Abstract
Purpose of Review:
This review summarizes (1) recent trends in delta-9-tetrahydrocannabionol [THC] and cannabidiol (CBD) content in cannabis products, (2) neurobiological correlates of cannabis use on the developing adolescent brain, (3) effects of cannabis on psychiatric symptoms and daily functioning in youth (i.e., academic performance, cognition, sleep and driving), (4) cannabis products used to relieve or treat medical issues in youth, and (5) available treatments for cannabis use disorder in adolescence.
Recent findings:
Despite marked increases in THC content and availability of cannabis, there has been a decline in perceived risk and an increase in use of THC extract products among youth in the United States. The primary psychiatric symptoms associated with cannabis use in youth are increased risk for addiction, depressive, and psychotic symptoms. Cannabis alters endocannabinoid system function which plays a central role in modulating the neurodevelopment of reward and stress systems. To date, few studies have examined neurobiological mechanisms underlying the psychiatric sequalae of cannabis exposure in youth. Adolescent cannabis exposure results in impaired cognition, sleep, and driving ability. There are very limited FDA-approved cannabinoid medications, none of them supporting their use for the treatment of psychiatric symptoms. Behavioral therapies are currently the mainstay of treating cannabis misuse, with no pharmacotherapies currently approved by the FDA for cannabis use disorder in youth.
Summary:
Here, we summarize the most up-to-date knowledge on the neurobiological psychiatric, and daily function effects of the most commonly used cannabinoids, delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD). We then review FDA approved medical use of cannabinoid treatments as well as pharmacological and psychological treatments for cannabis use disorder in youth. Our current understanding of the effects of cannabis on the developing brain and treatments for cannabis misuse in youth remain limited. Future research aimed at examining the neurobiological effects of cannabis, with objective measures of exposure, over the course of pediatric development and in relation to psychiatric symptoms are needed.
Keywords: Adolescence, cannabis, delta-9-tetrahydrocannabinol (THC), cannabidiol (CBD), neurodevelopment, cannabis use disorder
Introduction
Cannabis is the most widely used illicit substance by adolescents and young adults worldwide.[1] An estimated 45% of adolescents have a lifetime history of cannabis use by age 18, with 37% of high-school seniors reporting cannabis use within the past year.[2, 3] In the United States, over 6,000 people are first-time cannabis users per day, over 60% of whom are under age 18.[4, 5] Over the past decade, 4% of college students have consistently been regular cannabis users (i.e., using on 20 or more occasions in the past month), while the rate has doubled from 6% to 13% among same-age non-college transition-age youth.[3]
Adolescents tend to use cannabis more frequently than alcohol or other drugs.[6] The Monitoring the Future Study, surveying trends in legal and illicit drug use among approximately 50,000 American adolescents annually, reported a substantial decline in perceived risk associated with regular cannabis use over the past couple of decades, corresponding with increased marijuana use rates.[3] The belief among many teens that marijuana is benign relative to other recreational drugs is in contrast to a preponderance of evidence that adolescent marijuana use is linked to psychiatric symptoms including psychosis and suicidality,[7, 8] as well cognitive impairment in learning, memory, and executive function.[7, 8] Cannabis use also results in compromised motor coordination contributing to an increased risk of motor vehicle collisions, which is a leading cause of morbidity and mortality in adolescents.[9]
Cannabis ‘Potency’
Marijuana is made from dried flowers and leaves of the cannabis sativa plant. Although cannabis contains over 500 identified compounds, delta-9-tetrahydrocannabinol (THC) is the primary psychoactive ingredient of the plant that induces a “high.” While a wide range of potencies are available across cannabis products, the average THC content has significantly increased over the past two decades: In states with legal recreational and medicinal cannabis use, cannabis products contain an average of 16%−35% THC.[10] Conversely, concentrations of cannabidiol (CBD), a component in cannabis found to have antipsychotic properties, has decreased, such that the ratio of THC:CBD has increased 80-fold.[11] Remarkably, THC extract products containing upwards of 60% THC (e.g., “wax,” “crumble, “shatter”) are now easily accessible, especially in states which have legalized marijuana.[12] Purified CBD is also available in a wide array of consumable commercial products, including food products, oils, lotions, balms, bath products, and “vape pen” cartridges. Moreover, teens perceive vaping these cannabis extract preparations as safer than traditionally smoked joints.[3, 13] Use of cannabis extract products are increasingly popular among youth[3, 14] despite limited understanding of the potential consequences on neurodevelopment. [3, 13]
Information concerning the effects of cannabis use on adolescent health remain limited. The overwhelming majority of prior studies have assessed cannabis effects on health using self-report measures of use (primarily frequency of use), and have not incorporated objective measures of potency (i.e., THC content).[15, 16] Studies that included measures of potency have primarily assessed the acute effects of THC administration in adults using standardized cannabis preparations supplied by the National Institute on Drug Abuse which contained much lower THC levels (~3–5%) compared to present-day cannabis formulations.[17] Our limited understanding of the neurobiological and psychiatric sequelae of adolescent cannabis use poses an important public health concern, given the widespread use of cannabis products among youth during a period of brain development.
Neurobiological Effects of Cannabis Use in Adolescence
Adolescence is a critical period of brain maturation and neurodevelopment that is vulnerable to perturbations induced by cannabis exposure.[18] Cannabis exerts its effects via altering signaling within the endocannabinoid system (ECS), which serves a crucial modulatory role in regulating neurodevelopment of reward and stress circuitry in the brain.[19, 20] The ECS is primarily comprised of two G-protein coupled transmembrane receptors: cannabinoid type 1 receptors (CB1R, primarily expressed in the central nervous system), and cannabinoid type 2 receptors (CB2R, implicated in peripheral immune system function). CB1Rs are located on presynaptic terminals, and modulate GABA, glutamate, and dopamine neurotransmitter release and neuronal firing[21] CB1Rs are dynamically expressed and following activation are internalized into endosomes, such that the balance between rate of expression and internalization regulates the number of active receptors in synaptic membranes.[22] Upon CB1R activation, the receptors’ associated G-protein subunits uncouple; these subunits interact with ion channels to modulate cyclic adenosine monophosphate synthesis, downstream protein kinase A (PKA), and extracellular signal–regulated kinase (ERK) pathways. The ion channel modulation allows endocannabinoids to rapidly alter neural signaling.[21]
Anandamide and 2-arachidonoylglycerol (2-AG) are the two primary endocannabinoids that intrinsically activate these cannabinoid receptors.[21] Exogenous THC functions as an allosteric agonist of CB1 Rs. Amongst all G-protein receptors in the brain, CB1Rs are the most abundant, highlighting the widespread effects that exogenous cannabis can have, particularly in the developing brain. The degree to which endogenous and exogenous CB1 receptor agonists induces downstream signaling varies. THC is not as efficacious as anandamide or 2-AG at activating PKA or ERK signaling pathways,[23] but is significantly more effective at receptor internalization, resulting ultimately in tolerance to its effects.[21]
Cannabis may acutely contribute to an increase in pleasure and decrease in perceived stress by increasing dopamine with reward circuitry and decreasing cortisol released by the hypothalamic-pituitary-adrenal (HPA) axis in response to stress. Chronic cannabis use, however, results in cannabinoid receptor type 1 (CB1-R) downregulation and reductions in endocannabinoids levels [21] that impair sensitivity to reward and stress.[24] In animal models, disruption of ECS signaling results in a depressive phenotype with impaired reward sensitivity.[25, 26] In humans, the CB1-R antagonist rimonabant produced a significant increase in depressive symptoms in individuals with no history of mental illness in a double-blind placebo-controlled clinical trial. Depressive symptoms were so severe that they resulted in withdrawal of this medication from the European market, and prevented FDA approval.[27] The acute psychoactive and rewarding effects of cannabis are largely attributed to its modulatory effects on dopamine (DA) signaling via CB1Rs that are most highly expressed in key regions of meso-cortico-striatal reward circuitry, summarized in Figure 1. CB1R agonist exposure acutely results in alterations in neurotransmitters that are similar to those produced by other drugs of abuse via attenuation of evoked GABA release that results in downstream increases in DA within fronto-limbic brain circuitry, especially within the nucleus accumbens (NAc), a central hub of reward processing.[28] Tolerance that develops with chronic cannabis exposure is attributed to disrupted reward-related signaling mechanisms in this system by reducing DA cell density in the ventral tegmental area (VTA), as well as decreasing VTA DA cell firing and downstream DA release in the NAc and the medial prefrontal cortex (PFC).[21, 29] Reductions in CB1-R expression and function as well as decreased DA are more pronounced with adolescent cannabis use (relative to use in adulthood), contributing to disrupted reward signaling, impaired reward sensitivity, and ultimately depressive symptoms of anhedonia, depressed mood and decreased motivation.
Figure 1: Modulatory effects of THC on dopamine signaling within brain reward circuitry.
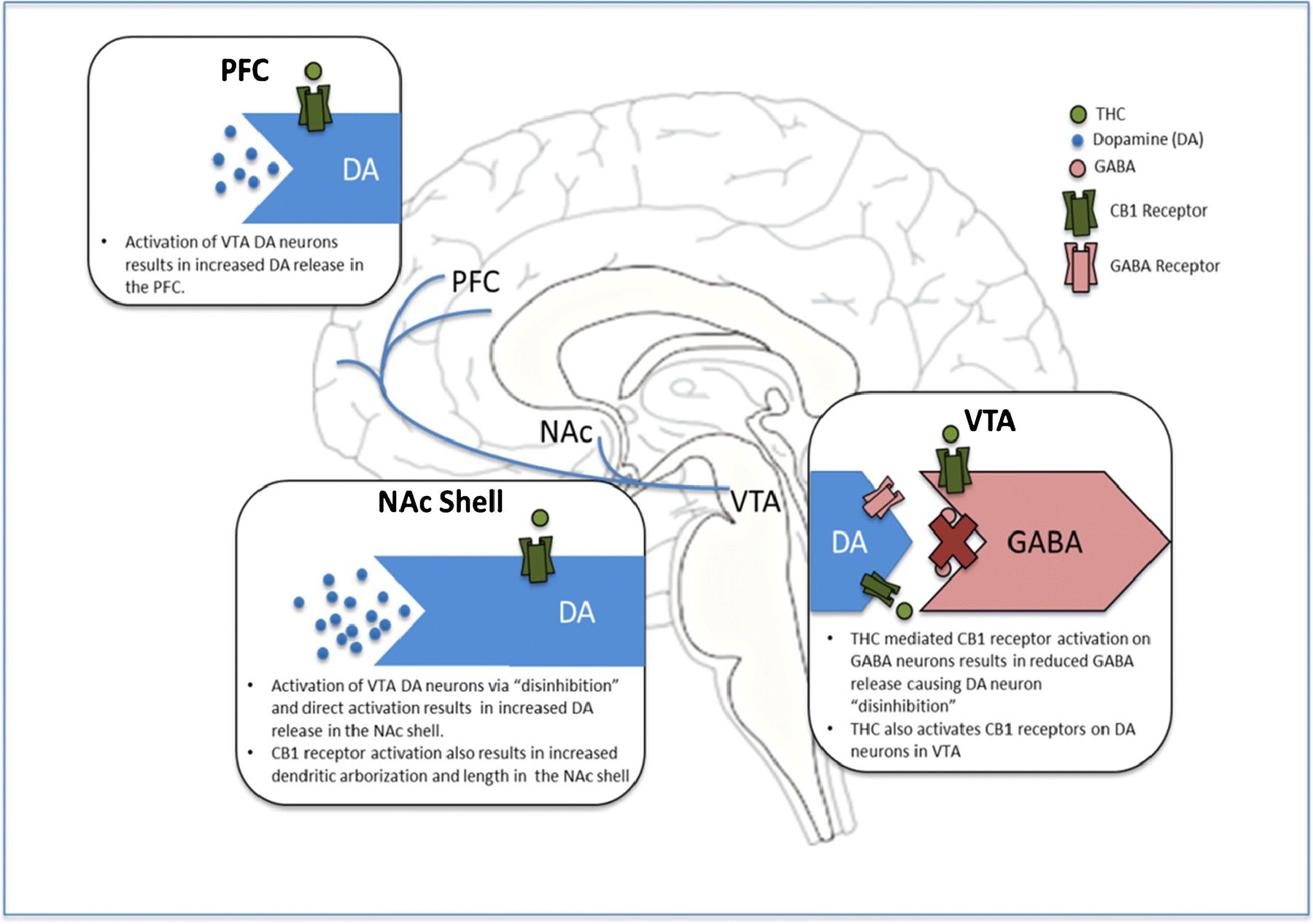
Figure Legend: Neuromodulatory effects of THC on dopamine signaling within key regions of brain reward circuitry. Acutely, THC increases DA synthesis within the VTA and downstream release within brain reward centers (i.e., NAc and PFC), similar to other drugs of abuse. With Chronic use, CB1-R expression and function decreases and DA release and cell density is reduced (more pronounced with adolescent exposure) resulting in disrupted reward signaling and impaired reward sensitivity and motivation. DA: dopamine, GABA: gamma-aminobutyric acid, VTA: ventral tegmental area, NAc: nucleus accumbens, PFC: prefrontal cortex.
Cannabidiol (CBD) also interacts with cannabinoid receptors but its mechanism of action is not fully understood, with discrepancies between in vitro and in vivo studies. CBD has a low affinity for CB1 receptors, [30, 31] and acts as a negative allosteric modulator of CB1R, which may reduce the potency and efficacy of CB1 agonists.[32–34] This interaction is posited to explain the role of CBD in attenuating the psychoactive adverse effects of THC.[35] While 65 discrete molecular targets of CBD have been identified, the majority only interact at supraphysiological concentrations.[36] Adenosine A1, the serotonin transporter (5-HT1a), G protein-coupled receptor 55 (GPR55), peroxisome proliferator-activated receptor (PPARγ),[36] and transient potential vanilloid receptor type-1 (TPVR-1) receptor, have been identified as plausible and potential targets in physiologic conditions.[35, 36]
Despite our limited understanding of the neurobiological effects of CBD, CBD products are increasingly marketed for ‘treating’ anxiety and insomnia, further highlighting the need for improved understanding of the neurobiological effects of cannabis exposure, particularly during critical periods of neurodevelopment.
Adolescent Cannabis Use and Psychiatric Symptoms
The primary psychiatric symptoms associated with adolescent cannabis are increased risk for addiction, depressive symptoms, and psychosis. Despite the significant associations found between adolescent cannabis use and psychiatric symptoms, a causal link between exposure and symptom manifestation is difficult to establish for several reasons: First, common risk factors such as family history of addiction and other psychopathology as well as environmental stressors may predispose an individual toward both substance use and psychopathology. Second, use may be directly associated with the risk of psychopathology through shared neurobiological mechanisms. Third, youth may be attempting to abate psychiatric symptoms through self-administration of cannabis.[48] Fourth, certain peer groups may influence cannabis use and be linked to psychopathology risk.[49] Regardless of the etiology, the co-occurrence of psychiatric symptoms and cannabis use are well documented, and should be a standard part of patient psychoeducation and motivational interviewing.
Substance use disorders.
Cannabis use significantly increases risk for addiction,[50] with adolescents being four times as likely to develop cannabis dependence within two years after use onset.[51] About 20% of individuals who start using marijuana in adolescence and up to 50% of teens who smoke marijuana daily will develop an addiction.[9] Moreover, use of cannabis preparations with higher THC content show a dose-dependent increase in risk of developing a substance use disorder.[52] Recent cannabis use trends among adolescents raise concerns because they account for the majority of substance abuse treatment admissions in adolescence.[53, 54] Importantly, adolescent cannabis use also confers increased risk for other substance use disorders. A recent meta-analysis demonstrated significant associations between the frequency of adolescent cannabis use and increased risk of cannabis use disorder (adjusted OR = 4.2 monthly, 8.7 weekly, 17.9 daily use), as well as use of opiates and other illicit drugs (adjusted OR = 2.8, 4.7, 7.8) by young adulthood.[55] Cannabis has recently been proposed as a potential treatment for opioid use disorder based on a study that found that states with medical cannabis laws experienced slower increases in opioid analgesic overdose mortality (−21%).[56] However, the association between legalization of cannabis for medicinal purposes and opioid-related mortality has increased 23% on longitudinal follow up.[57]
Depression.
While developing a substance use disorder is the most common long-term psychiatric diagnosis associated with adolescent cannabis use,[55] depressive symptoms are the most common psychiatric symptoms associated with cannabis use in adolescence.[58] Depressive symptoms in adolescence are particularly concerning given that suicide, often attributable to depression, is a leading cause of death in this age group.[59] A recent meta-analysis assessing the effects of adolescent cannabis use found an increased risk of major depressive disorder (OR 1.37, 95% CI 1.16–1.62), suicidal ideation (OR 1.50, 95% CI 1.11–2.03) and suicide attempts (OR 3.46 CI 1.53 – 7.83), but not anxiety symptoms by young adulthood.[8] A longitudinal study prospectively followed a community sample of 662 adolescents from 2003–2013 to examine the strength of association between cannabis use frequency and psychiatric symptoms. From ages 15–19 years, more frequent cannabis use lead to greater depressive, but not anxiety or psychotic symptoms, after controlling for age of onset of use, sex, socioeconomic status, and other drug use.[58] Of note, there were no reported changes in anxiety symptoms with more frequent cannabis use.[58] Notably, depressive symptoms during adolescence do not predict subsequent cannabis use in young adults, suggesting that this relation was not simply due to premorbid differences.[4, 60] In addition, reductions in cannabis use are associated with reductions in depressive symptoms.[61] In Colorado, the rate of increase in cannabis potency directly correlates with increased rates of cannabis related emergency department visits by adolescents.[50, 62] In a study of over 4,000 adolescents who presented for emergency and urgent care visits between 2005 and 2015, the most common ICD codes other than cannabis use were depression (39%) and unspecified mood disorder (22%), with a significant increase in marijuana-related visits following legalization of medicinal and recreational marijuana.[63]
Psychosis.
A broad-based literature has established a link between adolescent cannabis use and risk of psychosis. Cannabis use is considered a preventative risk factor for psychotic disorders, including schizophrenia, especially in those with a pre-existing genetic vulnerability.[20] Epidemiological studies have consistently reported an association between cannabis use and schizophrenia in which cannabis use precedes psychosis independent of other substance use.[20] This effect, however, is not immediate with adolescent cannabis use, portending an increased risk of psychotic disorders that manifest primarily in young adulthood.[58] In a sample of 6,534 subjects from the general population Northern Finland Birth Cohort of adolescents that were prospectively followed until age 30, adolescent cannabis use was significantly associated with developing a psychotic disorder after controlling for baseline prodromal symptoms, daily smoking, alcohol and other substance use, with a significant ‘doseresponse’ effect with respect to frequency of cannabis use (HR = 3.0, 95% CI 1.1–8.0).[64] In a study of over 400 first episode patients with psychosis, adolescents who had started cannabis at age 15 or younger, daily cannabis users, and use of cannabis with higher THC content, significantly advanced the timeline of when they experienced first psychotic episode by 2–6 years.[65] Increasing evidence demonstrates a dose-response relation between cannabis use and risk for psychotic outcomes.[64, 66] In a recent meta-analysis of all available published studies examining the relation between cannabis use and psychosis, regular cannabis users had a 2-fold increased risk, and heavy cannabis users a 4-fold increase in risk, for psychosis relative to nonusers.[7] For a comprehensive meta-analytic review of the association between cannabis use and psychosis, please see Marconi et al. [7]
Effects of Cannabis on Daily Functioning in Youth
Adolescent cannabis use is also linked to cognitive impairment, sleep disturbance, and increased risk of motor vehicle collisions. Teens who start using cannabis at an earlier age appear to have greater susceptibility to long-lasting consequences of cannabis use than those with a later onset of cannabis exposure.
Cognition and academic performance.
Although some controversy exists in the adult literature with respect to the lasting effects of cannabis on cognitive function, regular cannabis use in adolescence is significantly associated with cognitive impairment within the domains of attention, processing speed, verbal learning and memory, and executive functioning.[67–69] Longitudinal studies have demonstrated that increased adolescent cannabis use significantly predicts poorer verbal memory[70] and attention.[71] These deficits are also more likely persist following abstinence with adolescent (as compared to adult) cannabis use.[72] Earlier age of onset of use and heavier use during adolescence are associated with increased rates of cognitive impairment in adulthood.[73] In a prospective longitudinal study of over 1000 youth followed from birth to adulthood, those who regularly used cannabis during adolescence demonstrated the greatest reductions in IQ.[74] Individuals that started using cannabis in early adolescence demonstrated the greatest reductions in IQ (i.e., from ‘average’ in childhood to ‘low-average’ in adulthood). Moreover, they did not return to their predicted intellectual trajectory, and cognitive impairments remained evident following over one year of abstinence.[74] It is also important to note that an earlier age of onset has also been associated with greater cognitive impairments even when the total duration of use is relatively short as demonstrated by Solowij and colleagues.[75] Of note, there is some evidence to suggest that greater CBD content may serve a protective role against some THC-induced cognitive deficits.[67] Long term functional consequences include poor educational outcome, with increased likelihood of dropping out of school, and diminished long term academic and occupational achievement.[9]
Sleep.
While it has been shown that cannabis alters sleep architecture, [76] few studies have examined the effects of cannabis on sleep over the course of adolescent development. Studies examining the relation between sleep quality and substance abuse have demonstrated a bi-directional effect, with sleep disturbance repeatedly linked to substance use in adolescence.[77, 78] Adolescent cannabis use was found to predict subsequent sleep problems in a prospective study of approximately 250 12 year olds that were longitudinally followed until age 18.[78] Whereas lifetime alcohol use was also an important predictor of poor sleep quality at 18 years of age, cannabis showed greater contribution to this outcome. Conversely, shorter sleep duration and poorer quality of sleep in early adolescence is significantly associated with earlier and repeated cannabis (and alcohol) misuse later in adolescence.[77] A separate prospective longitudinal study demonstrated that self-reported ‘overtiredness’ and ‘trouble falling asleep’ predicted subsequent cannabis use.[79] This highlights the importance of focusing on educating pediatric patients and their families regarding the importance of good sleep and sleep hygiene as an important and underutilized preventive approach with respect to adolescent substance abuse, especially in light of the fact that almost half of all adolescents report sleep problems reach clinical levels.[80] Longitudinal studies examining the mechanisms linking cannabis use and sleep are warranted.
Driving.
Driving under the influence of cannabis is now more common than driving under the influence of alcohol among adolescent drivers in the United States, [81] and cannabis is the illicit drug most frequently reported in connection with impaired driving and accidents, a leading cause of death in adolescence.[82] Greater THC concentration in blood is directly related to greater impairment in motor coordination.[83] In adults, both immediate and long-term exposure to marijuana are found to impair driving ability.[50, 82] Indeed, meta-analyses have found that cannabis increases collision risk (pooled OR: 1.5 – 2.5). [84, 85] Simulated driving studies have demonstrated that driving under the influence of cannabis results in significantly poorer lane control and reduced driving speed, similar to effects observed when using a cell phone or texting while driving [85, 86]. This is particularly relevant to young drivers who are more likely to be involved in distraction related motor vehicle accidents. [87] While studies are lacking with adolescent drivers, simulated driving studies of young adult drivers have shown that driving performance in conditions of divided attention and increased driving complexity significantly worsened following cannabis use. Participants were significantly more likely to be classified as having a high crash risk (OR 4.31) after cannabis use lasting up to 5 hours after use. [88] Further research is needed to assess the impact of the cannabis on driving behavior and collision risk in youth, which to date has received little attention.
Adolescent Use of Cannabis Products to Relieve or Treat Medical Issues
While use of cannabis-derived products are increasingly marketed for medicinal purposes, the few Food and Drug Administration (FDA) approved indications are classified as Schedule I substances by the United States federal government. Some states have laws decriminalizing cannabis and cannabis-derived products such as THC and CBD, but no current regulations with respect to THC and CBD content are in place. In one study, CBD extracts were tested and only 31% were found to actually contain the percentage of CBD advertised. In fact, THC was detected in 21% of the ‘purified’ CBD products.[89] In 2016, the FDA sent warning letters to CBD vendors stating that CBD products are not to be classified as dietary supplements, and therefore cannot make medical claims without FDA approval.[90] This is disturbing in light of increasing rates of use among individuals with psychiatric symptoms of depression, anxiety, post-traumatic stress disorder, and psychosis, who report self-medication of symptoms as a driver of cannabis use.[91].
Currently, two medications containing THC have been approved by the FDA, dronabinol and nabilone. Both are indicated for nausea/vomiting in cancer chemotherapy, and as an appetite stimulant in the setting of weight loss due to AIDS. Outside of the United States, the United Kingdom, Canada, and several European countries have approved the THC and CBDcontaining drug nabiximols (Sativex®) for cancer pain and spasticity caused by multiple sclerosis. The most commonly reported adverse reactions include: central nervous system symptoms including dizziness (~18%), fatigue or drowsiness (~10), confusion (~7%), and psychiatric symptoms including psychotic symptoms, depressed mood and suicidal ideation (~10%); gastrointestinal symptoms including nausea (~10%), vomiting (5%), diarrhea (~7%); cardiovascular symptoms including Hypotension (~5%), palpitations (~1%), syncope (~1%), tachycardia (~1%), and increased risk for substance dependent and abnormal hepatic function (~5%). [92, 93] CBD, in the purified pharmaceutical form Epidiolex, is currently FDA-approved for the treatment of two forms of severe childhood epilepsy: Dravet syndrome and LennoxGaustaut syndrome. Both trials demonstrated efficacy in significantly reducing the number of seizures.[94, 95] These studies also noted CBD-related side effects that included diarrhea, vomiting, fatigue, pyrexia, anorexia, upper respiratory tract infections, convulsions, lethargy, somnolence and abnormal hepatic function.[94 [68] In one study, 12 patients in the CBD group had liver aminotransferase elevations greater than 3 times the upper limit, compared to 1 in the placebo group.{Devinsky, 2017 #67, 95, 96]
These studies, however, are limited to short-term efficacy and do not include the potential longer-term effects of receptor and/or neuronal changes in developing brains. Both Lennox-Gastaut and Dravet syndrome are refractory forms of epilepsy frequently involving severe developmental delays, such that more subtle effects on cognition and perception, for example, are likely to go unnoticed.
Although not FDA approved, three independent RCTs have been conducted on CBD therapy in adults with schizophrenia.[97–99] All three have shown a reduction in psychotic symptoms, but the measures which improved differed in each study. One study conducted an RCT of 88 participants with schizophrenia, randomizing participants to CBD 1000 mg/day or placebo for six weeks of treatment.[97] At the study’s end, patients who received CBD showed greater improvement in positive symptoms as measured by the PANSS (p = 0.019), but showed no significant differences in the other domains of the PANSS (0.133, 0.196, and 0.965). The CBD group showed greater improvement in cognition, as measured by the BACS composite score (p = 0.068), and particularly in motor speed. Finally, a higher proportion (78.6% vs 54.6%) of patients receiving CBD were scored by their physicians as being “improved” on the CGI-I, compared to placebo (p = 0.018). A similar six-week study compared 36 stable participants with schizophrenia in an RCT, randomizing them to CBD 600 mg/day vs placebo.[98] The PANSS total score showed a significant decrease over time (p <0.0001), but there was no significant drug × time interaction (p = 0.18). The MCCB Composite score showed no main effect from drug or time, but there was a significant drug × time effect (p = 0.02). The last study was a four-week double-blind RCT of CBD versus amisulpride in 42 participants with schizophrenia.[99] The dose was started at 200 mg per day and increased stepwise by 200 mg per day up to 800 mg per day. Both groups showed significant improvement from baseline in the PANSS, but there were no significant differences between the two (p = 0.884). Non-inferiority of CBD could not be demonstrated (p = 0.27). CBD patients did however have significantly fewer symptoms of EPS, less weight gain, and lower prolactin increase.
Few studies have examined the therapeutic potential of cannabinoids for the treatment of psychiatric symptoms. A recent meta-analysis and systematic review found insufficient evidence to suggest that cannabinoids improve depressive or anxiety symptoms or disorders, as well as other psychiatric disorders including attention-deficit hyperactivity disorder, Tourette syndrome, post-traumatic stress disorder, and psychosis.[91] Cannabis products may indeed have viable therapeutic potential, and existing clinical trials of CB are encouraging in adults with psychotic disorders.[97, 100] However, there remains inadequate evidence for their use in treating psychopathology. High-quality studies directly examining the effect of cannabinoids on treating mental disorders are needed, and the effects of exogenous cannabinoid administration in pediatric populations remain unknown. Therefore, until further research is available, caution in exposing adolescents and pediatric patients to cannabinoids remains warranted.
Treatments for Adolescent Cannabis Use Disorder
While cannabis derived products are increasingly used and investigated for therapeutic use, few effective treatments for cannabis use disorder are available to adolescents. Substance use treatment rates for youth who meet criteria for cannabis (or other substance) use disorder are very low (approximately 5%).[101] Importantly, cannabis use often co-occurs with anxiety and depression in youth, and treating these conditions may be helpful in minimizing cannabis use.[102] The majority of regular cannabis users remain unsuccessful at changing use patterns in the absence of treatment.[103] To date, no Food and Drug Administration approved pharmacotherapies are available for treating cannabis use disorder. Psychotherapy including motivational enhancement therapy (MET), cognitive behavioral therapy (CBT), and contingency management are the mainstay of treatment for cannabis misuse.[104] Randomized controlled trials, open-label trials, case-series, and case reports have been reported. Here we summarize evidence-based treatments for cannabis use disorder in adolescence that are supported by randomized controlled trials.
Behavioral Therapy.
Behavioral therapies are the mainstay of treating cannabis use disorder in adolescence. Motivational enhancement therapy using educational feedback has been shown to significantly lower cannabis use in a study of approximately 300 non-treatment seeking adolescents at 3 month (21% vs. 8%), but not at 12 month (16% vs 9%) follow-up.[105] Studies comparing the efficacy of CBT and multidimensional therapy (MDT) found comparable decreases in adolescent cannabis use, with approximately 30% reduction in use by 1 year follow up for both interventions. Older adolescents and those with co-occurring psychiatric disorders were significantly more likely to benefit from CBT, whereas younger adolescents were more likely to benefit from MDT.[106] A recent study demonstrated that Approach-Avoidance Training designed to reduce automatic approach bias for cannabis-related cues was effective in reducing cannabis use in 80 non-treatment-seeking adolescents.[107]
Available psychotherapies for cannabis use disorder in adolescence have modest effect sizes in improving abstinence rates, i.e., the primary outcome measure, however the majority of patients in these studies returned to using cannabis within 6–12 months.[101, 108] A greater number of studies have demonstrated reductions in use patterns, which may be a better clinical endpoint for treatment of cannabis misuse in adolescence than abstinence.
Pharmacotherapy.
A randomized 8 week double-blinded, placebo-controlled clinical trial of 116 cannabis dependent adolescents found that those randomized to receive N-acetylcysteine (NAC) demonstrated a significantly greater likelihood of negative urine toxicology versus placebo (41% versus 27%). While NAC is thought to alter glutamate transmission and reduce oxidative stress, the mechanism by which it may lower cannabis use is not well understood, and abstinence was not sustained at 4-week follow up.[109]
In a randomized double-blinded, placebo-controlled pilot study in adolescents, 66 heavy cannabis users were randomized to MET + topiramate versus MET plus placebo. Adolescents in both treatment arms demonstrated significant reductions in frequency of cannabis use during study participation, with no significant advantage of topiramate. Although the overall amount of cannabis that participants smoked was significantly less in the topiramate group, topiramate was poorly tolerated. Less than half of those randomized to topiramate completed the 6-week trial due to adverse medication side effects.[54]
In a randomized double-blind placebo-controlled study, 70 adolescents with major depressive disorder and cannabis use disorder were randomized to a 12-week course of MET and CBT plus fluoxetine or placebo. Although fluoxetine was well tolerated, it did not demonstrate greater efficacy than placebo with respect to reduction in depressive or cannabis-use related symptoms.[110]
While no trials have been conducted in adolescents, a 12 week randomized, double-blind, placebo-controlled trial of Gabapentin found statistically significant reductions in frequency and amount of cannabis use, as well as reduction in craving and symptoms of withdrawal in 50 adults with cannabis use disorder.[111]
Remaining issues in addressing adolescent cannabis use.
No consensus yet defines clinically meaningful reductions in adolescent cannabis use. Key challenges include heterogeneity by which substance use treatment outcomes are measured and reported across studies and limited studies that include both self-report and biological assays to assess change in use. The majority of studies have used self-report measures of cannabis use which presents several challenges, such as obtaining accurate measurements of exposure and route of administration. Improved clinical and functional outcome measures such as assessing change in clinical symptom severity using a dimensional versus categorical DSM or ICD diagnostic approach, as well as assessing ‘functional’ quality of life measures will assist in determining meaningful therapeutic effect in future treatment trials. Further, urine assays of cannabinoid levels are currently the only biological assay that have been employed in treatment trials.[104] Future research that incorporates objective measures of cannabis (e.g. plasma, saliva assays), as well as measures of exposure such as potency (THC) and changes in endocannabinoid levels would provide a more comprehensive understanding of the treatment induced changes with respect to changes in cannabis use and in the endocannabinoid system and help inform treatment development. Integrating neurobiological markers into the design of clinical trials may advance mechanistic understanding of how treatments work and identify focal treatment targets for cannabis misuse in adolescence.
Conclusions
In recent years, we have observed an increase in youth presenting for consultation and treatment for mood and psychotic disorders in the context of regular high potency cannabis use.[58] Teens report growing popularity of such use among their peers, and the ease of access to which they are able to obtain high potency cannabis preparations. This is consistent with what is being reported in states that have legalized marijuana, that have seen a 25% increase in problematic cannabis use by adolescents relative to states that have not legalized marijuana.[112] Recent surveys have demonstrated a steep rise in the rate of adolescent vaping of cannabis products, and a decline in public perception of harms associated with regular cannabis use.[14] Moreover, cannabinoid products are increasingly used to ‘self-medicate’ psychiatric symptoms despite available evidence demonstrating that they are ineffective for this indication and can worsen psychiatric symptoms.[91]
The endocannabinoid system (ECS) serves an integral role in regulating neurodevelopment of reward and stress systems during adolescence.[19, 20] Thus, adolescence is a developmental period that is particularly sensitive to perturbations induced by cannabis exposure.[18] Acutely, THC activates CB1R resulting in increased DA synthesis contributing to an increase in perceived pleasure. With chronic use, however, CB1Rs are downregulated and endocannabinoid levels are reduced[21] thereby impairing sensitivity to reward and stress.[24] CBD interacts with cannabinoid receptors are complex, with discrepancies between in vitro and in vivo studies. Studies suggest the CBD has a low affinity for CB1 receptors, [30, 31] however recent evidence has found that CBD also acts as a negative allosteric modulator of CB1R, and may reduce the potency and efficacy of CB1 agonists. [32–34] This interaction is posited to explain the role of CBD in attenuating or ameliorating the psychoactive adverse effects of THC.[35]
In addition, available diagnostic tools designed to assist in the assessment of cannabis and other substance use disorders may not adequately capture use patterns, including alternative means of cannabis use, such as vaping. Improved diagnostic tools are needed to better capture contemporary problematic substance use and their psychiatric sequelae including longitudinal assessment for progression toward co-occurring disorders in youth. In the context of decreased perceived risk of cannabis use, improved knowledge of the biological effects of cannabis use on neurodevelopment is key, especially with regard to high potency cannabis use. This will assist in identifying novel targets for future prevention and intervention research. We have found that patients often present with severe depressive symptoms, and occasionally psychosis or mania, in the context of regular use of high-potency cannabis. Vaping cannabis products are also increasing in popularity,[113], with approximately 1 in 10 adolescents reporting vaping cannabis.[3] This is concerning given our limited understanding of the effects of vaping high potency cannabis preparations in adolescence. As clinicians, it is important that we continue to educate our patients on the nuances associated with cannabis potency and use, and why cannabis and other substance use is particularly harmful during critical periods of development when the brain is particularly vulnerable to the negative effects of cannabis use.
Scientific research and public awareness of various cannabis preparations, potencies, and risk of psychiatric sequelae are needed due to increased availability, prevalence, and shifting perceptions of cannabis use. Studies to date have characterized the effects of cannabis according to self-report measures or acute effects of much lower potency preparations in adult samples. Future research is needed to examine the neurobiological effects of cannabis exposure, with objective measures of potency, and over the course of pediatric development and transitions into adulthood. The increase in cannabis potency and reliance on self-report assessments that do not incorporate quantifiable measures of potency may contribute to our limited understanding of the complex relation between the effects of cannabis on developing neurobiological symptoms modulated by the endocannabinoid system and psychiatric symptom onset and severity in adolescence. Future work examining biological effects of increasingly potent cannabis exposure in adolescence will help identify ‘bottom up’ neurobiologically informed treatment targets, as well as inform public policy and prevention to mitigate access to high potency cannabis and develop other harm reduction approaches.
Human and Animal Rights and Informed Consent.
This article does not contain any studies with human or animal subjects performed by any of the authors.
Funding
This work was supported by the Klingenstein Third Generation Foundation to ASF and the National Institute of Mental Health T32 MH019938 to ASF and R01MH106581 to MKS.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of Interest
Dr. Singh receives research support from Stanford’s Maternal Child Health Research Institute and Department of Psychiatry, National Institute of Mental Health, National Institute of Aging, Allergan, Johnson and Johnson, and the Brain and Behavior Research Foundation. She is on the advisory board for Sunovion and is a consultant for Google X and Limbix and receives royalties from the American Psychiatric Association Publishing.
Dr. Fischer received grants from the Klingenstein Third Generation Foundation.
Drs. Louie, Tapert, and Schatzberg report no conflicts of interest.
References
- 1.(WHO) WHO. WHO Substance Abuse Facts and Figures: Cannabis 2018. [Available from: http://www.who.int/substance_abuse/facts/cannabis/en/.
- 2.(NIDA) NIoDA. Marijuana 2018. [cited 2018 11/28/2018]. Available from: https://www.drugabuse.gov/drugs-abuse/marijuana.
- 3**.Johnston LD, O’Malley PM, Miech RA, Bachman JG, Schulenberg JE Monitoring the Future National Survey Results on Drug Use, 1975–2017: Overview, Key Findings on Adolescent Drug Use.2017. Available from: https://deepblue.lib.umich.edu/bitstream/handle/2027.42/142406/Overview%202017%20FINAL.pdf?sequence=1&isAllowed=y. [Google Scholar]; This overview highlights findings from a longitudinal annual survey that documents trends in increased use of cannabis along with declining perception of risk among United States Youth.
- 4.Chadwick B, Miller ML, Hurd YL. Cannabis Use during Adolescent Development: Susceptibility to Psychiatric Illness. Front Psychiatry. 2013;4:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Administration. SAaMHS. Results from the 2010 National Survey on Drug Use and Health: Summary of National Findings.. Rockville, MD: HHS Publication; 2011: p. 11–4658. [Google Scholar]
- 6.Wu LT, Zhu H, Swartz MS. Trends in cannabis use disorders among racial/ethnic population groups in the United States. Drug Alcohol Depend. 2016;165:181–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marconi A, Di Forti M, Lewis CM, Murray RM, Vassos E. Meta-analysis of the Association Between the Level of Cannabis Use and Risk of Psychosis. Schizophr Bull. 2016;42(5):1262–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8**.Gobbi G, Atkin T, Zytynski T, Wang S, Askari S, Boruff J, et al. Association of Cannabis Use in Adolescence and Risk of Depression, Anxiety, and Suicidality in Young Adulthood: A Systematic Review and Meta-analysis. JAMA Psychiatry. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]; A meta-analysis assessing the association between adolescent cannabis use and risk of depressive disorder, suicidal ideation and suicide attempts, and anxiety disorders in youth.
- 9.Volkow ND, Compton WM, Weiss SR. Adverse health effects of marijuana use. N Engl J Med. 2014;371(9):879. [DOI] [PubMed] [Google Scholar]
- 10.Smart R, Caulkins JP, Kilmer B, Davenport S, Midgette G. Variation in cannabis potency and prices in a newly legal market: evidence from 30 million cannabis sales in Washington state. Addiction. 2017;112(12):2167–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11**.ElSohly MA, Mehmedic Z, Foster S, Gon C, Chandra S, Church JC. Changes in Cannabis Potency Over the Last 2 Decades (1995–2014): Analysis of Current Data in the United States. Biol Psychiatry. 2016;79(7):613–9. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study examined change in cannabinoid [including delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD)] content of illicit cannabis confiscated by the Drug Enforcement Administration (DEA) over the last 2 decades. Findings from this study highlight the marked increase in potency (i.e. THC content) and decrease in CBD in contemporary cannabis products.
- 12.Stogner JM, Miller BL. Assessing the Dangers of “Dabbing”: Mere Marijuana or Harmful New Trend? Pediatrics. 2015;136(1):1–3. [DOI] [PubMed] [Google Scholar]
- 13.Wackowski OA, Sontag JM, Hammond D, O’Connor RJ, Ohman-Strickland PA, Strasser AA, et al. The Impact of E-Cigarette Warnings, Warning Themes and Inclusion of Relative Harm Statements on Young Adults’ E-Cigarette Perceptions and Use Intentions. Int J Environ Res Public Health. 2019;16(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hulvershorn LA. Editorial: Understanding the Child at Risk for Substance Use Disorders: Neuroimaging Addiction Risk. J Am Acad Child Adolesc Psychiatry. 2019;58(7):663–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prince MA, Conner BT, Pearson MR. Quantifying cannabis: A field study of marijuana quantity estimation. Psychol Addict Behav. 2018;32(4):426–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Casajuana C, Lopez-Pelayo H, Balcells MM, Miquel L, Colom J, Gual A. Definitions of Risky and Problematic Cannabis Use: A Systematic Review. Subst Use Misuse. 2016;51(13):1760–70. [DOI] [PubMed] [Google Scholar]
- 17.NIDA. 2016. [Available from: https://www-drugabusegov.laneproxy.stanford.edu/researchers/research-resources/nida-drug-supply-program-dsp/marijuana-plant-material-available-nida-drug-supply-program.
- 18.Keeley RJ, Trow J, McDonald RJ. Strain and sex differences in puberty onset and the effects of THC administration on weight gain and brain volumes. Neuroscience. 2015;305:328–42. [DOI] [PubMed] [Google Scholar]
- 19.Meyer HC, Lee FS, Gee DG. The Role of the Endocannabinoid System and Genetic Variation in Adolescent Brain Development. Neuropsychopharmacology. 2018;43(1):21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Volkow ND, Swanson JM, Evins AE, DeLisi LE, Meier MH, Gonzalez R, et al. Effects of Cannabis Use on Human Behavior, Including Cognition, Motivation, and Psychosis: A Review. JAMA Psychiatry. 2016;73(3):292–7. [DOI] [PubMed] [Google Scholar]
- 21*.Volkow ND, Hampson AJ, Baler RD. Don’t Worry, Be Happy: Endocannabinoids and Cannabis at the Intersection of Stress and Reward. Annu Rev Pharmacol Toxicol. 2017;57:285–308. [DOI] [PubMed] [Google Scholar]; This review summarizes the complex physiology of the endocannabinoid system and the effects of exogenous THC administration, with a particular focus on brain reward and stress circuitry.
- 22.Dudok B, Barna L, Ledri M, Szabo SI, Szabadits E, Pinter B, et al. Cell-specific STORM super-resolution imaging reveals nanoscale organization of cannabinoid signaling. Nat Neurosci. 2015;18(1):75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khajehali E, Malone DT, Glass M, Sexton PM, Christopoulos A, Leach K. Biased Agonism and Biased Allosteric Modulation at the CB1 Cannabinoid Receptor. Mol Pharmacol. 2015;88(2):368–79. [DOI] [PubMed] [Google Scholar]
- 24.Ceccarini J, Kuepper R, Kemels D, van Os J, Henquet C, Van Laere K. [18F]MK-9470 PET measurement of cannabinoid CB1 receptor availability in chronic cannabis users. Addict Biol. 2015;20(2):357–67. [DOI] [PubMed] [Google Scholar]
- 25.Gorzalka BB, Hill MN. Putative role of endocannabinoid signaling in the etiology of depression and actions of antidepressants. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(7):1575–85. [DOI] [PubMed] [Google Scholar]
- 26.Shen CJ, Zheng D, Li KX, Yang JM, Pan HQ, Yu XD, et al. Cannabinoid CB1 receptors in the amygdalar cholecystokinin glutamatergic afferents to nucleus accumbens modulate depressive-like behavior. Nat Med. 2019;25(2):337–49. [DOI] [PubMed] [Google Scholar]
- 27.Christensen R, Kristensen PK, Bartels EM, Bliddal H, Astrup A. Efficacy and safety of the weight-loss drug rimonabant: a meta-analysis of randomised trials. Lancet. 2007;370(9600):1706–13. [DOI] [PubMed] [Google Scholar]
- 28.Curran HV, Freeman TP, Mokrysz C, Lewis DA, Morgan CJ, Parsons LH. Keep off the grass? Cannabis, cognition and addiction. Nat Rev Neurosci. 2016;17(5):293–306. [DOI] [PubMed] [Google Scholar]
- 29.Rubino T, Vigano D, Premoli F, Castiglioni C, Bianchessi S, Zippel R, et al. Changes in the expression of G protein-coupled receptor kinases and beta-arrestins in mouse brain during cannabinoid tolerance: a role for RAS-ERK cascade. Mol Neurobiol. 2006;33(3):199–213. [DOI] [PubMed] [Google Scholar]
- 30.Schonhofen P, Bristot IJ, Crippa JA, Hallak JEC, Zuardi AW, Parsons RB, et al. Cannabinoid-Based Therapies and Brain Development: Potential Harmful Effect of Early Modulation of the Endocannabinoid System. CNS Drugs. 2018;32(8):697–712. [DOI] [PubMed] [Google Scholar]
- 31.Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9-tetrahydrocannabinol, cannabidiol and delta9tetrahydrocannabivarin. Br J Pharmacol. 2008;153(2):199–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laprairie RB, Bagher AM, Kelly ME, Denovan-Wright EM. Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. Br J Pharmacol. 2015;172(20):4790–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tham M, Yilmaz O, Alaverdashvili M, Kelly MEM, Denovan-Wright EM, Laprairie RB. Allosteric and orthosteric pharmacology of cannabidiol and cannabidioldimethylheptyl at the type 1 and type 2 cannabinoid receptors. Br J Pharmacol. 2019;176(10):1455–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McPartland JM, Duncan M, Di Marzo V, Pertwee RG. Are cannabidiol and Delta(9) -tetrahydrocannabivarin negative modulators of the endocannabinoid system? A systematic review. Br J Pharmacol. 2015;172(3):737–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pisanti S, Malfitano AM, Ciaglia E, Lamberti A, Ranieri R, Cuomo G, et al. Cannabidiol: State of the art and new challenges for therapeutic applications. Pharmacol Ther. 2017;175:133–50. [DOI] [PubMed] [Google Scholar]
- 36.Ibeas Bih C, Chen T, Nunn AV, Bazelot M, Dallas M, Whalley BJ. Molecular Targets of Cannabidiol in Neurological Disorders. Neurotherapeutics. 2015;12(4):699–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gee DG, Fetcho RN, Jing D, Li A, Glatt CE, Drysdale AT, et al. Individual differences in frontolimbic circuitry and anxiety emerge with adolescent changes in endocannabinoid signaling across species. Proc Natl Acad Sci U S A. 2016;113(16):4500–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gray JM, Vecchiarelli HA, Morena M, Lee TT, Hermanson DJ, Kim AB, et al. Corticotropin-releasing hormone drives anandamide hydrolysis in the amygdala to promote anxiety. J Neurosci. 2015;35(9):3879–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hill MN, McLaughlin RJ, Morrish AC, Viau V, Floresco SB, Hillard CJ, et al. Suppression of amygdalar endocannabinoid signaling by stress contributes to activation of the hypothalamic-pituitary-adrenal axis. Neuropsychopharmacology. 2009;34(13):2733–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morena M, Patel S, Bains JS, Hill MN. Neurobiological Interactions Between Stress and the Endocannabinoid System. Neuropsychopharmacology. 2016;41(1):80–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heng L, Beverley JA, Steiner H, Tseng KY. Differential developmental trajectories for CB1 cannabinoid receptor expression in limbic/associative and sensorimotor cortical areas. Synapse. 2011;65(4):278–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cyr M, Tau GZ, Fontaine M, Levin FR, Marsh R. Deficient Functioning of Frontostriatal Circuits During the Resolution of Cognitive Conflict in Cannabis-Using Youth. J Am Acad Child Adolesc Psychiatry. 2019;58(7):702–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lichenstein SD, Musselman S, Shaw DS, Sitnick S, Forbes EE. Nucleus accumbens functional connectivity at age 20 is associated with trajectory of adolescent cannabis use and predicts psychosocial functioning in young adulthood. Addiction. 2017;112(11):1961–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Robinson TE, Berridge KC. Incentive-sensitization and addiction. Addiction. 2001;96(1):103–14. [DOI] [PubMed] [Google Scholar]
- 45.Filbey FM. Weeding Through Marijuana’s Effects on the Brain. JAMA Psychiatry. 2016;73(8):773–4. [DOI] [PubMed] [Google Scholar]
- 46.Blest-Hopley G, Giampietro V, Bhattacharyya S. Residual effects of cannabis use in adolescent and adult brains - A meta-analysis of fMRI studies. Neurosci Biobehav Rev. 2018;88:26–41. [DOI] [PubMed] [Google Scholar]
- 47.Hurd YL, Manzoni OJ, Pletnikov MV, Lee FS, Bhattacharyya S, Melis M. Cannabis and the Developing Brain: Insights into Its Long-Lasting Effects. J Neurosci. 2019;39(42):8250–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sarvet AL, Wall MM, Keyes KM, Olfson M, Cerda M, Hasin DS. Self-medication of mood and anxiety disorders with marijuana: Higher in states with medical marijuana laws. Drug Alcohol Depend. 2018;186:10–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Caouette JD, Feldstein Ewing SW. Four Mechanistic Models of Peer Influence on Adolescent Cannabis Use. Curr Addict Rep. 2017;4(2):90–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Volkow ND, Baler RD, Compton WM, Weiss SR. Adverse health effects of marijuana use. N Engl J Med. 2014;370(23):2219–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen CY, Storr CL, Anthony JC. Early-onset drug use and risk for drug dependence problems. Addict Behav. 2009;34(3):319–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gray KM, Carpenter MJ, Baker NL, DeSantis SM, Kryway E, Hartwell KJ, et al. A double-blind randomized controlled trial of N-acetylcysteine in cannabis-dependent adolescents. Am J Psychiatry. 2012;169(8):805–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.SAMHSA. Treatment Episode Data Set (TEDS): 2000–2010 National Admissions to Substance Abuse Treatment Services. DASIS Series S-61. Administration SAaMHS (ed). Substance Abuse and Mental Health Services Administration, Rockville, MD: 2012. [Google Scholar]
- 54.Miranda R Jr., Treloar H, Blanchard A, Justus A, Monti PM, Chun T, et al. Topiramate and motivational enhancement therapy for cannabis use among youth: a randomized placebo-controlled pilot study. Addict Biol. 2017;22(3):779–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Silins E, Horwood LJ, Patton GC, Fergusson DM, Olsson CA, Hutchinson DM, et al. Young adult sequelae of adolescent cannabis use: an integrative analysis. Lancet Psychiatry. 2014;1(4):286–93. [DOI] [PubMed] [Google Scholar]
- 56.Bachhuber MA, Saloner B, Cunningham CO, Barry CL. Medical cannabis laws and opioid analgesic overdose mortality in the United States, 1999–2010. JAMA Intern Med. 2014;174(10):1668–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shover CL, Davis CS, Gordon SC, Humphreys K. Association between medical cannabis laws and opioid overdose mortality has reversed over time. Proc Natl Acad Sci U S A. 2019;116(26):12624–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58*.Leadbeater BJ, Ames ME, Linden-Carmichael AN. Age-varying effects of cannabis use frequency and disorder on symptoms of psychosis, depression and anxiety in adolescents and adults. Addiction. 2019;114(2):278–93. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study examined the relation between cannabis use frequency in adolescence and psychiatric symptoms using data from the Victoria Health Youth Survey (V-HYS). More frequent cannabis use lead to greater depressive symptoms in adolescence, greater psychotic symptoms in young adulthood, and no significant change in anxiety symptoms.
- 59.National Center for Injury Prevention and Control CfDCaP. 10 leading causes of death by age group, United States 2019. [Available from: https://www-cdcgov.laneproxy.stanford.edu/injury/images/lccharts/leading_causes_of_death_age_group_2015_1050w740h.gif
- 60.Patton GC, Coffey C, Carlin JB, Degenhardt L, Lynskey M, Hall W. Cannabis use and mental health in young people: cohort study. BMJ. 2002;325(7374):1195–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hser YI, Mooney LJ, Huang D, Zhu Y, Tomko RL, McClure E, et al. Reductions in cannabis use are associated with improvements in anxiety, depression, and sleep quality, but not quality of life. J Subst Abuse Treat. 2017;81:53–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Network DAW. national estimates of drug-related emergency department visits. Rockville, MD: Substance Abuse and Mental Health Services Administration;. 2011. [Google Scholar]
- 63*.Wang GS, Davies SD, Halmo LS, Sass A, Mistry RD. Impact of Marijuana Legalization in Colorado on Adolescent Emergency and Urgent Care Visits. J Adolesc Health. 2018;63(2):239–41. [DOI] [PubMed] [Google Scholar]; This retrospective review of marijuana-related emergency and urgent care visits by adolescents before and after legalization of marijuana in Colorado demonstrated significant increases in marijuana-related visits following commercialization of medicinal and recreational marijuana. The most common ICD codes were cannabis use, depression, and unspecified mood disorder.
- 64.Mustonen A, Niemela S, Nordstrom T, Murray GK, Maki P, Jaaskelainen E, et al. Adolescent cannabis use, baseline prodromal symptoms and the risk of psychosis. Br J Psychiatry. 2018;212(4):227–33. [DOI] [PubMed] [Google Scholar]
- 65.Di Forti M, Sallis H, Allegri F, Trotta A, Ferraro L, Stilo SA, et al. Daily use, especially of high-potency cannabis, drives the earlier onset of psychosis in cannabis users. Schizophr Bull. 2014;40(6):1509–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Moore TH, Zammit S, Lingford-Hughes A, Barnes TR, Jones PB, Burke M, et al. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet. 2007;370(9584):319–28. [DOI] [PubMed] [Google Scholar]
- 67.Broyd SJ, van Hell HH, Beale C, Yucel M, Solowij N. Acute and Chronic Effects of Cannabinoids on Human Cognition-A Systematic Review. Biol Psychiatry. 2016;79(7):557–67. [DOI] [PubMed] [Google Scholar]
- 68.Dougherty DM, Mathias CW, Dawes MA, Furr RM, Charles NE, Liguori A, et al. Impulsivity, attention, memory, and decision-making among adolescent marijuana users. Psychopharmacology (Berl). 2013;226(2):307–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Harvey MA, Sellman JD, Porter RJ, Frampton CM. The relationship between non-acute adolescent cannabis use and cognition. Drug Alcohol Rev. 2007;26(3):309–19. [DOI] [PubMed] [Google Scholar]
- 70.Tait RJ, Mackinnon A, Christensen H. Cannabis use and cognitive function: 8-year trajectory in a young adult cohort. Addiction. 2011;106(12):2195–203. [DOI] [PubMed] [Google Scholar]
- 71.Tapert SF, Granholm E, Leedy NG, Brown SA. Substance use and withdrawal: neuropsychological functioning over 8 years in youth. J Int Neuropsychol Soc. 2002;8(7):873–83. [DOI] [PubMed] [Google Scholar]
- 72.Jacobus J, Bava S, Cohen-Zion M, Mahmood O, Tapert SF. Functional consequences of marijuana use in adolescents. Pharmacol Biochem Behav. 2009;92(4):559–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lubman DI, Cheetham A, Yucel M. Cannabis and adolescent brain development. Pharmacol Ther. 2015;148:1–16. [DOI] [PubMed] [Google Scholar]
- 74.Meier MH, Caspi A, Ambler A, Harrington H, Houts R, Keefe RS, et al. Persistent cannabis users show neuropsychological decline from childhood to midlife. Proc Natl Acad Sci U S A. 2012;109(40):E2657–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Solowij N, Jones KA, Rozman ME, Davis SM, Ciarrochi J, Heaven PC, et al. Verbal learning and memory in adolescent cannabis users, alcohol users and non-users. Psychopharmacology (Berl). 2011;216(1):131–44. [DOI] [PubMed] [Google Scholar]
- 76.Schierenbeck T, Riemann D, Berger M, Hornyak M. Effect of illicit recreational drugs upon sleep: cocaine, ecstasy and marijuana. Sleep Med Rev. 2008;12(5):381–9. [DOI] [PubMed] [Google Scholar]
- 77.Mike TB, Shaw DS, Forbes EE, Sitnick SL, Hasler BP. The hazards of bad sleep-Sleep duration and quality as predictors of adolescent alcohol and cannabis use. Drug Alcohol Depend. 2016;168:335–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ogeil RP, Cheetham A, Mooney A, Allen NB, Schwartz O, Byrne ML, et al. Early adolescent drinking and cannabis use predicts later sleep-quality problems. Psychol Addict Behav. 2019;33(3):266–73. [DOI] [PubMed] [Google Scholar]
- 79.Wong MM, Robertson GC, Dyson RB. Prospective relationship between poor sleep and substance-related problems in a national sample of adolescents. Alcohol Clin Exp Res. 2015;39(2):355–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Waters KA, Suresh S, Nixon GM. Sleep disorders in children. Med J Aust. 2013;199(8):S31–5. [DOI] [PubMed] [Google Scholar]
- 81.O’Malley PM, Johnston LD. Driving after drug or alcohol use by US high school seniors, 2001–2011. Am J Public Health. 2013;103(11):2027–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Brady JE, Li G. Trends in alcohol and other drugs detected in fatally injured drivers in the United States, 1999–2010. Am J Epidemiol. 2014;179(6):692–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lenne MG, Dietze PM, Triggs TJ, Walmsley S, Murphy B, Redman JR. The effects of cannabis and alcohol on simulated arterial driving: Influences of driving experience and task demand. Accid Anal Prev. 2010;42(3):859–66. [DOI] [PubMed] [Google Scholar]
- 84.Rogeberg O, Elvik R. The effects of cannabis intoxication on motor vehicle collision revisited and revised. Addiction. 2016;111(8):1348–59. [DOI] [PubMed] [Google Scholar]
- 85.Brands B, Mann RE, Wickens CM, Sproule B, Stoduto G, Sayer GS, et al. Acute and residual effects of smoked cannabis: Impact on driving speed and lateral control, heart rate, and self-reported drug effects. Drug Alcohol Depend. 2019;205:107641. [DOI] [PubMed] [Google Scholar]
- 86.Hartman RL, Brown TL, Milavetz G, Spurgin A, Pierce RS, Gorelick DA, et al. Cannabis effects on driving lateral control with and without alcohol. Drug Alcohol Depend. 2015;154:25–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Buckley L, Chapman RL, Sheehan M. Young driver distraction: state of the evidence and directions for behavior change programs. J Adolesc Health. 2014;54(5 Suppl):S16–21. [DOI] [PubMed] [Google Scholar]
- 88.Ogourtsova T, Kalaba M, Gelinas I, Korner-Bitensky N, Ware MA. Cannabis use and driving-related performance in young recreational users: a within-subject randomized clinical trial. CMAJ Open. 2018;6(4):E453–E62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bonn-Miller MO, Loflin MJE, Thomas BF, Marcu JP, Hyke T, Vandrey R. Labeling Accuracy of Cannabidiol Extracts Sold Online. JAMA. 2017;318(17):1708–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mead A The legal status of cannabis (marijuana) and cannabidiol (CBD) under U.S. law. Epilepsy Behav. 2017;70(Pt B):288–91. [DOI] [PubMed] [Google Scholar]
- 91.Black N, Stockings E, Campbell G, Tran LT, Zagic D, Hall WD, et al. Cannabinoids for the treatment of mental disorders and symptoms of mental disorders: a systematic review and meta-analysis. Lancet Psychiatry. 2019;6(12):995–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lexicomp I Nabiximols Drug Information 2019 [Topic 9457 Version 111.0:[Available from: https://www-uptodatehttps://www-uptodate-com.laneproxy.stanford.edu/contents/nabiximols:druginformationcom.laneproxy.stanford.edu/contents/nabiximols:druginformation.
- 93.MacDonald E, Adams A. The Use of Medical Cannabis with Other Medications: A Review of Safety and Guidelines - An Update. CADTH Rapid Response Reports. Ottawa (ON) 2019. [PubMed] [Google Scholar]
- 94.Devinsky O, Cross JH, Laux L, Marsh E, Miller I, Nabbout R, et al. Trial of Cannabidiol for Drug-Resistant Seizures in the Dravet Syndrome. N Engl J Med. 2017;376(21):2011–20. [DOI] [PubMed] [Google Scholar]
- 95.Devinsky O, Patel AD, Cross JH, Villanueva V, Wirrell EC, Privitera M, et al. Effect of Cannabidiol on Drop Seizures in the Lennox-Gastaut Syndrome. N Engl J Med. 2018;378(20):1888–97. [DOI] [PubMed] [Google Scholar]
- 96.Biosciences G EPIDIOLEX (Cannabidiol) Oral Solution Prescribing Information. Carlsbad, CA: 2018. [Google Scholar]
- 97.McGuire P, Robson P, Cubala WJ, Vasile D, Morrison PD, Barron R, et al. Cannabidiol (CBD) as an Adjunctive Therapy in Schizophrenia: A Multicenter Randomized Controlled Trial. Am J Psychiatry. 2018;175(3):225–31. [DOI] [PubMed] [Google Scholar]
- 98.Boggs DL, Nguyen JD, Morgenson D, Taffe MA, Ranganathan M. Clinical and Preclinical Evidence for Functional Interactions of Cannabidiol and Delta(9)Tetrahydrocannabinol. Neuropsychopharmacology. 2018;43(1):142–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Leweke FM, Piomelli D, Pahlisch F, Muhl D, Gerth CW, Hoyer C, et al. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl Psychiatry. 2012;2:e94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Boggs DL, Surti T, Gupta A, Gupta S, Niciu M, Pittman B, et al. The effects of cannabidiol (CBD) on cognition and symptoms in outpatients with chronic schizophrenia a randomized placebo controlled trial. Psychopharmacology (Berl). 2018;235(7):1923–32. [DOI] [PubMed] [Google Scholar]
- 101.Squeglia LM, Fadus MC, McClure EA, Tomko RL, Gray KM. Pharmacological Treatment of Youth Substance Use Disorders. J Child Adolesc Psychopharmacol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kaminer Y, Connor DF, Curry JF. Comorbid adolescent substance use and major depressive disorders: a review. Psychiatry (Edgmont). 2007;4(12):32–43. [PMC free article] [PubMed] [Google Scholar]
- 103.Hughes JR, Naud S, Budney AJ, Fingar JR, Callas PW. Environmental cues and attempts to change in daily cannabis users: An intensive longitudinal study. Drug Alcohol Depend. 2016;161:15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Brezing CA, Levin FR. The Current State of Pharmacological Treatments for Cannabis Use Disorder and Withdrawal. Neuropsychopharmacology. 2018;43(1):173–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Walker DD, Stephens R, Roffman R, Demarce J, Lozano B, Towe S, et al. Randomized controlled trial of motivational enhancement therapy with nontreatment-seeking adolescent cannabis users: a further test of the teen marijuana check-up. Psychol Addict Behav. 2011;25(3):474–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hendriks V, van der Schee E, Blanken P. Treatment of adolescents with a cannabis use disorder: main findings of a randomized controlled trial comparing multidimensional family therapy and cognitive behavioral therapy in The Netherlands. Drug Alcohol Depend. 2011;119(1–2):64–71. [DOI] [PubMed] [Google Scholar]
- 107.Jacobus J, Taylor CT, Gray KM, Meredith LR, Porter AM, Li I, et al. A multi-site proof-of-concept investigation of computerized approach-avoidance training in adolescent cannabis users. Drug Alcohol Depend. 2018;187:195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Silvers JA, Squeglia LM, Romer Thomsen K, Hudson KA, Feldstein Ewing SW. Hunting for What Works: Adolescents in Addiction Treatment. Alcohol Clin Exp Res. 2019;43(4):578–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tomko RL, Gilmore AK, Gray KM. The role of depressive symptoms in treatment of adolescent cannabis use disorder with N-Acetylcysteine. Addict Behav. 2018;85:26–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cornelius JR, Bukstein OG, Douaihy AB, Clark DB, Chung TA, Daley DC, et al. Double-blind fluoxetine trial in comorbid MDD-CUD youth and young adults. Drug Alcohol Depend. 2010;112(1–2):39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mason BJ, Crean R, Goodell V, Light JM, Quello S, Shadan F, et al. A proof-ofconcept randomized controlled study of gabapentin: effects on cannabis use, withdrawal and executive function deficits in cannabis-dependent adults. Neuropsychopharmacology. 2012;37(7):1689–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cerda M, Mauro C, Hamilton A, Levy NS, Santaella-Tenorio J, Hasin D, et al. Association Between Recreational Marijuana Legalization in the United States and Changes in Marijuana Use and Cannabis Use Disorder From 2008 to 2016. JAMA Psychiatry. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kowitt SD, Osman A, Meernik C, Zarkin GA, Ranney LM, Martin J, et al. Vaping cannabis among adolescents: prevalence and associations with tobacco use from a cross-sectional study in the USA. BMJ Open. 2019;9(6):e028535. [DOI] [PMC free article] [PubMed] [Google Scholar]


