This editorial refers to ‘Delineating the molecular and histological events that govern right ventricular recovery using a novel mouse model of PA de-banding’ by M. Boehm et al., pp. 1700–1709.
Right ventricular (RV) function is increasingly recognized as a key independent indicator of clinical outcome in all forms of heart disease, and a focus for current research in pulmonary hypertension (PH).1 In response to aberrant pulmonary vasoconstriction and vascular remodelling that is the basis for disease, RV hypertrophy (RVH) can compensate for the increase in afterload and maintain cardiac output in normal range. However, this compensatory function deteriorates over time with the inexorable progression of pulmonary vascular remodelling, and RV failure (RVF) ensues. Closer study reveals a surprising phenotypic diversity of RV function among PH patients. For reasons that remain unclear, some patients with chronic PH develop RVH but with normal ejection fraction (adaptive remodellers) while others, with similar pulmonary arterial pressures, rapidly progress to RVF (maladaptive remodellers).1,2 RV failure is characterized by molecular and structural remodelling of the ventricle, including alterations in gene expression, metabolism, fibrosis, and contractility. Yet rather than an inevitable progression towards failure, clinical experience shows that this severe RV remodelling and dysfunction may be reversible: a striking observation in cases of end-stage PH requiring lung transplant or pulmonary endarterectomy is that RV dysfunction can recover, often rapidly, upon normalization of pulmonary haemodynamics.3 How does this happen?
Understanding the molecular and physiological basis of RV recovery requires development of improved animal models. The article by Boehm et al.,4 published in this issue of Cardiovascular Research, utilizes a novel murine model of PH reversal with defined kinetics to address this question. The authors imposed RV pressure overload by partial pulmonary artery (PA) occlusion using sutures with defined kinetics of degradation to examine the reversal of RV remodelling and recovery of RV function at specific intervals. The results showed that PH and parameters of cardiac function and remodelling were indeed reversed, but with telling differences in their kinetics. Specifically, RVH and exercise capacity recovered in concert with the fall in PA pressure, whereas RV fibrosis and capillary remodelling recovered more slowly. These results point, first, to the centrality of the cardiac myocyte as the determinant of RV function; with the important implication that the diminished function under pressure overload is the result of a process of encryption or hibernation of a viable myofibrillar contractile reserve, rather than outright loss of contractile units, or structural fibrotic or angiogenic remodelling.5 This conclusion is consistent with our findings that, in a neonatal calf model of severe PH, organ-level RV dysfunction was not mirrored by reduced contractile function or loss of mitochondrial integrity of isolated cardiomyocytes.6 From this perspective, fibrotic RV remodelling actually may be a compensatory process aimed at reinforcing ventricular wall stiffness to normalize pressure overload, rather than a cause of decreased myocardial contractility. Thus, attenuation of cardiac fibrosis by pharmacological or genetic manipulation may not improve, but rather worsen RV functional parameters in experimental PH.7 Furthermore, cardiac fibroblasts, which are likely primary drivers of the fibrotic responses isolated from the right ventricle in the same calf model, have the capability of reprogramming myocytes towards a more foetal-like phenotype exhibiting de-differentiation and dysfunction. These changes could be initially protective against cardiac pressure overload but if continued, they could likely drive the maladaptive phase.8 Similarly, stereological measures of capillary density in rat PH models have shown that the apparent capillary rarefaction is more accurately a reflection of increased myocyte size relative to existing capillaries.9 Collectively, these findings suggest an under-appreciated plasticity of the RV cardiac myocyte to adapt and survive pathological insult independent of other remodelling processes, opening an exciting new direction for translational research. In our view, this is the seminal conclusion from the study.
As the authors note, the remarkable capacity of the right ventricle to recover is predicated on the reduction of afterload. The kinetics of afterload reversal in this model may more closely reflect the clinical situation with initiation of therapy in newly diagnosed patients rather than the abrupt reversal upon lung transplant. The results emphasize the critical role of a reduction in PVR, either by vasodilator therapy or anti-remodelling agents or both as a cornerstone of clinical management of PH. Moreover, the results parallel the emerging clinical focus on early and aggressive vasodilator therapy in high-risk patients, including upfront combination therapy, to promote reverse remodelling of dysfunctional RV and improve PH outcomes (Figure 1).10
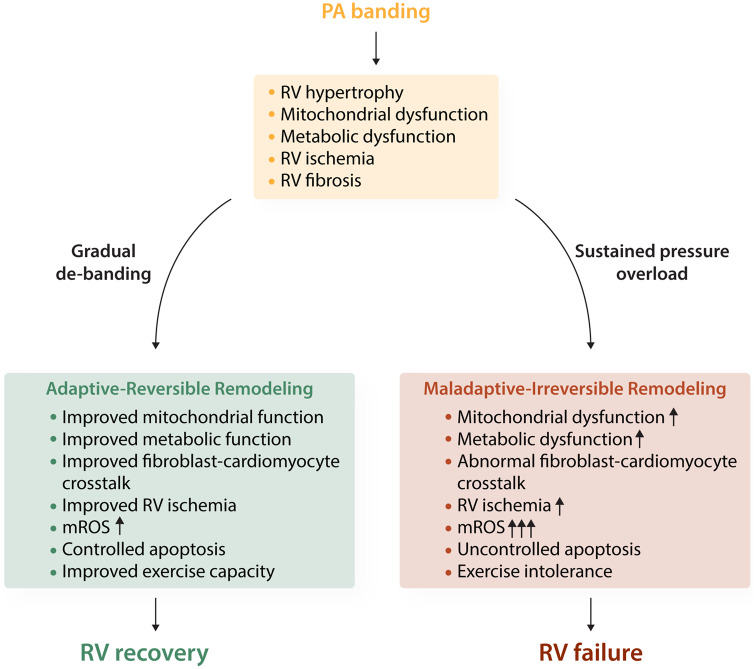
Figure 1.
Modulation of mitochondrial metabolism in right ventricular reverse remodelling. Impaired mitochondrial metabolism in a pressure overload model of RVH (PA banding) leads to metabolic dysfunction, cardiac fibroblast activation, and adaptive hypertrophy/fibrosis of the myocardium. Gradual de-banding and the resulting afterload reduction induced improvement in RV function involving modulation in energy metabolism and apoptosis as major regulators of functional RV improvement. In the compensated (adaptive-reversible) remodelling form of RVH, these changes include increases in glycolysis and angiogenesis, while mitochondrial integrity remains intact despite a slight increase in mitochondrial ROS due to improved blood flow and mitochondrial respiration. In decompensated (maladaptive-Irreversible) RVH, however, the increase in mitochondrial ROS appears to be associated with decreased angiogenesis, pathological fibroblast-cardiomyocyte crosstalk, and redox sensitive activation of apoptosis which, if unchecked, can lead to RVF. The conceptual model shown here indicates a smooth metabolic correction and potential balance between pro-apoptotic and anti-apoptotic genes during RV recovery, which could shed light on the potential targets of RV support during this transition.
At the same time, it is recognized that additional therapeutic approaches are needed in PH. Currently approved vasodilator therapies are mainly effective in Group 1 PAH, while approved agents are lacking for the vast majority of patients with Group 2/3 PH. Therapies directly targeted to preserve RV function and/or reverse pathological RV remodelling are also lacking; however, research efforts towards this goal are steadily increasing.11 In the future, these approaches may yield novel therapies that act co-ordinately on the RV–PA axis. In this regard, we and others have demonstrated excessive activation of Class I and IIb histone deacetylases (HDACs) in heart and PA of human PAH and experimental PH models. HDAC inhibition reverses PA cell phenotypes in vitro, and attenuates development of PH and pathological RV remodelling in animal models in vivo.12 Similarly, Drake et al.,13 identified down-regulation of the peptide receptor ligand apelin as a distinguishing feature of decompensated RV failure. Apelin and its cognate APJ receptor regulate MEF2, a master transcription factor, through a pathway mediated by Class IIa HDACs to regulate vascular angiogenesis, and cardiac development and contractility. Inhibition of Class IIa HDACs rescues PA endothelial cell phenotypes in human PAH and reverses experimental rodent PH.14
Important questions remain for continued research. Specifically, insight is critically lacking into the mechanistic determinants and clinical predictors for the transition from adaptive-reversible to maladaptive-irreversible phenotypes of RV remodelling that occur in human PH. In this regard, it may be of interest to examine the progression of RV remodelling to end-stage failure in non-reversible PA banding compared to the reversal paradigm in the present model to explore the determinants of irreversible RVF. Furthermore, the uniformity of the mouse as experimental model and the resilience of the mouse cardiovascular system suggest the need for continued exploration of novel animal models to address this gap in knowledge. It will be important to evaluate adaptive-maladaptive remodelling in animal models of severe PH progressing to RVF, as it is likely that circulating factors from the lung such as TNF, IL-6, IL-1β, could have significant modifying effects on the pressure overloaded cardiomyocyte or fibroblast. For example, beef cattle raised in montane environments of the USA exhibit phenotypic diversity of adaptive vs. maladaptive responses to chronic hypoxic exposure similar to human PH. Transport to lower elevations normalizes PH symptoms in most animals, whereas a subset progress to irreversible RVF.15 Despite the challenges of field studies, this naturally occurring large animal model provides interesting parallels with human PH.
The current era of vasodilator therapy has measurably increased life expectancy and quality of life for PH patients, from 50% mortality within 3 years to 7–8 years at present.1 Nonetheless, PH remains a progressive and incurable disease where pathognomonic pulmonary vascular remodelling results in RV failure as its most common outcome. Improved animal models such as those presented here and the insights they provide into preserving and improving cardiac function will be key to continued therapeutic progress.11
Conflict of interest: none declared.
The opinions expressed in this article are not necessarily those of the Editors of Cardiovascular Research or of the European Society of Cardiology.
Funding
K.R.S.: National Institutes of Health, Program Project Grant (NIH PPG HL014985); Department of Defense (DoD W81XWH1910259).
References
- 1. Lahm T, Douglas IS, Archer SL, Bogaard HJ, Chesler NC, Haddad F, Hemnes AR, Kawut SM, Kline JA, Kolb TM, Mathai SC, Mercier O, Michelakis ED, Naeije R, Tuder RM, Ventetuolo CE, Vieillard-Baron A, Voelkel NF, Vonk-Noordegraaf A, Hassoun PM; American Thoracic Society Assembly on Pulmonary Circulation. Assessment of right ventricular function in the research setting: knowledge gaps and pathways forward. An Official American Thoracic Society Research Statement. Am J Respir Crit Care Med 2018;198:e15–e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ryan JJ, Archer SL.. The right ventricle in pulmonary arterial hypertension: disorders of metabolism, angiogenesis and adrenergic signaling in right ventricular failure. Circ Res 2014;115:176–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Reesink HJ, Marcus JT, Tulevski II, Jamieson S, Kloek JJ, Vonk Noordegraaf A, Bresser P.. Reverse right ventricular remodeling after pulmonary endarterectomy in patients with chronic thromboembolic pulmonary hypertension: utility of magnetic resonance imaging to demonstrate restoration of the right ventricle. J Thorac Cardiovasc Surg 2007;133:58–64. [DOI] [PubMed] [Google Scholar]
- 4. Boehm M, Tian X, Mao Y, Ichimura K, Dufva MJ, Ali K, Prosseda SD, Shi Y, Kuramoto K, Reddy S, Kheyfets VO, Metzger RJ, Spiekerkoetter E.. Delineating the molecular and histological events that govern right ventricular recovery using a novel mouse model of PA de-banding. Cardiovasc Res 2020;116:1700–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ryan MJ, Perera D.. Identifying and managing hibernating myocardium: what’s new and what remains unknown? Curr Heart Fail Rep 2018;15:214–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bruns DR, Brown RD, Stenmark KR, Buttrick PM, Walker LA.. Mitochondrial integrity in a neonatal bovine model of right ventricular dysfunction. Am J Physiol Lung Cell Mol Physiol 2015;308:L158–L167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Crnkovic S, Egemnazarov B, Damico R, Marsh LM, Nagy BM, Douschan P, Atsina K, Kolb TM, Mathai SC, Hooper JE, Ghanim B, Klepetko W, Fruhwald F, Lassner D, Olschewski A, Olschewski H, Hassoun PM, Kwapiszewska G.. Disconnect between fibrotic response and right ventricular dysfunction. Am J Respir Crit Care Med 2019;199:1550–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bruns DR, Tatman PD, Kalkur RS, Brown RD, Stenmark KR, Buttrick PM, Walker LA.. The right ventricular fibroblast secretome drives cardiomyocyte dedifferentiation. PLoS One 2019;14:e0220573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Graham BB, Kumar R, Mickael C, Kassa B, Koyanagi D, Sanders L, Zhang L, Perez M, Hernandez-Saavedra D, Valencia C, Dixon K, Harral J, Loomis Z, Irwin D, Nemkov T, D’Alessandro A, Stenmark KR, Tuder RM.. Vascular adaptation of the right ventricle in experimental pulmonary hypertension. Am J Respir Cell Mol Biol 2018;59:479–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. D’Alto M, Badagliacca R, Argiento P, Romeo E, Farro A, Papa S, Sarubbi B, Russo MG, Vizza CD, Golino P, Naeije R.. Risk reduction and right heart reverse remodeling by upfront triple combination therapy in pulmonary arterial hypertension. Chest 2020;157:376–383. [DOI] [PubMed] [Google Scholar]
- 11. Groeneveldt JA, de Man FS, Westerhof BE.. The right treatment for the right ventricle. Curr Opin Pulm Med 2019;25:410–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jeong MY, Lin YH, Wennersten SA, Demos-Davies KM, Cavasin MA, Mahaffey JH, Monzani V, Saripalli C, Mascagni P, Reece TB, Ambardekar AV, Granzier HL, Dinarello CA, McKinsey TA.. Histone deacetylase activity governs diastolic dysfunction through a nongenomic mechanism. Sci Transl Med 2018;10:eaao0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Drake JI, Bogaard HJ, Mizuno S, Clifton B, Xie B, Gao Y, Dumur CI, Fawcett P, Voelkel NF, Natarajan R.. Molecular signature of a right heart failure program in chronic severe pulmonary hypertension. Am J Respir Cell Mol Biol 2011;45:1239–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sofer A, Lee S, Papangeli I, Adachi T, Hwangbo C, Comhair S, DaSilva-Jardine P, Kim J, Schwarz JJ, Erzurum SC, Chun HJ.. Therapeutic engagement of the histone deacetylase IIA-myocyte enhancer factor 2 axis improves experimental pulmonary hypertension. Am J Respir Crit Care Med 2018;198:1345–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rhodes J. Comparative physiology of hypoxic pulmonary hypertension: historical clues from brisket disease. J Appl Physiol (1985) 2005;98:1092–1100. [DOI] [PubMed] [Google Scholar]