Abstract
von Hippel–Lindau protein (pVHL) is the tumor suppressor responsible for ubiquitylating the hypoxia‐inducible factor (HIF) family of transcription factors for degradation under normoxic conditions. There are two major pVHL isoforms with the shorter isoform (pVHL19) lacking the acidic domain present in the N‐terminus of the longer isoform (pVHL30). Although both isoforms can degrade HIF and suppress tumor formation in experimental systems, previous research suggests that pVHL30 can undergo posttranslational modifications (PTM) and interact with unique proteins. Indeed, pVHL30 has long been observed to migrate as two species on a reducing polyacrylamide gel, indicating the presence of an uncharacterized PTM on the slower‐migrating pVHL30 without an identifiable biological consequence. Thus, there has been considerable effort to elucidate the exclusive biological activity of pVHL30, if any, by first defining the unique features of the slower‐migrating species. We show here that the migration of pVHL30, but not pVHL19, is retarded by 4‐(2‐aminoethyl)benzenesulfonyl fluoride hydrochloride (AEBSF), an irreversible serine protease inhibitor commonly found in protease inhibitor cocktails.
Keywords: AEBSF, gel electrophoresis, hypoxia‐inducible factor (HIF), isoform‐specific, serine protease inhibitor, von Hippel–Lindau (VHL)
1. INTRODUCTION
von Hippel–Lindau protein (pVHL) is the substrate‐conferring F‐box component of an E3 ubiquitin ligase complex. The best characterized target of this complex is the alpha subunit of the hypoxia‐inducible factor (HIF) transcription factor. Under normoxic conditions, HIFα is rapidly hydroxylated by prolyl hydroxylase (PHD) enzymes, which use molecular oxygen as a co‐substrate. 1 Hydroxylated HIFα is then bound by pVHL and targeted for degradation via the ubiquitin‐proteasome pathway. 2 , 3 Under hypoxic conditions, HIFα escapes recognition via pVHL and is consequently stabilized to dimerize with HIFβ to form a functional transcription factor that positively regulates factors governing cellular response and adaptation to hypoxia such as glycolysis, erythropoiesis and vasculogenesis. Mutations in the components of the HIF pathway, including pVHL, PHD2, and HIF2α, underlie numerous cancerous and non‐cancerous pathologies. 4 , 5 , 6 , 7
There are two isoforms of pVHL. pVHL30 represents the full‐length amino acid chain (1–213) encoded by the VHL gene. pVHL19 is a shorter isoform that is translated from an internal methionine (54–213). 8 , 9 It is still unclear whether there is a functional significance to having two isoforms. Both pVHL30 and pVHL19 possess the alpha domain necessary for forming an E3 complex and the beta domain that mediates HIFα binding. 10 , 11 Further, it has been shown that both isoforms can regulate HIFα levels and suppress tumor growth. 8 , 9 The first 53 amino acids of pVHL30, absent in pVHL19, encode a repetitive stretch of acidic residues. On the basis of multiple sequence alignments, it appears that the long form of pVHL lacks the acidic domain in non‐mammalian species. 12 Within kingdom mammalia, different orders possess a different number of acidic repeats; primates (7–8 units), cingulata (5–6 units), artiodactyla (4–5 units), proboscidea (2–3 units), and lagomorpha (1 unit). 12
Several unique properties have been attributed to pVHL30 and, by extension, to this acidic domain. First, several studies have suggested that pVHL30 is localized primarily to the cytoplasm and pVHL19 to the nucleus. 8 , 13 Second, three serine residues (33, 38, 43) present in the acidic domain have been shown to be phosphorylated by casein kinase II (CK2). 14 Phosphorylation by CK2 is suggested to be important for pVHL30 tumor suppressor function. Third, pVHL30 but not pVHL19 has been shown to interact with p14/ARF, a tumor suppressor that positively regulated p53 by inhibiting E3 ubiquitin ligase MDM2. 12 , 15 These bodies of work suggest that the acidic domain of pVHL30 may be biologically active.
In previous publications, it is apparent that pVHL30 can migrate as a doublet on an acrylamide gel. 2 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 The nature of the slower‐migrating band is unknown. We hypothesized that the elucidation of the posttranslational modification (PTM) of pVHL30 may help shed light on a potentially unique function of pVHL30.
2. RESULTS
2.1. pVHL30, but not pVHL19, migrates as a doublet
In previous publications, 17 , 18 we employed an in vitro transcription and translation (IVTT) system where rabbit reticulocyte lysate is used to produce HA‐pVHL30. The programmed rabbit reticulocyte is then incubated with biotinylated HIF1αOH peptides immobilized on streptavidin agarose beads. By pulling down on the beads, we can visualize levels of associated pVHL via western blotting. Curiously, we observed that a 10% input of HA‐pVHL30 migrated as a single species on 10% acrylamide gel while HA‐pVHL30 pulled down by HIF1αOH peptide migrated as a doublet (Figure 1a). In other experiments, we noted that HA‐pVHL19 does not migrate as a doublet when pulled down via HIF1αOH (Figure 1b). By conducting immunoprecipitation experiments with 3xFLAG‐pVHL30, we realized that this construct migrated as a singlet (Figure 1c). We considered multiple possibilities. One was that the HA tag promoted PTM of pVHL30. The second possibility was that the N‐terminal 3xFLAG tag, which is larger than an HA tag, prevented the PTM of pVHL30. To test the latter hypothesis directly, we cloned a C‐terminal 3xFLAG pVHL30. Indeed, this construct migrated as a doublet (Figure 1c). Further, when we added hydroxylated and unhydroxylated peptide to the pulldown buffer, we found no modulation in the proportion of the slower‐ and faster‐migrating species. These results suggested that the pVHL modification was independent of an interaction with HIFα (Figure 1c). To further interrogate the role of N‐terminal tags on the modification of the pVHL30 acidic domain, we cloned an untagged pVHL30 construct. Much like the HA‐tagged construct, untagged pVHL30 migrated as a doublet (Figure 1d).
FIGURE 1.
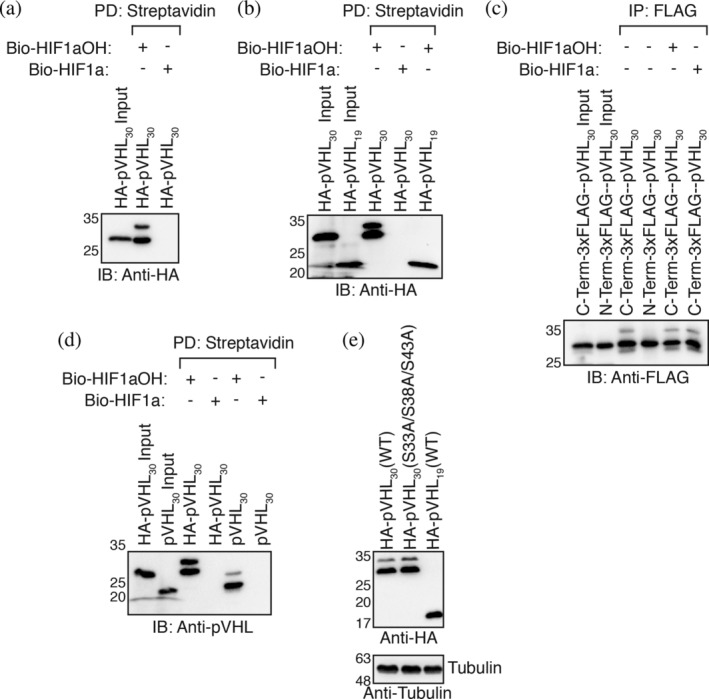
pVHL30 migrates as a doublet. (a, b, and d) Biotinylated HIF1α peptides were immobilized on streptavidin‐agarose beads and incubated with in vitro transcribed and translated (IVTT) pVHL in EBC buffer supplemented with protease inhibitors. Streptavidin beads were pulled down (PD), and levels of pVHL were visualized via immmunoblotting (IB). (c) IVTT pVHL was incubated in EBC buffer supplemented with protease inhibitors with or without HIF1α peptides. 3xFLAG‐pVHL was immunoprecipitated (IP) with an anti‐FLAG antibody, and levels of pVHL were visualized via IB. (e) HEK293a cells were transiently transfected HA‐pVHL constructs for 48 hr before being lysed in EBC buffer supplemented with protease inhibitors. Hundred micrograms of total protein was resolved via SDS‐PAGE, and levels of pVHL were visualized via immunoblotting
We observed a similar phenomenon when HEK293A cells were transiently transfected with either pVHL30 or pVHL19 (Figure 1e). Our first hypothesis was that the shift in apparent molecular weight may represent phosphorylation of serine residues 33, 38, and 43. 14 However, site‐directed mutagenesis of these three serine residues to alanine did not abolish the slower‐migrating band (Figure 1e). At this point in our investigation, we hypothesized that a novel PTM localized to the acidic domain of pVHL30 was being catalyzed by an active enzyme present in both rabbit reticulocyte lysate and HEK293A cells.
2.2. AEBSF retards pVHL30 migration
We further completed a series of biochemical experiments by omitting and altering certain conditions of the pVHL pulldown experiment. We found that the slower‐migrating band does not appear when the pulldown is conducted in a buffer with neutral pH (Figure S1). The slower‐migrating band becomes incrementally more prominent with increasing alkalinity of the pulldown buffer (Figure S1). Notably, pVHL30 migrated as a singlet when protease inhibitor cocktail was omitted from the pulldown buffer (Figure 2a). The addition of a protease inhibitor cocktail to the rabbit reticulocyte lysate yielded the slower‐migrating pVHL30 species when directly resolved on an acrylamide gel (Figure 2b). We hypothesized that the protease inhibitor cocktail may be inhibiting an enzyme that removes a PTM from pVHL30 or that the modified pVHL30 species is proteolytically degraded. However, further attempts to probe the conditions necessary for the modification of pVHL30 were hampered by the inability to modify the rabbit reticulocyte lysate without also directly affecting pVHL30 translation/stability. To circumvent this issue, we purified recombinant pVHL30 and pVHL19 in complex with elongin B and elongin C from BL21 Escherichia coli. pVHL19 migrated as a singlet even when incubated with protease inhibitor and rabbit reticulocyte lysate (Figure 2c). Conversely, pVHL30 was purified as a singlet but migrated as a doublet when incubated with protease inhibitor and rabbit reticulocyte (Figure 2c). This process was temperature dependent as the proportion of modified pVHL30 was increased when the incubation temperature was increased from 4°C to 30°C (Figure 2c). Unexpectedly, the presence of a protease inhibitor cocktail was both necessary and sufficient for the modification of pVHL30 (Figure 2c).
FIGURE 2.
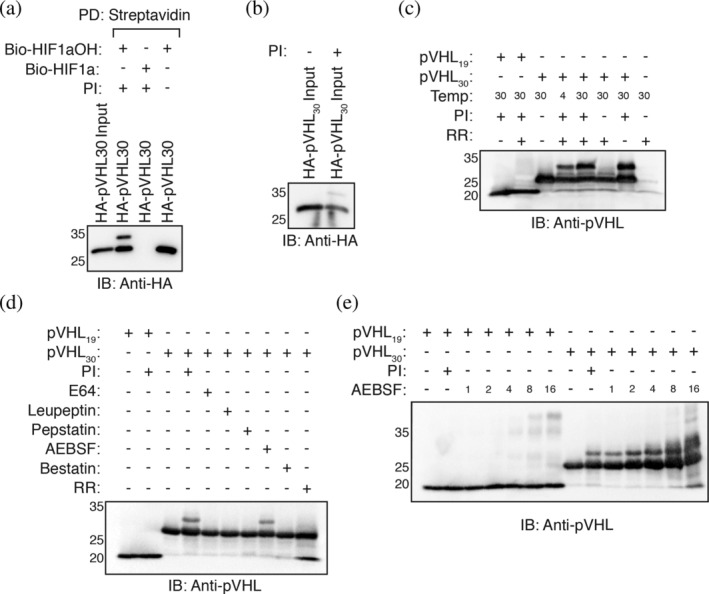
AEBSF covalently modifies pVHL30. (a) Biotinylated HIF1α peptides were immobilized on streptavidin‐ agarose beads and incubated with in vitro transcribed and translated (IVTT) pVHL in EBC buffer supplemented with or without protease inhibitors. Streptavidin beads were pulled down (PD), and levels of pVHL were visualized via immmunoblotting (IB). (b) Rabbit reticulocyte lystate was programmed with a HA‐pVHL30 construct in the presence or absence of protease inhibitors. Two microliter of rabbit reticulocyte lysate was resolved via SDS‐PAGE. (c–e) 2 μg of purified pVHL complex (pVHL19/pVHL30, elongin B, and elongin C) was incubated at 30°C for 2 hr with or without rabbit reticulocyte lysate and with or without protease inhibitors. One microgram of protein was resolved via SDS‐PAGE, and levels of pVHL were visualized via immunoblotting
We next screened individual protease inhibitors and observed that incubation with the serine protease inhibitor 4‐(2‐aminoethyl)benzenesulfonyl fluoride hydrochloride (AEBSF) alone is sufficient to obtain two migratory species of pVHL30 (Figure 2d). Incubation of pVHL30 with other protease inhibitors, including E64, leupeptin, bestatin, and pepstatin, did not induce a slower‐migratory shift in pVHL30 (Figure 2d). Under increasing concentrations of AEBSF, pVHL30 began to migrate in a laddering pattern (Figure 2e). pVHL19 also began to migrate as multiple species but only under higher concentrations of AEBSF. These results demonstrate that AEBSF covalently modifies pVHL30 at concentrations normally used in experiments. As AEBSF predominantly modifies serine and tyrosine residues, 27 and mutation of the three serine residues found in the acidic domain failed to prevent pVHL30 modification (Figure 1e), we hypothesized that AEBSF may be modifying the lone tyrosine residue found in the acidic domain (Tyr23). However, Y23F mutation did not prevent pVHL30 modification (Figure S2). The first 53 amino acids of pVHL30 lack lysine and histidine residues that are other possible sites of AEBSF modification. 27
We next aimed to identify the modified site by mass spectrometry. AEBSF‐modified pVHL30 was enzymatically digested using trypsin and samples were analyzed by LC–MS. As AEBSF treatment results in a modification with a calculated mass shift of 183.035, we used an open search with this mass shift as a potential modification on any amino acid, in addition to other typical post‐translational modifications (see Section 4). This resulted in the identification of 11 unique peptides, representing sequence coverage of approximately 50%. Based on the in silico tryptic digestion of pVHL30, the identified peptides correspond to the majority of predicted MS‐amenable peptides in the protein sequence. None of the identified peptides, however, mapped to the N‐terminal region of the protein. This was not surprising, considering that no tryptic cleavage site is present within the first 63 amino acid residues. Moreover, digestion with a different protease such as Glu‐C would be of little use considering that over one‐third (21/60) of the first 60 amino acids of the protein are glutamic acid residues. However, we did identify a peptide (IAHQ[+183]R) with a putative AEBSF‐modification near the C‐terminus of the protein (Figure S3). Interestingly, AEBSF appears to modify a glutamine residue at position 209 in this case. To the best of our knowledge, glutamine residues have not been reported to be covalently modified by AEBSF. As the C‐terminus region is common to both pVHL19 and pVHL30, this modification is unlikely to explain the slower migration seen specifically with pVHL30.
3. DISCUSSION
PTMs have long been recognized as an important means of regulating protein activity, localization, stability, and function. Numerous pVHL PTMs have been reported, including phosphorylation, 14 , 28 , 29 SUMOylation, 19 ubiquitylation, 30 neddylation, 16 , 31 and N‐terminal cleavage. 32 Of these potential PTMs, phosphorylation of serine residues 33, 38, and 43 and cleavage C‐terminal to Tyr23 are localized to the N‐terminal acidic domain unique to pVHL30. Thus, we were intrigued to study a slower migrating pVHL30 species (Figure 1a), especially once the potential modification was found to be localized to the acidic domain, and the possibility of serine phosphorylation was ruled out (Figure 1b,e). Experiments with both IVTT pVHL30 and purified pVHL30–elongin B–elongin C complex show that the slower migrating pVHL30 species is an AEBSF‐related artifact.
Other proteins, including amyloid beta, 33 transthyretin, 34 and the ζ of the T cell receptor, 33 , 34 , 35 have been found to be covalently modified by AEBSF. In the aforementioned cases, the modification by AEBSF was detected by mass spectrometry. In the case of the T cell receptor, the protein modification was not observable by SDS‐PAGE. Two‐dimensional electrophoresis studies of human plasma revealed that AEBSF alters the migration profile of several proteins. 27 AEBSF inhibits serine proteases through covalent modification of serine residues in the active site, thus forming a sulfonate ester. AEBSF has also been reported to modify serine residues in other chemical contexts in addition to tyrosine, histidine, lysine, and N‐terminal amine nucleophiles. 27 Interestingly, we identified a Gln209 as being covalently modified by AEBSF. This modification, however, is unlikely to cause the slower migration associated with pVHL30 as it also found in pVHL19. It is likely that modification of a residue unique to pVHL30 retards protein migration, but no such site was identified in our MS/MS experiment. It is also possible that the acidic domain of pVHL30 interacts with the core of the pVHL protein and activates a nucleophile that otherwise would not react with AEBSF.
AEBSF preferentially modifies active, nucleophilic serine residues, which has led to the development of AEBSF as an activity‐based probe (ABP) for serine proteases. 36 Interestingly, further use of AEBSF as an ABP uncovered the specific labeling of functional tyrosine residues in the active site of glutathione‐s‐transferase enzymes. 37 This work has been extended to designing ABPs that specifically react with specific tyrosine residues in the active site of the mRNA‐decapping scavenger enzyme DcpS. 38 Thus, AEBSF as ABP could be useful for specifically labeling pVHL30 and not pVHL19. However, this might be a challenge given that we found a residue common to both pVHL19 and pVHL30 that can react with AEBSF.
In addition to identifying a putitive and artifactual PTM of pVHL30, we uncovered several conditions that prevent this modification. The presence of modified pVHL30 is more pronounced under alkaline condition. This might explain why the pVHL30 doublet is not observable in experiments where buffer conditions are more neutral. 39 Further, the use of a N‐terminal 3xFLAG tag prevents modification of pVHL30 by AEBSF (Figure 1d). This observation suggests that the lengthy 3xFLAG tag interacts with the acidic domain. This notion is supported by the observation that intracellular truncation of the pVHL30 acidic domain, hypothesized to be catalyzed by chymotrypsin, is inhibited by N‐terminal HA and 3xFLAG tags. 32
Here, we show that the migration of pVHL30 is artifactually modified by the protease inhibitor AEBSF. This phenomenon is dependent on the first 53 amino acids unique to the longer isoform of pVHL. Since it is unknown whether the artifactual, slower‐migrating species of pVHL30 has or influences any biological activity, any investigation into the potential function of the acidic domain, which is predicted to be disordered, 12 should avoid the use of any N‐terminal tags.
4. MATERIALS AND METHODS
4.1. Plasmids
Construction of the following plasmids has been described previously; pcDNA3‐HA‐VHL30(WT), 40 pcDNA3‐3xFLAG‐VHL30(WT), pACYCDuet‐1 plasmid encoding untagged elongin B and elongin C(17–112), pGEX‐4T‐1‐GST‐VHL19. 41 pcDNA3‐HA‐VHL19(WT), pcDNA3‐VHL30(WT), and pGEX‐4T‐1‐GST‐VHL30 were subcloned from pcDNA3‐HA‐VHL30(WT). pcDNA3‐HA‐VHL30(S33A/S38A/S43A) was generated by site‐directed mutagenesis of pcDNA‐HA‐VHL30(WT). C‐terminal tagged pcDNA3‐3xFLAG‐VHL30(WT) was generated by amplifying VHL30 from pcDNA3‐HA‐VHL30(WT) with a reverse primer containing 3xFLAG and subcloning into an empty pcDNA3 vector.
4.2. Antibodies
Anti‐HA (C29F4; 1:2,000 dilution) was obtained from Cell Signaling Technology. Anti‐FLAG (F1804; 1:5,000) and Anti‐tubulin (T5168; 1:5,000) were obtained from Sigma‐Aldrich. Anti‐VHL (sc‐135657, 1:1,500) was obtained from Santa Cruz.
4.3. Peptides
HIF‐1α (556–564; DLDLEMLAPYIPMDDDFQL) peptides with N‐terminal biotinylation and C‐terminal amidation modifications were custom synthesized by Genscript. All peptides were reconstituted to 2 mg/mL, as measured by A280, using sterile DMSO, aliquoted, and stored at −80°C.
4.4. Protein expression and purification
BL21(DE3) E. coli cells were co‐transformed with a dual expression construct encoding untagged elongin B (full‐length, 1–118) and elongin C (17–112) and a construct encoding either N‐terminal GST‐tagged pVHL19 or N‐terminal GST‐tagged pVHL30. Bacterial cultures (1 L) were grown to an OD600 of approximately 0.6. Expression of the pVHL–elongin B–elongin C complex was induced via addition of IPTG (final concentration of 1 mM). Following induction, cells were grown for an additional 3.5 hr at a temperature of 37°C. Bacterial pellets were resuspended in 20 mM HEPES pH 7.4, 200 mM NaCl freshly supplemented with 10 mM DTT and lysed using a cell disruption unit at a pressure of 30 kPSI. Cell lysate was cleared via centrifugation (34,000g for 40 min). Cleared lysate was applied to a column of glutathione sepharose resin (GE Life Sciences). The column was washed with 20 mM HEPES pH 7.4, 200 mM NaCl and protein was eluted with 20 mM HEPES pH 7.4, 200 mM NaCl supplemented with 10 mM reduced glutathione. Protein solution was concentrated using a 4‐mL centrifugal concentrator with a molecular weight cut‐off of 3,500 Da (Pall Corporation). The concentrated protein solution was purified on a Superdex 200 10/300 size exclusion chromatography column equilibrated with 20 mM HEPES pH 7.4, 200 mM NaCl, and 1 mM DTT. The monomeric VBC complex was diluted to a concentration of 0.7 mg/mL. The GST‐tag was cleaved via incubation (60 hr) with 1 U thrombin per mg of VBC protein at 4°C. Free GST was removed by applying the protein solution to regenerated glutathione sepharose resin. The protein solution was concentrated as discussed above and size exclusion chromatography was employed to purify soluble, monomeric VBC complex. Purity was confirmed via SDS‐PAGE analysis. Aliquots of protein were frozen at −80°C at a concentration between 1 and 2 mg/mL.
4.5. In vitro binding assay
The in vitro pVHL‐HIF‐1α binding assay was performed according to a previously published protocol. 42 VHL constructs was expressed in a transcription and translation (TNT) rabbit reticulocyte lysate system (Promega, Cat. No. L1170) and incubated with 2 μg of biotinylated HIF‐1α peptide (hydroxylated or unhydroxylated), immobilized on streptavidin agarose beads, in either 500 μL of EBC buffer (50 mM Tris–HCl pH 8.0, 120 mM NaCl, 0.5% [v/v] NP‐40) supplemented with protease inhibitors for 2 hr at 4°C. Following incubation, beads were washed ×5 with NETN buffer (20 mM Tris–HCl pH 8.0, 100 mM NaCl, 1 mM EDTA, 0.5% [v/v] NP‐40). Biotinylated peptide was pulled down via streptavidin agarose beads and protein was eluted by boiling beads in sample buffer. Samples were resolved on a 10% acrylamide gel via SDS‐PAGE.
4.6. In vitro VHL modification assay
Two μg of purified pVHL–elongin B–elongin C was incubated in 20 μL of 50 mM Tris–HCl pH 8.0, 120 mM NaCl for 2 hr at 30°C. Two microliter of rabbit reticulocyte was added to indicated reaction conditions. For individual protease inhibitors, the following working concentrations were utilized; leupeptin (100 μM), pepstatin A (10 μM), E‐64 (10 μM), AEBSF (1 mM), bestatin hydrochloride (10 μM). After incubation, the reaction was stopped with sample buffer. Samples were resolved on a 10% acrylamide gel via SDS‐PAGE.
4.7. Cell culture and transfection
HEK293a cells were obtained from American Type Culture Collection (ATCC). Cells were maintained in DMEM (Invitrogen) supplemented with 10% (vol/vol) fetal bovine serum (Wisent) and were grown at 37°C in a humidified, 5% CO2 atmosphere. The indicated plasmids were transiently transfected into HEK293a cells using polyethylenimine.
4.8. Liquid chromatography–mass spectrometry
Purified pVHL was incubated with AEBSF as indicated above. To avoid inhibition of trypsin activity by excess AEBSF, samples were subjected to buffer exchange in Amicon Ultra 3 kDa MWCO columns (Millipore) using 50 mM NH4HCO3. Samples were next reduced with DTT (5 mM, 20 min, 60°C) and alkylated with iodoacetamide (10 mM, 30 min, room temperature, light protected). Protein digestion was performed with sequencing‐grade, TPCK‐treated, modified trypsin (Promega) for 16 hr at 37°C. The resulting peptide samples were desalted using C18 chromatography columns and lyophilized. Peptides were re‐suspended in 0.1% formic acid analyzed by LC–MS.
Liquid chromatography was conducted using a C18 pre‐column (Acclaim PepMap 100, 2 cm × 75 μm ID, Thermo Scientific) and a C18 analytical column (Acclaim PepMap RSLC, 50 cm × 75 μm ID, Thermo Scientific), running a 120 min reversed‐phase gradient (0–40% ACN in 0.1% formic acid) at 225 nL/min on an EASY‐nLC1200 pump (Thermo Scientific). Mass spectrometry was performed on a Q‐Exactive HF instrument (Thermo Scientific). An MS scan was performed with a resolution of 60,000 followed by up to 20 MS/MS scans (minimum ion count of 1,000 for activation) using higher energy collision induced dissociation (HCD) fragmentation. Dynamic exclusion was set for 5 s (10 ppm; exclusion list size = 500). Raw data was analyzed for identification of peptides and post‐translational modifications using the FragPipe platform. 43 Spectra were searched against the pVHL30 protein sequence. Search parameters specified a parent ion mass tolerance of 10 ppm, and an MS/MS fragment ion tolerance of 0.4 Da, with up to two missed cleavages allowed for trypsin (excluding K/RP). Variable modifications of 0.98 on N or Q, 15.99 on M, 57.02 on C and 183.04 (corresponding to the mass difference resulting from the AEBSF‐induced modification) on any amino acid were set in the search parameters. Spectra were manually inspected for quality assurance and confirmation of proper assignment of modifications.
AUTHOR CONTRIBUTIONS
Daniel Tarade: Conceptualization; data curation; formal analysis; investigation; methodology; supervision; validation; visualization; writing‐original draft; writing‐review and editing. Shelley He: Formal analysis; investigation; methodology; validation; visualization; writing‐review and editing. Jonathan St‐Germain: Data curation; formal analysis; investigation; methodology; validation; visualization; writing‐review and editing. Avi Petroff: Investigation; validation. Anya Murphy: Investigation. Brian Raught: Resources; supervision; writing‐review and editing. Michael Ohh: Conceptualization; formal analysis; funding acquisition; methodology; resources; supervision; writing‐original draft; writing‐review and editing.
Supporting information
Figure S1 Alkaline conditions promote covalent modification of pVHL30. Biotinylated HIF1α peptides were immobilized on streptavidin‐ agarose beads and incubated with in vitro transcribed and translated (IVTT) pVHL in EBC buffer supplemented with protease inhibitors. Streptavidin beads were pulled down (PD), and levels of pVHL were visualized via immmunoblotting (IB).
Figure S2 AEBSF does not modify pVHL30 Tyr23. HA‐pVHL30 was in vitro transcribed and translated (IVTT) and incubated with the indicated concentration of AEBSF for 2 hr at 30°C. Levels of pVHL were visualized via immmunoblotting (IB).
Figure S3 Covalent modification of pVHL Gln209 by AEBSF. Purified pVHL30 complex was incubated with or without AEBSF for 2 hr at 30°C. AEBSF was eliminated from the sample using an Amicon Ultra 3 kDa MWCO columns to buffer exchange. Protein was reduced (5 mM DTT) and alkylated (10 mM iodoacetamide) prior to incubation with trypsin for 16 hr at 37°C. Resultant peptides were desalted on a C18 column and lyophilized. Peptides were re‐suspended in 0.1% formic acid analyzed by LC–MS. Liquid chromatography was conducted using a C18 pre‐column and a C18 analytical column, running a 120 min reversed‐phase gradient (0–40% ACN in 0.1% formic acid) at 225 nL/min on an EASY‐nLC1200 pump. Mass spectrometry was performed on a Q‐Exactive HF instrument. An MS scan was performed with a resolution of 60,000 followed by up to 20 MS/MS scans (minimum ion count of 1,000 for activation) using higher energy collision induced dissociation (HCD) fragmentation. Dynamic exclusion was set for 5 s (10 ppm; exclusion list size = 500). Raw data was analyzed for identification of peptides and post‐translational modifications using the FragPipe platform. Spectra were searched against the pVHL30 protein sequence. Amino acid sequence of pVHL30 with AEBSF‐modified tryptic peptide highlighted is provided. Search parameters specified a parent ion mass tolerance of 10 ppm, and an MS/MS fragment ion tolerance of 0.4 Da, with up to two missed cleavages allowed for trypsin (excluding K/RP). Variable modifications of 0.98 on N or Q, 15.99 on M, 57.02 on C and 183.04 (corresponding to the mass difference resulting from the AEBSF‐induced modification) on any amino acid were set in the search parameters. Spectra were manually inspected for quality assurance and confirmation of proper assignment of modifications.
ACKNOWLEDGEMENTS
We thank the members of the Ohh lab for their helpful comments and discussions. S.H., A.P. and A.M. are recipients of the Undergraduate Research Opportunity Program Award at the University of Toronto. D.T. is a Vanier Scholar of the Canadian Institutes of Health Research (CIHR). This work was supported by funds from CIHR (PJT‐159773 to M.O.).
Tarade D, He S, St‐Germain J, et al. The long form of pVHL is artifactually modified by serine protease inhibitor AEBSF . Protein Science. 2020;29:1843–1850. 10.1002/pro.3898
Contributor Information
Daniel Tarade, Email: daniel.tarade@mail.utoronto.ca.
Michael Ohh, Email: michael.ohh@utoronto.ca.
REFERENCES
- 1. Epstein AC, Gleadle JM, McNeill LA, et al. C. elegans egl‐9 and mammalian homologs define a family of dioxygenases that regulate hif by prolyl hydroxylation. Cell. 2001;107:43–54. [DOI] [PubMed] [Google Scholar]
- 2. Ivan M, Kondo K, Yang H, et al. Hifalpha targeted for VHL‐mediated destruction by proline hydroxylation: Implications for o2 sensing. Science. 2001;292:464–468. [DOI] [PubMed] [Google Scholar]
- 3. Jaakkola P, Mole DR, Tian YM, et al. Targeting of HIF‐alpha to the von Hippel‐Lindau ubiquitylation complex by O2‐regulated prolyl hydroxylation. Science. 2001;292:468–472. [DOI] [PubMed] [Google Scholar]
- 4. Percy MJ, Furlow PW, Lucas GS, et al. A gain‐of‐function mutation in the hif2a gene in familial erythrocytosis. N Engl J Med. 2008;358:162–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhuang Z, Yang C, Lorenzo F, et al. Somatic hif2a gain‐of‐function mutations in paraganglioma with polycythemia. N Engl J Med. 2012;367:922–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ladroue C, Carcenac R, Leporrier M, et al. Phd2 mutation and congenital erythrocytosis with paraganglioma. N Engl J Med. 2008;359:2685–2692. [DOI] [PubMed] [Google Scholar]
- 7. Latif F, Tory K, Gnarra J, et al. Identification of the von Hippel‐Lindau disease tumor suppressor gene. Science. 1993;260:1317–1320. [DOI] [PubMed] [Google Scholar]
- 8. Iliopoulos O, Ohh M, Kaelin WG Jr. Pvhl19 is a biologically active product of the von Hippel‐Lindau gene arising from internal translation initiation. Proc Natl Acad Sci U S A. 1998;95:11661–11666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schoenfeld A, Davidowitz EJ, Burk RD. A second major native von Hippel‐Lindau gene product, initiated from an internal translation start site, functions as a tumor suppressor. Proc Natl Acad Sci U S A. 1998;95:8817–8822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stebbins CE, Kaelin WG Jr, Pavletich NP. Structure of the VHL‐ElonginC‐ElonginB complex: Implications for VHL tumor suppressor function. Science. 1999;284:455–461. [DOI] [PubMed] [Google Scholar]
- 11. Min JH, Yang H, Ivan M, Gertler F, Kaelin WG Jr, Pavletich NP. Structure of an hif‐1alpha‐pvhl complex: Hydroxyproline recognition in signaling. Science. 2002;296:1886–1889. [DOI] [PubMed] [Google Scholar]
- 12. Minervini G, Mazzotta GM, Masiero A, et al. Isoform‐specific interactions of the von Hippel‐Lindau tumor suppressor protein. Sci Rep. 2015;5:12605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hergovich A, Lisztwan J, Barry R, Ballschmieter P, Krek W. Regulation of microtubule stability by the von Hippel‐Lindau tumour suppressor protein pvhl. Nat Cell Biol. 2003;5:64–70. [DOI] [PubMed] [Google Scholar]
- 14. Lolkema MP, Gervais ML, Snijckers CM, et al. Tumor suppression by the von Hippel‐Lindau protein requires phosphorylation of the acidic domain. J Biol Chem. 2005;280:22205–22211. [DOI] [PubMed] [Google Scholar]
- 15. Lai Y, Song M, Hakala K, Weintraub ST, Shiio Y. Proteomic dissection of the von Hippel–Lindau (VHL) interactome. J Proteome Res. 2011;10:5175–5182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Russell RC, Ohh M. Nedd8 acts as a ‘molecular switch’ defining the functional selectivity of VHL. EMBO Rep. 2008;9:486–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tarade D, Robinson CM, Lee JE, Ohh M. Hif‐2α‐pvhl complex reveals broad genotype‐phenotype correlations in hif‐2α‐driven disease. Nat Commun. 2018;9:3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tarade D, Ohh M. The hif and other quandaries in VHL disease. Oncogene. 2018;37:139–147. [DOI] [PubMed] [Google Scholar]
- 19. Cai Q, Verma SC, Kumar P, Ma M, Robertson ES. Hypoxia inactivates the VHL tumor suppressor through PIASy‐mediated sumo modification. PLoS One. 2010;5:e9720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cai Q, Robertson ES. Ubiquitin/sumo modification regulates VHL protein stability and nucleocytoplasmic localization. PLoS One. 2010;5:e12636. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21. Yang H, Minamishima YA, Yan Q, et al. Pvhl acts as an adaptor to promote the inhibitory phosphorylation of the nf‐κb agonist card9 by ck2. Mol Cell. 2007;28:15–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lee SB, Frattini V, Bansal M, et al. An id2‐dependent mechanism for VHL inactivation in cancer. Nature. 2016;529:172–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yu X, Chen S, Hou P, Wang M, Chen Y, Guo D. Vhl negatively regulates sars coronavirus replication by modulating nsp16 ubiquitination and stability. Biochem Biophys Res Commun. 2015;459:270–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cho HK, Kim SY, Kim KH, Kim HH, Cheong J. Tumor suppressor protein VHL inhibits hedgehog–gli activation through suppression of gli1 nuclear localization. FEBS Lett. 2013;587:826–832. [DOI] [PubMed] [Google Scholar]
- 25. Yuen J, Cockman M, Sullivan M, et al. The VHL tumor suppressor inhibits expression of the igf1r and its loss induces igf1r upregulation in human clear cell renal carcinoma. Oncogene. 2007;26:6499–6508. [DOI] [PubMed] [Google Scholar]
- 26. Kim D, Choi Y, Han B, et al. Cancer cells promote survival through depletion of the von Hippel–Lindau tumor suppressor by protein crosslinking. Oncogene. 2011;30:4780–4790. [DOI] [PubMed] [Google Scholar]
- 27. Schuchard MD, Mehigh RJ, Cockrill SL, et al. Artifactual isoform profile modification following treatment of human plasma or serum with protease inhibitor, monitored by 2‐dimensional electrophoresis and mass spectrometry. Biotechniques. 2005;39:239–247. [DOI] [PubMed] [Google Scholar]
- 28. Ampofo E, Kietzmann T, Zimmer A, Jakupovic M, Montenarh M, Götz C. Phosphorylation of the von Hippel–Lindau protein (VHL) by protein kinase ck2 reduces its protein stability and affects p53 and hif‐1α mediated transcription. Intl J Biochem Cell Biol. 2010;42:1729–1735. [DOI] [PubMed] [Google Scholar]
- 29. Hergovich A, Lisztwan J, Thoma CR, Wirbelauer C, Barry RE, Krek W. Priming‐dependent phosphorylation and regulation of the tumor suppressor pvhl by glycogen synthase kinase 3. Mol Cell Biol. 2006;26:5784–5796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Metcalf J, Bradshaw P, Komosa M, Greer S, Meyn MS, Ohh M. K63‐ubiquitylation of VHL by socs1 mediates DNA double‐strand break repair. Oncogene. 2014;33:1055–1065. [DOI] [PubMed] [Google Scholar]
- 31. Stickle NH, Chung J, Klco JM, Hill RP, Kaelin WG Jr, Ohh M. Pvhl modification by nedd8 is required for fibronectin matrix assembly and suppression of tumor development. Mol Cell Biol. 2004;24:3251–3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. German P, Bai S, Liu X‐D, et al. Phosphorylation‐dependent cleavage regulates von Hippel Lindau proteostasis and function. Oncogene. 2016;35:4973–4980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Conboy JJ, Wood KG, Lame ME, Durham RA, Geoghegan KF. Modification of amyloid‐β (1–40) by a protease inhibitor creates risk of error in mass spectrometric quantitation of amyloid‐β (1–42). Analyt Biochem. 2008;382:147–149. [DOI] [PubMed] [Google Scholar]
- 34. Bergen HR III, Klug MG, Bolander ME, Muddiman DC. Informed use of proteolytic inhibitors in biomarker discovery. Rapid Commun Mass Spectrom. 2004;18:1001–1002. [DOI] [PubMed] [Google Scholar]
- 35. Sweeney B, Proudfoot K, Parton A, King L, Slocombe P, Perry M. Purification of the t‐cell receptor ζ‐chain: Covalent modification by 4‐(2‐aminoethyl)‐benzenesulfonyl fluoride. Anal Biochem. 1997;245:107–109. [DOI] [PubMed] [Google Scholar]
- 36. Shannon DA, Gu C, McLaughlin CJ, Kaiser M, van der Hoorn RA, Weerapana E. Sulfonyl fluoride analogues as activity‐based probes for serine proteases. Chembiochem. 2012;13:2327–2330. [DOI] [PubMed] [Google Scholar]
- 37. Gu C, Shannon DA, Colby T, et al. Chemical proteomics with sulfonyl fluoride probes reveals selective labeling of functional tyrosines in glutathione transferases. Chem Biol. 2013;20:541–548. [DOI] [PubMed] [Google Scholar]
- 38. Hett EC, Xu H, Geoghegan KF, et al. Rational targeting of active‐site tyrosine residues using sulfonyl fluoride probes. ACS Chem Biol. 2015;10:1094–1098. [DOI] [PubMed] [Google Scholar]
- 39. Guo J, Chakraborty AA, Liu P, et al. Pvhl suppresses kinase activity of akt in a proline‐hydroxylation–dependent manner. Science. 2016;353:929–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ohh M, Park CW, Ivan M, et al. Ubiquitination of hypoxia‐inducible factor requires direct binding to the beta‐domain of the von Hippel‐Lindau protein. Nat Cell Biol. 2000;2:423–427. [DOI] [PubMed] [Google Scholar]
- 41. Heir P, Srikumar T, Bikopoulos G, et al. Oxygen‐dependent regulation of erythropoietin receptor turnover and signaling. J Biol Chem. 2016;291:7357–7372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Heir P, Ohh M. Hydroxylation‐dependent interaction of substrates to the von hippel‐Lindau tumor suppressor protein (VHL). Tumor Microenviron Methods Protoc. 2016;1458:87–94. [DOI] [PubMed] [Google Scholar]
- 43. Kong AT, Leprevost FV, Avtonomov DM, Mellacheruvu D, Nesvizhskii AI. Msfragger: Ultrafast and comprehensive peptide identification in mass spectrometry–based proteomics. Nat Methods. 2017;14:513–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Alkaline conditions promote covalent modification of pVHL30. Biotinylated HIF1α peptides were immobilized on streptavidin‐ agarose beads and incubated with in vitro transcribed and translated (IVTT) pVHL in EBC buffer supplemented with protease inhibitors. Streptavidin beads were pulled down (PD), and levels of pVHL were visualized via immmunoblotting (IB).
Figure S2 AEBSF does not modify pVHL30 Tyr23. HA‐pVHL30 was in vitro transcribed and translated (IVTT) and incubated with the indicated concentration of AEBSF for 2 hr at 30°C. Levels of pVHL were visualized via immmunoblotting (IB).
Figure S3 Covalent modification of pVHL Gln209 by AEBSF. Purified pVHL30 complex was incubated with or without AEBSF for 2 hr at 30°C. AEBSF was eliminated from the sample using an Amicon Ultra 3 kDa MWCO columns to buffer exchange. Protein was reduced (5 mM DTT) and alkylated (10 mM iodoacetamide) prior to incubation with trypsin for 16 hr at 37°C. Resultant peptides were desalted on a C18 column and lyophilized. Peptides were re‐suspended in 0.1% formic acid analyzed by LC–MS. Liquid chromatography was conducted using a C18 pre‐column and a C18 analytical column, running a 120 min reversed‐phase gradient (0–40% ACN in 0.1% formic acid) at 225 nL/min on an EASY‐nLC1200 pump. Mass spectrometry was performed on a Q‐Exactive HF instrument. An MS scan was performed with a resolution of 60,000 followed by up to 20 MS/MS scans (minimum ion count of 1,000 for activation) using higher energy collision induced dissociation (HCD) fragmentation. Dynamic exclusion was set for 5 s (10 ppm; exclusion list size = 500). Raw data was analyzed for identification of peptides and post‐translational modifications using the FragPipe platform. Spectra were searched against the pVHL30 protein sequence. Amino acid sequence of pVHL30 with AEBSF‐modified tryptic peptide highlighted is provided. Search parameters specified a parent ion mass tolerance of 10 ppm, and an MS/MS fragment ion tolerance of 0.4 Da, with up to two missed cleavages allowed for trypsin (excluding K/RP). Variable modifications of 0.98 on N or Q, 15.99 on M, 57.02 on C and 183.04 (corresponding to the mass difference resulting from the AEBSF‐induced modification) on any amino acid were set in the search parameters. Spectra were manually inspected for quality assurance and confirmation of proper assignment of modifications.


