an approximately 4-fold reduction in hGBM bioluminescence signal compared to the control group at 4 weeks after injection, which was just prior to the start of measurable increase, and (ii) reduced the subsequent rate of signal increase by approximately 23% (Fig 4B).
To test whether the effect of paclitaxel treatment observed in Cohort 1 (Fig 4B) was robust, we performed an equivalent study on a second, larger group of animals (Cohort 2, n = 10). We conducted bioluminescence imaging over the time period of exponential signal increase from 5 to10 weeks after injection of hGBM cells. In this second, larger group of mice, the DCH-paclitaxel treatment also reduced the initial size of the of hGBM bioluminescence signal by approximately 4-fold, but the rate of the increase in signal was not altered (as shown by the similar gradients for the two curves in Fig 4C) and luminescence signals eventually reached similar levels to that of DCH-only controls. As expected, the high variability of luminescence signal among different animals precluded statistical evaluation and the values obtained can only be regarded as semi-quantitative estimates. Nevertheless, the clear shift to the right in the timing of the exponential increase in bioluminescence in the DCH-paclitaxel group (Δt in Fig 4C) suggested that DCH-paclitaxel treatment reduced the number of hGBM cells, but did not eliminate them and for this reason tumor expansion continued at a similar exponential rate but in a delayed manner. This suggestion was further supported by the observation that DCH-paclitaxel treatment was associated with a statistically significant 2.5 longer survival time in DCH-paclitaxel animals compared with DCH-only controls (p value = 0.0063 in log-rank test) (Fig 4D), representing a 23% extension of life in this hGBM xenotransplantation model. This prolonged survival time correlated well with the approximately 1.5-week delay in the onset and progression of the exponential increase of bioluminescence imaging (Δt, Fig 4C) in DCH-paclitaxel treated mice. Together, these data strongly suggested that our DCH-paclitaxel treatment initially reduced the hGBM tumor load and thereby delayed the expansion of hGBM growth, but eventually the hGBM cells were able to escape treatment. Finally, to test the efficacy of the DCH-paclitaxel on a larger more established tumor, we injected glioblastoma cells and allowed them to grow for two weeks before injecting DCH-paclitaxel through the same burr hole. Similar to the previous cohorts the tumor cells were eventually able to outgrow the hydrogel. DCH-paclitaxel was associated with a smaller survival benefit that did not reach statistical significance (Fig 4E and 4F).
DCH-paclitaxel depots reduce local hGBM cell survival but hGBM cells are able to migrate away from treatment zone
Although bioluminescence imaging provided a useful estimate of the effects of DCH-paclitaxel on overall hGBM tumor growth, it could not provide direct information about the effects of DCH-paclitaxel on hGBM cell number or location. To obtain and quantify this type of information, we conducted histological evaluations in particular at 5 weeks after hGBM cell and DCH injection (Fig 5), the time point at which hGBM tumors began to exhibit exponential growth as indicated by bioluminescence (Fig 4B). hGBM tumor cells were identified by immunofluorescence staining for GFP and human nuclear antigen (NA) antibodies.
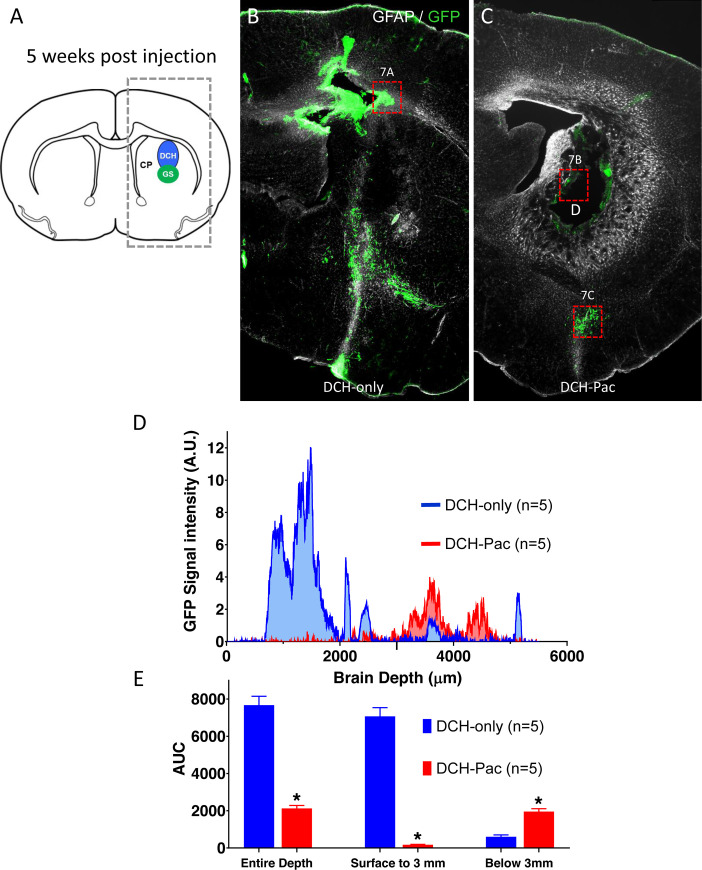
Fig 5. The DCH-paclitaxel system substantively ablates locally residing hGBM cells but does not prevent the migration of tumor cells within brain parenchyma.
A. Schematic of mouse forebrain showing the location of hGBM and DCH injections in the caudate putamen (CP). Box of dashed lines delineates the area quantitatively analyzed for the presence of GFP labeled cells in 5-week post injection tissue and presented in D and E. B,C. Survey immunofluorescent images of staining for GFP (hGBM cells) and GFAP show the location of hGBM cells in forebrain at 5 weeks post injection. B. Mouse that received hGBM cells and DCH-only. Note the high density of GFP-positive hGBM cells immediately above and below the injection site. C. Mouse that received hGBM cells and DCH-paclitaxel. Note the essential absence of GFP-positive hGBM cells immediately around the persisting DCH-paclitaxel depot (D). Note also the presence of hGBM cells that have migrated away from the injection site (box shown at higher magnification in 7C). D. Graph of quantification of GFP signal from hGBM cells. GFP staining intensity was measured as a function of brain depth in serial linear units in the boxed area shown in A. As expected, animals receiving DCH-only (blue) exhibited a high intensity of GFP signal in and immediately above the injection region (0.5 to 3 mm). In contrast, animals receiving DCH-paclitaxel (DCH-Pac, red) exhibited little or no GFP signal in this area, but did exhibit substantive signal at deeper levels (around 4mm). E. Bar graphs quantifying area under the Curve (AUC) for GFP staining intensity. Over the entire depth of the brain, DCH-paclitaxel treated animals (DCH-Pac, red) exhibited an over 72% reduction in hGBM-derived GFP signal compared with DCH-only (p<0.001). In the area immediately around the hGBM and DCH injections (surface to 3 mm), DCH-paclitaxel treated animals exhibited an over 97% reduction in hGBM-derived GFP signal (p<0.001). In contrast, in the deeper areas away from DCH depots (around 4 mm), DCH-paclitaxel treated animals exhibited a 65% greater hGBM-derived GFP signal (p<0.001). n = 5 per group for all measures.
Control animals receiving DCH-only consistently exhibited a focal large cluster of GFP-positive and HNA-positive cells in the dorsal striatum and cerebral cortex along the needle track above the striatal injection site, which represented the predominant hGBM cell population (Fig 5A and 5B). In addition, many hGBM cells had migrated along disrupted tissue planes and white matter tracts into deep subcortical regions and the contralateral hemisphere (Fig 5B). By contrast, DCH-paclitaxel treated animals showed only occasional hGBM cells in the dorsal striatum and overlying cerebral cortex while the bulk of GFP-positive and HNA signal in these treatment animals was observed in two main regions (i) at the margins of the DCH deposit and (ii) in deep subcortical regions well below the DCH deposit site (Fig 5C).
To quantify the number of hGBM cells in different brain locations at 5 weeks after injection we measured GFP staining intensity as a function of brain depth in serial linear units relative to the cortical surface at the injection coordinates (Fig 5D). These measurements showed that the DCH-paclitaxel treatment significantly reduced the number of hGBM cells in the area immediately around the injection site (i.e. cortical surface to 3 mm deep) by 97% (p<0.001) (Fig 5D and 5E). The number of total tumor cells across the entire depth of the brain was significantly reduced by approximately 72.3% (p<0.001) in the paclitaxel group compared to controls (Fig 5D and 5E). Interestingly, in ventral brain regions 1 or more mm distal from the injection site (Fig 5C), the number of tumor cells was somewhat higher in the paclitaxel group (Fig 5D and 5E), suggesting that tumor cells had migrated away from the paclitaxel. It should be noted that during the initial procedure the cells were placed first under the DCH and that may contribute to the deeper location of the resulting tumor.
Discussion
Extent of resection (EOR) has been highly correlated to improved survival in low and high-grade glioma [23–26]. However, the ability to perform near complete tumor resection is dependent on the location of the primary mass within the brain. Tumors located in deep sub-cortical or midline structures, in close proximity to ventricles, near large vessels or at eloquent brain regions, are often classified as inoperable or unsuitable for large volume tumor resection due to the high risk of severe postsurgical morbidity [27, 28]. These unresectable tumors are associated with shorter patient survival [26, 27]. The paclitaxel-loaded DCH system represents a potential approach for debulking these difficult to access tumors without causing significant post-surgical morbidity.
In this study we show that DCH can locally deliver a potent hydrophobic chemotherapeutic agent, paclitaxel, to a focal site within the brain and substantively ablate the fluorescent signal from hGBM tumor cells while minimizing the extent of damage to healthy host tissue. Although DCH-paclitaxel treatment massively depleted the fluorescence from hGBM cells, the surviving hGBM cells were able to avoid the treatment area and survive in deeper areas of the brain. This finding highlights the importance of using realistic cellular models. Previous studies using serum-derived glioma cells or utilizing in vitro or subcutaneous environments under-estimate the effect of cell migration and thus likely overstate their efficacy. Despite the difficulty of targeting migrating tumor cells with a focal treatment vehicle, the potential survival benefit of this technology should warrant further investigation and consideration. There has been some clinical trials using locally implantable paclitaxel-based gels. Most notably Oncogel (paclitaxel-based gel) was used clinically on esophageal cancer. After a promising phase II trial [29], the follow-up phase III trial was halted and no further trials are planned.
One potential solution to the problem of cell migration involves a combination of cell homing and cell ablation in which chemoattractants could be used to guide migration of glioblastoma cells towards a focal “kill zone” where they are destroyed by chemotherapeutic agents [30]. Many chemoattractant molecules and other physical cell guidance systems are being identified, including various canonical cytokines and growth factors [31]. Another potential use of this technology involves pairing it with other minimally invasive tumor ablation techniques. Devices using ultrasound [32] or thermo-ablation [33] are being developed to debulk the central tumor mass. These technologies leave a hole in the tumor mass that could potentially be filled with a hydrogel for extended tumor control.
One problem with delivering chemotherapeutic agents at the time of surgery is the concern for wound healing. The initial trials with gliadel were associated with an increase in wound break-down. It is for this reason, that many systemic chemotherapies are started several weeks after the surgery to allow for the wound to heal. One advantage of minimally invasive injection techniques is that they involve smaller wounds, which are less prone to wound complications and break-down.
In conclusion, this study demonstrates the potential for using DCH to deliver a potent chemotherapeutic agent to a focal site within the brain and effectively chemically debulk a central area of tumor with a resulting survival benefit. This technology brings hope to a population of patients for whom surgical debulking is not possible.
Acknowledgments
We thank the Microscopy Core Resource of the UCLA Broad Stem Cell Research Center-CIRM Laboratory.
Data Availability
All relevant information is in the paper and figures.
Funding Statement
We thank the Microscopy Core Resource of the UCLA Broad Stem Cell Research Center-CIRM Laboratory. This work was supported by the Dr. Miriam and Sheldon G. Adelson Medical Foundation (M.V.S., T.J.D. and H.I.K.), the US National Institutes of Health (NS084030 to M.V.S. and NS053563 to H.I.K), and a training grant from the California Institute for Regenerative Medicine (M.C.G.).
References
- 1.Wen PY, Kesari S. Malignant gliomas in adults. The New England journal of medicine. 2008;359(5):492–507. 10.1056/NEJMra0708126 . [DOI] [PubMed] [Google Scholar]
- 2.Gruber ML, Hochberg FH. Systematic evaluation of primary brain tumors. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 1990;31(6):969–71. . [PubMed] [Google Scholar]
- 3.Yonezawa H, Hirano H, Uchida H, Habu M, Hanaya R, Oyoshi T, et al. Efficacy of bevacizumab therapy for unresectable malignant glioma: A retrospective analysis. Molecular and clinical oncology. 2017;6(1):105–10. 10.3892/mco.2016.1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stewart LA. Chemotherapy in adult high-grade glioma: a systematic review and meta-analysis of individual patient data from 12 randomised trials. Lancet. 2002;359(9311):1011–8. 10.1016/s0140-6736(02)08091-1 . [DOI] [PubMed] [Google Scholar]
- 5.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. The New England journal of medicine. 2005;352(10):987–96. 10.1056/NEJMoa043330 . [DOI] [PubMed] [Google Scholar]
- 6.Johnson BE, Mazor T, Hong C, Barnes M, Aihara K, McLean CY, et al. Mutational analysis reveals the origin and therapy-driven evolution of recurrent glioma. Science. 2014;343(6167):189–93. 10.1126/science.1239947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kitange GJ, Carlson BL, Schroeder MA, Grogan PT, Lamont JD, Decker PA, et al. Induction of MGMT expression is associated with temozolomide resistance in glioblastoma xenografts. Neuro-oncology. 2009;11(3):281–91. 10.1215/15228517-2008-090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang SM, Kuhn JG, Robins HI, Schold SC Jr., Spence AM, Berger MS, et al. A Phase II study of paclitaxel in patients with recurrent malignant glioma using different doses depending upon the concomitant use of anticonvulsants: a North American Brain Tumor Consortium report. Cancer. 2001;91(2):417–22. . [DOI] [PubMed] [Google Scholar]
- 9.Moses MA, Brem H, Langer R. Advancing the field of drug delivery: taking aim at cancer. Cancer cell. 2003;4(5):337–41. 10.1016/s1535-6108(03)00276-9 . [DOI] [PubMed] [Google Scholar]
- 10.Rowland MJ, Parkins CC, McAbee JH, Kolb AK, Hein R, Loh XJ, et al. An adherent tissue-inspired hydrogel delivery vehicle utilised in primary human glioma models. Biomaterials. 2018;179:199–208. 10.1016/j.biomaterials.2018.05.054 . [DOI] [PubMed] [Google Scholar]
- 11.Puente P, Fettig N, Luderer MJ, Jin A, Shah S, Muz B, et al. Injectable Hydrogels for Localized Chemotherapy and Radiotherapy in Brain Tumors. Journal of pharmaceutical sciences. 2018;107(3):922–33. 10.1016/j.xphs.2017.10.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cui Y, Xu Q, Chow PK, Wang D, Wang CH. Transferrin-conjugated magnetic silica PLGA nanoparticles loaded with doxorubicin and paclitaxel for brain glioma treatment. Biomaterials. 2013;34(33):8511–20. 10.1016/j.biomaterials.2013.07.075 . [DOI] [PubMed] [Google Scholar]
- 13.Zhao M, Danhier F, Bastiancich C, Joudiou N, Ganipineni LP, Tsakiris N, et al. Post-resection treatment of glioblastoma with an injectable nanomedicine-loaded photopolymerizable hydrogel induces long-term survival. International journal of pharmaceutics. 2018;548(1):522–9. 10.1016/j.ijpharm.2018.07.033 . [DOI] [PubMed] [Google Scholar]
- 14.Tyler B, Fowers KD, Li KW, Recinos VR, Caplan JM, Hdeib A, et al. A thermal gel depot for local delivery of paclitaxel to treat experimental brain tumors in rats. Journal of neurosurgery. 2010;113(2):210–7. 10.3171/2009.11.JNS08162 . [DOI] [PubMed] [Google Scholar]
- 15.Hemmati HD, Nakano I, Lazareff JA, Masterman-Smith M, Geschwind DH, Bronner-Fraser M, et al. Cancerous stem cells can arise from pediatric brain tumors. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(25):15178–83. 10.1073/pnas.2036535100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laks DR, Crisman TJ, Shih MY, Mottahedeh J, Gao F, Sperry J, et al. Large-scale assessment of the gliomasphere model system. Neuro-oncology. 2016;18(10):1367–78. 10.1093/neuonc/now045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dull T, Zufferey R, Kelly M, Mandel RJ, Nguyen M, Trono D, et al. A third-generation lentivirus vector with a conditional packaging system. Journal of virology. 1998;72(11):8463–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang S, Anderson MA, Ao Y, Khakh BS, Fan J, Deming TJ, et al. Tunable diblock copolypeptide hydrogel depots for local delivery of hydrophobic molecules in healthy and injured central nervous system. Biomaterials. 2014;35(6):1989–2000. 10.1016/j.biomaterials.2013.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee SH, Yoo SD, Lee KH. Rapid and sensitive determination of paclitaxel in mouse plasma by high-performance liquid chromatography. Journal of chromatography B, Biomedical sciences and applications. 1999;724(2):357–63. 10.1016/s0378-4347(98)00566-0 . [DOI] [PubMed] [Google Scholar]
- 20.Faulkner JR, Herrmann JE, Woo MJ, Tansey KE, Doan NB, Sofroniew MV. Reactive astrocytes protect tissue and preserve function after spinal cord injury. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2004;24(9):2143–55. 10.1523/JNEUROSCI.3547-03.2004 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hafner M, Niepel M, Chung M, Sorger PK. Growth rate inhibition metrics correct for confounders in measuring sensitivity to cancer drugs. Nat Meth. 2016;13(6):521–7. 10.1038/nmeth.3853, http://www.nature.com/nmeth/journal/v13/n6/abs/nmeth.3853.html#supplementary-information. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang C-Y, Song B, Ao Y, Nowak AP, Abelowitz RB, Korsak RA, et al. Biocompatibility of amphiphilic diblock copolypeptide hydrogels in the central nervous system. Biomaterials. 2009;30(15):2881–98. 10.1016/j.biomaterials.2009.01.056 [DOI] [PubMed] [Google Scholar]
- 23.Lacroix Michel, Dima Abi-Said Daryl R. Fourney, Gokaslan Ziya L., Shi Weiming, Franco DeMonte, et al. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. Journal of Neurosurgery. 2001;95(2):190–8. 10.3171/jns.2001.95.2.0190 . [DOI] [PubMed] [Google Scholar]
- 24.Stummer W, Reulen H-J, Meinel T, Pichlmeier U, Schumacher W, Tonn J-C, et al. EXTENT OF RESECTION AND SURVIVAL IN GLIOBLASTOMA MULTIFORME: IDENTIFICATION OF AND ADJUSTMENT FOR BIAS. Neurosurgery. 2008;62(3):564–76. 10.1227/01.neu.0000317304.31579.17 . [DOI] [PubMed] [Google Scholar]
- 25.Sanai Nader, Polley Mei-Yin, Michael W. McDermott, Andrew T. Parsa, Mitchel S. Berger. An extent of resection threshold for newly diagnosed glioblastomas. Journal of Neurosurgery. 2011;115(1):3–8. 10.3171/2011.2.jns10998 . [DOI] [PubMed] [Google Scholar]
- 26.Kuhnt D, Becker A, Ganslandt O, Bauer M, Buchfelder M, Nimsky C. Correlation of the extent of tumor volume resection and patient survival in surgery of glioblastoma multiforme with high-field intraoperative MRI guidance. Neuro-Oncology. 2011;13(12):1339–48. 10.1093/neuonc/nor133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Orringer D, Lau D, Khatri S, Zamora-Berridi GJ, Zhang K, Wu C, et al. Extent of resection in patients with glioblastoma: limiting factors, perception of resectability, and effect on survival. J Neurosurg. 2012;117(5):851–9. Epub 2012/09/18. 10.3171/2012.8.JNS12234 . [DOI] [PubMed] [Google Scholar]
- 28.Kubben PL, ter Meulen KJ, Schijns OE, ter Laak-Poort MP, van Overbeeke JJ, van Santbrink H. Intraoperative MRI-guided resection of glioblastoma multiforme: a systematic review. The Lancet Oncology. 2011;12(11):1062–70. Epub 2011/08/27. 10.1016/S1470-2045(11)70130-9 . [DOI] [PubMed] [Google Scholar]
- 29.DuVall GA, Tarabar D, Seidel RH, Elstad NL, Fowers KD. Phase 2: a dose-escalation study of OncoGel (ReGel/paclitaxel), a controlled-release formulation of paclitaxel, as adjunctive local therapy to external-beam radiation in patients with inoperable esophageal cancer. Anticancer Drugs. 2009;20(2):89–95. Epub 2009/02/12. 10.1097/CAD.0b013e3283222c12 . [DOI] [PubMed] [Google Scholar]
- 30.van der Sanden B, Appaix F, Berger F, Selek L, Issartel J-P, Wion D. Translation of the ecological trap concept to glioma therapy: the cancer cell trap concept. Future Oncology. 2013;9(6):817–24. 10.2217/fon.13.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim Y. Regulation of cell proliferation and migration in glioblastoma: new therapeutic approach. Frontiers in oncology. 2013;3:53 10.3389/fonc.2013.00053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hersh DS, Kim AJ, Winkles JA, Eisenberg HM, Woodworth GF, Frenkel V. Emerging Applications of Therapeutic Ultrasound in Neuro-oncology: Moving Beyond Tumor Ablation. Neurosurgery. 2016;79(5):643–54. 10.1227/NEU.0000000000001399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patel P, Patel NV, Danish SF. Intracranial MR-guided laser-induced thermal therapy: single-center experience with the Visualase thermal therapy system. Journal of neurosurgery. 2016;125(4):853–60. 10.3171/2015.7.JNS15244 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant information is in the paper and figures.