This editorial refers to ‘Development of a new mouse model for coxsackievirus-induced myocarditis by attenuating coxsackievirus B3 virulence in the pancreas’ by S. Pinkert et al., pp. 1756–1766.
Over the past several years, the use of experimental animal models has enabled substantial improvement in understanding the pathogenesis of cardiovascular diseases in their management. Rodent models are commonly used in Cardiovascular Research because of several advantages including short lifespan, which allows following the natural history of the disease at an accelerated pace, and the large availability of genetically modified models, which permits rapid target validation. However, rodents are phylogenetically very distant from humans and their suitability to faithfully reproduce human disease and response to therapy may not be ideal.
Myocarditis refers to a spectrum of clinical conditions manifesting inflammation of the heart muscle, recognized as an emerging cause of morbidity and early mortality.1 The pathogenetic mechanisms remain largely elusive however both infections and non-infectious triggers have been identified.2 The study of myocardial inflammation is still to this day challenging due to the fact that the heart is a hard to reach organ for sampling purposes and clinical research relies on small pieces of tissue acquired through biopsy and advanced cardiac imaging or post-mortem studies. Elucidating the key mechanisms that underlie myocardial inflammation and its treatment have heavily relied upon experimental murine models including coxsackievirus B3 (CVB3)-induced myocarditis, considered the gold standard for modelling human viral myocarditis.2
In this model, myocarditis and pancreatitis typically co-exist3 and pancreatitis has always been considered an important component of this model in which the pancreas has been proposed to serve as a reservoir and conduit of the CVB that precedes cardiac infection. Specifically, induction in the pancreas of interferon-gamma was shown to protect mice from CVB3 infection of the heart and subsequent myocarditis.4 This concept has been challenged by experiments in mice with pancreas-specific deletion of the coxsackievirus and adenovirus receptor, which have shown that infection of the pancreas has very little effect on cardiac CVB3 infection and pathology.5 In addition, while CVB3 has been traditionally considered one of the major pathogens identified in human viral myocarditis,6 pancreatic involvement manifesting as clinical pancreatitis has been reported in a very limited number of cases with CVB myocarditis to date,7–9 raising questions on the reliability of this model to reproduce human disease.
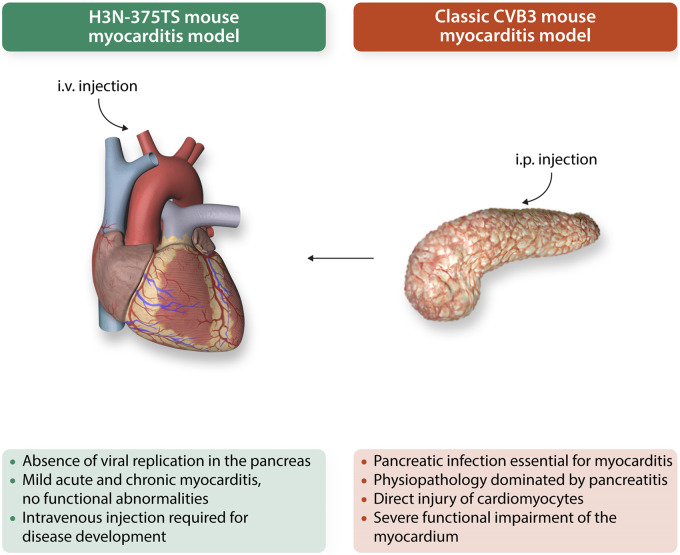
In an interesting and provocative paper, Pinkert et al.10 report the development of a new mouse model for coxsackievirus-induced myocarditis achieved by attenuating CVB3 virulence in the pancreas. Specifically, they generated a recombinant CVB variant H3N-375TS which was suppressed in pancreatic miR-375 expressing cells. Intravenous administration of the H3N-375TS variant to NMRI mice resulted in myocarditis in the absence of pancreatic viral infection or tissue damage, further supporting the concept that pancreatic infection is not required for viral myocarditis. It is important to note that although acute myocarditis was established in the hearts of these mice, which was characterized by myocardial injury, inflammatory infiltrates, and proinflammatory cytokines, there was no significant depression of cardiac function observed as compared to uninfected mice. This suggests a myocardial inflammatory syndrome of a milder form thank that of the classical CVB3 model, hence more similar to the human disease. Furthermore, signs of chronic myocarditis (Day 28) in the form of fibrosis in the affected areas, but in the absence of replicating virus in the heart was observed in these mice, another feature often observed in human myocarditis and inflammatory cardiomyopathies.
Another interesting observation arising from this model is that intraperitoneal administration of the variant to NMRI mice—as in the ‘classical’ CVB3 model—did not produce pancreatitis or myocarditis whereas intravenous infection with the variant led to myocardial infection and myocarditis. The mechanisms of this observation remain unclear, as both routes of administration are conventionally considered ‘systemic’. However, it is plausible to hypothesize that the intraperitoneal route might decrease the systemic viral titers via ‘exposure’ to phagocytes in the peritoneum itself or during draining of the peritoneal fluid into the lymphatic system and lymphoid organs. Further studies are needed to provide a mechanistic explanation for this finding as well as clarify its potential relevance for human disease.
Overall, this novel mouse model of ‘cardiac-selective’ CVB3-induced myocarditis is a step-forward for the accurate modelling of human myocarditis as it addresses an important clinical discrepancy by attenuating the biological ‘noise’ caused by pancreatitis (Figure 1). Creative approaches such as the one used in this study can be of significant help in calibrating organ involvement for the development of mouse models that faithfully recapitulate human disease.10 Hopefully, this model will help closing at least some of the substantial gaps that still remain in our understanding of human myocarditis.
Figure 1.
Schematic representation of the H3N-375TS mouse model as per Pinkert et al. (green characters) vs. the classic coxsackievirus B3 model (red characters) of myocarditis. i.p., intraperitoneal; i.v., intravenous.
That said, it has to be borne in mind that human inflammatory cardiomyopathies are a complex and heterogeneous spectrum of diseases whose pathogenesis includes infectious or other noxious agents interacting with genetic determinants as well as environmental factors.11 Therefore, experimental modelling can complement—but not replace—clinical studies, which allow genomic and molecular assessment as well as stratification of patients based on phenotypic description and co-morbidities, a plethora of fundamental variables that even the most faithful disease model cannot reproduce.
Conflict of interest: none declared.
Funding
This work was supported by the British Heart Foundation [FS/18/82/34024 to A.P. and RG/14/2/30616 and CH/15/2/32064 to F.M.B.].
The opinions expressed in this article are not necessarily those of the Editors of Cardiovascular Research or of the European Society of Cardiology.
References
- 1. Caforio AL, Pankuweit S, Arbustini E, Basso C, Gimeno-Blanes J, Felix SB, Fu M, Helio T, Heymans S, Jahns R, Klingel K, Linhart A, Maisch B, McKenna W, Mogensen J, Pinto YM, Ristic A, Schultheiss HP, Seggewiss H, Tavazzi L, Thiene G, Yilmaz A, Charron P, Elliott PM; European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 2013;34:2636–2648, 2648a–2648d. [DOI] [PubMed] [Google Scholar]
- 2. Blyszczuk P. Myocarditis in humans and in experimental animal models. Front Cardiovasc Med 2019;6:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tracy S, Höfling K, Pirruccello S, Lane PH, Reyna SM, Gauntt CJ.. Group B coxsackievirus myocarditis and pancreatitis: connection between viral virulence phenotypes in mice. J Med Virol 2000;62:70–81. [DOI] [PubMed] [Google Scholar]
- 4. Horwitz MS, La Cava A, Fine C, Rodriguez E, Ilic A, Sarvetnick N.. Pancreatic expression of interferon-gamma protects mice from lethal coxsackievirus B3 infection and subsequent myocarditis. Nat Med 2000;6:693–697. [DOI] [PubMed] [Google Scholar]
- 5. Kallewaard NL, Zhang L, Chen JW, Guttenberg M, Sanchez MD, Bergelson JM.. Tissue-specific deletion of the coxsackievirus and adenovirus receptor protects mice from virus-induced pancreatitis and myocarditis. Cell Host Microbe 2009;6:91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Magnani JW, Dec GW.. Myocarditis: current trends in diagnosis and treatment. Circulation 2006;113:876–890. [DOI] [PubMed] [Google Scholar]
- 7. Coplan NL, Atallah V, Mediratta S, Bruno MS, DePasquale NP.. Cardiac, pancreatic, and liver abnormalities in a patient with coxsackie-B infection. Am J Med 1996;101:325–326. [DOI] [PubMed] [Google Scholar]
- 8. Lau G. Acute fulminant, fatal coxsackie B virus infection: a report of two cases. Ann Acad Med Singapore 1994;23:917–920. [PubMed] [Google Scholar]
- 9. Persichino J, Garrison R, Krishnan R, Sutjita M.. Effusive-constrictive pericarditis, hepatitis, and pancreatitis in a patient with possible coxsackievirus B infection: a case report. BMC Infect Dis 2016;16:375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pinkert S, Pryshliak M, Pappritz K, Knoch K, Hazini A, Dieringer B, Schaar K, Dong F, Hinze L, Lin J, Lassner D, Klopfleisch R, Solimena M, Tschope C, Kaya Z, Beling A, Kurreck J, van Linthout S, Klingel K, Fechner H.. Development of a new mouse model for coxsackievirus-induced myocarditis by attenuating coxsackievirus B3 virulence in the pancreas. Cardiovasc Res 2020;116:1756–1766. [DOI] [PubMed] [Google Scholar]
- 11. Bondue A, Arbustini E, Bianco A, Ciccarelli M, Dawson D, De Rosa M, Hamdani N, Hilfiker-Kleiner D, Meder B, Leite-Moreira AF, Thum T, Tocchetti CG, Varricchi G, Van der Velden J, Walsh R, Heymans S.. Complex roads from genotype to phenotype in dilated cardiomyopathy: scientific update from the Working Group of Myocardial Function of the European Society of Cardiology. Cardiovasc Res 2018;114:1287–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]