Abstract
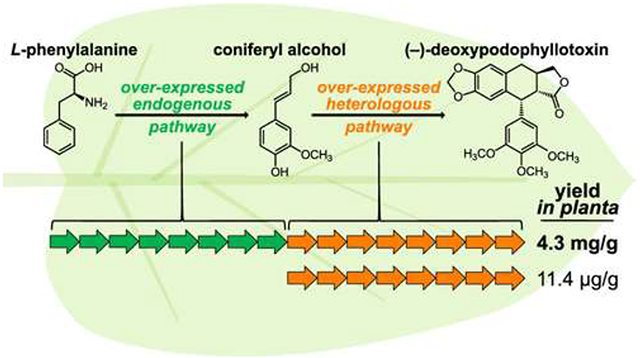
Etoposide is a plant-derived drug used clinically to treat several forms of cancer. Recent shortages of etoposide demonstrate the need for a more dependable production method to replace the semisynthetic method currently in place, which relies on extraction of a precursor natural product from Himalayan mayapple. Here we report milligram-scale production of (−)-deoxypodophyllotoxin, a late-stage biosynthetic precursor to the etoposide aglycone, using an engineered biosynthetic pathway in tobacco. Our strategy relies on engineering the supply of coniferyl alcohol, an endogenous tobacco metabolite and monolignol precursor to the etoposide aglycone. We show that transient expression of 16 genes, encoding both coniferyl alcohol and main etoposide aglycone pathway enzymes from mayapple, in tobacco leaves results in the accumulation of up to 4.3 mg/g dry plant weight (−)-deoxypodophyllotoxin, and enables isolation of high-purity (−)-deoxypodophyllotoxin after chromatography at levels up to 0.71 mg/g dry plant weight. Our work reveals that long (>10 step) pathways can be efficiently transferred from difficult-to-cultivate medicinal plants to a tobacco plant production chassis, and demonstrates mg-scale total biosynthesis for access to valuable precursors of the chemotherapeutic etoposide.
Graphical Abstract
The topoisomerase II inhibitor etoposide is used in chemotherapy regimens for the treatment of lung cancer, testicular cancer, lymphomas, and other malignancies.1 Currently, etoposide is produced semisynthetically from (−)-podophyllotoxin extracted from the plant Sinopodophyllum hexandrum.2,3 However, despite being on the World Health Organization’s list of essential medicines,4 there have been several etoposide shortages in the past decade5 and S. hexandrum is considered endangered.6 One alternative approach to the current production method is to isolate (−)-podophyllotoxin from plant tissue culture systems; such methods have been attempted with some success,7 but these techniques are technically challenging and not easily scalable.8 Recently, we reported the elucidation of the complete biosynthetic pathway to the etoposide aglycone (EA),9 a more direct precursor to etoposide than (−)-podophyllotoxin (Scheme 1, Figure S1), thereby opening up potential for a metabolic engineering approach to etoposide production. This approach could decrease the dependence on environmental factors10 and would allow for more facile access to analogues of the aglycone scaffold in a manner complementary to current synthetic and chemoenzymatic methods.11 Additionally, this approach is potentially more efficient and less labor intensive than other methods given the functional equivalence of total biosynthesis to a one-pot chemical synthesis.
Scheme 1. Engineered Biosynthetic Pathway for Production of EA and Late-Stage Pathway Intermediatesa.
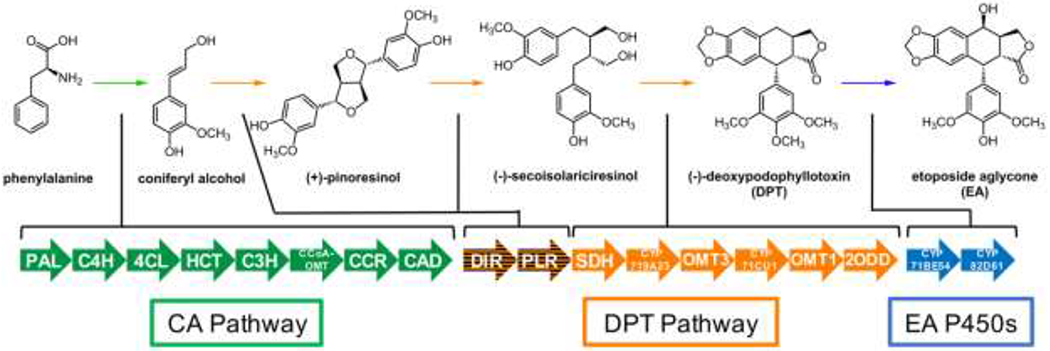
aArrows represent previously characterized biosynthetic genes. The coniferyl alcohol (CA) pathway is shared by all vascular plants, including tobacco, while the (−)-deoxypodophyllotoxin (DPT) and etoposide aglycone (EA) genes are less widespread (see Supporting Information). The latter five DPT enzymes and EA cytochromes P450 are thought to be more specific to EA production and are not found in tobacco.
Only a few complete biosynthetic pathways for plant natural products have been engineered in heterologous hosts, typically yeast, with important examples including artemisinic acid,12 the benzylisoquinoline13 and monoterpene indole alkaloids,14 and cannabinoids.15 Many of these efforts show promise for alleviating supply problems associated with the native plants. While yeast is appealing because of its scalability, engineering plant biosynthetic pathways in this organism remains challenging.16 It is estimated that engineering the four-step artemisinic acid pathway into yeast required roughly 150 person-years to complete, from discovery through optimization.17 Recently, the wild relative of tobacco Nicotiana benthamiana (referred to here as tobacco) has emerged as a platform for plant biosynthetic pathway discovery and engineering due to its ability to transiently express enzymes quickly via infiltration of Agrobacterium tumefaciens (Agro.) strains harboring enzyme-encoding genes as part of a rapid design−build−test cycle.9,18 Plant platforms may be advantageous over microbial platforms because plant enzymes, particularly cytochromes P450 (CYP), are often difficult to express in microbial hosts.16 Additionally, different plant species typically share the same subcellular compartments, cofactors, and metabolic precursors, many of which are absent in microbes, simplifying pathway transfer.18 In one recent example,19 Reed et al. demonstrated β-amyrin production in tobacco at the level of 3.3 mg/g dry leaf weight (DW) with a two-enzyme system, sufficient for isolation of near gram-scale quantities of the product from ∼460 plants. When coupled with expression of individual cytochromes P450, a variety of derivatives were produced at similar yields and tested for antiproliferative and anti-inflammatory activity in human cell lines, an exciting proof of concept that tobacco can be used for production of drug candidates difficult to access using traditional synthetic methods at the scale required for evaluation of their medicinal potential.
Precursor supply engineering has classically enabled high-level production of metabolites in microorganisms but has been widely unexplored in plants, the most notable exception being in terpene biosynthesis.19,20 Previously we reported production of (−)-deoxypodophyllotoxin (DPT), a late-stage intermediate in the EA pathway, in tobacco at a yield of 11.4 ± 3.8 μg/g DW from co-expression of the eight DPT pathway genes by Agro.-infiltration.9 Although products at these levels can be readily distinguished by mass spectrometry, a large increase would be required for facile isolation of pure DPT and other late-stage intermediates. Here, we report that by co-expressing eight precursor supply genes found in all vascular plants with eight other biosynthetic genes required for DPT production (Scheme 1), yields up to 4.3 mg/g DW can be achieved.
We began our efforts to increase the yield of the EA pathway in tobacco by determining the key pathway bottleneck. Previously, our work had shown that direct exogenous addition of (+)-pinoresinol to the plant chassis resulted in an 8-fold improvement in DPT yield.9 Therefore, we hypothesized that the laccase-catalyzed, dirigent protein (DIR)-directed coniferyl alcohol (CA) dimerization that generates (+)-pinoresinol was the yield-limiting step. This dimerization is thought to occur in the plant cell apoplast,21 necessitating the transport of metabolites out of and back into the cytoplasm. To test if CA coupling limits pathway yield, we infiltrated CA into tobacco leaves 4 days after infiltration of a mixture of Agro. strains harboring the DPT pathway genes, harvesting leaves 1 day later. This resulted in a 13-fold increase in DPT production, as quantified by liquid chromatography-mass spectrometry (LC-MS), compared to a parallel experiment where no CA was infiltrated (Figure 1). Notably, when the Agro. strain harboring the DIR gene was replaced with a strain harboring a GFP-encoding gene, the yield increase dropped to 2-fold. This suggests that while tobacco is able to produce (+)-pinoresinol endogenously, over-expression of the stereochemistry-mediating dirigent protein is important for limiting flux into off-target pathways.
Figure 1.
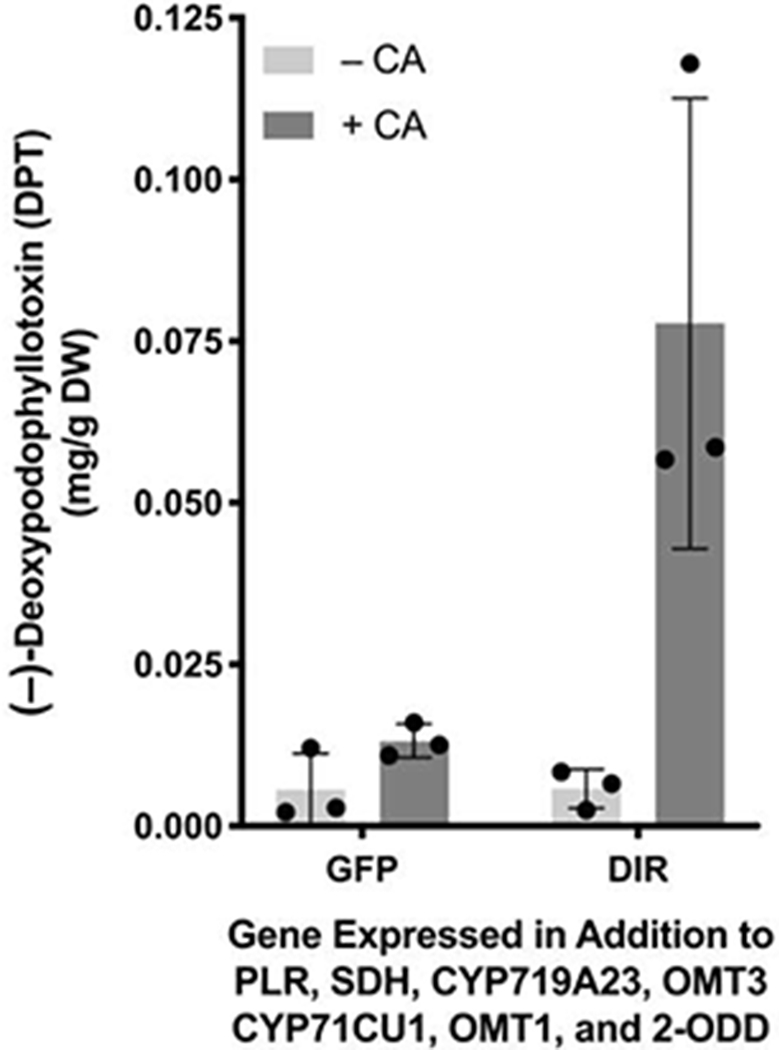
Impact of CA infiltration and DIR expression on DPT production in planta according to LC-MS of extracts from Agro.- infiltrated tobacco. Gray bar height indicates mean (error bars show ± SD); individual biological replicates are represented as dots. Colors indicate absence or presence of CA infiltration (−CA or +CA, respectively).
Because yield increases were only observed when CA was infiltrated, we hypothesized that, in addition to CA coupling, CA supply in the tobacco leaf might also limit pathway flux. A biological means for increasing the CA pool in leaves is attractive because it avoids the need for infiltration of synthetic CA, a costly and likely inefficient step in a large-scale production process and, furthermore, a strategy that is not broadly applicable to other plant natural products for which precursors may not be able to access the cytoplasm upon infiltration. Thus, candidate CA biosynthetic genes were selected from our previous S. hexandrum RNA-Seq data set9 based on homology to characterized Arabidopsis thaliana genes and co-expression with DPT pathway genes (Tables S1 and S2). The CA biosynthetic pathway has been extensively studied and characterized, and the most widely accepted pathway includes nine steps catalyzed by eight different enzymes: PAL, C4H, 4CL, HCT, C3H, CCoA-OMT, CCR, and CAD (Scheme S1).22 The top co-expression candidate for each was selected, and these genes were incorporated into plasmids in separate strains of Agro. When these strains were co-infiltrated alongside the others harboring the DPT pathway, a 680-fold increase in DPT yield was observed compared to the GFP control (Figure S2). The yield for this initial attempt was approximately 3.5 ± 1.2 mg/g DW, compared to 5.2 ± 0.6 μg/g DW in the non-engineered control.
To investigate the impacts of individual CA biosynthetic genes on the increased yield, we examined yields after sequential addition of each gene to the pathway. From this analysis, it was determined that PAL plays an important role, with PAL expression being responsible for a 5- to 6-fold increase in yield (Figures 2 and S3). Additionally, CCoA-OMT, CCR, and CAD were found to collectively be responsible for a 95- to 160-fold yield increase, though the individual contributions of each enzyme were not consistent between different experimental batches (Figures 2 and S3–S5). While the other four enzymes do not appear to increase flux significantly based on sequential addition, replacing these four with GFP diminished yields significantly (Figure S6). In certain experiments, it appears that a subset of these eight genes is sufficient to generate the overall yield increase; however, the most consistent increase is observed when all eight are included (Figures S7 and S8). Interestingly, PAL does not appear to have much impact on yield when expressed alone with the DPT pathway (Figure S8), suggesting the intermediary steps that connect the resulting cinnamic acid to CA are necessary for the increased production.
Figure 2.
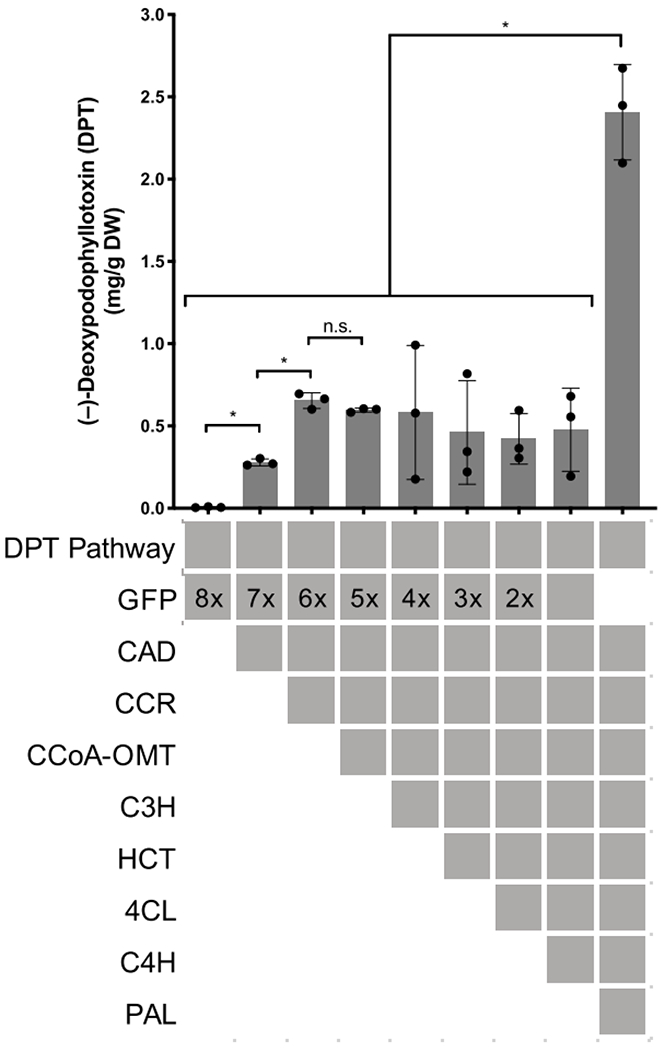
Impact of individual CA pathway enzyme expression on DPT yield in planta according to LC-MS of extracts from Agro.- infiltrated tobacco. Gray bar height indicates mean (error bars show ± SD); individual biological replicates are represented as dots. Asterisks indicate a significant difference between sets, p < 0.05. Gray-shaded boxes indicate enzymes expressed via Agro.-infiltration, with numbers showing the amount of the GFP-expressing strain used relative to the other strains.
To further improve DPT yields in the tobacco system, total Agro. mixture OD600 and infiltration-to-harvest time were both optimized. It was determined that a total Agro. mixture OD600 of 3.0 and an infiltration-to-harvest time of 7−9 days were optimal, yielding up to 4.3 mg/g DW (Figures S9 and S10). We next used the optimized conditions and scaled production up to 15−20 plants. High-purity DPT was successfully isolated at yields up to 0.71 mg/g DW using silica gel flash chromatography and preparative HPLC (Table S3A).
Having achieved yield boosts enabling isolation and NMR characterization of DPT, we next investigated the applicability of CA pathway over-expression in tobacco for EA and EA analogue biosynthesis. In our previous work, EA was only detected in planta when the full biosynthetic pathway was expressed along with exogenous addition of the precursor (−)-matairesinol.9 Here we observe that, with CA pathway expression, EA can now be detected without precursor infiltration (Figure 3A).
Figure 3.
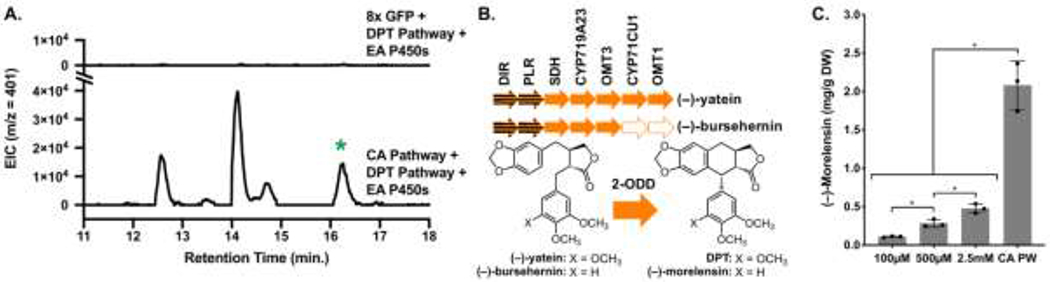
EA and (−)-morelensin production. (A) LC-MS extracted ion chromatograms (EIC) at 401 m/z, [EA + H]+. Green asterisk indicates EA; other peaks are likely derived from in-source fragmentation of EA glycosides. Both traces are from experiments where noted genes were expressed in tobacco via Agro.-infiltration. (B) Engineered pathway for (−)-morelensin production. Dotted arrows depict omitted enzymes. (C) (−)-Morelensin production with exogenous addition of varying CA levels or co-infiltration with CA pathway (PW)-expressing Agro. strains. Gray bar height indicates mean (error bars show ± SD); individual biological replicates are represented as dots. Asterisks indicate a significant difference between sets, p < 0.05.
Given the high levels of EA produced, we next tested whether EA analogues not previously reported to accumulate in mayapple could be made using our engineered pathway. One potential bottleneck is the stereospecific C−C bond formation step catalyzed by 2-ODD. We had previously tested 2-ODD substrate specificity with metabolites other than (−)-yatein.9 These tests showed that the enzyme can also utilize (−)-bursehernin, indicating flexibility in processing alternative substrates. Here, by co-expressing the entire CA pathway and a subset of the DPT pathway enzymes lacking CYP71CU1 and OMT1 (Figure 3B), 2.2 mg/g DW yield (1.4 mg/g DW isolated yield) of the alternative 2-ODD product, (−)-morelensin, was obtained in planta without exogenous precursor addition (Figure 3C, Table S3B). Importantly, this yield was significantly higher than what could be obtained by exogenous addition of a saturated CA solution (2.5 mM), supporting the value of engineering the production of CA in planta. We further report here that the EA P450 enzymes, CYP82D61 and CYP71BE54, catalyze hydroxylation and demethylation, respectively, of (−)-morelensin, producing (−)-4′-demethyl-5′-desmethoxy-epipodophyllotoxin (5′-desmethoxy-EA, not known to accumulate in mayapple) in planta. Co-expression of the CA pathway resulted in an order of magnitude yield increase of 5′-desmethoxy-EA compared to exogenous CA addition (Figures S11 and S12).
In this work, we have demonstrated the applicability of precursor supply engineering for high-yield plant natural product biosynthesis in tobacco. By co-expressing eight genes that enable CA production from intracellular phenylalanine alongside eight genes that generate DPT from CA—to our knowledge, the longest natural product pathway reconstituted in a plant heterologous host to date—a 2 orders of magnitude DPT yield increase was observed compared to when the CA pathway genes were excluded. Additionally, we demonstrated a significant increase in the yield of EA from the system and showed how this method allows for access to analogues of etoposide pathway intermediates.
We anticipate this work will be important for development of tobacco as a platform for medicinal plant natural product production. By our count, 10% of WHO essential medicines are plant natural products or derivatives,4 and a majority of these medicines still rely on the native plant for production (e.g., etoposide, Taxol23). Our work shows that by rewiring central metabolism in situ to meet the demands of a given biosynthetic pathway, tobacco transient expression can be used to obtain isolatable milligram-scale quantities of natural plant small molecules and biosynthetically accessible analogues.
Supplementary Material
Acknowledgments
We would like to thank all members of the Sattely lab for helpful discussions and Amita Gupta for initial efforts in etoposide analogue biosynthesis. We acknowledge financial support from an AAAS Marion Milligan Mason Award for Women in the Chemical Sciences and NIH R01GM121527 (E.S.S.). Summer support for B.J.S. was provided by a Stanford Chemistry Department Undergraduate Summer Research Fellowship and a Stanford Undergraduate Advising and Research Major Grant. S.K. acknowledges support from the National Science Foundation Graduate Research Fellowship Program (DGE-1656518).
Footnotes
Supporting Information: The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.9b10717. Supplementary discussion, methods, Tables S1–S5, Scheme S1, Figures S1–S13, spectra, and references (PDF).
Notes: The authors declare no competing financial interest.
References
- (1).Hande KR Etoposide: Four Decades of Development of a Topoisomerase II Inhibitor. Eur. J. Cancer 1998, 34 (10), 1514–1521. [DOI] [PubMed] [Google Scholar]
- (2).Stähelin HF; von Wartburg A The Chemical and Biological Route from Podophyllotoxin Glucoside to Etoposide: Ninth Cain Memorial Lecture. Cancer Res. 1991, 51, 5–15. [PubMed] [Google Scholar]
- (3).In previous work,9 we referenced the native plant genus as Podophyllum; we have updated the naming to Sinopodophyllum to reflect the current preferred nomenclature.
- (4).WHO Model List of Essential Medicines, 2017. World Health Organization Web site https://apps.who.int/iris/bitstream/handle/10665/273826/EML-20-eng.pdf?ua=1. (accessed August 8, 2018).
- (5).Current and Resolved Drug Shortages and Discontinuations Reported to FDA. U. S. Food and Drug Administration Web site https://www.accessdata.fda.gov/scripts/drugshortages/default.cfm. (accessed August 8, 2018).
- (6).Checklist of CITES Species. Convention on International Trade in Endangered Species of Wild Fauna and Flora Web site http://checklist.cites.org/#/en (accessed August 8, 2018).
- (7).(a) Jiang W; Chen L; Pan Q; Qiu Y; Shen Y; Fu C An efficient regeneration system via direct and indirect organogenesis for the medicinal plant Dysosma versipellis (Hance) M. Cheng and its potential as a podophyllotoxin source. Acta Physiol. Plant 2012, 34 (2), 631–639 [Google Scholar]; (b) Samadi A; Jafari M; Nejhad NM; Hossenian F Podophyllotoxin and 6-methoxy podophyllotoxin Production in Hairy Root Cultures of Linum mucronatum ssp. mucronatum. Pharmacogn. Mag 2014, 10 (38), 154–160 [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Rajesh M; Sivanandhan G; Jeyaraj M; Chackravarthy R; Manickavasagam M; Selvaraj N; Ganapathi A An efficient in vitro system for somatic embryogenesis and podophyllotoxin production in Podophyllum hexandrum Royle. Protoplasma 2014, 251, 1231–1243 [DOI] [PubMed] [Google Scholar]; (d) Rajesh M; Sivanandhan G; Subramanyam K; Kapildev G; Jaganath B; Kasthurirengan S; Manickavasagam M; Ganapathi A Establishment of somatic embrogenesis and podophyllotoxin production in liquid shake cultures of Podophyllum hexandrum Royle. Ind. Crops Prod 2014, 60, 66–74. [Google Scholar]
- (8).(a) Srivastava S; Srivastava AK Hairy Root Culture for Mass-Production of High-Value Secondary Metabolites. Crit. Rev. Biotechnol 2007, 27 (1), 29–43 [DOI] [PubMed] [Google Scholar]; (b) Ochoa-Villarreal M; Howat S; Hong S; Jang MO; Jin Y-W; Lee E-K; Loake GJ Plant cell culture strategies for the production of natural products. BMB Rep. 2016, 49 (3), 149–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Lau W; Sattely ES Six enzymes from mayapple that complete the biosynthetic pathway to the etoposide aglycone. Science 2015, 349 (6253), 1224–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Alam MA; Naik PK Impact of Soil Nutrients and Environmental Factors on Podophyllotoxin Content among 28 Podophyllum Hexandrum Populations of Northwestern Himalayan Region Using Linear and Nonlinear Approaches. Commun. Soil Sci. Plant Anal 2009, 40 (15-16), 2485–2504. [Google Scholar]
- (11).Select recent examples of traditional and chemoenzymatic syntheses of (−)-podophyllotoxin and various analogues:; (a) Ting CP; Maimone TJ C-H Bond Arylation in the Synthesis of Aryltetralin Lignans: A Short Total Synthesis of Podophyllotoxin. Angew. Chem., Int. Ed 2014, 53 (12), 3115–3119 [DOI] [PubMed] [Google Scholar]; (b) Hajra S; Garai S; Hazra S Catalytic Enantioselective Synthesis of (–)-Podophyllotoxin. Org. Lett 2017, 19 (24), 6530–6533 [DOI] [PubMed] [Google Scholar]; (c) Xiao J; Cong X-W; Yang G-Z; Wang Y-W; Peng Y Divergent Asymmetric Syntheses of Podophyllotoxin and Related Family Members via Stereoselective Reductive Ni-Catalysis. Org. Lett 2018, 20 (6), 1651–1654 [DOI] [PubMed] [Google Scholar]; (d) Lazzarotto M; Hammerer L; Hetmann M; Borg A; Schmermund L; Steiner L; Hartmann P; Belaj F; Kroutil W; Gruber K; Fuchs M Chemoenzymatic Total Synthesis of Deoxy-, epi-, and Podophyllotoxin and a Biocatalytic Kinetic Resolution of Dibenzylbutyrolactones. Angew. Chem., Int. Ed 2019, 58 (24), 8226–8230 [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Li J; Zhang X; Renata H Asymmetric Chemoenzymatic Synthesis of (–)-Podophyllotoxin and Related Aryltetralin Lignans. Angew. Chem., Int. Ed 2019, 58 (34), 11657–11660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Ro D-K; Paradise EM; Ouellet M; Fisher KJ; Newman KL; Ndungu JM; Ho KA; Eachus RA; Ham TS; Kirby J; Chang MCY; Withers ST; Shiba Y; Sarpong R; Keasling JD Production of the antimalarial drug precursor artemisinic acid in engineered yeast. Nature 2006, 440, 940–943. [DOI] [PubMed] [Google Scholar]
- (13).Galanie S; Thodey K; Trenchard IJ; Filsinger Interrante M; Smolke CD Complete biosynthesis of opioids in yeast. Science 2015, 349 (6252), 1095–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Brown S; Clastre M; Courdavault V; O’Connor SE De novo production of the plant-derived alkaloid strictosidine in yeast. Proc. Natl. Acad. Sci. U. S. A 2015, 112 (11), 3205–3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Luo X; Reiter MA; d’Espaux L; Wong J; Denby CM; Lechner A; Zhang Y; Grzybowski AT; Harth S; Lin W; Lee H; Yu C; Shin J; Deng K; Benites VT; Wang G; Baidoo EEK; Chen Y; Dev I; Petzold JC; Keasling JD Complete biosynthesis of cannabinoids and their unnatural analogues in yeast. Nature 2019, 567, 123–126. [DOI] [PubMed] [Google Scholar]
- (16).Trenchard IJ; Smolke CD Engineering strategies for the fermentative production of plant alkaloids in yeast. Metab. Eng 2015, 30, 96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Kwok R Five hard truths for synthetic biology. Nature 2010, 463, 288–290. [DOI] [PubMed] [Google Scholar]
- (18).(a) Klein AP; Sattely ES Biosynthesis of cabbage phytoalexins from indole glucosinolate. Proc. Natl. Acad. Sci. U. S. A 2017, 114 (8), 1910–1915 [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Reed J; Osbourn A Engineering terpenoid production through transient expression in Nicotiana benthamiana. Plant Cell Rep. 2018, 37, 1431–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Reed J; Stephenson MJ; Miettinen K; Brouwer B; Leveau A; Brett P; Goss RJM; Goossens A; O’Connell MA; Osbourn A A translational synthetic biology platform for rapid access to gram-scale quantities of novel drug-like molecules. Metab. Eng 2017, 42, 185–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Lee A-R; Kwon M; Kang M-K; Kim J; Kim S-U; Ro D-K Increased sesqui- and triterpene production by co-expression of HMG-CoA reductase and biotin carboxyl carrier protein in tobacco (Nicotiana benthamiana). Metab. Eng 2019, 52, 20–28. [DOI] [PubMed] [Google Scholar]
- (21).(a) Kwon M; Burlat V; Davin LB; Lewis NG Localization of dirigent protein involved in lignan biosynthesis: implications for lignification at the tissue and subcellular level. In Plant Polyphenols 2; Gross GG, Hemingway RW, Yoshida T, Branham SJ, Eds.; Basic Life Sciences, Vol. 66; Springer: Boston, MA, 1999; pp 393–411 [Google Scholar]; (b) Pickel B; Constantin M-A; Pfannstiel J; Conrad J; Beifuss U; Schaller A An Enantiocomplementary Dirigent Protein for the Enantioselective Laccase-Catalyzed Oxidative Coupling of Phenols. Angew. Chem. Int. Ed 2010, 49 (1), 202–204 [DOI] [PubMed] [Google Scholar]; (c) Gasper R; Effenberger I; Kolesinski P; Terlecka B; Hofmann E; Schaller A Dirigent Protein Mode of Action Revealed by the Crystal Structure of AtDIR6. Plant Physiol. 2016, 172 (4), 2165–2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).(a) Barros J; Serk H; Granlund I; Pesquet E The cell biology of lignification in higher plants. Ann. Bot 2015, 115 (7), 1053–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Weng J-K; Chapple C The origin and evolution of lignin biosynthesis. New Phytol. 2010, 187 (2), 273–285. [DOI] [PubMed] [Google Scholar]
- (23).Liu WC; Gong T; Zhu P Advances in exploring alternative Taxol sources. RSC Adv. 2016, 6, 48800–48809. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


