Abstract
With healthcare becoming digital, patients today are more empowered than ever before. As a result, digital health solutions have become the need of the hour to keep up with an increasing number of empowered patients participating in their own treatment decisions. Digital health encompasses various platforms and systems that apply technological solutions to enhance healthcare delivery. Digital therapeutics (DTx) is one such category of digital health solutions that provides evidence-based software-driven therapeutic interventions for the prevention and management of a medical disorder or disease. This review aims to provide a comprehensive overview of DTx, its functions and applications in healthcare, and associated regulatory aspects, among others.
Keywords: App-based healthcare, digital medicine, digital therapeutics, regulatory aspects
Introduction
Drug development is considered to be a lengthy, expensive, and high-risk venture in today's world.[1] Despite massive investment by global players, the overall research and development (R and D) productivity in the pharmaceutical and life sciences industry has substantially declined across the world.[1] As a result, various industry stakeholders, academia, and health regulatory authorities are continuously trying to improve, encourage, and devise the best scientific tools to modernize drug development. For instance, the US Food and Drugs Administration (USFDA), in an attempt to identify critical areas for scientific improvement, is increasingly encouraging novel ideas. Examples of such ideas include- development and qualification of new biomarkers, implementation of more efficient clinical trial designs (such as adaptive designs and bioinformatics), the advancement of quantifiable disease models, and the augmentation of drug manufacturing processes.[2] With concepts such as personalized and precision medicine taking the lead, the landscape of healthcare is increasingly becoming more digital.
The world has witnessed the explosion of digital health over the last decade with the emergence of social media platforms, smartphones and mobile applications, wearable devices, cloud-based data platforms, real-world evidence studies, and the likes. As a consequence, general well-being and health monitoring are gradually extending from being space-bound activities restricted to hospitals and clinics to the widespread digital world through various smartphone applications. In this context, the term “Digital Health” applies to all the technologies that engage patients in their health and well-being and includes concepts such as mobile health (mHealth), telehealth (i.e. telemedicine), smart devices, sensors and wearable's, health information technology, and personalized medicine.[3] Innovative models are being studied in the digitized health domain, utilizing open source engineering and optimizing the potential of professionals from traditional healthcare settings. This emergent phenomenon within the digital health sphere is called “Digital Therapeutics” (DTx).[3,4]
What is “Digital Therapeutics” (DTx)?
Digital Therapeutics (DTx) is a subdivision of digital health, which represents a collection of technologies, products, and services across healthcare and wellness industries. Sepah et al., in 2015, mentioned the term “digital therapeutics” for the first time in their peer-reviewed publication, and formally defined it as, “evidence-based behavioral treatments delivered online that can increase accessibility and effectiveness of healthcare.”[5] The Digital Therapeutics Alliance (DTA) defines the DTX as “delivering evidence-based therapeutic interventions to patients that are driven by software to prevent, manage, or treat a medical disorder or disease. They are used independently or in concert with medications, devices, or other therapies to optimize patient care and health outcomes.”[6] The DTA further elaborates that the products of DTx integrate advanced technology with the best practices on design, clinical support, usability, and data security. These products are reviewed and approved by regulators to supplement the product claims regarding risk, efficacy, and intended use. The DTA also empowers all the stakeholders including patients, healthcare providers, and payers with clever and easily available tools for approaching a variety of conditions through data-based interventions that are high quality, safe, and effective.[7]
The value of the global DTx market is estimated at USD 1.8 billion in 2018, which is expected to reach USD 7.1 billion by 2025.[7] A recent report estimates the biggest applications for DTx to be diabetes and weight loss shortly, with other applications likely to be observed in conditions such as chronic obstructive pulmonary disorder (COPD), developmental disorders (with the use of computer games), and post-traumatic stress disorder [PTSD, with the use of virtual reality (VR)].[8]
History of DTx
In 1995, Dr. Joseph Kvedar from Boston, USA, led a program to learn the development and application of technology for delivering care outside the traditional setup of a hospital or a doctor's office while suggesting the “one-to-many model of care.” The idea was to expand the physicians’ scope by overcoming time, place, and personnel limitations that restrict healthcare delivery while also taking better care of patients with fewer resources by providing access, convenience, and efficiency.[9] This attempt by Dr. Kvedar could be attributed to being among the firsts made by researchers in the digital health/DTx domain.
Evidence from literature depicting the use of digital products to yield enhanced health outcomes dates as far back as 2000.[10] The term “E-patient” was coined by Dr. Tom Ferguson in 1999[11] but gained awareness only after nearly a decade.[12] The formal use of the term “DTx” has been noted since 2012.[13]
“E-patients” are patients who are “equipped, enabled, empowered, and engaged in their health and healthcare decisions.”[14] Today, the number of e-patients is on the rise. They are better engaged and want to participate in informed decision-making about their care. They expect informed answers for their medical and health technology-related questions from their physicians.[8] In such an aware world, the need for digital health and related tools becomes inevitable.
Relationship between Digital Health, Digital Medicine, and DTx
Digital health can be broadly defined as fitting together healthcare and technology. Differentiating between digital health, digital medicine, and DTx is essential to avoid confusion among digital health stakeholders as well as the manufacturers and developers of these products for better placement and utility of these products in the market.[15] The relationship between these three related terms is depicted in Figure 1.[15]
Figure 1.
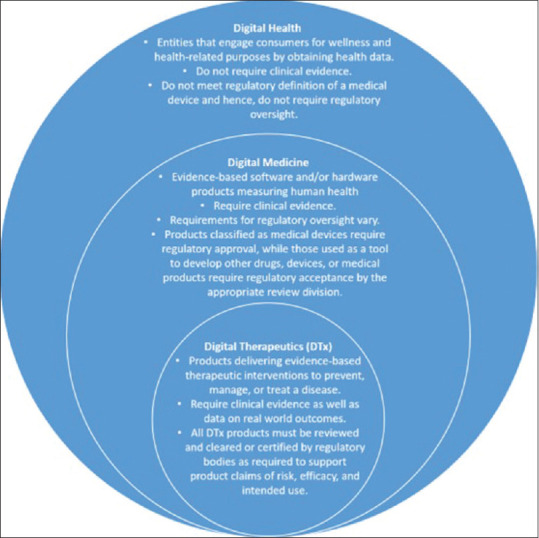
Difference between digital health, digital medicine, and DTx products concerning different risks and corresponding levels of necessary evidence and regulatory oversight.[5,15,16]
Digital health acts as an umbrella entity that encompasses digital medicine which includes DTx. Products categorized under all these categories convey different levels of claims and risks. Moreover, their requirements for clinical evidence and regulatory oversight are different too. Digital health is a broad category comprising of technologies, platforms, and systems that engage consumers for lifestyle, wellness, and health-related purposes. Digital health entities can capture, store, and transmit health data to support clinical operations. Examples of digital health systems include health information technologies, telehealth systems, systems that use consumer health information, and clinical care administration tools, among others.
Digital medicine, on the other hand, consists of software or hardware products, typically supported by evidence, to measure or intervene in the service of human health. Digital diagnostics, digital biomarkers, and remote patient monitoring devices are a few examples of digital medicines.
DTx, as defined earlier, encompasses evidence-based therapeutic interventions that prevent, manage, or treat a medical disorder or a disease.[6] Examples of DTx applications include digital sensors, wearable devices, certain VR, and artificial intelligence (AI) devices.
However, assuming that digital health products to have no possible risks compared to digital medicine or DTx products may be wrong. While the latter two rely on a higher degree of evidence and regulatory oversight owing to greater clinical risk, commercial digital health tools may carry their own set of risks. Therefore, the classification of products under digital health, medicine, and therapeutics warrants further work to explore their potential to enhance healthcare delivery as it may help all the stakeholders to thoroughly understand the purpose and clinical value of these products.[15]
How Does DTx Work?
DTx is a new category of applications that help treat diseases through changes in patient behavior and remote monitoring to yield enhanced and long-term health outcomes. These applications are devised to achieve favorable outcomes; for example, they can encourage patients to adhere to a particular diet and exercise routine or drug regimens. The primary difference between DTx and wellness applications is that the DTx applications are developed to target specific disease conditions, particularly major chronic diseases such as diabetes, cardiovascular conditions such as hypertension, and pulmonary diseases like COPD.[17] The arrival of DTx is reforming the landscape for new medicines, product reimbursement, and regulatory surveillance. As a result, new data-sharing processes and payment models will be formed soon to incorporate these products into the broader treatment plans and regulatory structure for drug and device approvals. Recently, there has been a massive investment of USD 12.5 billion in digital health ventures.[18]
Services available under DTx either complement and add on to the value of the traditional healthcare delivery system or have the potential to significantly replace the existing system. Examples for the former include mobile applications and devices, which help patients with reminders for time and dosage of medication, thus improving their medication adherence. Examples for the latter are platforms that substitute medications with sensory stimuli delivered via an application on the mobile device.[3]
To distinguish DTx from other digital health innovations, the DTA has elaborated on what makes a product classified as a DTx. Even though DTx is a part of the digital health domain, it is different from diagnostics, telehealth, and other digital health products. According to DTA, a DTx product not only has to be software-driven and backed by evidence but should also be able to prevent and manage a disease or a disorder.[9] DTx industry often refers to DTX applications as “clinically validated,” “rigorous,” “focused,” and “FDA-approved,”[19] depending on the situation. Therapeutics mimicking the product development path of pharmaceutical drugs are often referred to as “software as medicine” or “behavioral medicine in a digital format,” which indicates online or mobile delivery of skills-based psychotherapy techniques. Diagnostic and therapeutic use of DTx can be categorized under one class while many companies are trying to transform several behavioral interventions in digital formats. DTx relies exclusively on algorithms for its mechanism of action as well as the algorithmic information management, both of which make it easier to generate, collate, and analyze the health data.[4]
DTx often targets conditions that are poorly addressed by the healthcare system including chronic diseases or neurological disorders. Evidence is also emerging that shows the value of DTx in treating substance addiction. The USFDA recently approved a mobile application to help treat alcohol, marijuana, and cocaine addiction, referring to the findings of clinical trials, which showed abstinence in 40% patients using a DTx application for 3 months, compared with 17.6% of those who used standard therapy alone.[20,21] Besides, DTx can often deliver relatively cost-effective treatments when compared with traditional therapy by reducing demands on clinicians’ time.
An area of great interest for the DTx industry is how these therapeutics can drive behavioral change at large scales. For instance, a 2002 study showed that a systematic behavioral intervention targeting diet and exercise, through face-to-face communication, substantially reduced people's risk of developing type 2 diabetes.[22] This finding led to the development of similar lifestyle-change programs in the US, which are approved by the US Centers for Disease Control and Prevention (CDC). Subsequently, several DTx companies are developing ways to deliver such behavioral programs digitally to reach more people. For instance, one of the DTx companies in the US offers behavioral intervention for diabetes using a social network, which has a year-long educational curriculum, personalized health coaching, and support through a small peer group. This program also uses connected devices to track people's nutrition, activity, and weight by using tools such as hardware, software, and human coaching over a long period.[23] The CDC also recognizes many such DTx-based lifestyle-change and diabetes-prevention programs.[9]
An increasing number of DTx applications are emerging for the treatment of mental health disorders. The digital application of cognitive-behavioral therapy (CBT) is showing promising results in the treatment of depression and anxiety disorders. CBT is also showing rapid diversification in other areas, e.g. substance abuse disorders. Similar products for the treatment of schizophrenia and insomnia are also under development.[24] For instance, an online self-care program based on CBT for insomnia has shown improvements in both insomnia symptoms as well as mental well-being.[25]
DTx is also showing promising results in medication adherence by helping people optimize the benefits of conventional pharmaceutical therapies. For example, a DTx company in the US has developed a robot to nudge people to take their medications on time, intending to overcome the challenges of medication nonadherence. This robot uses artificial intelligence (AI), paired with psychology modeling, to tailor conversations to keep them adhering to the recommended dosing regimens for a longer duration. This robot is currently functional in therapy areas such as renal disease, rheumatoid arthritis (RA), and congestive heart failure; the company is trying to adapt it for several other conditions.[26] Another company has used a new approach to tackle medication nonadherence by developing a pill with an ingestible sensor, as small as the size of a grain of sand, and coated on either side with copper and magnesium. After the pill is swallowed and hits the stomach connecting the two sides, it generates a signal for the sensor patch worn on the person's skin. Finally, this process sends a digital record a mobile app to the users, and with their consent, to their healthcare providers. This sensor patch can also monitor the users’ activity, heart rate, sleep quality, and temperature, suggesting the ability to record people's responses to the medication. The sensor currently monitors type 2 diabetes, hypertension, and hepatitis C patients while further studies are ongoing to determine its use in HIV prevention and treatment as well as its potential use in oncology.[27] In a pilot study of 28 high-risk hepatitis C patients incorporating this technology showed 94% adherence with 26 patients cured.[28] To track medication adherence in individuals living with bipolar II disorder and schizophrenia, another DTx company in the US has developed an ingestible sensor.[29]
In addition to the digital drugs and applications, DTx is also increasingly incorporating VR technology owing to the recent advancements paired with falling costs. The technology has been in use for over the last two decades but only for very few conditions and in specialist centers. It has shown significant use as a leading application in providing exposure therapy for PTSD. Studies to assess its potential application in other areas, including depression, anxiety, phobias, obsessive-compulsive disorder (OCD), eating disorders, addiction, and psychosis, among others, are also underway. The VR technology in DTx applies simulation to enable people to learn by experiencing real-world situations. For example, through VR, patients go into situations and learn how to think, feel, and act differently.[30]
Regulatory Aspects of DTx
DTx and USFDA
The USFDA considers DTx to be mobile medical applications (MMAs). However, a substantial number of MMAs come under the USFDA's enforcement discretion, wherein they do not need to submit premarket review applications or register and list their applications with the USFDA. Examples of MMAs under such enforcement discretion include[2,31]:
Applications helping patients organize and track their health information
Applications providing easy access to information related to conditions or treatments, and
Applications helping patients self-manage their disease or condition (e.g. wellness applications) without providing specific treatment.
Furthermore, with the need to modernize and encourage better innovation, the USFDA's new “Digital Health Innovation Action Plan” outlines its effort to make high-quality, safe, and effective digital health products accessible to all patients.[19] However, its product category for many DTx applications leaves several grey areas, thus making it confusing for products unfit within a specific risk category. These loopholes may lead to companies overseeing the regulatory environment, especially now when the time is quite ripe for regulatory reforms. Owing to recent changes in regulations, the USFDA has started revamping its evaluation pathway for digital health products. The action plan further outlines the pathways for evaluating digital health products based on company characteristics instead of individual product efficacy.[19] Such an assessment is new and aimed at better assessment of the repetitive nature of software development that may proceed faster and inexpensively than carrying a product through prototyping procedures.[4]
While the new regulatory pathway lifts barriers to market for DTx products, in a way serving as an incentive for technological innovation, regulatory authorities have not entirely developed specific methodologies to evaluate the companies formally. Thus, for many DTx entities, it is still not clear as to how their exclusive algorithmic mechanisms will be evaluated.[4] Figure 2 represents the USFDA's regulatory framework for DTx.
Figure 2.
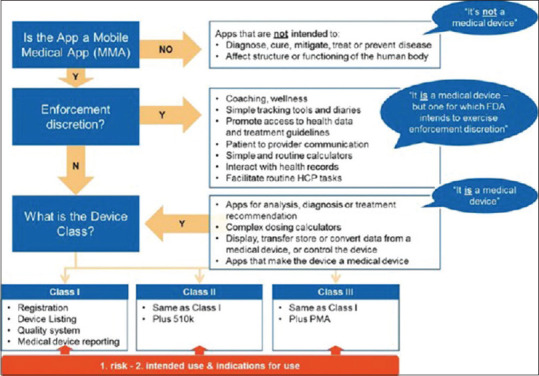
The regulatory framework for digital therapeutics, as implemented by the USFDA.[2]
With the proliferation of digital health, the need for scrutinizing various medical claims has become more critical than ever. With so many DTx products awaiting approval, it is becoming difficult for the regulators to keep up with the volume to ensure there are no false claims. The DTA industry group expects new member companies to implement certain principles and best practices, to reassure users of robust evidence and adherence to regulatory clearance. These adherence standards consist of peer-reviewed publication of findings and clinically meaningful outcomes from trials, and appropriate protection for privacy and security.[9]
DTx incorporation guidance by other regulators
The National Institute for Health and Care Excellence (NICE), together with NHS England and NHS Digital in the UK, is also trying ways to accelerate the uptake of digital innovations. As a result, NICE has recently published guidelines to help manufacturers understand the required evidence.[32]
In a recent forum to address current market challenges and opportunities in the field of DTx, the Academy of Managed Care Pharmacy (AMCP) set some guidelines for the entities to qualify as DTx.[33] Apart from fitting into the DTA's definition of DTx, a product requires “approval as well as third-party validation for safety and efficacy claims” by a regulatory authority, such as the USFDA. Furthermore, payers will base their coverage decisions on a detailed assessment of the safety, efficacy, and utility of a product, along with the medical claim or function of the treatment. For instance, therapeutics aimed at replacing a pharmaceutical intervention may entail more evidence for coverage than those aimed at monitoring a condition or complementing a pharmaceutical intervention. Findings and recommendations from this forum will soon be published to help stakeholders find innovative and collaborative solutions for adopting and utilizing DTx products in healthcare practice.[33]
Barriers in the Adoption of DTx
Notwithstanding significant solutions offered by DTX to poorly addressed conditions and substantial evidence proving their clinical value, DTx entities have not yet significantly entered the mainstream healthcare. The two main obstacles to broader adoption of DTx are[21]:
Difficulty in distinguishing DTx from the more general health and well-being applications in the general digital health market
Uneven incentives in the healthcare environment.
With a massive investment of millions of dollars, more than 315,000 (as noted in 2017) health-related applications are currently available in the market.[34] For consumers as well as medical professionals, it is tough to separate unproven or low-value applications from those with proven therapeutic value. Today, many digital therapies demand changes in healthcare provider workflows, thus increasing physician burden by an overload of data and required interpretation. Moreover, payers would prefer to offer reimbursement for digital therapy upon achieving better clinical outcomes within a time frame, a vital thought considering the limitations of the US private health insurance market.[3]
Although the widespread adoption of DTx may take longer than the industry had hoped for, solutions to the main challenges are evolving. The need for defining the standards of safety and efficacy, for collaboration among stakeholders, and swifter regulatory approval exists.[34]
DTx in India
Many Indian pharmaceutical companies are now modifying their business models, strategies, product portfolio to enhance their market presence to face the changing market dynamics better. Companies have realized the tremendous value proposition offered by DTx products/services. It is believed that DTx services could reduce the drug manufacturing costs, while adding value for insurers, too, allowing them to customize products based on the patients’ needs. However, DTx is still not prevalent in India, and a few companies are planning to invest and venture into the DTx domain.[35] For instance, an Indian pharmaceutical giant is aiming to simplify the management of cardiovascular disorders using prescription drugs and AI-driven digital therapies.[36]
DTx and Practice of Primary Care
DTx has the potential to improve primary care practice. A major advantage of DTx is that it offers the physicians an option to provide treatment anywhere and anytime, transcending the physical borders of a clinic or a hospital. Thus, primary care physicians can make the most out of the innovations in technology to offer low-cost treatment options that fill the current gaps in treatment, thereby encouraging patients to work together for achieving better health outcomes.[37] DTx applications can help primary care physicians to make timely, informed, and accurate clinical diagnoses and patient care decisions. Further, DTx applications can enable primary care physicians to monitor and track response to the prescribed therapies, thereby aiding to provide personalized treatment.[38] DTx applications can also be used by primary care physicians to monitor medication adherence and thereby prevent complications.[39] There are DTx applications which can assist the primary care physician in monitoring chronic conditions such as diabetes. There are DTx programs that can monitor blood sugar readings, medication intake, diet, and exercise, and the records can be reviewed by the primary care physician for getting a holistic picture of the patient's health.[40] Efficacy of DTx applications in imparting behavioral modifications required for lifestyle therapy in diabetes have been studied and reported recently.[41] Similarly, DTx can help the primary care physician to personalize the therapy and monitor the outcomes in conditions such as depression and anxiety.[40]
Conclusion
DTx is here to stay. It is a category of emerging treatment approaches, ready to address chronic and other difficult-to-treat conditions. DTx is expected to expected to significantly influence healthcare delivery and its consumption across the globe. These therapies have the potential to change what the pharmaceutical industry sells, catering to both providers and patients both by selling not just a drug, but a combination of drugs and digital services. An increasing number of pharmaceutical companies are taking a thoughtful approach to DTx. More rigorous testing is required through randomized trials to achieve reliable evidence on the safety and efficacy of DTx. Health authorities including USFDA and NHS, are also recognizing the potential of DTX and encouraging innovations. Although the efforts of DTx companies, stakeholders, and regulators are still in the preliminary stage, growing R and D investment will undoubtedly show the enormous potential impact of DTx very soon.
Financial support and sponsorship
Nil.
Conflicts of interest
The authors are founders of “KYT Adhere“, a prescription Digital Therapeutics (DTx), that helps to improve patient adherence via AI enabled remote patient monitoring technology.
References
- 1.Lendrem D, Senn S, BC L, JD I. R and D productivity rides again. Pharm Stat. 2015;14:1–3. doi: 10.1002/pst.1653. [DOI] [PubMed] [Google Scholar]
- 2.Sverdlov O, van Dam J, Hannesdottir K, Thornton-Wells T. Digital therapeutics: An integral component of digital innovation in drug development. Clin Pharmacol Ther. 2018;104:72–80. doi: 10.1002/cpt.1036. [DOI] [PubMed] [Google Scholar]
- 3.Rastegayeva I. The rise of digital therapeutics [monograph on the Internet] Dassault Systèmes Dassault Systèmes North America. 2019. Available from: https://blogs 3dscom/northamerica/the-rise-of-digital-therapeutics/ Updated 2019 Sept 10; Cited 2020 Jan 09.
- 4.Martin M. The dawn of digital therapeutics [monograph on the Internet] Platypus. Available from: http://blogcastacorg/2019/04/the-dawn-of-digital-therapeutics/ Updated 2019 Apr 16; Cited 2020 Jan 09.
- 5.Sepah S, Jiang L, Peters A. Long-term outcomes of a Web-based diabetes prevention program: 2-year results of a single-arm longitudinal study. J Med Internet Res. 2015;17:e92. doi: 10.2196/jmir.4052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DTA. Digital therapeutics alliance [homepage on the Internet] 2017. Available from: https://wwwdtxallianceorg/ Cited 2020 Jan 09.
- 7.Catalani C, Farese C. Can digital therapeutics revolutionize medicine [monograph on the Internet] Ideo Journal. Available from: https://wwwideocom/journal/can-digital-therapeutics-revolutionize-medicine . Updated 2019 Oct 02; Cited 2020 Jan 09.
- 8.Moar J. Digital therapeutics and wellness [homepage on the Internet] Juniper Research. Available from: https://wwwjuniperresearchcom/researchstore/innovation-disruption/digital-therapeutics-wellness . Updated 2019 May 15; Cited 2020 Jan 09.
- 9.Makin S. The emerging world of digital therapeutics. Nature. 2019;573:S106–9. doi: 10.1038/d41586-019-02873-1. [DOI] [PubMed] [Google Scholar]
- 10.Capobianco E. On Digital Therapeutics. Front Digit Humanit. 2015;2:6. [Google Scholar]
- 11.Tom Ferguson M. D [homepage on the internet] Doctom. Available from: http://wwwdoctomcom/ published 2006 Apr 14; Cited 2020 Jan 09.
- 12.Fox S. The engaged e-patient population [monograph on the internet] Pew Research Centre. Available from: https://wwwpewresearchorg/internet/2008/08/26/the-engaged-e-patient-population/ Published 2008 Aug 26; Cited 2020 Jan 09.
- 13.United States Patent and Trademark Office. homepage on the internet. 2012. Available from: http://tsdr.uspto.gov/documentviewer?caseId=sn85765357&docId=FTK20121101070417#docIndex=4&page= Cited 2020 Jan 09.
- 14.Society for Participatory Medicine. homepage on the internet. Available from: https://participatorymedicineorg/epatients/about-e-patientsnet .
- 15.Goldsack J, Coder M, Fitzgerald C, Navar-Mattingly N, Coravos A, Atreja A. HealthXL Digital health, digital medicine, digital therapeutics (DTx): What's the difference [monograph on the interent] HealthXLcom. Available from: https://wwwhealthxlcom/blog/digital-health-digital-medicine-digital-therapeutics-dtx-whats-the-difference . Updated 2019 Nov 07; Cited 2020 Jan 09.
- 16.Defining digital medicine [monograph on the [internet] Digital Medicine Society- DiMe. Available from: https://wwwdimesocietyorg/indexphp/defining-digital-medicine . Cited 2020 Jan 09.
- 17.Natanson E. Digital therapeutics: The future of health care will be app-based [monograph on the internet] Forbes. Available from: https://wwwforbescom/sites/eladnatanson/2017/07/24/digital-therapeutics-the-future-of-health-care-will-be-app-based/#5738a9876372 . Updated 2017 Jul 24; Cited 2020 Jan 09.
- 18.Zweig M, Tecco H, Huang M. 2018 midyear funding review: Digital health déjà vu in yet another record breaking half [monograph on the internet] Rock Health. Available from: https://rockhealthcom/reports/2018-midyear-funding-review-digital-health-deja-vu- Cited 2020 Jan 09.
- 19.Digital health innovation action plan. monograph on the internet] USFDA centre for devices and radiological health, Digital health program. Available from: https://wwwfdagov/media/106331/download . Cited 2020 Jan 09.
- 20.Mukherjee S. FDAFDA clears the first-ever mobile app to treat alcohol, marijuana, cocaine addiction [monograph on the internet] Fortune. Available from: https://fortunecom/2017/09/14/fda-alcohol-marijuana-cocaine-mobile-app/ Updated 2017 Sept 14; Cited 2020 Jan 09.
- 21.Joyce M, Leclerc O, Westhues K, Xue H. Digital therapeutics: Preparing for take-off [monograph on the internet] McKinsey and Company. Available from: https://wwwmckinseycom/industries/pharmaceuticals-and-medical-products/our-insights/digital-therapeutics-preparing-for-takeoff . Updated 2018 Feb; Cited 2020 Jan 09.
- 22.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weiss P. Tips for designing for behaviour change [monograph on the internet] Omada Health- Omada Updates. Available from: https://wwwomadahealthcom/news/tips-for-designing-for-behavior-change . Updated 2018 Sept 26; Cited 2020 Jan 09.
- 24.reSET® and reSET-O® [homepage on the internet] Pear Therapeutics Pear Therapeutics. Available from: https://peartherapeuticscom/products/reset-reset-o/ Cited 2020 Jan 09.
- 25.Sleep well, feel happier, worry less [homepage on the internet] Big Health. Available from: https://www.bighealth.com/sleepio . Cited 2020 Jan 20.
- 26.The Catalia Health platform: How it works [homepage on the internet] Catalia Health. Available from: http://wwwcataliahealthcom/how-it-works/ Cited 2020 Jan 20.
- 27.Better insights. Better treatment Better care [homepage on the internet] Proteus Digital Health. Available from: https://wwwproteuscom/ Cited 2020 Jan 09.
- 28.Bonacini M, Kim Y, Pitney C, McKoin L, Tran M, Landis C. 2220. Wirelessly observed therapy with a digital medicines program to optimize adherence and target interventions for oral hepatitis C treatment. Open Forum Infect Dis. 2018;5(Suppl 1):S656. doi: 10.2196/15532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abilify mycite® (aripiprazole tablets with sensor): A component of the Abilify mycite® system to record drug ingestion [homepage on the interent] Abilify Mycite. 2017. Available from: https://www.abilifymycite.com/ Cited 2020 Jan 09.
- 30.gameChange: Improving lives through VR therapy [homepage on the internet] gameChangeVR. Available from: https://gamechangevrcom/ Cited 2020 Jan 09.
- 31.Examples of software functions for which the FDA will exercise enforcement discretion [monograph on the internet] USFDA. Available from: https://wwwfdagov/medical-devices/device-software-functions-including-mobile-medical-applications/examples-software-functions-which-fda-will-exercise-enforcement-discretion . Updated 2019 Sept 26; Cited 2020 Jan 09.
- 32.Evidence standards framework for digital health technologies. [monograph on the internet] NICE. Available from: https://wwwniceorguk/Media/Default/About/what-we-do/our-programmes/evidence-standards-framework/digital-evidence-standards-frameworkpdf . Cited 2020 Jan 09.
- 33.[monograph on the internet] AMCP- Academy of Managed Care Pharmacy. Digital therapeutics: What are they and where do they fit in pharmacy and medical benefits. Available from: https://wwwamcporg/sites/default/files/2019-10/PF-SEPT2019_execsummary%20%28oct15%29pdf . Cited 2020 Jan 09.
- 34.The growing value of digital health [monograph on the internet] IQVIA Institute Reports. Available from: https://wwwiqviacom/insights/the-iqvia-institute/reports/the-growing-value-of-digital-health . Updated 2017 Nov 07; Cited 2020 Jan 09.
- 35.KYT Adhere. Digital Therapeutic Platform Optimizing Patient Engagement And Treatment Adherence. [Last accessed on 2020 Apr 06]. Available from: https://kytai/
- 36.Somvanshi KK. Cipla may raise bar with digital therapeutics push [monograph on the internet] The Economic Times. Available from: https://economictimesindiatimescom/industry/healthcare/biotech/pharmaceuticals/cipla-may-raise-bar-with-digital-therapeutics-push/articleshow/71752197cmsfrom=mdr . Updated Oct 25; Cited 2020 Jan 09.
- 37.Bier M. Digital therapeutics: Treating patients outside hospital walls [monograph on the internet] Available from: https://wwwclaconnectcom/resources/articles/2019/digital-therapeutics-treating-patients-outside-hospital-walls . Updated 2019 Nov 12; Cited 2020 Mar 13.
- 38.Taraman S. Digital therapeutics can solve the greatest unmet need in behavioral healthcare: Early intervention [monograph on the internet] MedCity News. Available from: https://medcitynewscom/2020/01/digital-therapeutics-can-solve-the-greatest-unmet-need-in-behavioral-healthcare-early-intervention/rf=1 . Updated 2020 Jan 22; Cited 2020 Mar 13.
- 39.Digital therapeutics. Improving patient outcomes through convergence [monograph on the internet] Available from: https://www2deloittecom/us/en/pages/life-sciences-and-health-care/articles/digital-therapeuticshtml Deloittecom . Cited 2020 Mar 13.
- 40.Anderes M. Digital therapeutics: The future of health care [monograph on the internet] Network Health Connections. Available from: https://wwwnetworkhealthconnectionscom/tech-and-health/digital-therapeutics-the-future-of-health-care/ Cited 2020 Mar 13.
- 41.Berman MA, Guthrie NL, Edwards KL, Appelbaum KJ, Njike VY, Eisenberg DM, et al. Change in glycemic control with use of a digital therapeutic in adults with type 2 diabetes: Cohort study. JMIR Diabetes. 2018;3:e4. doi: 10.2196/diabetes.9591. [DOI] [PMC free article] [PubMed] [Google Scholar]


