Abstract
Claudin-5 is a tight junction protein expressed in endothelial cells and in some epithelial cells. It has been shown as a marker in canine angiosarcoma; however, data on human mesenchymal tumors are limited. In this study, we examined claudin-5 in selected normal tissues, in 280 benign and malignant vascular tumors, and in 448 other epithelial, mesenchymal, and neuroectodermal tumors. Early human embryos showed limited claudin-5 expression in endothelia of large truncal vessels, in liver sinusoids, and in the epidermis. In adult human tissues, claudin-5 was widely present in the endothelia of vessels of different calibers. However, neovascular capillaries in carcinomas and other tumors were often negative. Claudin-5 was also present in many glandular and ductal epithelia, hair shafts, and glomerular podocytes. Capillary and cavernous hemangiomas and lymphangiomas generally showed endothelial positivity; however, many vessels, especially those with poorly formed lumina, were negative in juvenile capillary hemangiomas, and fewer vessels were highlighted in lobular capillary hemangiomas. Hemangioendotheliomas of retiform, kaposiform, epithelioid, and epithelioid sarcoma-like types showed positivity, the latter in a diffuse cytoplasmic manner. Most angiosarcomas (115 of 119) and Kaposi sarcomas (28 of 29) showed strong labeling, but rare cases only contained positive cytoplasmic dots. Claudin-5 was commonly present in carcinomas (except in sarcomatoid ones), but most tumors showed heterogenous labeling weaker than that in angiosarcomas. Seminomas and renal cell, hepatocellular, and signet ring cell carcinomas were negative. Among non-vascular mesenchymal tumors, biphasic synovial sarcoma was the only tumor to contain claudin-5-positive nonvascular elements. In hemangiopericytomas, glomus tumor, and melanomas, claudin-5 was expressed in endothelial cells only. Claudin-5 is a promising new marker for angiosarcomas and hemangioendotheliomas, but widespread expression in carcinomas and biphasic synovial sarcoma should be considered in the differential diagnosis and addressed with the use of an antibody panel including keratins, especially the more epithelial-specific AE1/AE3 and epithelial membrane antigen.
Keywords: claudin-5, angiosarcoma, tight junction protein, immunohistochemistry
Claudins are a family of cell junction proteins, and claudin-5 is a transmembrane tight junction (zonula occludens) protein contributing to epithelial and endothelial barrier function. It is expressed in various endothelia and in some, especially juxtaluminal, glandular and ductal epithelial cells.4,14–16 Claudin-5 is also required in cardiovascular development, and monoallelic loss of a chromosome 22 segment including claudin-5 locus causes velocardio-facial syndrome, including cardiac malformations.14
Although claudin-5 expression has been extensively examined in various carcinomas, especially those of the lung,6,13,17,19 there is limited information on claudin-5 expression in vascular tumors. In canine hemangiomas and (hem)angiosarcomas, claudin-5 was consistently expressed and found absent in all other benign mesenchymal tumors and sarcomas, including fibroblastic and smooth muscle tumors, hemangiopericytomas, and synovial sarcoma.5 A recent abstract suggested high specificity for claudin-5 for human vascular tumors, with no reactivity in sarcomas and carcinomas of different types, except pancreatic adenocarcinomas.20
In this study, we examined claudin-5 in human vascular tumors and found claudin-5 to be expressed in most angiosarcomas and hemangioendotheliomas: a highly sensitive marker for malignant vascular tumors. Other neoplasms, including epithelial, neuroectodermal, and nonendothelial, were also examined to assess the value of claudin-5 in the differential diagnosis of vascular tumors, especially angiosarcoma and hemangioendotheliomas.
MATERIALS AND METHODS
A range of normal human tissues and tumors (n = 727) derived from surgical pathology specimens were analyzed in this study. All angiosarcomas (n = 119) were previously verified as CD31 positive, and Kaposi (n = 29) sarcomas were verified as HHV8 positive. The tissues were arranged in multitumor blocks containing 5 to 50 samples. Immunostaining (especially in angiosarcomas) was examined semiquantitatively as the approximate percentage of positive cells. The location of immunostaining (membrane, cytoplasmic) was also recorded.
A rabbit polyclonal antibody to claudin-5 (Abcam, Cambridge, MA) was used in a dilution of 1:750. The immunostaining was performed with a Leica Bondmax immunostainer using the Bondmax detection system with diaminobenidizine as the chromogen. Only cases that showed positive vascular endothelial staining were considered valid. Another, mouse monoclonal claudin-5 IgG1 antibody (clone 4C3C2, Invitrogen, Camarillo, CA), diluted at 1:500, was used in parallel testing for selected vascular tumors with a similar protocol as described above.
Results from immunostains previously carried out and reported for ERG and CD31 from a previous study were available for comparative purposes. The technical details of those immunostains have been previously described.12 Von Willebrand factor (factor VIII-related antigen) and CD34 were not studied here because of their known heterogenous expression in vascular tumors, especially angiosarcoma.10
RESULTS
Normal Human Tissues
In an early first trimester human embryo, claudin-5 immunoreactivity was limited to the epidermis and endothelia of a large thin-walled vessel close to the developing spine, possibly inferior vena cava, and to hepatic sinusoids. Peripheral blood vessels were not detectable (Figs. 1A, B). In a late trimester human fetus, capillary endothelia in soft tissues were positive, whereas there was no positivity in the epidermis.
FIGURE 1.

Claudin-5 in normal human tissues. A, Embroynic epidermis is positive but mesenchymal elements are negative. B, A large embryonic truncal vessel shows endothelial claudin-5 positivity. C, Adult capillary endothelia in brain are claudin-5 positive, along with some cell processes. D, Adult renal glomerular podocytes and some tubular elements are claudin-5 positive.
In normal adult human tissues, claudin-5 was detected in the vascular endothelia of various calibers but was strongest in capillaries, such as those in the brain (Fig. 1C). The lymph node sinusal endothelium (retothelium) and high endothelial venules of reactive lymphoid tissue were also strongly positive. Lymphatics, for example, in the intestinal mucosae, typically showed faint, discontinuous labeling. In the skin, claudin-5 was expressed in the inner hair shaft epithelia in a membrane pattern and in some sweat glands that showed luminal staining. There was no detectable staining in the epidermis, except in round hollow structures, consistent with sweat gland orifices.
Epithelial positivity was present in especially apical and lateral membranes in the surface epithelia of the gastrointestinal tract: the stomach, small intestine, gallbladder, colon, and ducts of pancreas and some acinar lumina. In the liver, extravascular positivity was restricted to bile ducts that showed predominant luminal membrane staining. Prostate glandular epithelia and thyroid follicular epithelia were also positive (the latter weakly and variably). Renal glomeruli showed podocyte labeling, with positivity also in the glomerular capsular epithelium and some tubuli or ducts (Fig. 1D). Tonsillar crypt epithelia were positive, and surface squamous epithelia were variably (mostly basally) positive. Ductal epithelia of the breast showed delicate positivity. Monoclonal claudin-5 antibody studied for comparison showed similar endothelial staining but slightly lesser epithelial reactivity (negative in pancreatic acini and delicately positive in some renal tubules). There was no claudin-5 immunoreactivity beyond the endothelia in the parathyroid, (postmenopausal) ovarian stroma, and testis. No lymphoid or histiocytic elements were claudin-5 positive.
Vascular Tumors
Claudin-5 immunoreactivity in 280 vascular tumors has been summarized in Table 1. In hemangiomas, such as cavernous, venous, and intramuscular ones, the endothelial component was typically positive, and intravascular microthrombosis was also sometimes positive (Fig. 2A). In spindle cell hemangiomas, the endothelial cells were positive, and spindle cells were negative (Fig. 2B). An unusual hemangioma with a sieve-like pattern was strongly positive (Fig. 2C). However, juvenile capillary hemangiomas had variable labeling, with most cases containing only small numbers of vessels with positive endothelia (Fig. 2D). In particular, the primitive vessels with inconspicuous lumina were negative. Similarly partial, although somewhat greater, endothelial positivity was seen in lobular capillary hemangiomas.
TABLE 1.
Summary of Claudin-5 Expression in 280 Vascular Tumors
| Hemangioma | |
| Capillary | 14/14* |
| Cavernous | 4/4 |
| Intamuscular capillary | 7/7 |
| Juvenile | 5/5† |
| Spindle cell | 16/16 |
| Venous hemangioma | 4/4 |
| Verrucous | 1/1 |
| Lymphangioma | 7/7 |
| Hemangioendothelioma | |
| Kaposiform | 5/5 |
| Retiform | 10/10 |
| Epithelioid | 51/58 |
| Epithelioid sarcoma-like | 1/1‡ |
| Kaposi sarcoma | 28/29 |
| Angiosarcoma | 114/119 |
| ERG negative | 2/3 |
| ERG invalid | 3/3 |
| Location of claudin-5-negative angiosarcomas | |
| Deep soft tissue | 3 |
| Gastrointestinal | 1 |
| Lung metastasis | 1 |
Heterogenous staining in lobular capillary hemangiomas.
Positivity limited to a small number of vessels.
Prominent, diffuse cytoplasmic staining.
FIGURE 2.
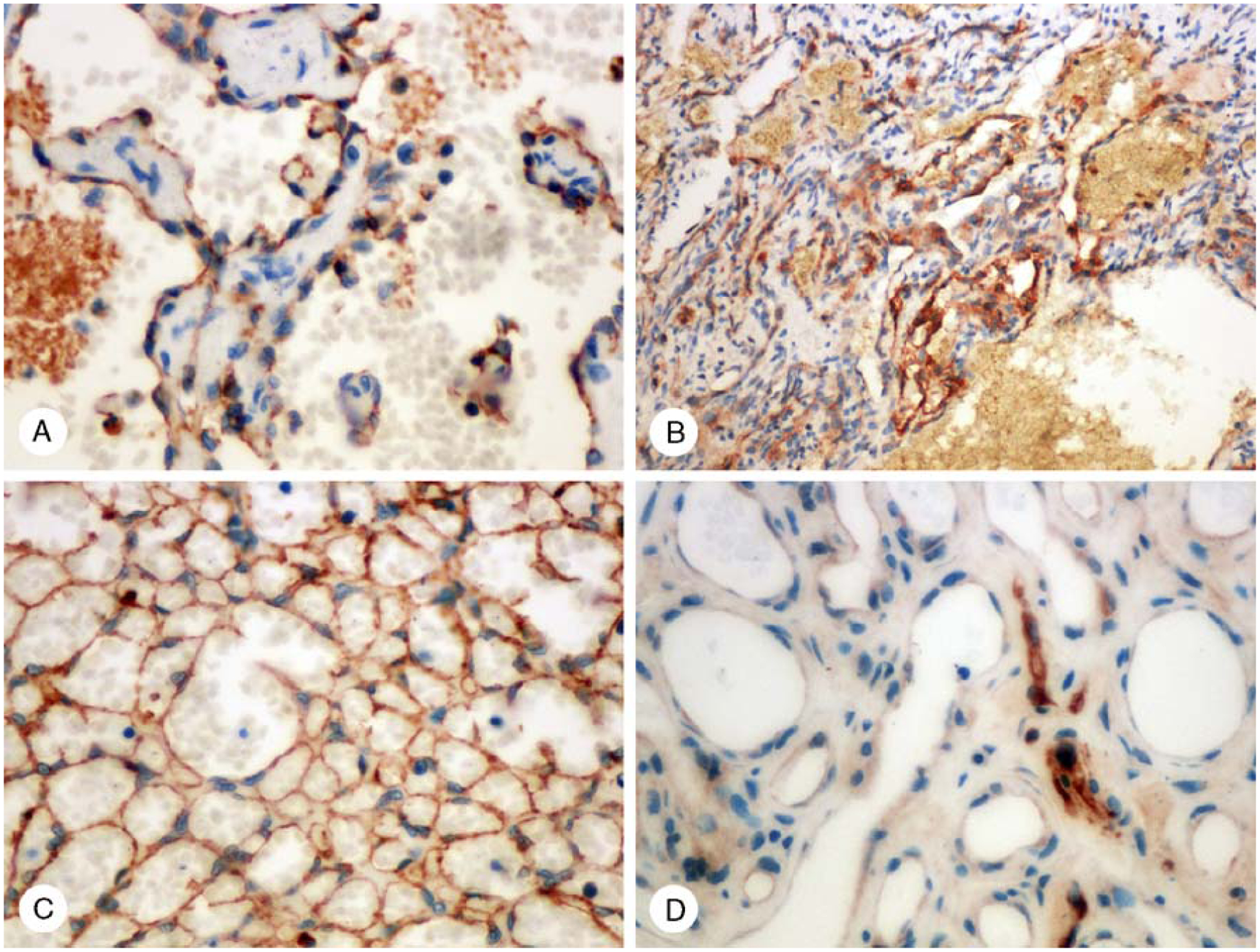
Claudin-5 in hemangiomas. A, Endothelial positivity in a capillary hemangioma. Some intraluminal positivity is also present. B, Spindle cell hemangioma contains claudin-5-positive capillaries, but the spindle cells are negative. C, A hemangioma with an unusual sieve-like pattern is claudin-5 positive. D, In juvenile capillary hemangioma, only a few vessels are claudin-5 positive.
The endothelial elements of retiform hemangioendothelioma were positive in all cases (Fig. 3A). A great majority of epithelioid hemangioendotheliomas showed positivity in tumor cells, but this varied from one region to another, with both membrane and cytoplasmic staining (Fig. 3B). In kaposiform hemangioendotheliomas, the endothelial elements showed immunoreactivity, and the pericytic spindle cells were negative (Fig. 3C). One epithelioid sarcoma-like hemangioendothelioma composed of irregular cords of discohesive epithelioid cells (also CD31 positive) showed prominent, diffuse cytoplasmic claudin-5 immunoreactivity (Fig. 3D).
FIGURE 3.
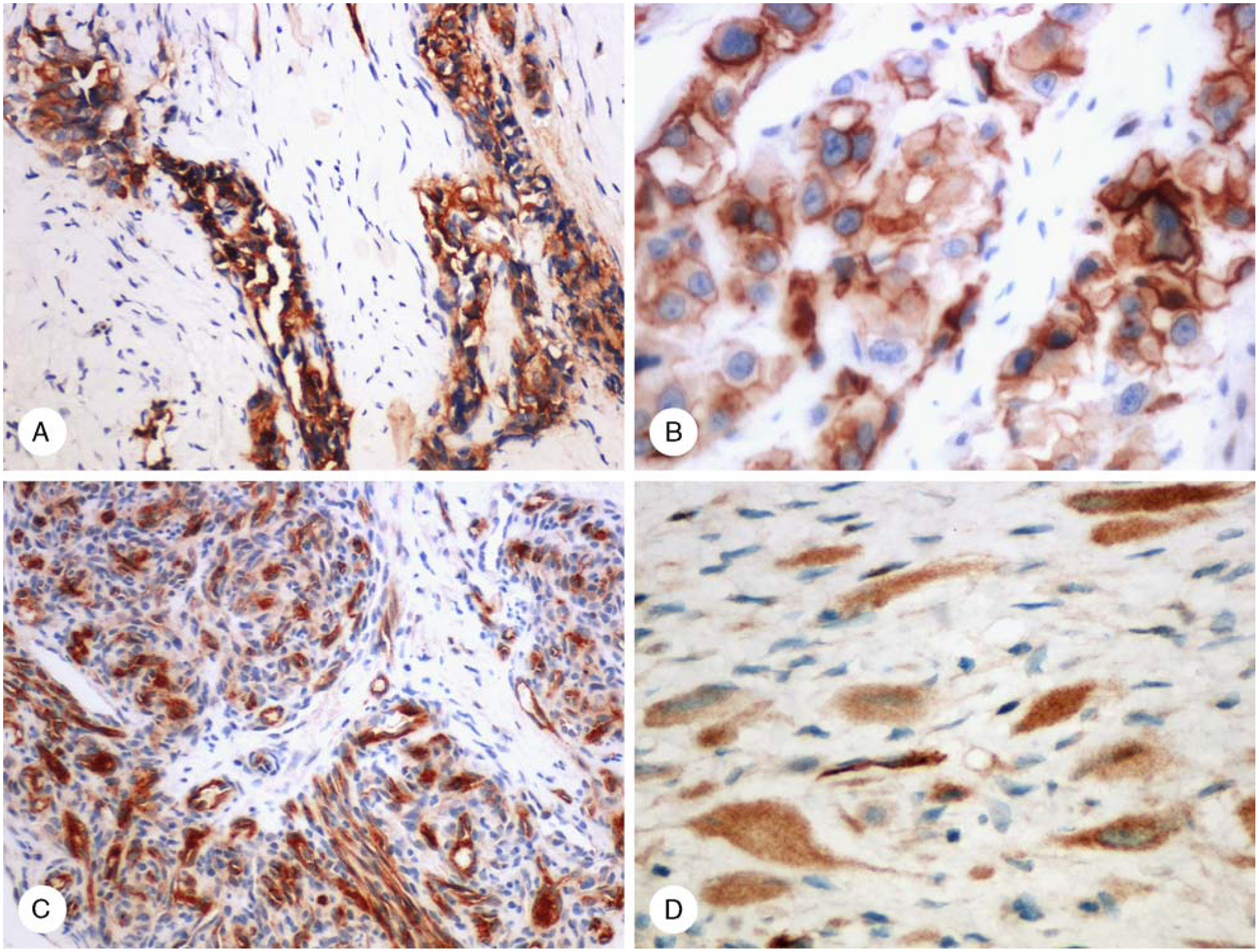
Claudin-5 positivity in vascular endothelia of hemangioendotheliomas. A, Retiform hemangioendothelioma. B, Epithelioid hemangioendothelioma. C, Kaposiform hemangioendothelioma contains positive endothelial and negative pericytic components. D, Epithelioid sarcoma-like hemangioendothelioma cells show diffuse cytoplasmic claudin-5 immunostaining.
All but 5 of 119 angiosarcomas (96%) showed claudin-5 positivity, which was typically strong and uniform throughout the tumor, irrespective of differentiation, including both vasoformative and solid areas (Fig. 4). In these strongly positive tumors, subcellular localization was difficult to determine, although some tumors showed membrane accentuation. Although epithelioid angiosarcomas were generally equally positive, 2 showed only trace positivity, including 1 example with focal dot-like cytoplasmic positivity. This tumor also showed membrane staining for CD31 and nuclear staining for ERG (Fig. 5). In all, only 3 angiosarcomas showed claudin-5 positivity in <10% of tumor cells, 12 cases showed positivity in 10% to 50% of tumor cells, whereas the remaining cases showed positivity in >50% of tumor cells, usually near to 100%. Slides from selected blocks containing angiosarcomas and hemangioendothelioma variants showed practically identical results with the mouse monoclonal antibody to claudin-5.
FIGURE 4.
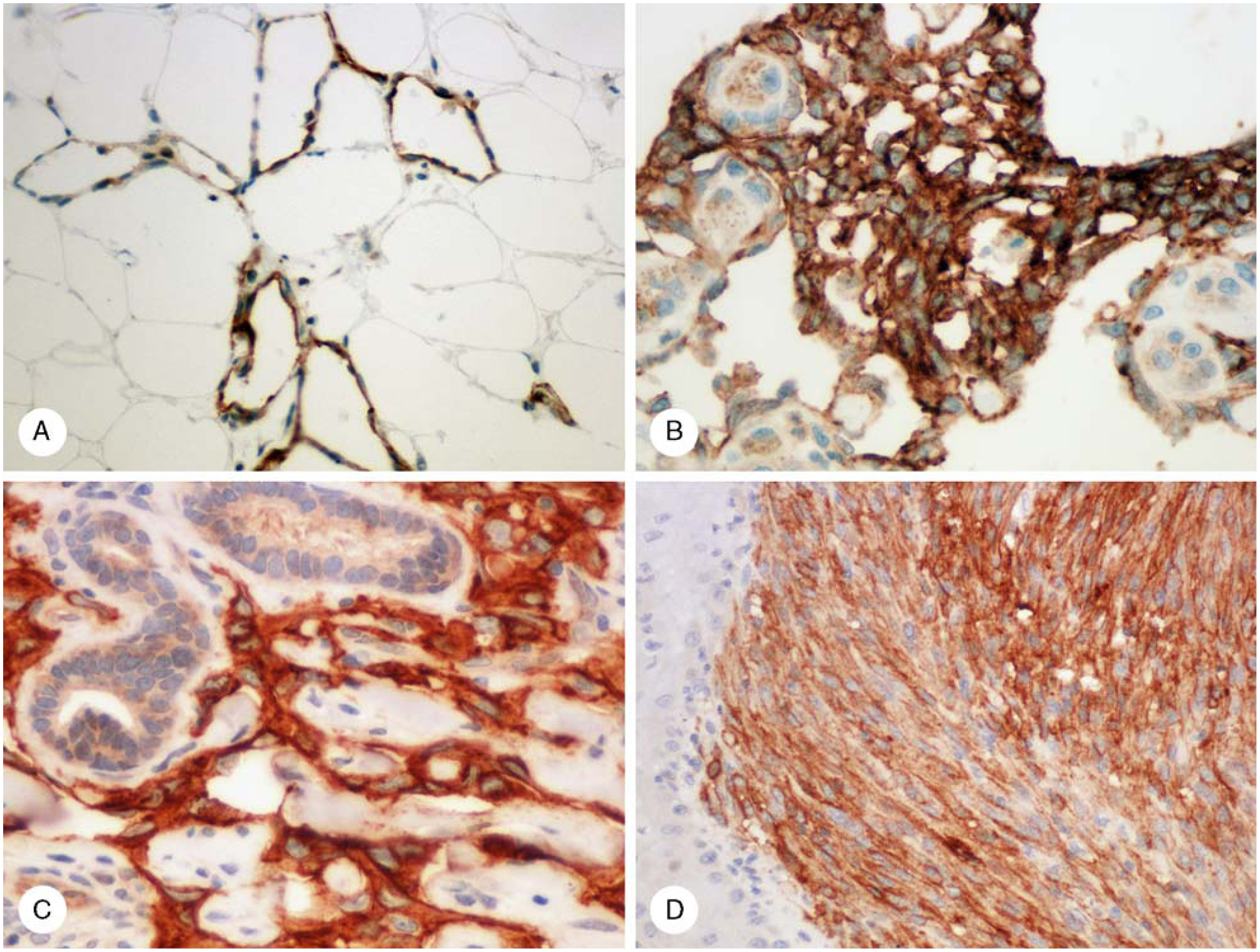
Claudin-5-positivity in angiosarcomas and Kaposi sarcoma. A, Well-differentiated vasoformative angiosarcoma involving adipose tissue. B, Thorothrast-associated hepatic angiosarcoma. Residual hepatocytes contain lipofuscin pigment. C, Cutaneous angiosarcoma cells are positive, and claudin-5 immunoreactivity is also present in some sweat glands. D, Kaposi sarcoma spindle cells are strongly positive, and the epidermis is negative.
FIGURE 5.

Poorly differentiated gluteal epithelioid angiosarcoma involving skeletal muscle. Many tumor cells contain dot-like claudin-5 positivity. Membranous and cytoplasmic positivity is present for CD31, and nuclear ERG positivity is seen in most tumor cells.
Of the angiosarcomas that were previously found to be ERG negative,11 2 of 3 cases were positive. Also positive were all 3 cases with invalid ERG staining related to poor antigen preservation. The claudin-5 negative angiosarcomas included 3 in deep soft tissue, 1 in the gastrointestinal tract, and 1 metastatic in the lung. Kaposi sarcomas typically showed strong claudin-5 positivity in neoplastic spindle cells (Fig. 4D).
Epithelial Neoplasms
Claudin-5 was variably expressed in pulmonary, gastric, colonic, pancreatic, ovarian, prostatic, and endometrial adenocarcinomas, often with a heterogenous membrane staining pattern with alternating positive and negative areas (Table 2). It was present in low-grade ductal carcinomas and was generally absent in high-grade ductal and lobular carcinomas of the breast. In squamous cell carcinomas of the skin, positivity was limited to better-differentiated areas with keratin pearls (Figs. 6A–C).
TABLE 2.
Claudin-5 Expression in 448 Nonendothelial Tumors
| Carcinomas and mesothelioma | |
| Basal cell, skin | 0/6 |
| Breast, ductal carcinoma | 3/5* |
| Colon, adenocarcinoma | 12/12 |
| Endometrial adenocarcinoma | 13/13 |
| Hepatocellular carcinoma | 0/6 |
| Lung, large cell undifferentiated | 5/18 |
| Lung, adenocarcinoma | 7/8 |
| Lung, small cell carcinoma | 7/9 |
| Malignant mesothelioma (epithelial) | 0/7 |
| Ovarian serous | 7/8 |
| Pancreatic, ductal | 3/4 |
| Prostatic adenocarcinoma | 3/6* |
| Renal cell carcinoma | 0/8 |
| Sarcomatoid carcinoma (small intestine) | 0/11 |
| Squamous cell, skin | 3/9 |
| Squamous cell, esophagus | 4/7 |
| Stomach, adenocarcinoma with glands | 8/9 |
| Stomach, signet ring cell carcinoma | 0/8 |
| Testicular seminoma | 0/6 |
| Testicular embryonal carcinoma | 1/1* |
| Metastatic carcinoma from an unknown site | 2/5 |
| Total for epithelial tumors | 78/166 |
| *Delicate focal positivity | |
| Nonepithelial tumors | |
| Clear cell sarcoma of soft parts | 0/6 |
| Epithelioid sarcoma | 0/17 |
| Ewing sarcoma | 0/14 |
| Extraskeletal myxoid chondrosarcoma | 3/10† |
| GIST, gastric | 0/15 |
| GIST, small intestinal | 0/33 |
| Glomus tumor | 0/12 |
| Hemangiopericytoma/solitary fibrous tumor | 0/38 |
| Leiomyosarcoma | 0/14 |
| Lymphoma, anaplastic large cell | 0/5 |
| Lymphoma, small cell lymphocytic | 0/8 |
| Melanoma, metastatic | 0/35 |
| Meningioma | 0/34 |
| MFH/Undifferentiated sarcoma | 0/23 |
| Synovial sarcoma, biphasic | 5/6 |
| Synovial sarcoma, monophasic | 0/12 |
| Total for nonepithelial tumors | 8/282 |
Delicate positivity in a minority of tumor cells.
Weak cytoplasmic staining.
GIST indicates gastrointestinal stromal tumor.
FIGURE 6.

Claudin-5 in tumors with epithelial differentiation. A, Well-differentiated squamous cell carcinoma contains membranous positivity in keratin pearls. B, Metastatic adenocarcinoma of unknown origin is strongly positive. C, Endometrial carcinoma cells and stromal capillaries are positive. D, Biphasic synovial sarcoma contains claudin-5 immunoreactivity in the epithelial elements.
Regionally variable expression was noted in squamous cell carcinomas of the esophagus. Large cell undifferentiated carcinomas of the lung often showed patchy but strong positivity (5 of 18 cases). No claudin-5 positivity was detected in malignant epithelial mesothelioma (including pleural and peritoneal ones), hepatocellular carcinoma, renal cell carcinoma, gastric signet ring cell carcinoma, sarcomatoid carcinoma in the small intestine (many of them metastatic from the lung), and testicular seminoma.
Nonepithelial Tumors, Other Than Vascular Tumors
Biphasic synovial sarcoma was the only nonendothelial sarcoma with distinct claudin-5 positivity, which was observed in the epithelial element. The staining varied from luminal and membrane (Fig. 6D) to diffuse cytoplasmic pattern.
In hemangiopericytoma and glomus tumor, claudin-5 was restricted to endothelial cells, and the main cell population was negative (Table 2). In undifferentiated sarcoma (malignant fibrous histiocytoma), malignant melanoma, various sarcomas, and lymphomas, only endothelial cells were positive. A variety of other sarcomas that can mimic hemangioendothelioma or angiosarcoma were negative, among them were epithelioid sarcoma and Ewing sarcoma. Weak cytoplasmic staining of unknown significance was occasionally noted in extraskeletal myxoid chondrosarcoma.
DISCUSSION
In this study, we examined expression of claudin-5, a tight junction protein, in vascular tumors, especially in angiosarcomas. We also examined normal tissues and reproduced previously described expression patterns, such as vascular and lymph node sinus endothelial,14–16 and glomerular podocytic positivity7 as an indication of validity of immunostaining. Claudin-5 is a sensitive marker for angiosarcoma and hemangioendothelioma but is not very specific, considering its relatively widespread expression in carcinomas. Claudin-5 is significantly expressed in most angiosarcomas, indicating that this cell junction protein is generally conserved in endothelia with malignant transformation.
Although claudin-5 is a membrane-associated cell junction protein, subcellular localization is difficult to judge on the basis of immunostains that typically produce strong, often apparently diffuse, cytoplasmic staining. However, this may be contributed by a strong membrane expression, in addition to cytoplasmic presence by high protein turnover. In some tumors, notably epithelioid vascular tumors such as some epithelioid angiosarcomas and most epithelioid hemangioendotheliomas, a more expected membrane staining is apparent. Two different antibodies, one polyclonal and another monoclonal, gave virtually identical results in angiosarcomas and hemangioedotheliomas.
For sensitivity (96% in this study), claudin-5 compares well with other endothelial markers, especially CD31, platelet-endothelial cell adhesion molecule-1, a membrane protein,2,10 and ERG1, a nuclear transcription factor.12 In some cases, it may be superior to other markers. For example, ERG may be susceptible to poor antigen preservation. Indeed, all 3 angiosarcomas with invalid ERG immunostain and 2 of 3 ERG-negative angiosarcomas were claudin-5 positive.
Similar to angiosarcomas, hemangioendotheliomas of different types are also positive for claudin-5, although in many cases epithelioid hemangioendotheliomas showed a weaker and less uniform, often more, membrane-associated labeling. In some hemangioendotheliomas, notably epithelioid sarcoma-like hemangioendothelioma (a newly described rare entity),1 there was prominent diffuse cytoplasmic claudin positivity without membrane staining. This may reflect dysregulated and aberrant claudin-5 expression. We have seen similar cytoplasmic staining in epithelioid clusters in some epithelioid hemangiomas (Miettinen et al, unpublished).
For sensitivity, claudin-5 is clearly superior to von Willebrand factor (factor VIII-related antigen) that frequently fails to detect endothelial differentiation in less-differentiated angiosarcomas and also has the problem of extracellular staining because of its presence in serum and exudates.10 This marker is largely replaced by newer ones. Claudin-5 also lacks histiocytic and plasma cell labeling, which may occur for CD31 as a potential pitfall in the differential diagnosis of angiosarcoma.9,10 However, some hemangiomas with thrombosis also seem to show extracellular claudin-5 immunoreactivity. This could be derived from claudin-5 being released after endothelial cell damage during thrombosis.
Specificity of claudin-5 for angiosarcoma, among other sarcomas, and for nonvascular mesenchymal tumors is high. Only rare sarcomas, specifically those with true epithelial differentiation, such as biphasic synovial sarcoma, were positive. Even most of those tumors show more limited expression compared with the extensive endothelial claudin-5 labeling observed in most angiosarcomas.
Many mesenchymal mimics of angiosarcoma, such as hemangiopericytoma, epithelioid sarcoma, and melanoma, are consistently claudin-5 negative; hence, claudin-5 is useful for separating these highly vascular nonendothelial tumors from angiosarcoma and other endothelial neoplasms. Our results are in agreement with a recent study on canine angiosarcomas.5
However, there is fairly extensive epithelial expression in claudin-5 in various types of carcinomas, in agreement with previous studies.19 In particular, commonly positive are adenocarcinomas with delicate membrane staining, which, however, differs from the diffuse strong staining seen in most angiosarcomas. Nevertheless, some undifferentiated carcinomas, such as those of the lung, can show focally strong claudin-5 expression. Therefore, epithelial markers such as keratin cocktail AE1/AE3 and EMA, which are rarely expressed in angiosarcomas but usually present in carcinomas, must be evaluated in the differential diagnosis of strongly claudin-5-positive tumors to rule out carcinoma.
The potential keratin 18, and to some degree keratin 8 and 7, immunoreactivity in angiosarcoma can be a further pitfall in the differential diagnosis between angiosarcoma and carcinoma.11,21 Keratin cocktail AE1/AE3 has the advantage of not reacting with keratin 18 and then to less commonly react with angiosarcomas and is more useful in the differential diagnosis of most carcinomas and angiosarcomas.
Lack of claudin-5 expression in epithelial mesothelioma may be another differentiating feature between epithelial mesothelioma and pulmonary or other adeno-carcinoma. Our findings on fairly extensive claudin-5 immunoreactivity in different adenocarcinomas (and also in other carcinomas) differ from a recent abstract, where only limited expression was reported in carcinomas (apparently limited to pancreatic and gastric ones), as studied with the same polyclonal antibody.20
In benign vascular tumors, claudin-5 is consistently expressed in cavernous, intramuscular, and venous hemangiomas, which may correlate with their more mature endothelial phenotype. Indeed, some of these hemangiomas are now increasingly clinically considered vascular malformations rather than ordinary hemangiomas.3 However, some capillary hemangiomas, especially juvenile capillary hemangiomas, are essentially negative for claudin-5, probably reflecting immature endothelial phenotype. To some degree, this seems to be true for lobular capillary hemangiomas as well. This immaturity may be parallel to the finding that early embryonic capillary endothelia are also largely claudin-5 negative.
The process of detection and quantification of tumor neovascularization has become an important application for endothelial markers. However, for this purpose, claudin-5 is not expected to be useful because of its limited labeling of neovascular endothelia—an endothelial cell subset that also shows many functionally immature features. Previous studies have also indicated low claudin-5 expression in neovascular endothelia of glioblastoma and hepatocellular carcinoma.8,18
In conclusion, claudin-5, an endothelial and epithelial tight junction protein, is strongly expressed in most cases of angiosarcoma and hemangioendothelioma. Because of the limited claudin-5 expression in other mesenchymal tumors and normal cell components, claudin-5 may be a valuable supplemental marker for angiosarcoma and related tumors and an addition to markers such as CD31 and ERG. However, variable, but sometimes extensive, claudin-5 expression in various carcinomas and biphasic synovial sarcomas must be considered in the immunohistochemical differential diagnosis by additional use of epithelial markers, especially keratin cocktail AE1/AE3 and EMA.
Conflicts of Interest and Source of Funding:
Supported by NIH/NCI intramural research program. The authors have disclosed that they have no significant relationships with, or finanical interest in, any commerical companies pertaining to this article.
REFERENCES
- 1.Billings SD, Folpe AL, Weiss SW. Epithelioid sarcoma-like hemangioendothelioma. Am J Surg Pathol. 2003;27:48–57. [DOI] [PubMed] [Google Scholar]
- 2.de Young BR, Wick MR, Fitzgibbon JF, et al. CD31: an immunospecific marker for endothelial differentiation in human neoplasms. Appl Immunohistochem. 1993;1:97–100. [Google Scholar]
- 3.Hassanein AK, Mulliken JB, Fishman, et al. Evaluation of terminology for vascular anomalies in current literature. Plast Reconstr Surg. 2011;127:347–351. [DOI] [PubMed] [Google Scholar]
- 4.Heiskala M, Peterson PA, Yang Y. The roles of claudin superfamily proteins in paracellular transport. Traffic. 2001;2:92–98. [DOI] [PubMed] [Google Scholar]
- 5.Jakab C, Halasz J, Kiss A, et al. Claudin-5 protein is a new differential marker for histopathological diagnosis of canine hemangiosarcoma. Histol Histopathol. 2009;24:801–813. [DOI] [PubMed] [Google Scholar]
- 6.Jung JH, Jung CK, Choi HJ, et al. Diagnostic utility of expression of claudins in non-small cell lung cancer : different expression profiles in squamous cell carcinomas and adenocarcinomas. Pathol Res Pract. 2009;205:409–416. [DOI] [PubMed] [Google Scholar]
- 7.Koda P, Zhao L, Yaoita E, et al. Novel expression of claudin-5 in glomerular podocytes. Cell Tissue Res. 2011;343:637–648. [DOI] [PubMed] [Google Scholar]
- 8.Liebner S, Fischmann A, Rascher G, et al. Claudin-1 and claudin-5 expression and tight junction morphology are altered in blood vessels of glioblastoma multiforme. Acta Neuropathol. 2000;100: 323–331. [DOI] [PubMed] [Google Scholar]
- 9.McKenney JK, Weiss SW, Folpe AL. CD31 expression in intratumoral macrophages: a potential diagnostic pitfall. Am J Surg Pathol. 2001;25:1167–1173. [DOI] [PubMed] [Google Scholar]
- 10.Miettinen M, Lindenmayer AE, Chaubal A. Endothelial cell markers CD31, CD34, and BNH9 antibody to H- and Y-antigens: evaluation of their specificity and sensitivity in the diagnosis of vascular tumors and comparison with von Willebrand’s factor. Mod Pathol. 1994;7:82–90. [PubMed] [Google Scholar]
- 11.Miettinen M, Fetsch JF. Distribution of keratins in normal endothelial cells and in a spectrum of vascular tumors: implications in tumor diagnosis. Hum Pathol. 2000;31:1062–1067. [DOI] [PubMed] [Google Scholar]
- 12.Miettinen M, Wang ZF, Paetau A, et al. ERG transcription factor as n immunohistochemical marker for vascular endothelial tumors and prostatic carcinoma. Am J Surg Pathol. 2011;35:432–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moldvay J, Jackel M, Paska C, et al. Distinct claudin expression profile in histologic subtypes of lung cancer. Lung Cancer. 2007; 57:159–167. [DOI] [PubMed] [Google Scholar]
- 14.Moll R, Sievers E, Hammerling B, et al. Endothelial and virgultar cell formations in the mammalian lymph node sinus:endothelial differentiation morphotypes characterized by a special kind of junction (complexus adherens). Cell Tissue Res. 2009; 335:109–141. [DOI] [PubMed] [Google Scholar]
- 15.Morita K, Sasaki H, Furuse M, et al. Endothelial claudin : claudin-5 TMVCF constitutes tight junction strands in endothelial cells. J Cell Biol. 1999;147:185–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morita K, Sasaki H, Furuse K, et al. Expression of claudin-5 in dermal vascular endothelia. Exp Dermatol. 2003;12:289–295. [DOI] [PubMed] [Google Scholar]
- 17.Paschoud S, Bongiovanni M, Pache JC, et al. Claudin-1 and claudin-5 expression patterns differentiated lung squamous cell carcinomas from adenocarcinomas. Mod Pathol. 2007;20:947–954. [DOI] [PubMed] [Google Scholar]
- 18.Sakaguchi T, Suzuki S, Higashi H, et al. Expression of tight junction protein claudin-5 in tumor vessels and in patients with hepatocellular carcinoma. J Surg Res. 2008; 147:123–131. [DOI] [PubMed] [Google Scholar]
- 19.Soini Y Expression of claudins 1,2,3,4,5 and 5 in various types of tumors. Histopathology. 2005;46:551–560. [DOI] [PubMed] [Google Scholar]
- 20.Shon W, Popp LA, Folpe AL. Claudin-5: a novel, highly sensitive and specific endothelial marker [Abstract]. Mod Pathol. 2011; 24(suppl 1):21A. [Google Scholar]
- 21.Wenig BM, Abbondanzo SL, Heffess CS. Epithelioid angiosarcoma of the adrenal glands: a clinicopathologic study of nine cases with a discussion of the implications of finding “epithelial specific” markers. Am J Surg Pathol. 1994;18:62–73. [PubMed] [Google Scholar]


